Abstract
There are numerous types of junctions in the seminiferous epithelium which are integrated with, and critically dependent on the Sertoli cell cytoskeleton. These include the basal tight junctions between Sertoli cells that form the main component of the blood–testis barrier, the basal ectoplasmic specializations (basal ES) and basal tubulobulbar complexes (basal TBC) between Sertoli cells; as well as apical ES and apical TBC between Sertoli cells and the developing spermatids that orchestrate spermiogenesis and spermiation. These junctions, namely TJ, ES, and TBC interact with actin microfilament-based cytoskeleton, which together with the desmosomal junctions that interact with the intermediate filament-based cytoskeleton plus the highly polarized microtubule-based cytoskeleton are working in concert to move spermatocytes and spermatids between the basal and luminal aspect of the seminiferous epithelium. In short, these various junctions are structurally complexed with the actin- and microtubule-based cytoskeleton or intermediate filaments of the Sertoli cell. Studies have shown toxicants (e.g., cadmium, bisphenol A (BPA), perfluorooctanesulfonate (PFOS), phthalates, and glycerol), and some male contraceptives under development (e.g., adjudin, gamendazole), exert their effects, at least in part, by targeting cell junctions in the testis. The disruption of Sertoli–Sertoli cell and Sertoli–germ cell junctions, results in the loss of germ cells from the seminiferous epithelium. Adjudin, a potential male contraceptive under investigation in our laboratory, produces loss of spermatids from the seminiferous tubules through disruption of the Sertoli cell spermatid junctions and disruption of the Sertoli cell cytoskeleton. The molecular and structural changes associated with adjudin administration are described, to provide an example of the profile of changes caused by disturbance of Sertoli-germ cell and also Sertoli cell-cell junctions.
Keywords: adjudin, cell junctions, germ cells, indazole-carboxylic acid, lonidamine, seminiferous epithelium, seminiferous epithelial cycle, Sertoli cells, spermatogenesis, testis
Abbreviations
- AJ
adherens junction
- BPA
bisphenol A
- BTB
blood-testis barrier
- CAR
coxsackievirus and adenovirus receptor
- c-Yes
cellular Yamaguchi sarcoma viral oncogene homolog 1, a non-receptor protein tyrosine kinase
- c-Src
cellular transforming gene of Rous sarcoma, a non-receptor protein tyrosine kinase
- ES
ectoplasmic specialization
- FAK
focal adhesion kinase
- JAM
junctional adhesion molecule
- MAPK
mitogen activated protein kinase
- PFOS
perfluorooctanesulfonate
- SHP2
SH2-domain-containing protein tyrosine phosphatase also known as PTPN11, tyrosine-protein phosphatase non-receptor type 11
- SFK
Src family kinase
- SSC
spermatogonial stem cell
- TBC
tubulobulbar complex
- TJ
tight junction
- ZO-1
zonula occludens 1
Introduction
Studies have shown many environmental toxicants, such as cadmium and bisphenol A, exert their effects by targeting cell junctions between testicular cells and also synapses between neurons in both rodents and humans.1-11 Several toxicants also perturb gap junction-based cell–cell communication.12-15 In the testis, Sertoli and germ cell junctional proteins, as well as the tight junction (TJ)-permeability barrier, are the early targets of toxicants based on studies in vitro14,16-22 and/or in vivo.22-28 Accumulating evidence indicates that toxicants activate and/or disrupt mitogen activated protein kinase (MAPK); focal adhesion kinase (FAK); Src family kinases (SFKs), such as c-Yes and c-Src; and phosphatases, such as tyrosine phosphatase SHP2, which are signaling proteins that function downstream of adhesion protein complexes at cell junctions1,22,23,29-32 (also see Mathur this issue). These signaling molecules alter the phosphorylation states of proteins at the Sertoli–Sertoli cell and/or Sertoli–germ cell interface, which in turn disrupts protein localization (often, but not always, resulting in the re-localization of proteins from the cell membrane into the cytosol via endocytic vesicle-mediated protein trafficking), damages cell junctions and the blood–testis barrier, and results in germ cell loss from the seminiferous epithelium.2,33–35 Furthermore, a disruption of kinase and/or phosphatase activity by environmental toxicants, such as cadmium, bisphenol A, phthalates, and other androgen disruptors can affect androgen action, including the transcriptional regulation of androgen-related genes necessary for the maintenance of spermatogenesis.34,36-40 Because the disruption of cell junctions in the testis2,4,6,33 and other organs13,41 by environmental toxicants has been the subject of several reviews, we will use this short review to describe the main features of the different types of junctions in the testis as summarized in Table 1, and discuss recent findings on adjudin, a potential male contraceptive that affects the testis similar to that of various toxicants.42-45
Table 1.
Cell junctions in the seminiferous epithelium of mammalian testes that are targets of adjudin and other environmental toxicants*
| Junction Type | Location | Adhesion protein complex |
|---|---|---|
| Anchoring Junction: | ||
| Apical ES (testis-specific cell-cell actin-based) | Sertoli cell-spermatid (step 8–19) in the adluminal compartment | α6ß1-integrin-laminin α3ß3γ3; N-cadherin-ß-catenin; Nectin-2/3-afadin; JAM-C-ZO-1; CAR-ZO-1 |
| Desmosome (cell-cell intermediate filament-based) | Sertoli cell-spermatocyte/spermatogonium in the basal compartment | |
| Sertoli cell-spermatid (step 1–7) Desmoglein-desmocolin | Desmoglein-desmocollin | |
| Hemidesmosome (cell-basement membrane intermediate filament-based) | Sertoli cell-basement membrane in the tunica propria | ß1-integrin/laminin α2 |
| Communicating Junction | ||
| Gap junction (a cell-cell actin-based) | Sertoli cell-spermatid (step 1–7) in the adluminal compartment** | Connexin 43-plakophilin-2 |
| Sertoli cell-spermatogonium in the basal compartment or stem cell niche | Connexins (e.g., connexin 43, connexin 33) | |
| Blood-testis barrier: | ||
| Tight junction (cell-cell actin-based) | Sertoli-Sertoli cell | Occludin-ZO-1; JAM-A-ZO-1; JAM-B-ZO-1; CAR-ZO-1 |
| Basal ES (cell-cell actin-based) | Sertoli-Sertoli cell | N-cadherin-ß-catenin; Nectin-2-afadin |
