Abstract
Testes of salamanders or urodeles are paired elongated organs that are attached to the dorsal wall of the body by a mesorchium. The testes are composed of one or several lobes. Each lobe is morphologically and functionally a similar testicular unit. The lobes of the testis are joined by cords covered by a single peritoneal epithelium and subjacent connective tissue. The cords contain spermatogonia. Spermatogonia associate with Sertoli cells to form spermatocysts or cysts. The spermatogenic cells in a cyst undergo their development through spermatogenesis synchronously. The distribution of cysts displays the cephalo-caudal gradient in respect to the stage of spermatogenesis. The formation of cysts at cephalic end of the testis causes their migration along the lobules to the caudal end. Consequently, the disposition in cephalo-caudal regions of spermatogenesis can be observed in longitudinal sections of the testis. The germ cells are spermatogonia, diploid cells with mitotic activity; primary and second spermatocytes characterized by meiotic divisions that develop haploid spermatids; during spermiogenesis the spermatids differentiate to spermatozoa. During spermiation the cysts open and spermatozoa leave the testicular lobules. After spermiation occurs the development of Leydig cells into glandular tissue. This glandular tissue regressed at the end of the reproductive cycle.
Keywords: salamanders, lobular testis, cyst, cephalo-caudal spermatogenesis, spermatophores
Introduction
The basic progression of sperm development in urodeles proceeds similarly to the rest of vertebrates. Spermatogenesis occurs in the testes and is maintained by a stem cell population, the spermatogonia, which permit constant supplies of abundant male gametes. Spermatogonial stem cells divide through mitosis to produce generations of spermatogonia which enter the spermatogenic cycle. Spermatogonia develop into spermatocytes that undergo meiosis through primary and secondary spermatocytes to produce haploid cells, the spermatids. Spermatids differentiate into mature sperm through morphological and functional changes during spermiogenesis, which results in the mature spermatozoa.
Testicular Structure
The testes are paired and elongated organs, attached to the dorsal side of the body cavity by the mesorchium. The testes are aligned in parallel position to the long axis of the body, and consequently, they are parallel to the mesonephros, Wolffian or mesonephric ducts, and rudimentary Müllerian or paramesonephric ducts.1-10
The testes of urodeles are composed of one or several lobes. Each lobe is morphologically and functionally a similar testicular unit. In some species, sexually immature males have a testis containing only one lobe, whereas mature males have a testis containing multiple lobes.11 The number of testicular lobes depends on the age of the animal.3,12 Testis with multiple lobes were described in various species: Desmognathus fuscus, Diemyctylus viridescens, Diemyctylus torosus, and Salamandra atra11; Triturus viridescens1; Taricha granulosa2; Trituroides hongkongenesis13; Triturus cristatus14; Diemyctylus viridescens, Cynops pyrrhogaster4; Triturus marmoratus15; Salamandra salamandra3,16,12,17, Bolitoglossa occidentalis, B. rostrata, Dendrotriton bromeliacia, Pseudoeurycea goebeli18, Pleurodeles waltl.19 Other species have simple testes, formed by one lobe, regardless of their age, as Eurycea quadridigitata,20 Salamandrina terdigitata5 and Lissotriton italicus.21
The lobes of the testis are joined by narrow interlobar cords covered by a single peritoneal epithelium and subjacent vascularized connective tissue. These cords contain spermatogonia among the connective tissue.4,11,12,22 The lobes and interlobar cords are illustrated in Taricha granulosa (Fig. 1A).
Figure 1.
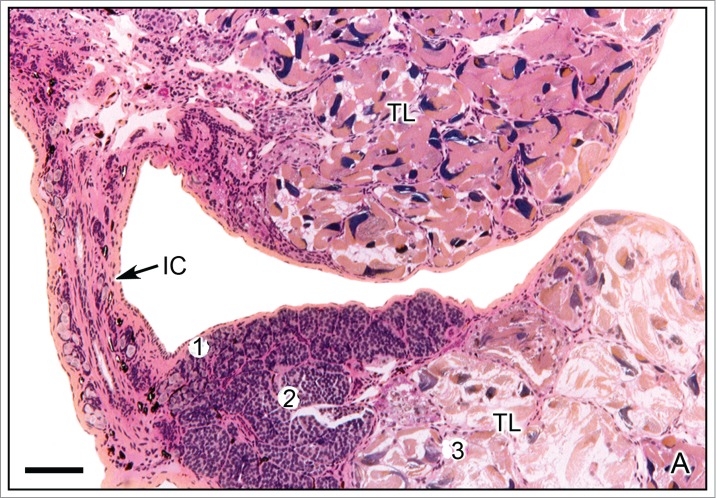
A. Testicular lobes of Taricha granulosa. The testicular lobes (TL) are joined by an interlobar cord (IC). The cephalic region of the lobe contains spermatogonia (1) and spermatocytes (2) and the caudal region contain spermatozoa (3). Hematoxylin-eosin. Bar = 200 μm. Courtesy of Dr. Harry J. Grier.
Several authors have described the structure of testicular lobes in urodeles.4,7-10,14,23-26,27-30, The testicular lobes are surrounded by fibrous connective tissue which forms the tunica albuginea. The internal structure of the testicular lobes consists of abundant longitudinal lobules, separated by trabeculae of thin and vascularized connective tissue, that is a continuation of the tunica albuginea. Among the trabeculae of connective tissue and near the capillaries there are Leydig cells. Each lobule contains several cysts with spermatogenic cells in different stages of development. The spermatogenic cells in a cyst undergo their development through spermatogenesis synchronously.
The distribution of cysts displays the cephalo-caudal gradient in respect to the stage of spermatogenesis (Figs. 1A, 2A,B). This type of spermatogenesis process has been termed the spermatogenesis wave.31 The cephalic edge of the testis contains spermatogonia, either as individual cells or in small groups as hollow chambers lined by the spermatogonia. Progressively, there are lobules within which primary and secondary spermatocytes, spermatids, and spermatozoa are formed.19,20,29,30,32,33 The continuous formation of cysts at cephalic end of the testis causes their migration along the lobules to the caudal end. Consequently, the disposition in cephalo-caudal regions of spermatogenesis can be observed in longitudinal sections of the testis (Fig. 2A), meanwhile, in transversal sections, all cysts are approximately at the same stage, resulting in a spatial and temporal segregation of differentiating germ cells.
Figure 2.