| Desmosome (cell-cell Intermediate filament- based) | Sertoli-Sertoli cell | Desmoglein-2-desmocollin-2 |
| Gap junction (cell-cell actin-based) | Sertoli-Sertoli cell | Connexin 43-plakophilin-2 |
*This Table was prepared based on studies in the rat testis, and updated from recent reviews from our laboratory.85,86,108 Apical ES, basal ES, tight junction and gap junction are junctions at the cell-cell interface using actin for their attachment; desmosome and hemidesmosome, however, are junctions at the cell-cell and cell-matrix interface, respectively, using intermediate filament for their attachment. In the testis, basement membrane is a modified form of extracellular cell matrix (ECM).54,55 While there is no specific adhesion protein complex known to use microtubule (MT) for their attachment in Sertoli and/or germ cells, the polarized MTs are found near the polarized actin microfilaments in Sertoli cells. Focal contact (also known as focal adhesion complex), a cell-matrix anchoring junction using actin for its attachment is not found in the testis. Abbreviations used: EC, ectoplasmic specialization; CAR, coxsackievirus and adenovirus receptor; JAM, junctional adhesion molecule; ZO-1, zonula occludens-1. **, it is noted that once apical ES is established at the Sertoli-spermatid (step 8) interface, it persists until step 19 spermatids when they are transformed to spermatozoa to prepare for spermiation, replacing desmosome and gap junction, becoming the only anchoring device that adhere developing spermatids onto the Sertoli cells during spermiogenesis. However, connexins, such as connexin 43 is found at the apical ES at the Sertoli-spermatid interface from step 8–19 spermatids, while no gap junction ultrastructures are detected, but connexin 43 alone can form hemichannels for the transport of signals as recently reviewed.115
Signature Lesion
The most common manifestation of disrupted junctions between Sertoli and germ cells is sloughing of germ cells into the tubular lumen. The germ cells often retain relatively normal cytological features and may be present in considerable numbers. Specific germ cells may be shed, depending on which junctions have been damaged. It is noted that apical ectoplasmic specialization (apical ES, a testis-specific anchoring junction) is highly susceptible to the male contraceptive adjudin.46,47 While apical ES is considered to be one of the strongest anchoring junctions, such as when compared to desmosome at the Sertoli-spermatid (pre-step 8) interface48 that utilizes intermediate filament for attachment (Table 1), it is most susceptible to adjudin treatment49 because adjudin effectively perturbs the spatiotemporal expression of actin regulatory proteins that governs the organization of actin microfilaments at the apical ES based on recent studies in our laboratory reviewed in.50 Thus, following adjudin, a male contraceptive (Fig. 1), treatment, such as a single oral dose at 50 mg/kg b.w., elongating/elongated spermatids are rapidly depleted from the seminiferous epithelium into the tubule lumen, and more than 50% of the tubules display signs of spermatid loss within ∼6–9 hr following treatment. This is followed by round spermatid and spermatocyte depletion, which takes place by ∼3- and ∼6.5-day after adjudin treatment, respectively.51 However, basal ES/BTB is not disrupted until after 2 wk of adjudin treatment,52 likely due to the presence of 2 arrays of actin microfilament bundles on both sides of the Sertoli cells at the basal ES vs. a single array of actin microfilament bundles on the Sertoli cells at the apical ES.
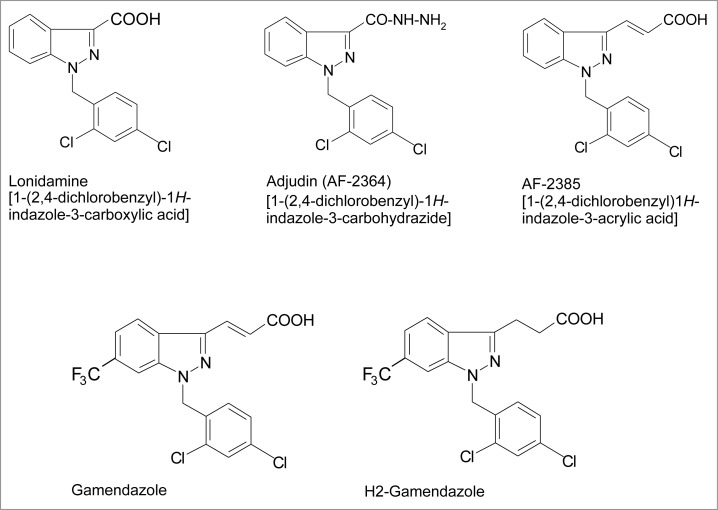
Figure 1.
Structural formulae of adjudin and other indazole-based compounds that are being explored as potential male contraceptives. Detailed chemical synthesis of adjudin can be found in an earlier report.134
The actin cytoskeleton and anchoring junctions
In mammalian epithelia/endothelia such as the seminiferous epithelium of the testis, cell junctions are broadly classified based on their function (such as occluding, anchoring or communicating function), relative location (such as at either the cell-cell or cell-matrix interface) and the cytoskeletal element that serves as attachment site for the constituting adhesion protein complex.53 These include: (i) tight (or occluding) junction (TJ), (ii) anchoring junction, and (iii) communicating junction. TJ is constituted by adhesion protein complexes occludin-ZO-1, JAM-A-ZO-1, CAR-ZO-1 and others (see Table 1), utilizing actin microfilaments for attachment, in which each adhesion protein complex is composed of an integral membrane protein (e.g., occludin, JAM-A, CAR) and an adaptor protein that tethers the complex to the cytoskeleton. In the testis, TJ is restricted to the Sertoli cell-cell interface at the BTB.47 While no TJ ultrastructures are visible at the Sertoli-germ cell interface, TJ proteins, however, are present at the Sertoli-spermatid (step 8–19) interface such as JAM-C and CAR. Anchoring junction is found either at the cell-cell interface or at the cell-matrix interface (basement membrane is a modified form of extracellular matrix in the testis54,55). For cell-cell anchoring junctions that use F-actin or intermediate filament for attachment, they are known as adherens junction (AJ) or desmosome, respectively. For cell-matrix anchoring junctions that use F-actin or intermediate filament for attachment, they are focal contact (or focal adhesion) or hemidesmosome, respectively. For communicating junction, gap junction is the best studied cell-cell communicating junction, and the other less studied communicating junction is intercellular bridge (also known as tunneling nanotube) which is used to transmit chemical/biological signaling molecules of larger molecular sizes.56,57 In most epithelia, TJ usually lies at the apex between cells, underneath the TJ is the adhesion belt formed by aggregates of AJs, to be followed by the desmosome, and these junctional ultrastructures constitute the junctional complex.53 Gap junction, and hemidesmosome or focal adhesion are then found behind the junctional complex.53 In the testis, a testis-specific cell-cell adherens junction (AJ) called the ectoplasmic specialization (ES)58 is limited to the Sertoli-spermatid (step 8–19 spermatids in the rat testis) and designated apical ES vs. the basal ES at the Sertoli cell-cell interface at the BTB.
In short, the anchoring junction at the Sertoli-spermatid (step 8–19 spermatids) interface in the rat testis is an F-actin-rich and a testis-specific adherens junction (AJ) known as apical ectoplasmic specialization (apical ES). The apical ES is limited to the apical (adluminal) compartment of the seminiferous epithelium.58-60 Besides apical ES, ES is also found at the Sertoli cell-cell interface but restricted to the basal compartment known as the basal ES, and together with the actin-based tight junction (TJ) and gap junction, and the intermediate filament-based desmosome, they constitute the blood-tubule barrier (BTB),47,60,61 which in turn physically divides the epithelium into the basal and the adluminal (apical) compartments. Basal ES shares similar ultrastructural features as of the apical ES.47,62,63 For instance, both types of ES have bundles of actin microfilaments in the Sertoli cell that lie perpendicular to the Sertoli cell plasma membrane and are sandwiched either between cisternae of endoplasmic reticulum (ER) and the apposing plasma membranes of the Sertoli cell and the spermatid at the apical ES or between the ER and the apposing plasma membranes of the adjacent Sertoli cells at the basal ES.47,58,60,64 Thus, basal ES has 2 arrays of actin filament bundles found on both sides of the adjacent Sertoli cells vs. just a single array of microfilament bundles at the apical ES, likely making basal ES structurally stronger. These actin filament bundles also confer unusual adhesive strength to the ES.48 Interestingly, these actin microfilaments at the ES are rapidly reorganized from their “bundled” to “un-bundled/branched” configuration and vice versa. This is thought to give plasticity to the ES in order to accommodate the transport of either spermatids across the epithelium in the apical compartment during the epithelial cycle or transport of the preleptotene spermatocytes across the BTB at stage VIII of the cycle. Since adhesion protein complexes that confer adhesive function at the ES are using F-actin for their attachment, rapid re-organization of F-actin at the apical ES during the epithelial cycle also confers changes in spermatid adhesion and de-adhesion to facilitate spermatid transport during spermiogenesis. We have noted in earlier studies that adjudin is effective in disrupting actin microfilaments at the apical ES.44,65,66 For instance, F-actin organization at the apical ES, but not basal ES/BTB, begins to show signs of disruption by ∼12-hr after adjudin treatment; microfilaments are no longer well-organized surrounding the spermatid head,67,68 and defragmentation of actin microfilaments is also detected.65 Probably because of the 2 arrays of actin filament bundles that are found on both sides of the Sertoli cells at the basal ES, the BTB integrity remains undisturbed until after ∼2-wk following adjudin treatment, and the disrupted BTB can be resealed thereafter and spermatogenesis rebounds52,67 (Fig. 2). Based on these earlier studies using adjudin-treated rats as a study model, alongside with the use of RNAi to selectively disrupt the expression of target genes pertinent to the regulation of F-actin cytoskeleton at the ES to monitor the function of ES, it is becoming increasingly clear that apical ES restructuring is mediated by the spatiotemporal expression of actin bundling/barbed end capping protein Eps8,69 actin bundling/cross-linking protein palladin,68 and barbed end nucleation protein Arp2/3 complex 70 at the apical ES junction between Sertoli cells and elongated spermatids. For instance, in stage VI-II tubules, Eps8 that confers actin bundling and prevents barbed end nucleation (i.e., it effectively prevents branching of an existing actin microfilament) is highly expressed at the apical and basal ES50,69 to maintain the integrity of actin microfilament at both sites. In stage VIII tubules, the expression of Eps8 diminishes considerably to a level virtually undetectable at the apical and basal ES when these structures undergo degeneration and/or remodeling to facilitate the release of sperm at spermiation and the transport of preleptotene spermatocytes, respectively.50,69 Treatment of rats with adjudin was found to down-regulate Eps8 expression at the apical ES in stage VI-VII tubules.69,71 Additionally, branched actin-inducing protein Arp3, which effectively turns bundled microfilaments to an unbundled/branched configuration is highly expressed at the apical ES but confined to the concave (ventral) side of spermatid head to facilitate endocytic vesicle-mediated protein trafficking, was found to become mis-localized, surrounding other parts of the spermatid heads. The combined down-regulation of Eps8 and the improper localization of Arp3 at the apical ES following adjudin treatment thus impedes spermatid adhesion to the Sertoli cell in the seminiferous epithelium. Studies have shown that the changes at the ES regarding the spatiotemporal expression of Eps8 and Arp3 during the epithelial cycle of spermatogenesis are mediated, at least in part, via the action of FAK72 that serves as the molecular switch that regulates the organization of actin microfilaments at the Sertoli cell-spermatid interface.50,71 In short, adjudin perturbs the spatiotemporal expression of these actin regulatory proteins, thereby disrupting the proper organization of F-actin by compromising the conversion of actin microfilaments between their “bundled” to “un-bundled/branched” configuration. This destabilizes the apical ES, leading to its disruption which is accompanied by the premature loss of spermatids from the seminiferous epithelium.45,46,71 Based on histological analysis, many of these prematurely depleted germ cells following adjudin treatment were detected in the tubule lumen and the relative number of phagosomes in the tubules was not considerably induced (Figs. 3–5), so it is not likely that these germ cells undergo apoptosis and get phagocytosed by the Sertoli cell.42,44,46 These findings are consistent with the underlying concept that adjudin exerts its effects at the Sertoli cell F-actin-based cytoskeleton via its effects on the spatiotemporal expression of actin regulatory proteins, which leads to apical ES disruption and germ cell loss.
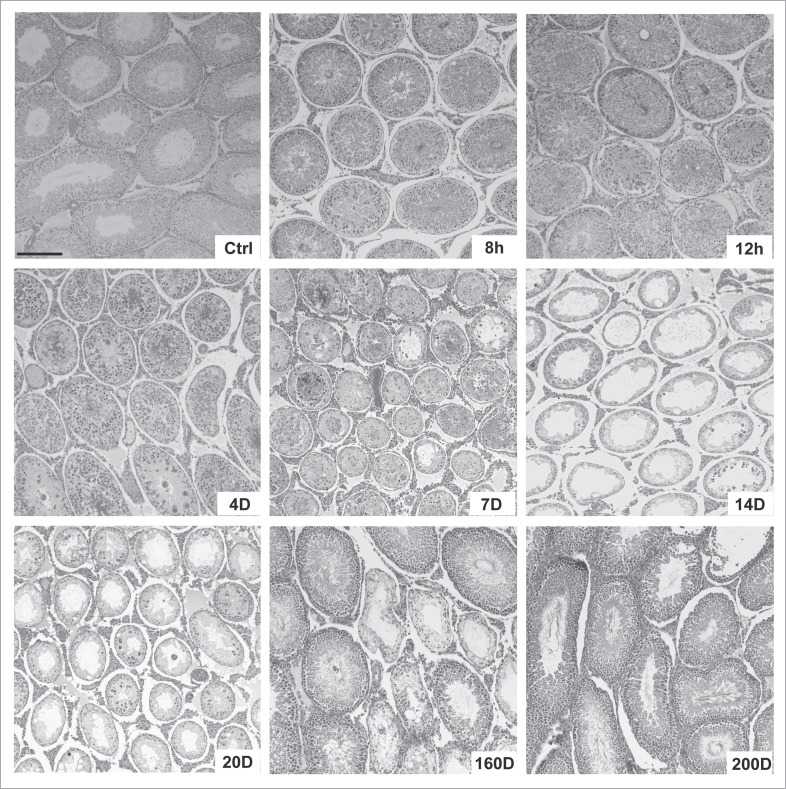
Figure 2.
Changes in the seminiferous epithelium of testes following treatment of adult rats with adjudin (50 mg/kg b.w., by oral gavage). Adult Sprague-Dawley rats (n = 4 - 6 rats per time point) at 275–300 g b.w. received an oral dose of adjudin (50 mg/kg b.w., by oral gavage) suspended in 0.05% methylcellulose (0.05 g methylcellulose in 100 ml double distilled water, containing adjudin at 20 mg/ml) as earlier described.42,52 At specified time points at 8 h (hour), 12 h, 4 D (day), 7D, 14D, 20D, 160D and 200D, rats were euthanized by CO2 asphyxiation, testes removed, fixed in Bouin's fixative and embedded in paraffin for histological analysis following hematoxylin and eosin staining as described.42,52 Scale bar, 150 μm which applies to other micrographs.
Figure 3.