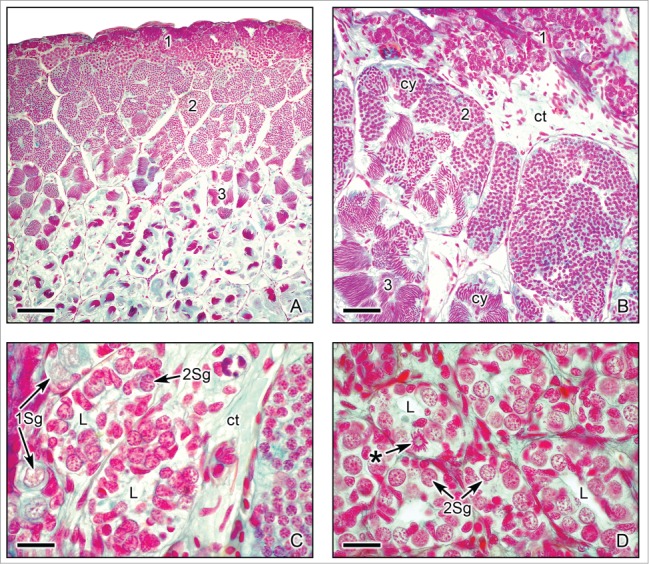
Cephalo-caudal disposition of the spermatogenic stages in longitudinal sections of the testes of Ambystoma dumerilii. (A,B). Three testicular regions of spermatogenesis advance progressively along the cephalo-caudal axis. (1) Starting at the cephalic region, the testis contains numerous lobules with early stages of spermatogenesis, which include spermatogonia, primary and secondary spermatocytes. (2) Subsequently, middle stages of spermatogenesis with spermatids. (3) Followed by late stages of spermatogenesis with spermatozoa. The testicular lobules enclose several cysts (cy) with germinal cells in synchronous stages of spermatogenesis. Alcian blue. Bar = 200 μm. Bar = 50 μm. (C,D). Sections of the peripheral cephalic region of the testis with cysts of proliferating spermatogonia. Primary spermatogonia (1Sg) and secondary spermatogonia (Sg2) are seen. The secondary spermatogonia form clusters surrounding a central lumen (L), structures that originate the testicular lobules. The clusters of secondary spermatogonia are delimited by loose connective tissue (ct). One secondary spermatogonium shows mitotic activity (*). Alcian blue. Bar = 20 μm.
During the reproductive cycle the type of spermatogenic cells and the number of cysts differ.2,4,12,34,35 For instance, the endemic urodeles of Mexico, Ambystoma dumerilii from Patzcuaro Lake, and A. mexicanum from Xochimilco Lake, have a spring breeding season. In this annual cycle sperm mature in summer and autumn and occupy the most of testicular lobe and only a small area, at the cephalic end of the lobe, contains spermatogonia and some cysts with spermatocytes and spermatids. Spermatozoa are stored during several months in the testis and in the ducts system. When females spawn in the next spring, then, the males release spermatozoa and the fertilization occurs. In contrast to this cycle, Cryptobranchus alleganiensis and Necturus maculosus, spermatozoa are produced only shortly before spawning by a very rapid phase of spermiogenesis.3,4 We illustrate the testis and spermatogenesis in urodeles here with the species Ambystoma dumerilii and A. mexicanum.
A cyst is formed when a primary spermatogonium becomes surrounded by a Sertoli cell (Figs. 2C,D). Thus, the Sertoli cell forms the wall of the cyst.3,12,15,36,37,38 The synchronous development of spermatogenic cells inside a cyst, during spermatogenesis is a result of persistent intercellular cytoplasmic bridges between germ cells, and these bridges are due to incomplete cytokinesis during the mitotic divisions of germ cells. The Sertoli cells have complex and essential functional relationships with the spermatogenic cells. Sertoli cells maintain a permeability barrier to spermatogenic cells during spermatogenesis, determine the endocrine activity that controls spermatogenesis, phagocytose degenerating spermatogenic cells and residual bodies during the spermiogenesis, and form specific antigens.25,31,37,39,40 During spermiation, when the cysts open and spermatozoa leave the testis, Sertoli cells (Fig. 2D) remain inside the lobule (Fig. 2D) and undergo morphological changes (Figs. 3C,D) consistent with the acquisition of steroid secreting behavior. Sertoli cells then degenerate and disappear. According to the cell cycle of Sertoli cells, they are not a permanent cell type in the testis; consequently, their cyclic supply is of great importance for the development of cysts and maintaining spermatogenesis in the males.38
Figure 3.
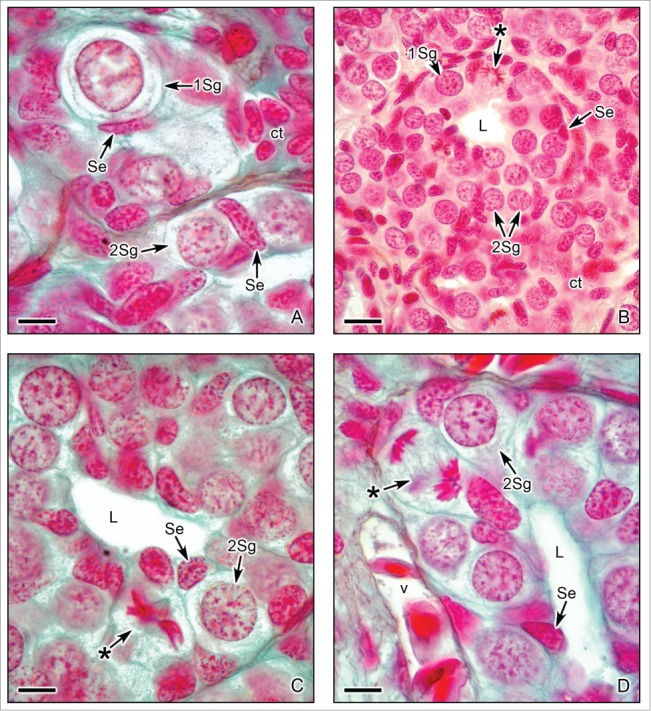
Cephalic region of the testis of Ambystoma dumerilii with primary and secondary spermatogonia. (A). A primary spermatogonium (1Sg) and several secondary spermatogonia (Sg2) surrounded by Sertoli cells (Se). Sertoli cells have elongated nuclei with granular chromatin and bordered by connective tissue (ct). Alcian blue. Bar = 10 μm. (B,C,D). The proliferation of spermatogonia is evident by chromosomes during mitotic phases, as metaphase (*) in Figs. B and C and anaphase (*) in Fig. D. The fibers of the spindle are observed in mitotic phases in Figs. C and D. The clusters of secondary spermatogonia (2Sg) have a central lumen (L). The spermatogonia are surrounded by Sertoli cells (Se). The connective tissue contains blood vessels (v). Hematoxylin-eosin. Bar = 20 μm. Alcian blue. Bar = 10 μm.
The Leydig cells are in the testicular interstitial tissue, between the lobules. These cells present cyclic morpho-physiological changes according to the stage of spermatogenesis. The Leydig cells are small and irregular in shape during spermatogenesis, but initiate proliferation, hypertrophy and maturation, developing a glandular tissue around the lobules, during and after spermiation.39,41,42-44 The cephalo-caudal disposition of spermatogenesis in the testis defines the spermiation at the caudal region; consequently, the development of Leydig cells at this region involves a complex interaction that demonstrates local regulatory control of testicular function.10 Leydig cells attain 35–55 μm in diameter7,9 and contain lipids, becoming sites of androgen synthesis (testosterone and 5α-dihydrotestosterone). During the regression period, Leydig cells suffer rapid involution.28 The morpho-physiological changes of Leydig cells are described in several species: Ambystoma tigrinum26,28; Trituroides hongkongensis13; Cynops pyrrhogaster pyrrhogaster45; A. mexicanum32,39; and Necturus maculosus31,43; Triturus marmoratus.36,44
Spermatogenesis
In most urodeles, spermatogenesis has cyclic changes under the control of the neuroendocrine system and is influenced by environmental factors, such as temperature, rainy season and photoperiod.27,28,29,46,47 Spermatogenesis is regulated by follicle-stimulated hormone (FSH) released by the adenohypophysis. Sertoli cells express FSH receptors from early spermatogenesis through the spermatid stage. FSH, through its actions on Sertoli cells, is involved in the mitotic activity of the spermatogonia, the meiotic divisions of primary and secondary spermatocytes during the formation of spermatids and spermiogenesis throughout the development of the spermatozoa.32,38 Luteinizing hormone (LH) plays a role in spermiation and testosterone secretion.28,31 Additionally, spermatogonia and spermatocytes of Triturus marmoratus marmoratus showed a positive reaction to androgen, estrogen α, and estrogen β receptors during the annual reproductive cycle, suggesting that there is an androgen-estrogen regulation of the function and development of the newt testis.48
As discussed previously, during the cyclic changes a longitudinal wave of spermatogenesis occurs along the length of the testis.11,31 Spermatozoa are formed by mitotic multiplication of spermatogonia followed by meiosis in spermatocytes and spermiogenesis in spermatids. According to these observations, the testicular cycle of urodeles advances from a period of spermatogenesis to a period of maturation, spermiation and regression.