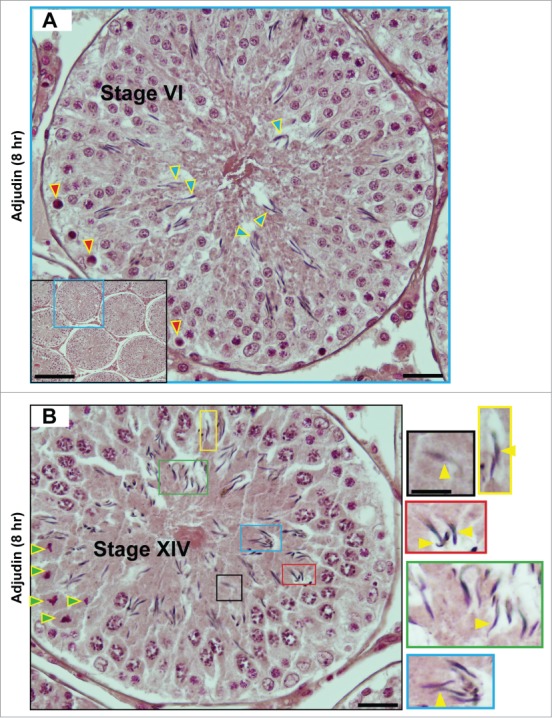
A, B. Sloughing of elongating/elongated spermatids from the seminiferous epithelium of rat testes by 8 hr after a single dose of adjudin (50 mg/kg b.w., by oral gavage). A stage VI (A) and a stage XIV (B) tubule are shown by H&E (hematoxylin and eosin) staining of paraffin-embedded testis sections. It is noted that elongating spermatids (step 18 spermatids in (A) and step 14 spermatids in (B)) are detected in the tubule lumen. These 2 micrographs illustrate as if the tubular lumen has closed, which may possibly be due to a shutdown of fluid secretion by Sertoli cells rather than physical shedding of spermatids, because the spermatid heads still look to be well embedded between the round spermatids, such as in (A) except for a few step 18 spermatids that are obviously found in the lumen, away from round spermatids as annotated by blue arrowheads. Also, there appears to be a layer of apoptotic pachytene spermatocytes around the basal layer of the tubule (annotated by red arrowheads). However, it is still likely that there is a disruption of spermatid adhesion to the Sertoli cell, at least an onset of apical ES disruption by 8 hr after adjudin treatment, so that spermatids are depleted at later time points. This possibility is supported by studies that have illustrated a disruption on the spatiotemporal expression of actin regulatory proteins Arp3, Eps8, and palladin in ∼5- to 24-hr following adjudin treatment,68-70 which subsequently perturbs F-actin organization, leading to eventual apical ES breakdown. In (B), this is a stage XIV tubule because meiosis is detected (meiotic germ cells are annotated by green arrowheads). Also, many spermatids have lost their polarity, recognized by heads, which are no longer pointing toward the basement membrane (annotated by yellow arrowheads in color-boxed areas which are the corresponding magnified images shown on the left panel). Scale bars: (A), 40 μm, and 150 μm in inset; (B), 40 μm, and 20 μm in inset, which applies to other insets in this panel.
Figure 4.
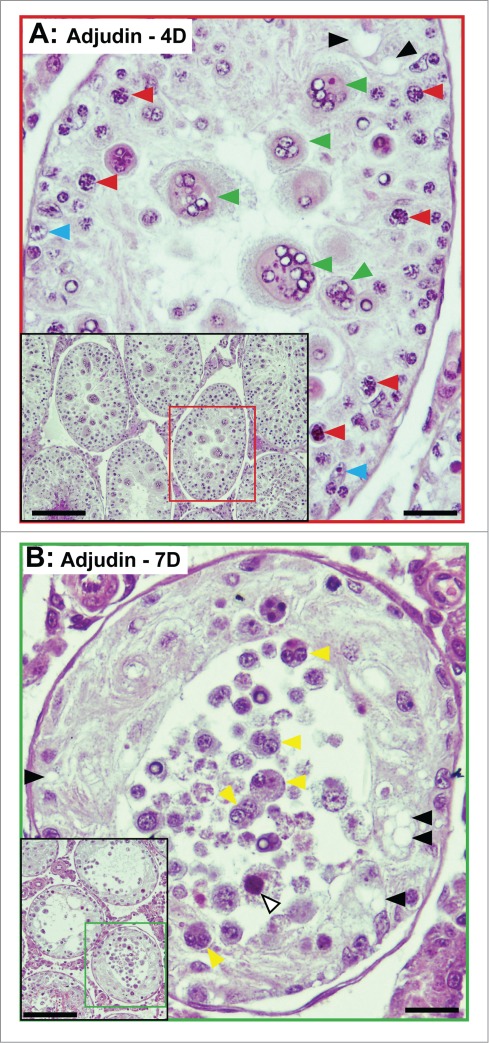
Sloughing of round spermatids (A) and spermatocytes (B) from the seminiferous epithelium of rat testes by 4- and 7-day after a single dose of adjudin (50 mg/kg b.w., by oral gavage). Following the loss of elongating/elongated spermatids which begins to take place in hours following exposure to adjudin, the sloughing of round spermatids (A) and spermatocytes is noted by (B) ∼4D (day) and 7D, respectively. In (A), multinucleate round spermatid cells (green arrowheads), illustrating degenerating germ cells are shown. Spermatocytes (red arrowheads) and Sertoli cells (blue arrowheads) are also noted. In (B), multinucleate spermatocytes (yellow arrowheads), illustrating degenerating spermatocytes (annotated by white arrowhead) are shown. Sertoli cell vacuoles (annotated by black arrowheads) are also noted in both (A) and (B), illustrating Sertoli cell focal injury has occurred. These micrographs are magnified images of the corresponding tubules shown in insets. Scale bars, 40 μm; 150 μm in insets.
Figure 5.
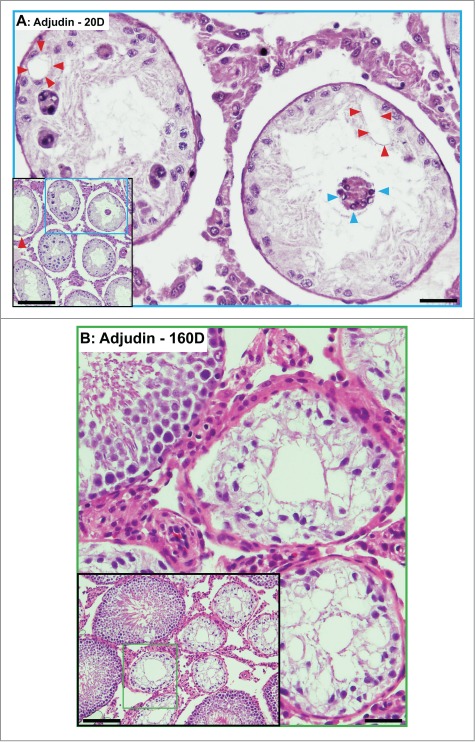
Phenotypes of the seminiferous epithelium in the testes of adult rats by day 20 (A) and day 160 (B) after treatment with a single dose of adjudin (50 mg/kg b.w., by oral gavage). (A) By day 20, tubules are virtually devoid of advanced germ cells. Only Sertoli cells and spermatogonia are found in the seminiferous epithelium. Occasionally, some multinucleate giant cells, such as multinucleate round spermatids are found (blue arrowheads). Vacuolization of seminiferous epithelium, illustrating Sertoli cell focal injury, is noted (red arrowheads). (B) By day 160, signs of rebounding of spermatogenesis is detected. As noted in the inset, at least 4 out of 10 tubules shown display signs of spermatogenesis with notable presence of elongating/elongated spermatids. Micrographs are magnified images from the corresponding boxed area shown in inset. Scale bars, 150 μm and 40 μm in insets.
Ectoplasmic specialization (ES), tubulobulbar complex (TBC), actin- and microtubule-based cytoskeletons
Once apical ES appears at the interface of step 8 spermatids and Sertoli cells at stage VIII of the epithelial cycle, it replaces the desmosome and gap junction, and it is the only anchoring device for spermatids until the step 19 spermatids are transformed to spermatozoa shortly before their release at spermiation at late stage VIII of the epithelial cycle.58,60,64,73,74 However, at late stage VII, the concave (ventral) side, but not the convex (dorsal) side, of the step 19 spermatid head begins to undergo extensive endocytic vesicle-mediated trafficking, converting apical ES at this site to a transitional structure known as the apical tubulobulbar complex (apical TBC).75-77 In short, apical TBC is an invagination of the plasma membranes of Sertoli cell and spermatid, which represents giant endocytic vesicles to facilitate the events of endocytosis, transcytosis and recycling so that “old” Sertoli apical ES proteins can be recycled to assemble “new” apical ES that will arise around the upcoming generation of elongating spermatids in stage VIII tubules.58,76 This concept is supported by the abundant presence of proteins known to be involved in endocytic vesicle-mediated trafficking events, including clathrin, cortactin, N-WASP, vinculin, zyxin and others at the apical TBC.70,78-81 Similar ultrastructure known as basal TBC derived from basal ES is also detected at the BTB, which is also used to facilitate endocytic vesicle-mediated trafficking to recycle adhesion proteins from the “old” to the “new” BTB during the transport of preleptotene spermatocytes across the immunologic blood-tubule barrier.82,83 Thus, proteins can be rapidly “recycled” without requiring de novo synthesis of proteins to assemble new apical or basal ES during the epithelial cycle.
Besides serving as an anchoring device, ES is crucial to facilitate spermatid transport across the adluminal compartment during spermiogenesis and also preleptotene spermatocyte transport at the BTB.64,84-86 Since late spermatocytes and post-meiotic spermatids are non-motile cells, they rely on the Sertoli cell for their transport to move from the base of the adluminal compartment to the edge of the tubule lumen while differentiating into more advanced germ cell types. It is now generally accepted that the cargoes (i.e., spermatids or preleptotene spermatocytes) are being transported by the vehicle (i.e., actin-based cytoskeleton with the motor proteins which likely serve as the engine) along the track (i.e., microtubules and the associated motor proteins) in Sertoli cells.87-91 Thus, it is not surprising that microtubules are tightly associated with actin microfilaments in the Sertoli cell and they are 2 inseparable entities which support spermatid transport. It is noted that microtubules in the Sertoli cell are a highly polarized cytoskeleton in which their plus (+) and minus (−) ends are located to the basal and the apical region of the seminiferous epithelium, respectively, and they are stabilized by various microtubule-associated proteins (MAP) such as MARK4 (microtubule affinity regulating kinase 4).87,92 Studies have shown that germ cell sloughing induced by some toxicants, such as colchicine and carbendazim, follows Sertoli cell microtubule disruption,93-97 in which carbendazim exerts its effects by blocking tubulin polymerization98 (also see Johnson, this issue). Furthermore, this effect is stage-specific, and spermatids that are embedded deep inside the seminiferous epithelium, such as stages I-V, are less susceptible to carbendazim-induced loss.99 Treatment of rats with glycerol via intratesticular injection100,101 is also known to disrupt microtubules and actin microfilaments.26 In fact, studies have shown that microtubules are a primary target of numerous toxicants.93,102,103 Adjudin also induces mis-localization of MARK4 (a microtubule stabilizing protein) in the rat testis, so that MARK4 at the apical ES in adjudin-treated rats no longer localizes to the concave (ventral) side of spermatid heads. Instead MARK4 diffuses away from the apical ES within 6–12 hr following adjudin exposure, and by 24 hr, the expression of MARK4 is considerably down-regulated.104 Also, MARK4 is considerably diminished and virtually non-detectable in spermatid heads that have lost their polarity 12–24 hrs following adjudin exposure, and these spermatids also begin their sloughing from the epithelium.104 Much work is needed to better understand the role of microtubule in anchoring junction integrity and spermatid transport during spermatogenesis and toxicant-induced aspermatogenesis. Also, the molecule(s) and the molecular mechanism(s) that provide the proper cross-talk between microtubules and microfilaments to elicit spermatid transport remain to be investigated.
Desmosome and intermediate-filament based cytoskeleton
The desmosome is considered to be one of the strongest adhesive junctions at the cell-cell interface in mammals,105 most notably in the skin106,107 when the force that required to disrupt ES vs. desmosome was quantified using a micropipette pressure transducing system.48 In the testis, adhesion of spermatogonia, spermatocytes and pre-step 8 spermatids to the Sertoli cell in the seminiferous epithelium is largely dependent on desmosomes which utilize intermediate filaments for attachment.108-111 The desmosome is also a structural component of the BTB.47,85 In the Sertoli cell, intermediate filaments are constituted almost exclusively by vimentin112 vs. keratins found in other cells and/or tissues such as the skin, neurons and intestines.106 Desmosome is also an emerging platform for cell signaling functions.106,113 Despite the presumed structural significance of desmosome, mice lacking vimentin are known to develop and reproduce normally without impaired spermatogenesis,114 illustrating that its function can be superseded by other junctions in the testis, such as the gap junction which is an actin-based communicating junction.115,116 These findings are not entirely unexpected since studies have shown that gap junction in the testis also shares some of the common features of desmosomes,111,117 suggesting that gap junction in the testis can be more than an intercellular communication junction. Furthermore, these findings suggest that the vimentin-based intermediate filaments that constitute desmosome in the testis are structurally and functionally engaged with the actin-based gap junction. In the adult rat testis, vimentin is localized intensely near the basement membrane, encircling the entire Sertoli cell nucleus, but relatively weak staining of vimentin is detected in the adluminal compartment.89,118 In fact, desmosome is an integrated component of the BTB, and its function is likely to be tightly coupled with the actin-based cytoskeleton to confer the immunological barrier function via signaling proteins that are recruited to the desmosome.109 Interestingly, few studies are found in the literature, in particular the molecular mechanism(s), that specifically investigate changes in the intermediate filaments following exposure of animals to toxicants including adjudin except for studies based on the exposure of rodents to 2,5-hexanedione,118 a known microtubule stabilizing toxicant.119
Lonidamine, a Derivative of Indazole-Carboxylic Acid and a Sibling of Adjudin
Adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide (Fig. 1), is a derivative of lonidamine, an indazole-based compound first synthesized in the 1970s that was shown to have potent antispermatogenic activity.120,121 Adjudin was selected from more than 2-dozen lonidamine analogs that were synthesized in the 1990s. The goal was to use an in vivo assay to identify a compound that could disrupt spermatid adhesion in the mammalian testis and result in infertility without the toxicity of lonidamine, serving as a potential non-hormonal male contraceptive. The assay was developed based on the observation that the level of testin, a signaling molecule highly expressed in the testis and ovary, was transiently up-regulated (usually within 4–6 h after treatment) whenever spermatid adhesion was disrupted; however, if the testin level remained elevated past ∼6 hr, that chemical entity usually produced considerable toxicity.42,121-123 Adjudin was selected based on this unique activity. Indeed, a subsequent study has shown that adjudin disrupts apical ES selectively since it preferentially perturbs apical ES adhesion and leaves the desmosomes unaffected, at least for a later time.49