During spermiation, spermatozoa are progressively released from the testes to the efferent ducts.13,28,39,34,49,44 The urodele testicular cycle is synthesized in several phases: proliferative phase, characterized by mitotic divisions, when proliferation of spermatogonia forms new cysts where spermatocytes advance in spermatogenesis; meiotic phase, characterized by meiotic divisions, when primary and second spermatocytes are formed and develop spermatids; spermiogenesis phase when spermatids differentiate to spermatozoa; and, spermiation when cysts of spermatozoa open and spermatozoa leave the testicular lobules. After spermiation occurs the development of Leydig cells into glandular tissue. This glandular tissue regressed at the end of the reproductive cycle.10
Morphology of germ cells during spermatogenesis
The stages of spermatogenic cell maturation, in a variety of species of urodeles, was described by several authors: in Desmognathus fusca23; in Necturus maculosus24,31; in Ambystoma tigrinum25,26,28; in Pleurodeles waltlii50; in Tritutoides hongkongensis13; in A. mexicanum27,49; in Salamandrina terdigitata5; in Triturus marmoratus44,47; in Salamandra salamandra12,16,17; in A. dumerilii29; in Batrachuperus pinchonii51; Uribe in A. mexicanum and A. dumerilii8,9; in Lissotriton italicus.21 A cinematographic study of meiosis in salamander spermatocytes in vivo was done.52 This study put in relevance the movements of bivalents homologous chromosomes during the first meiotic division.
Primary and secondary spermatogonia
The primary spermatogonia are situated in the cephalic region of the testis as individual cells surrounded by connective tissue (Fig. 3A), and in multilobular testis, also between lobes, in the interlobar cords among the connective tissue (Fig. 1A). Some spermatogonia are also observed between the connective tissue that surrounds lobules in other regions of the testis, not only in the cephalic region but in the direction of the middle part of the testis. The spermatogonia are the biggest germinal cells (40–55 μm in diameter). Primary spermatogonia are spherical cells with light granular cytoplasm and spherical or irregular nuclei containing diploid granular chromatin and one or 2 nucleoli (Figs. 3A,B). Primary spermatogonia proliferate mitotically (Figs. 3B–D), thereby renewing the pool of germ cells and giving rise to secondary spermatogonia.
Secondary spermatogonia attain 35–45 μm in diameter and the nucleus is similar to those of the primary spermatogonia (Figs. 3A–D). They form clusters during several mitosis. Clusters are interconnected by cytoplasmic bridges. Sertoli cells, progressively, surround the secondary spermatogonia (Figs. 3A–D); in consequence, secondary spermatogonia become enclosed within a cyst. During the development of a cyst, the number of both spermatogonia and Sertoli cells increases dividing mitotically several times before the initiation of meiosis. A central lumen appears in the center of the clusters of secondary spermatogonia (Figs. 3B–D). Into the cyst, secondary spermatogonia enter meiosis and transform into primary spermatocytes.
Primary and secondary spermatocytes
Primary spermatocytes are spherical cells, similar in size to secondary spermatogonia (35–45 μm in diameter). Primary spermatocytes contain duplicated chromosomes that present a gradual thickening along with prophase I of meiosis. different stages of prophase I can be easily observed within primary spermatocytes: leptonema (fine reticular chromatin with irregular distribution of deep-stained granules of varying size), zygonema (fine fibrillar pattern of duplicated homologous chromosomes in synapsis) (Fig. 4A), pachynema (duplicated chromosomes in crossing-over) (Figs. 4A,B, 5A–C), diplonema (separation of homologous duplicated chromosomes, that remain attached in some regions which form chiasmata) (Figs. 4A, 5B) and diakinesis (preparation for cell division). The cells during these meiotic stages of prophase I are named as leptotene, zygotene, pachytene and diplotene spermatocytes. The primary spermatocytes at pachynema stage are relatively abundant, whereas those in leptonema, zygonema, and especially in diplonema are rarely seen. This reflects the duration of meiotic stages, among which pachynema is the longest, leptonema and zygonema are shorter, and diplonema is the shortest stage.53 The primary spermatocytes enter the first division of meiosis (Figs. 4B, 5B,C) through metaphase I, anaphase I, and telophase I. Two secondary spermatocytes are the result of the first meiotic.
Figure 4.
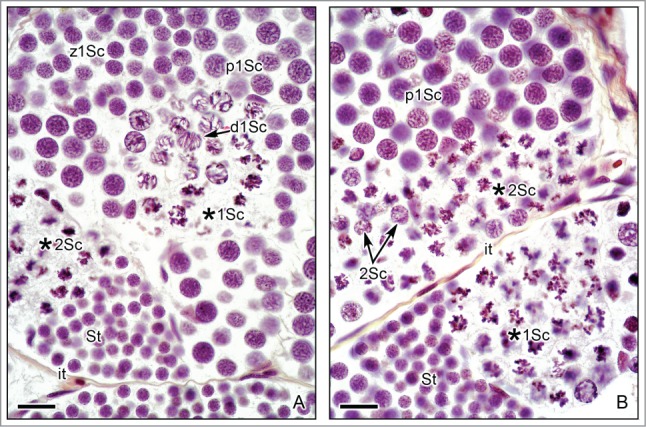
Spermatogenesis in the testis of Ambystoma dumerilii with cysts of primary and secondary spermatocytes. (A,B). Primary spermatocytes in several stages of the prophase I of meiosis are seen, as: zygotene (z1Sc) with fibrillar chromosomes; pachytene (p1Sc) also with fibrillar chromosomes and the cell diameter increses; diplotene (d1Sc), the chromosomes have characteristic chiasmata; first meiotic division (*1Sc) with dense chromosomes in the spindle. Secondary spermatocytes (*2Sc) in the second meiotic division also contain dense chromosomes in the spindle. Compare the different size diameter of the primary and secondary spermatocytes in division, being smaller the secondary spermatocytes. Cysts with spermatids (St), near the secondary spermatocytes. Elongated nuclei of Sertoli cells (Se) surround the different cysts. Hematoxylin-eosin. Bar = 20 μm.
Figure 5.