Adjudin Disrupts the Cytoskeleton and Sertoli–Germ Cell Junctions
Adjudin initially affects the Sertoli cell actin cytoskeleton, which is followed by the disassembly of adhesion protein complexes at the Sertoli cell–spermatid interface and the sloughing of premature germ cells, most notably elongating/elongated spermatids, from the seminiferous epithelium.44,46,66,68,71 With continued dosing, non-specific morphological changes such as germ cell degeneration and sloughing, seminiferous tubule atrophy, and focal Sertoli cell injury develop (Figs. 2, 3, 4 and 5). These non-specific changes are analogous to those seen after repeated exposure of rodents to other testicular toxicants, such as cadmium, phthalates, 2,5-hexandione, and glycerol, at both cellular and molecular levels.2,24-26,28,93,102,103,124-126 In addition, the use of adjudin-treated rats has become a valuable in vivo model to study the regulation of spermatid adhesion to the Sertoli cell, and the transport of spermatids across the adluminal compartment during the epithelial cycle of spermatogenesis, as well as spermatid polarity.58,73,127
Studies have shown that adjudin effectively induces germ cell loss from the seminiferous epithelium121,123 (Fig. 2). In fact, ∼6 to 8 hr following exposure of rats to a single dose of adjudin at 50 mg/kg b.w. by oral gavage, over 50% of the tubules display signs of elongating/elongated spermatid loss from the seminiferous epithelium46,51,85 (Figs. 2, 3). While adjudin preferentially and effectively disrupts the apical ES, non-apcial ES anchoring junctions at the Sertoli-spermatocyte/round spermatid interface are also found to be perturbed but at a later time. This notion is supported by the observation that depletion of round spermatids and spermatocytes occurs by day 3–4 and day 7, respectively,51 instead of within ∼6–8 hr; and by day 3–7, elongating/elongated spermatids are no longer seen in virtually any tubule examined (Fig. 4), consistent with the postulate that apical ES is an early target whereas desmosome/gap junction may be a later target. It is noted that the adhesion of spermatocytes and step 1–7 spermatids to the Sertoli cell in the seminiferous epithelium is supported by intermediate filament-based desmosome and actin-based gap junction.85,108-110 The unusual adhesive strength of the apical ES vs. desmosome is supported by studies in which the force required to pull post step 8 spermatids from the Sertoli cell is at least twice as much as that required to pull pre-step 8 spermatids or spermatocytes away from the Sertoli cell.48 Yet following exposure of Sertoli-germ cell cocultures to adjudin, less than half of the force is needed to disrupt the apical ES vs. desmosome,49 illustrating apical ES is most sensitive to adjudin treatment. These findings thus support the notion that apical ES is more susceptible to adjudin vs. the desmosome and/or gap junction even though ES is considered to be one of the strongest anchoring junctions due to the presence of the array of actin filament bundles.59,128
Interestingly, spermatogonial adhesion is largely unaffected in rats treated with adjudin and the population of spermatogonia in the seminiferous tubule remains relatively unaltered following adjudin exposure.52 A possible explanation for the lack of response of spermatogonia/spermatogonial stem cell (SSC) adhesion to adjudin is because these cells are located under the Sertoli cell BTB, or at the stem cell niche, namely at the juncture where three seminiferous tubules meet, adjacent to the microvessels in the interstitial space. But it is also possible that there are some yet-to-be defined adhesion protein complexes at the Sertoli-spermatogonia/SSC interface that are not susceptible to adjudin. For instance, studies by molecular modeling have identified α6β1-integrin, one of the best studied apical ES adhesion protein,129-132 possesses a putative docking domain for adjudin,133 suggesting that there is specific interaction of adjudin and adhesion proteins at the apical ES. If the predominant adhesion protein(s) at the Sertoli-spermatogonia/SSC interface is lacking a docking domain for adjudin, it becomes non-responsive to adjudin treatment. This postulate also explains the lack of cell depletion found in other organs, such as liver and kidney, in subchronic toxicity studies of adjudin in both male and female rats.134
As shown in Figure 2, within 8–12 hr following adjudin treatment, the tubule lumen in virtually all the seminiferous tubules is filled with elongating/elongated spermatids regardless of the stage of the epithelial cycle (see also Fig. 3), suggesting an onset of the apical ES disruption that eventually leads to spermatid depletion from the epithelium that is clearly visible by 4 d. This possibility is supported by findings in which the spatiotemporal expression of branched actin inducing protein Arp, and actin microfilament bundling proteins Eps8, and palladin, are all perturbed at the apical ES within 5- to 24-hr following adjudin treatment,68-70 which in turn facilitate the subsequent disorganization of actin microfilament bundles at the apical ES, leading to spermatid sloughing. In this context, it is of interest to note that the histological appearance at 8- to 12-h following adjudin treatment in which the tubular lumens have all closed down is plausible due to reduced fluid secretion by the Sertoli cell in the tubule, rather than the lumen being occluded by sloughed germ cells (Fig. 3). This possibility must be carefully evaluated in future studies to examine changes in fluid secretion by Sertoli cells following adjudin treatment. Also, there are no signs of an increase in phagocytic activity, such as an increase in the number of phagosomes in the epithelium, illustrating these depleting germ cells are not subjected to phagocytosis, at least not extensively. In this context, it is of interest to note that this phenotype of actin microfilament defragmentation at the ES also mimics the cadmium-induced defragmentation of actin filaments at the Sertoli cell-cell interface in the rat testis,28 also known as basal ES that constitute the BTB.47 This latter finding is also consistent with earlier reports demonstrating a disruption of occludin-based TJ-fibrils (note: TJ is also an actin-based occluding junction using F-actin for its attachment, see Table 1) at the Sertoli cell-cell interface at the basal ES/BTB in rats exposed to an acute dose of either CdCl2 (via i.p.)25 or glycerol (via intratesticular administration).26 Due to the adjudin-induced defects at the apical ES, which is known to confer spermatid polarity, many spermatids also become mis-oriented and the heads of elongating/elongated spermatids are no longer pointing toward the basement membrane, but are either parallel to the basement membrane or point toward the tubule lumen (Fig. 3B). A recent report using human Sertoli cells cultured in vitro has also demonstrated toxicant-induced actin microfilament disruption in these cells, which is likely mediated by changes in the localization of actin bundling/barbed end capping protein Eps8 and branched actin polymerization protein Arp3 following exposure of human Sertoli cells to either cadmium or BPA,11 consistent with a study using rat primary Sertoli cells following exposure to PFOS.14 Collectively, these findings thus support the concept that at least some toxicants likely mediate germ cell sloughing via a disruption on the spatiotemporal and/or distribution of actin regulatory proteins (e.g.,, branched actin inducing protein Arp3, and actin bundling proteins Eps8, palladin and others) at the apical ES.