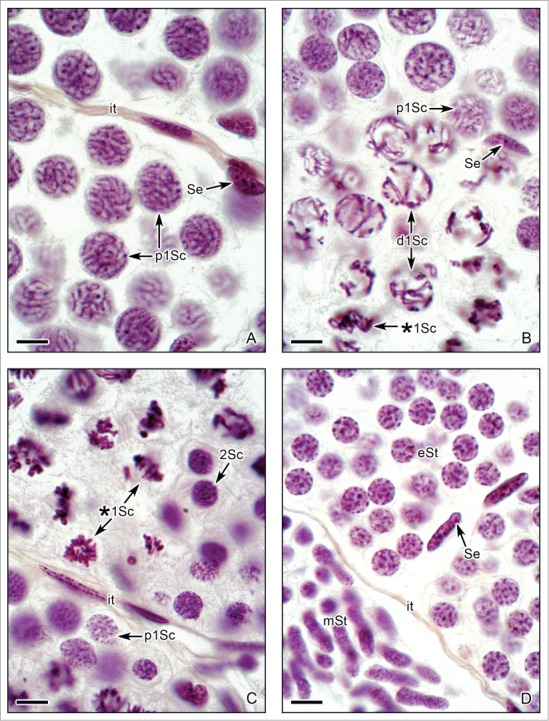
Spermatogenesis in Ambystoma dumerilii with morphological details of primary spermatocytes and spermatids. (A). Primary spermatocytes during pachytene (p1Sc). The nuclei contain dense paired chromosomes. Sertoli cell nucleus (Se) and the interstitial tissue (it) are seen. (B). Primary spermatocytes during diplotene (d1Sc). The separation of the paired chromosomes is evident, remaining united in the chiasmata. Other spermatocyte is in metaphase of the first meiotic division (*1Sc). A Sertoli cell nucleus (Se) is seen. (C). Primary spermatocytes during metaphase of the first meiotic division (*1Sc). The division of the primary spermatocytes originates the secondary spermatocytes (2Sc). The secondary spermatocytes contain filamentous chromosomes previous to the second division of meiosis. Compare the smaller size of the secondary spermatocytes with the primary spermatocytes. A primary spermatocyte during pachytene (p1Sc) and the limit of the lobule with interstitial tissue (it) are also seen. (D). Early spermatids (eSt) with round nucleus, and middle spermatids (mSt) with elongation of the nucleus are evident. Sertoli cell nuclei (Se) and interstitial tissue (it). (A-D): Hematoxylin-eosin. Bar = 10 μm.
Secondary spermatocytes are spherical cells and have an average diameter of 18–20 μm. Their nuclei contain haploid chromosomes, but duplicated. Their chromosomes have fibrillar shape due to their immediately entrance to the second division of meiosis (Figs. 4B, 5C). They are seen less frequent, as they enter the second meiotic division (Figs. 4A,B) after a short interphase, rapidly giving rise to 2 spermatids. Therefore, secondary spermatocytes are often observed in the same lobule along with dividing primary spermatocytes (Fig. 5C).
Spermatids and spermatozoa
The result of meiosis is 4 haploid spermatids. Early spermatids are spherical in shape, and attain a diameter of 14–17 μm (Figs. 6A,B). Their nuclei stain denser than the nuclei of secondary spermatocytes. The spermatids transform into spermatozoa through spermiogenesis and become progressively elongated cells (Figs. 6A–D). As spermiogenesis proceeds, the nuclei of spermatids become very long and the chromatin shows increasing degree of condensation (Figs. 6D, 7A–D), defining the head and a large flagellum (Figs. 7B,D).
Figure 6.
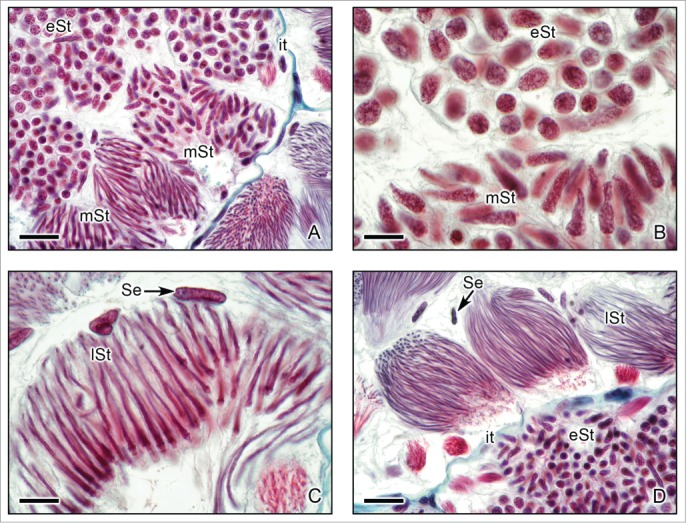
Caudal region of the testis of Ambystoma dumerilii (A,C,D) and A. mexicanum (B) with details of morphological changes of the spermatids during spermiogenesis. (A,B,C,D). Lobules with cysts that contain early spermatids (eSt) with round nucleus, and middle spermatids (mSt) with different levels of elongation of the nucleus and late spermatids (lSt) with evident and progressive elongation. Sertoli cell nuclei (Se) surround the cysts, interstitial tissue (it). (A): Masson's trichrome. Bar = 10 μm. (B): Hematoxylin-eosin. Bar = 10 μm. (C,D): Masson's trichrome. Bar = 10 μm.
Figure 7.
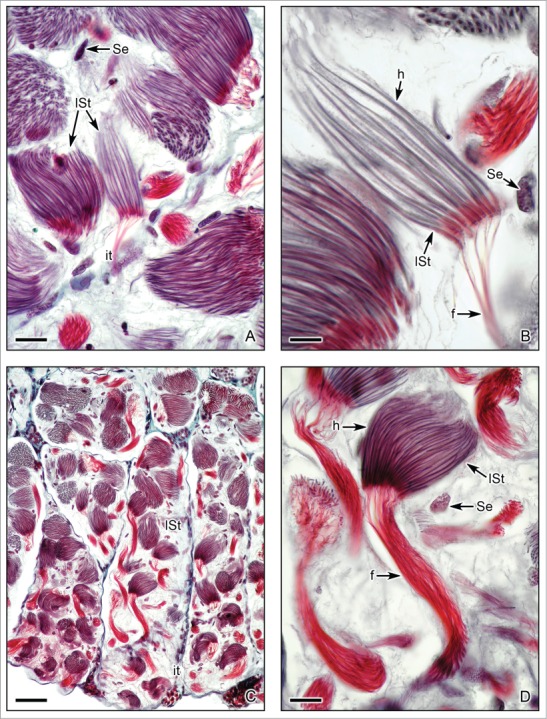
Caudal region of the testis of Ambystoma dumerilii with details of morphological changes of the late spermatids during spermiogenesis. (A,B,C,D). Lobules with cysts that contain late spermatids (lSt) with final elongation of the nucleus on the head (h) and development of a thin and large flagella (f). Nuclei of Sertoli cells (Se) and interstitial tissue (it) are seen. Masson's trichrome. (A,B,D): Bar = 10 μm. (C): Bar = 50 μm.
The morphology of spermatozoa is relatively uniform. A detailed review of the structure of mature spermatozoa of urodeles was done.54 The spermatozoa consist of an elongated large head, a neck and a flagellum (Figs. 7B,D, 8A,B). The head of the spermatozoa contains the acrosome and nucleus. The nucleus has an elongated shape, with narrower cephalic part. The proximal centriole lies close to the nucleus, and the distal one is a part of the basal body of the axonema. Throughout the long middle piece of the spermatozoa, mitochondria, that provide the energy for locomotion, are closely applied. The basal body is formed by a dense ring around the distal centriole.29,30,55 The spermatozoa of salamanders posses undulating membrane.51,54,55,56,57,58,
Figure 8.
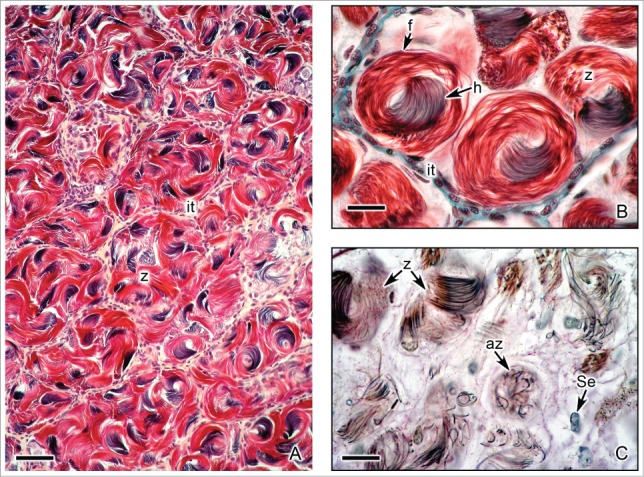
Caudal region of the testis of Ambystoma mexicanum with spermatozoa. (A). Lobules containing abundant cysts with spermatozoa (z). Interstitial tissue (it) surrounds the lobules. Hematoxylin-eosin. Bar = 50 μm. (B). Detail of cysts with spermatozoa (z) formed by the head (h) and flagellum (f). At the end of spermiogenesis the spermatozoa are swirled into the cysts. Masson's trichrome. Bar = 10 μm. (C). During spermiation, abnormal spermatozoa (az), showing irregular morphology, may remain into the cysts. Nuclei of Sertoli cells (Se) are seen. Alcian blue. Bar = 10 μm.
Spermatozoa have a swirl arrangement inside the cyst, with their heads oriented in the same direction as observed in Lissotriton italicus21 and Ambystoma dumerilii illustrated here (Figs. 8A,B). The total length of spermatozoa of urodeles is usually longer than those of other amphibians, and other vertebrates (for review see54). The shortest spermatozoa were reported for Hynobius nebulosus with a length of 156 μm, whereas the longest with about a length of 1mm was observed in Necturus maculosus.59 The lengths of spermatozoa were also measured in several species: Lissotriton italicus (360 μm),21 Ambystoma mexicanum (444 μm), A. dumerilii (451 μm),58 E. bislineata (459 μm), E. lucifuga (523 μm), Desmognathus wrighti (504 μm), D. aeneus (388 μm), Plethodon cinereus (507 μm), P. dorsalis (535 μm), and P. dunni (626 μm).54 The biological significance of differences in the lengths of spermatozoa is unknown.
After completion of spermiogenesis, the cysts open and spermatozoa are released from Sertoli cell in a process termed spermiation. In several cysts remain some abnormally sized and shaped spermatozoa (Fig. 8C). These spermatozoa eventually are phagocytized by Sertoli cells.17 The Sertoli cells remain in the lobules, gradually degenerate and are absorbed.31 Among the Sertoli cells some spermatogonia may be seen. Around the lobules, Leydig cells hypertrophy, attaining columnar or polyhedral shape (Figs. 9A,B). At the end of spermiation, this tissue regresses constantly and consists of small and irregular cells with pycnotic nuclei and amorphous and lightly stained cytoplasm.9,31,36
Figure 9.
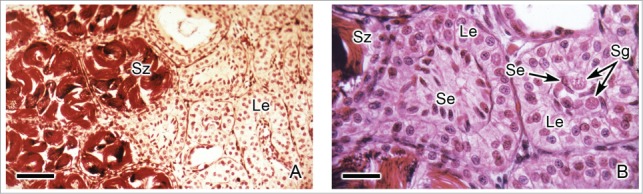
Caudal region of the testis of Ambystoma mexicanum during spermiation. (A). There are lobules with spermatozoa (Sz) and, more caudally, there are lobules after spermiation without spermatozoa. Leydig cells (Le) hypertrophy around the lobules. Hematoxylin-eosin. Bar = 50 μm. (B). Detail of the Fig. 9A. During hypertrophy, Leydig cells (Le) become cuboidal, columnar or polyhedral in shape. The Leydig cells surround the lobules, where residual Sertoli cells (Se) and some spermatogonia (Sg) may be seen. Hematoxylin-eosin. Bar = 10 μm.
Reproductive ducts
Down stream of the intratesticular ducts, the spermatozoa continue to a ducts system which provides secretions that form a favorable condition for the spermatozoa and transport the spermatozoa to the cloaca.6,8,34, Sperm transport from the testicular lobules to the Wolffian ducts occurs through the nephrons of the genital kidney, which form transversal ducts. Analysis of the genital kidney of the salamanders Ambystoma maculatum60 and Notophthalmus viridescens61 described that these nephrons have reduced reabsorptive capacity and aid in the transportation of sperm. The transversal ducts run to the anterior region of the kidney (Figs., 10A,B). They have a cuboidal epithelium with long stereocilia at the apical end, surrounded by connective tissue, thin smooth muscle and squamous peritoneal epithelium (Figs. 10C,D). Wolffian ducts have a wide lumen that may contain abundant spermatozoa after spermiation. The epithelium has irregular cuboidal or low columnar cells, with adjacent connective tissue which may have some melanocytes, and surrounding, there are some smooth muscle fibers and a squamous peritoneal epithelium (Figs. 10C,D).
Figure 10.
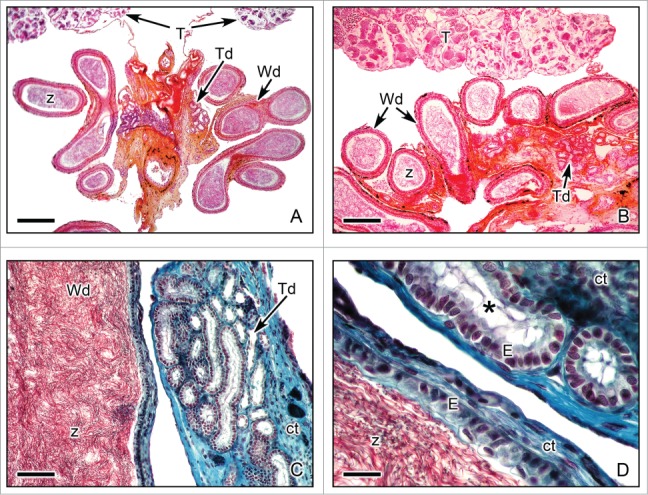
Efferent ducts of Ambystoma dumerilii after spermiation. (A,B). Panoramic view of sections with 2 types of efferent ducts: transversal ducts (Td) and Wolffian ducts (Wd). The lumen of the Wolffian ducts contains abundant spermatozoa (z). Near the ducts, the periphery of both testes (T) is seen. Hematoxylin-eosin. Bar = 50 μm. Bar = 40 μm. (C,D). Details of the efferent ducts of Ambystoma dumerilii. Both types of ducts, transversal ducts (Td) and Wolffian ducts (Wd) have columnar epithelium (E). The epithelium of the transversal ducts presents long stereocilia (*) in the apical end. Wolffian ducts contain abundant spermatozoa (z). Connective tissue (ct) surrounds the ducts. Masson's trichrome. Bar = 20 μm. Bar = 10 μm.