Disruption of the Sertoli cell blood-testis barrier (BTB)
When rats are exposed to acute doses of cadmium (administered via i.p.)25,27,28 or glycerol26 (via intratesticular injection), the BTB is irreversibly damaged via disruption of the actin microfilaments, microtubules and also TJ-fibrils at the site, causing irreversible infertility. However, a generally accepted view regarding cadmium toxicity in the testis is that cadmium mediates its effects through the vascular system by reducing blood flow, increasing microvessel permeability, causing interstitial edema and leading to ischemic damage to the Sertoli cells, thereby causing breakdown of the BTB,135 and the toxicity to the cytoskeleton is likely to be an indirect effect due to shut down of blood flow. However, it was first reported that cadmium induced BTB disruption prior to microvessel damage in the rat.27 A subsequent kinetics study in adult rats (cadmium chloride at 3 mg/kg b.w.; i.p.) based on histological analysis that monitored erythrocyte leakage into the interstitial space, coupled with electron microscopy to assess endothelial TJ-barrier disruption vs. the Sertoli cell BTB has shown that the BTB was damaged at least ∼12–14 hr prior to endothelial TJ-barrier disruption in microvessels in the interstitium.28 These findings thus suggest that the Sertoli cell is somehow highly susceptible to cadmium toxicity. Interestingly, in adjudin-treated rats, the BTB integrity remains robust until at least 2-wk after treatment, perhaps due to the presence of 2 arrays of actin microfilament bundles on both sides of the adjacent Sertoli cells at the basal ES that create the immunologic barrier.52,85 Studies have shown that the BTB is disrupted after 6-wk following adjudin exposure, but the damage is transient because the disrupted BTB is resealed by 20-wk, unless a high dose of adjudin, such as 250 mg/kg b.w. is used vs. 50 mg/kg b.w., and this high acute dose of adjudin renders the BTB irreversibly disrupted, and BTB damage is detected as early as 2-wk.52 Even though the population of spermatogonial stem cells/undifferentiated spermatogonia in the rats that are subjected to a high acute dose of adjudin remains comparable to control rats, spermatogenesis fails to resume possibly due to a permanently damaged BTB,52 consistent with findings of rats exposed to acute doses of either cadmium or glycerol. It is of interest to note that even though the BTB was transiently compromised by adjudin, resident macrophages were not detected in the seminiferous epithelium (see Figs. 3–5),52 unlike autoimmune orchitis that occurs spontaneously or induced by vasectomy or by immunization with testis antigens, in which macrophages are capable of entering the adluminal compartment following a disruption of the BTB.136-139 These findings seemingly suggest that the nature of disruption at the BTB, such as a transient vs. a permanent disruption, determines if macrophages are freely permeable to the disrupted immunological barrier.
Concluding Remarks
As noted herein, many of the pathological findings in the testis induced by exposure of rodents to toxicants are also detected in the rat testis following exposure to adjudin – a male contraceptive actively under investigation in our laboratory.42,44,46,85,108 These observations illustrate that these pathological changes are likely the physiological consequences in response to agents that exert their effects in the seminiferous epithelium behind the BTB. While many toxicants appear to exert their effects at the cell junction level, it is likely that these changes are secondary to the disruption of the actin-, intermediate filament- and/or microtubule-based cytoskeleton since adhesion protein complexes at the Sertoli cell-cell or Sertoli-germ cell interface use either actin or intermediate filament for their attachment, whereas microtubules are most notably used for spermatid transport and the transport of other essential organelles in the Sertoli cell cytosol during the epithelial cycle including endosome-based vesicles.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author is grateful to the critical reading and comments of Dr. Dolores Mruk during the preparation of this manuscript. The author also thanks all the former and current members of his laboratory for their contribution through countless hours of dedicated research in the laboratory to understand the effects of toxicants including adjudin on cell junctions and cytoskeletons in the testis, many of their original research papers are also cited herein. The author are also indebted to the constructive and thoughtful comments of Drs. Dianne Creasy and Robert Chapin during the preparation and revision of this manuscript.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5; RO1 HD056034).
References
- 1. Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci 2011; 32:290-9; PMID:21324536; http://dx.doi.org/ 10.1016/j.tips.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong EWP, et al. Cell junctions in the testis as targets for toxicants. In: Comprehensive Toxicology. 2nd Edition (McQueen CA, Ed; Series Editor); Vol. 11 Reproductive and Endocrine Toxicology; Hoyer PB, Richburg JH. (Ed.); Oxford: Academic Press, Elseiver, 2010; pp. 167-88. [Google Scholar]
- 3. Atchison WD. Effects of neurotoxicants on synaptic transmission: lessons learned from electrophysiological studies. Neurotoxicol Teratol 1988; 10:393-416; PMID:2854607; http://dx.doi.org/ 10.1016/0892-0362(88)90001-3 [DOI] [PubMed] [Google Scholar]
- 4. Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol 2009; 238:240-9; PMID:19236889; http://dx.doi.org/ 10.1016/j.taap.2009.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mruk DD, Cheng CY. Environmental contaminants. Is male reproductive health at risk? Spermatogenesis 2011; 1:283-90; PMID:22332111; http://dx.doi.org/ 10.4161/spmg.1.4.18328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng CY, Wong EW, Lie PP, Li MW, Su L, Siu ER, Yan HH, Mannu J, Mathur PP, Bonanomi M, Silvestrini B, et al. Environmental toxicants and male reproductive function. Spermatogenesis 2011; 1:2-13; PMID:21866273; http://dx.doi.org/ 10.4161/spmg.1.1.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prozialeck W. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol 2000; 164:231-49; PMID:10799334; http://dx.doi.org/ 10.1006/taap.2000.8905 [DOI] [PubMed] [Google Scholar]
- 8. Prozialeck WC, Edward JR. Cell adhesion molecules in chemical-induced renal injury. Pharmacol Ther 2007; 114:74-93; PMID:17316817; http://dx.doi.org/ 10.1016/j.pharmthera.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prozialeck WC, Grunwald GB, Dey PM, Reuhl KR, Parrish AR. Cadherins and NCAM as potential targets in metal toxicity. Toxicol Appl Pharmacol 2002; 182:255-65; PMID:12183105; http://dx.doi.org/ 10.1006/taap.2002.9422 [DOI] [PubMed] [Google Scholar]
- 10. Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and b-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol 2003; 189:180-95; PMID:12791303; http://dx.doi.org/ 10.1016/S0041-008X(03)00130-3 [DOI] [PubMed] [Google Scholar]
- 11. Xiao X, Mruk DD, Tang EI, Wong CK, Lee WM, John CM, Turek PJ, Silvestrini B, Cheng CY. Environmental toxicants perturb human Serotli cell adhesive function via changes in F-actin organization medicated by actin regulatory proteins. Hum Reprod 2014; 29:1279-91; PMID:24532171; http://dx.doi.org/ 10.1093/humrep/deu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trosko JE. Commentary on “Toxicity testing in the 21st century: a vision and a strategy”: stem cells and cell-cell communication as fundamental targets in assessing the potential toxicity of chemicals. Hum Exp Toxicol 2010; 29:21-9; PMID:20061464; http://dx.doi.org/ 10.1177/0960327109354663 [DOI] [PubMed] [Google Scholar]