Spermatophores
The spermatophore is a characteristic structure of most male urodeles which have internal fertilization, more than 90%, with the exception of species belonging to the families Hynobiidae and Cryptobran-chidae, which have external fertilization.3 Spermatophores are formed by the cloacal glands and consist of a gelatinous base and a stalk surmounted by a sperm-containing gelatinous cap. Spermatophores contain a package of spermatozoa released from the Wolffian duct and the gelatinous capsule formed by secretions of the cloacal gland complex. Sever described and compared the anatomy and evolution of the cloacal glands in several species.46,62-64 The cloacal region exhibits a glandular complex structure. The types of cloacal glands are dorsal, pelvic, ventral and Kingsbury glands.46,62-64,65 The spermatophores may present differences in size and shape.66 The spermatophores are formed during courtship, deposited by a male in the exterior and picked up by cloacal labia of the female.3 The structure and histochemistry of spermatophores of several species of the families Ambystomatidae, Salamandridae, and Plethodontidae are described.66,67 In Necturus, the spermatophores are deposited by a male to a female directly by cloacal apposition and the stalk may be absent in these species.3
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Gerardo Gómez-Ríos for processing some histological specimens and valuable discussions during the early progress of this work; we are grateful to Adriana García-Alarcón and Gabino De la Rosa-Cruz for helpful technical assistance with digital photography; and José Antonio Hernández Gómez who kindly assisted with the digital preparation of figures.
References
- 1. Adams EA. Sexual conditions in Triturus viridescens. III. The reproductive cycle of the adult aquatic form of both sexes. Amer J Anat 1940;66:235-276; http://dx.doi.org/ 10.1002/aja.1000660205 [DOI] [Google Scholar]
- 2. Baker CL. The male urogenital system of the Salamandridae. J Tennessee Acad Sci 1965;40:1-5. [Google Scholar]
- 3. Lofts B. Amphibians. In Lamming G.E. (ed.). Marshall's Physiology of Reproduction. Vol. One. Reproductive Cycles of Vertebrates Churchill Livingstone, Edinburgh: 1984. pp:127-05. [Google Scholar]
- 4. Lofts B. Testicular function. In Norris D.O. and Jones R.E. (eds.), Hormones and Reproduction in Fishes, Amphibians and Reptiles. Plenum Press, New York: 1987. pp:288-98. [Google Scholar]
- 5. Brizzi R, Calloni C, Vanni S. Spermatogenetic cycle in Salamandrina terdigitata (Lacepede, 1788) (Amphibia: Salamandridae). Z Mikrosk Anat Forsh Leipzig 1985;99:271-92; PMID:4024700 [PubMed] [Google Scholar]
- 6. Norris DO. Vertebrate Endocrinology. Academic Press, New York: 1997. pp:634. [Google Scholar]
- 7. Uribe MC. Reproductive systems of caudata amphibians, In Dutta H.M. and Datta Munshi J.S. (eds.). Vertabrate Functional Morphology. Horizon of Research in the 21st. Century. Science Publishers, Enfield, USA: New Hampshire 2001. pp:267-293. [Google Scholar]
- 8. Uribe MC. The testes, spermatogenesis and male reproductive ducts, In Sever D. (volume ed.), and Jamieson B.G.M. (Series ed.). Reproductive Biology and Phylogeny of Urodela. Science Publishers, Enfield: (NH: ), USA: Plymouth, UK 2003. pp:183-202. [Google Scholar]
- 9. Uribe MC. Chapter 3. Spermatogenesis and male reproductive system. Urodela. In Ogielska M. (ed.). “Reproduction of Amphibians.” Science Publishers, Inc; Enfield, NH, USA; Plymouth, UK 2009. pp:100-124. [Google Scholar]
- 10. Propper CR. Chapter 3. Testicular structure and control of sperm development in Amphibians. In: David O Norris. and Kristin H Lopez. (Editors). Volume 2: Amphibians, “Hormones and Reproduction in Vertebrates." Ac Press, Elsevier; 2011. pp:39-53. [Google Scholar]
- 11. Humphrey RR. The multiple testis in urodeles. Biol Bull 1922;43:45-67; http://dx.doi.org/ 10.2307/1536690 [DOI] [Google Scholar]
- 12. Bergmann M. The morphology of the testis in Salamandra salamandra (L.). In: Thiesmeier Greven HB. (eds.). Mertensiella. Supplement zu Salamandra. Proceedings of the Symposium “Biology of Salamandra and Mertensiella” Nummer 4. Bonn: 1994. pp:75-82. [Google Scholar]
- 13. Tso ECF, LOFTS B. Seasonal changes in the newt, Trituroides hongkongenesis testis. I. A histological and histochemical study. Acta Zool-Stockholm 1977;58:1-8; http://dx.doi.org/ 10.1111/j.1463-6395.1977.tb00230.x [DOI] [Google Scholar]
- 14. Franchi E, Camatini M, de Curtis I. Morphological evidence of a permeability barrier in urodele testis. J Ultra Mol Struct R 1982;80:253-63; http://dx.doi.org/ 10.1016/S0022-5320(82)80038-5 [DOI] [PubMed] [Google Scholar]
- 15. Fraile B, Paniagua R, Rodríguez MC, Saez FJ. Jiménez A. Annual changes in the number, testosterone content and ultrastructure of glandular tissue cells of the testis in the marbled newt, Triturus marmoratus. J Anat 1989;167:85-94; PMID:26305436882150 [PMC free article] [PubMed] [Google Scholar]
- 16. Schindelmeiser J, Greven H, Bergmann M. The immature part of the testis in Salamandra salamandra, (L.) (Amphibia, Urodela). Arch Histol Jap 1983;46(2):159-72; PMID:6882150 [DOI] [PubMed] [Google Scholar]
- 17. Schindelmeiser J, Bergmann M, Greven H. Cellular differentiation in the urodele testis, In Duncker HR, Fleischer G. (eds.). Functional Morphology in Vertebrates. Proceedings of the 1st International Symposium on Vertebrate Morphology Giessen, 1983 Fortschritte der Zoologie, Band 30 Gustav Fischer Verlag, Stuttgart, New York: 1985. pp: 445-7. [Google Scholar]
- 18. Chan LM. Seasonality, microhabitat and cryptic variation in tropical salamander reproductive cycles. Biol J Linn Soc 2003;78:489-496; http://dx.doi.org/ 10.1046/j.0024-4066.2002.00157.x [DOI] [Google Scholar]
- 19. Flament S, Dumond H, Chardard D, Chesnel A. Lifelong testicular differentiation in Pleurodeles waltl (Amphibia, Caudata). Reprod Biol Endocrinol 2009. 7:21; PMID:19265523; http://dx.doi.org/ 10.1186/1477-7827-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trauth SE. Reproductive biology and spermathecal anatomy of the dwarf salamander (Eurycea quadridigitata), in Alabama. Herpetologica 1983;39:9-15. [Google Scholar]
- 21. Sperone E, Bonacci A, Brunelli E, Tripepi S, Jamieson BGM. Male reproductive system in the Italian newt Lissotriton italicus (Peracca 1898) (Amphibia, Urodela): ultrastructural and morphological study with description of spermiogenesis, spermatozoon and spermatophore. Zoomorphology 2009;128:183-195; http://dx.doi.org/ 10.1007/s00435-009-0087-7 [DOI] [Google Scholar]
- 22. Takahashi H, Hanaoka KI. Cultivation in vitro of the testis cord of the newt, Triturus pyrrhogaster. Dev Growth Differ 1969;11:186-202; PMID:5392793; http://dx.doi.org/ 10.1111/j.1440-169X.1969.00186.x [DOI] [PubMed] [Google Scholar]
- 23. Kingsbury BF. The spermatogenesis of Desmognathus fusca. Am J Anat 1901;1:99-135; http://dx.doi.org/ 10.1002/aja.1000010202 [DOI] [Google Scholar]
- 24. Humphrey RR. The interstitial cells of the urodele testis. Am J Anat 1921;29:213-79; http://dx.doi.org/ 10.1002/aja.1000290204 [DOI] [Google Scholar]
- 25. Carrick R. The spermatogenesis of the axolotl (Ambystoma tigrinum). Trans Royal Society Edinburgh 1934;58:63-76; http://dx.doi.org/ 10.1017/S0080456800023048 [DOI] [Google Scholar]
- 26. Moore FL. Spermatogenesis in larval Ambystoma tigrinum: Positive and negative interactions of FSH and testosterone. Gen Comp Endocrinol 1975;26:525-33; PMID:1181244; http://dx.doi.org/ 10.1016/0016-6480(75)90175-6 [DOI] [PubMed] [Google Scholar]
- 27. Miltner MJ, Armstrong JB. Spermatogenesis in the Mexican axolotl, Ambystoma mexicanum. J Exp Zool 1983;227:255-63; http://dx.doi.org/ 10.1002/jez.1402270209 [DOI] [Google Scholar]
- 28. Norris DO, Norman MF, Pankak MK, Duvall D. Seasonal variation in spermatogenesis, testicular weights, vasa deferentia and androgen levels in neotenic tiger salamander, Ambystoma tigrinum. Gen Comp Endocrinol 1985;60:51-7; PMID:4054587; http://dx.doi.org/ 10.1016/0016-6480(85)90291-6 [DOI] [PubMed] [Google Scholar]
- 29. Uribe MC, Gómez ríos G, Brandon RA. Spermatogenesis in the urodele Ambystoma dumerilii. J Morphol 1994;222:287-99; http://dx.doi.org/ 10.1002/jmor.1052220306 [DOI] [PubMed] [Google Scholar]
- 30. Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 1995;32:459-97; PMID:8605396; http://dx.doi.org/ 10.1002/jemt.1070320602 [DOI] [PubMed] [Google Scholar]
- 31. Pudney J, Canick JA, Mak P, Callard GV. The differentiation of Leydig cells, steroidogenesis, and the spermatogenetic wave in the testis of Necturus maculosus. Gen Comp Endocrinol 1983;50:43-66; PMID:6852522; http://dx.doi.org/ 10.1016/0016-6480(83)90241-1 [DOI] [PubMed] [Google Scholar]
- 32. Lecouteux A, Garnier DH, Bassez T, Joly J. Seasonal variations of androgens, estrogens, and progesterone in the different lobules of the testis and in the plasma of Salamandra salamandra. Gen Comp Endocrinol 1985;58:211-221; PMID:3996890; http://dx.doi.org/ 10.1016/0016-6480(85)90337-5 [DOI] [PubMed] [Google Scholar]
- 33. Uribe MCA, GÓMEZ RÍOS G, LÓPEZ ARRIAGA C. Cambios morfológicos del testículo de Ambystoma dumerilii durante un ciclo anual. Bol Soc Herpetol Mex 1991;3:13-8. [Google Scholar]
- 34. Williams AA, Brandon RA, Martan J. Male genital ducts in the salamanders Eurycea lucifuga and Eurycea longicauda. Herpetologica 1984;40(3):322-30. [Google Scholar]
- 35. Eisthen HL, Krause BC. Ambiguities in the relationship between gonadal steroids and reproduction in axolotls (Ambystoma mexicanum). Gen Comp Endocrinol 2012;176(3):472-80; PMID:22245262; http://dx.doi.org/ 10.1016/j.ygcen.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 36. Fraile B, Paniagua R, Saez FJ, PANIAGUA R. The cycle of follicular and interstitial cells (Leydig cells) in the testis of the marbled newt, Triturus marmoratus. J Morphol 1990;204:89-101; PMID:2338719; http://dx.doi.org/ 10.1002/jmor.1052040110 [DOI] [PubMed] [Google Scholar]
- 37. Grier HJ. Comparative organization of Sertoli cells including the Sertoli cell barrier, In Russell LD, Griswold MD. (eds.). The Sertoli Cell. Cache River Press, Clearwater, Florida: 1993. pp:704-39. [Google Scholar]
- 38. Bouma J, Cloud JG. Chapter 5. Sertoli cell biology in fishes and amphibians. In: Skinner MK, Griswold MD. (Eds.). Sertoli Cell Biology. Elsevier, Ac Press, San Diego, CA, USA: 2005. pp:71-9. [Google Scholar]
- 39. Lazard L. Steroidogenesis in axolotl testis. Histochemistry of two major enzymes related to cell type, spermatogenesis, and substrate. Gen Comp Endocrinol 1979;39:381-87; PMID:499762; http://dx.doi.org/ 10.1016/0016-6480(79)90135-7 [DOI] [PubMed] [Google Scholar]
- 40. Jin Y, Uchida I, Eto K, Kitano T, Abe S. Size-selective junctional barrier and Ca2+-independent cell adhesion in the testis of Cynops pyrrhogaster: Expression and Function of Occludin. Mol Reprod Dev 2008;75:202-16; PMID:17342736; http://dx.doi.org/ 10.1002/mrd.20662 [DOI] [PubMed] [Google Scholar]
- 41. Lazard L. Spermatogenesis and 3b-HSDH activity in the testis of the axolotl. Nature 1976;264:796-97; PMID:1012324; http://dx.doi.org/ 10.1038/264796a0 [DOI] [PubMed] [Google Scholar]
- 42. Callard GV, Canick JA, Pudney J. Estrogen synthesis in Leydig cells: structural-functional correlations in Necturus testis. Biol Reprod 1980;23:461-79; PMID:6968228; http://dx.doi.org/ 10.1095/biolreprod23.2.461 [DOI] [PubMed] [Google Scholar]
- 43. Pudney J, Callard GV. Organization of interstitial tissue in the testis of the salamander Necturus maculosus (Caudata: Proteidae). J Morphol 1984;181:87-95; PMID:6471107; http://dx.doi.org/ 10.1002/jmor.1051810108 [DOI] [PubMed] [Google Scholar]
- 44. Fraile BR, Paniagua MC, Rodríguez Saez FJ. Effect of photoperiod and temperature on spermiogenesis in the marbled newt, Triturus marmoratus marmoratus. Copeia 1989:357-63; http://dx.doi.org/ 10.2307/1445432 [DOI] [Google Scholar]
- 45. Imai K, Tanaka S. Histochemical and electron microscopic observations on the steroid hormone-secreting cells in the testis of the Japanese red-bellied newt, Cynops pyrrhogaster pyrrhogaster. Dev Growth Differ 1978;20 N°2:151-67; http://dx.doi.org/ 10.1111/j.1440-169X.1978.00151.x [DOI] [PubMed] [Google Scholar]
- 46. Sever DM. Morphology and seasonal variation of the mental hedonic glands of the dwarf salamander (Eurycea quadridigitata), (Holbrook). Herpetologica 1975;31:241-51 [Google Scholar]
- 47. Ricote M, Alfaro JM, Garcia-Tuñon I, Arenas MI, Fraile B, Paniagua R, Royuela M. Control of the annual testicular cycle of the marbled-newt by p53, p21, and Rb gene products. Mol Reprod Dev 2002;63:202-9; PMID:12203830; http://dx.doi.org/ 10.1002/mrd.10167 [DOI] [PubMed] [Google Scholar]
- 48. Arenas MI, Royuela M, Lobo MVT, Alfaro JM, Fraile B, Paniagua R. Androgen receptor (AR), estrogen receptor-α (ER-α) and estrogen receptor-β (ER-β) expression in the testis of the newt, Triturus marmoratus marmoratus during the annual cycle. J Anat 2001;199:465-72; PMID:11693307; http://dx.doi.org/ 10.1046/j.1469-7580.2001.19940465.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Armstrong JB. Spermatogenesis, In Armstrong JB, Malacinski GM. (eds.), Developmental Biology of the Axolotl. Columbia University Press, New York: 1989. pp:36-41. [Google Scholar]
- 50. Picheral B. Les elements cytoplasmiques au cours de la spermiogenese du Triton Pleurodeles waltlii Michah. III. L’évolution des formations caudales. Z Zellforsch 1972;131:399-416; PMID:5074143; http://dx.doi.org/ 10.1007/BF00582858 [DOI] [PubMed] [Google Scholar]
- 51. Wang Hong Y. Studies on microstructure and infrastructure of testis of little salamander, Batrachuperus pinchonii. Master's thesis. Shaanxi Normal University; Xi'an, China: 2002. [Google Scholar]
- 52. Seto T, Kezer J, Pomerat CM. A cinematographic study of meiosis in salamander spermatocytes in vivo. Z Zellforsch 1969;94:407-24; PMID:5791454; http://dx.doi.org/ 10.1007/BF00319185 [DOI] [PubMed] [Google Scholar]
- 53. Cobb J, Handel MA. Dynamics of meiotic prophase I during spermatogenesis: from pairing to division. Semin Cell Dev Biol 1998;9:445-50; PMID:9813191; http://dx.doi.org/ 10.1006/scdb.1998.0202 [DOI] [PubMed] [Google Scholar]
- 54. Scheltinga DM, Jamieson BGM. The mature spermatozoa, In Sever D. (volume ed.), Jamieson BGM. (Series ed.). Reproductive Biology and Phylogeny of Urodela. Science Publishers, Enfield: (NH: ), USA: Plymouth, UK 2003. pp:203-74. [Google Scholar]
- 55. Wortham JWE, Brandon RA, Martan J. Comparative morphology of some Plethodontid salamander spermatozoa. Copeia. 1977(4):666-80; http://dx.doi.org/ 10.2307/1443165 [DOI] [Google Scholar]
- 56. Fawcett DW. A comparative view of sperm ultrastructure. Biol Reprod Suppl 1970;2:90-127; PMID:12254595; http://dx.doi.org/ 10.1095/biolreprod2.Supplement_2.90 [DOI] [PubMed] [Google Scholar]
- 57. Bedford JM, Calvin HI. Changes in -S-S- linked structures of the sperm tail during epididymal maturation, with comparative observations in sub-mammalian species. J Exp Zool 1974. 187(2):181-203.; PMID:4205051; http://dx.doi.org/ 10.1002/jez.1401870202 [DOI] [PubMed] [Google Scholar]
- 58. Brandon RA, Martan J, Wortham JWE, Englert DC. The influence of interspecific hybridization on the morphology of the spermatozoa of Ambystoma (Caudata, Ambystomatidae). J Reprod Fert 1974;41:275-84; http://dx.doi.org/ 10.1530/jrf.0.0410275 [DOI] [PubMed] [Google Scholar]
- 59. Wortham JWE, Jr, Murphy JA, Martan J, Brandon RA. Scanning electron microscopy of some salamander spermatozoa. Copeia 1982(1):52-60; http://dx.doi.org/ 10.2307/1444267 [DOI] [Google Scholar]
- 60. Siegel DS, Aldridge RD, Rheubert JL, Gribbins KM, Sever DM, Trauth SE. The testicular sperm ducts and genital kidney of male Ambystoma maculatum (Amphibia, Urodela, Ambystomatidae). J Morphol 2013;274(3):344-60; PMID:23192852; http://dx.doi.org/ 10.1002/jmor.20100 [DOI] [PubMed] [Google Scholar]
- 61. Nicholson AE, Siegel DS. Modifications of the genital kidney proximal and distal tubules for sperm transport in Notophthalmus viridescens (Amphibia, Urodela, Salamandridae). J Morphol 2014;275(8):914-22; PMID:24643856; http://dx.doi.org/ 10.1002/jmor.20268 [DOI] [PubMed] [Google Scholar]
- 62. Sever DM. Cloacal anatomy of male salamanders in the families Ambystomatidae, Salamandridae and Plethodontidae. Herpetologica 1981;37:142-55. [Google Scholar]
- 63. Sever DM. Comparative anatomy and phylogeny of the cloacae of salamanders (Amphibia: Caudata). VII. Plethodontidae, Herpetological Monographs. 1994.8 pp:276-337. [Google Scholar]
- 64. Sever DM. Courtship and mating glands, In Sever D. (volume ed.) Jamieson BGM. (Series ed.). Reproductive Biology and Phylogeny of Urodela. Science Publishers, Enfield: (NH: ), USA: Plymouth, UK 2003. pp:323-81. [Google Scholar]
- 65. Brizzi R, Calloni C. Male cloacal region of the spotted salamander, Salamandra salamandra gigliolii (Amphibia, Salamandridae). Boll Zool 1992;59:377-85; http://dx.doi.org/ 10.1080/11250009209386697 [DOI] [Google Scholar]
- 66. Zalisko EJ, Brandon RA, Martan J. Microstructure and histochemistry of salamander spermatophores (Ambystomatidae, Salamandridae and Plethodontidae). Copeia. 1984(3):739-47; http://dx.doi.org/ 10.2307/1445158 [DOI] [Google Scholar]
- 67. Russell LD, Brandon RA, Zalisko EJ, Martan J. Spermatophores of the salamander Ambystoma texanum. Tissue Cell 1981;13(3):609-21; PMID:6172883; http://dx.doi.org/ 10.1016/0040-8166(81)90031-8 [DOI] [PubMed] [Google Scholar]