- 13. Trosko JE, Chang CC, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular signalling leading to altered gap junctional intercellular communication. Toxicol Lett 1998; 102-103:71-8; PMID:10022235; http://dx.doi.org/ 10.1016/S0378-4274(98)00288-4 [DOI] [PubMed] [Google Scholar]
- 14. Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407 - an in vitro study. Endocrinology 2014; 155:249-62; PMID:24169556; http://dx.doi.org/ 10.1210/en.2013-1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pointis G, Gilleron J, Carette D, Segretain D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis 2011; 1:303-17; PMID:22332114; http://dx.doi.org/ 10.4161/spmg.1.4.18392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol 2004; 18:413-21; PMID:15082077; http://dx.doi.org/ 10.1016/j.reprotox.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 17. Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 2009; 41:2302-14; PMID:19497385; http://dx.doi.org/ 10.1016/j.biocel.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures - a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol 1992; 112:51-7; PMID:1733048; http://dx.doi.org/ 10.1016/0041-008X(92)90278-Z [DOI] [PubMed] [Google Scholar]
- 19. Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 2001; 142:1878-88; PMID:11316753 [DOI] [PubMed] [Google Scholar]
- 20. Steinberger A, Klinefelter G. Sensitivity of Sertoli and Leydig cells to xenobiotics in in vitro model. Reprod Toxicol 1993; 7 (Suppl 1):23-37; PMID:8400637; http://dx.doi.org/ 10.1016/0890-6238(93)90066-G [DOI] [PubMed] [Google Scholar]
- 21. Grima J, Cheng C. Testin induction: the role of cyclic 3’,5’-adenosine monophosphateprotein kinase A signaling in the regulation of basal and lonidamine-induced testin expression by rat Sertoli cells. Biol Reprod 2000; 63:1648-60; PMID:11090432; http://dx.doi.org/ 10.1095/biolreprod63.6.1648 [DOI] [PubMed] [Google Scholar]
- 22. Qiu L, Zhang X, Zhang X, Zhang Y, Gu J, Chen M, Zhang Z, Wang X, Wang SL. Sertoli cell is a potential target for perfluorooctane sulfonate-induced reproductive dysfunction in male mice. Toxicol Sci 2013; 135:229-40; PMID:23761298; http://dx.doi.org/ 10.1093/toxsci/kft129 [DOI] [PubMed] [Google Scholar]
- 23. Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-b3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 2003; 144:1139-42; PMID:12639893; http://dx.doi.org/ 10.1210/en.2002-0211 [DOI] [PubMed] [Google Scholar]
- 24. Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci 2004; 117:783-98; PMID:14734653; http://dx.doi.org/ 10.1242/jcs.00900 [DOI] [PubMed] [Google Scholar]
- 25. Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod 1993; 49:840-9; PMID:8218650; http://dx.doi.org/ 10.1095/biolreprod49.4.840 [DOI] [PubMed] [Google Scholar]
- 26. Wiebe J, Kowalik A, Gallardi R, Egeler O, Clubb B. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl 2000; 21:625-35; PMID:10975408 [PubMed] [Google Scholar]
- 27. Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol 1970; 47:81-6; PMID:5428920; http://dx.doi.org/ 10.1677/joe.0.0470081 [DOI] [PubMed] [Google Scholar]
- 28. Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by a2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology 2005; 146:1893-908; PMID:15618353; http://dx.doi.org/ 10.1210/en.2004-1464 [DOI] [PubMed] [Google Scholar]
- 29. Puri P, Walker WH. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol Reprod 2013; 88:59; PMID:23325809; http://dx.doi.org/ 10.1095/biolreprod.112.104414 [DOI] [PubMed] [Google Scholar]
- 30. Lui WY, Lee WM, Cheng CY. Transforming growth factor-b3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 2003; 68:1597-612; PMID:12606350; http://dx.doi.org/ 10.1095/biolreprod.102.011387 [DOI] [PubMed] [Google Scholar]
- 31. Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 2011; 43:651-65; PMID:21256972; http://dx.doi.org/ 10.1016/j.biocel.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol 2012; 763:295-317; PMID:23397631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan HT, Mruk DD, Wong CKC, Cheng CY. The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol Med 2013; 19:396-405; PMID:23643465; http://dx.doi.org/ 10.1016/j.molmed.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wan HT, Mruk DD, Wong CKC, Cheng CY. Targeting testis-specific proteins to inhibit spermatogenesis - lession from endocrine disrupting chemicals. Expert Opin Ther Targets 2013; 17:839-55; PMID:23600530; http://dx.doi.org/ 10.1517/14728222.2013.791679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li JCH, Mruk DD, Cheng CY. The inter-Sertoli tight junction permeability barrier is regulated by the inter-play of protein phosphatases and kinases: an in vitro study. J Androl 2001; 22:847-56; PMID:11545299 [PubMed] [Google Scholar]
- 36. Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 2014; 30:2-13; PMID:24598768; http://dx.doi.org/ 10.1016/j.semcdb.2014.02.012 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1557-69; PMID:20403869; http://dx.doi.org/ 10.1098/rstb.2009.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li MWM, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med 2009; 15:159-68; PMID:19303360; http://dx.doi.org/ 10.1016/j.molmed.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lie PPY, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem Sci 2009; 34:366-73; PMID:19535250; http://dx.doi.org/ 10.1016/j.tibs.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis - a molecular and biochemical perspective. Cytokine Growth Factor Rev 2007; 18:299-311; PMID:17521954; http://dx.doi.org/ 10.1016/j.cytogfr.2007.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LoPachin RM, Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci 2006; 94:240-55; PMID:16880199; http://dx.doi.org/ 10.1093/toxsci/kfl066 [DOI] [PubMed] [Google Scholar]
- 42. Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception 2005; 72:251-61; PMID:16181968; http://dx.doi.org/ 10.1016/j.contraception.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 43. Cheng CY, Mruk DD. New frontiers in non-hormonal male contraception. Contraception 2010. 82:476-82; PMID:20933122; http://dx.doi.org/ 10.1016/j.contraception.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab 2004; 15:439-47; PMID:15519891; http://dx.doi.org/ 10.1016/j.tem.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 45. Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminifeorus epithelium of mammalian testes. Reproduction 2011; 141:571-80; PMID:21307270; http://dx.doi.org/ 10.1530/REP-10-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 2008; 60:146-80; PMID:18483144; http://dx.doi.org/ 10.1124/pr.107.07105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 2012; 64:16-64; PMID:22039149; http://dx.doi.org/ 10.1124/pr.110.002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 2005; 26:354-9; PMID:15867003; http://dx.doi.org/ 10.2164/jandrol.04142 [DOI] [PubMed] [Google Scholar]
- 49. Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by adjudin. J Androl 2006; 27:790-4; PMID:16809272; http://dx.doi.org/ 10.2164/jandrol.106.000422 [DOI] [PubMed] [Google Scholar]
- 50. Cheng CY, Lie PPY, Wong EWP, Mruk DD. Focal adhesion kinase and actin regulatorybinding proteins that modulate F-actin organization at the tissue barrier. Lession from the testis. Tissue Barriers 2013; 1:e24252; PMID:24665388; http://dx.doi.org/ 10.4161/tisb.24252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinaseFer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod 2003; 69:656-72; PMID:12700184; http://dx.doi.org/ 10.1095/biolreprod.103.016881 [DOI] [PubMed] [Google Scholar]
- 52. Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 2012; 35:86-101; PMID:21696392; http://dx.doi.org/ 10.1111/j.1365-2605.2011.01183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell, New York: Garland Science, 2002. [Google Scholar]
- 54. Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev 1994; 15:102-15; PMID:8156935 [DOI] [PubMed] [Google Scholar]
- 55. Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays 2004; 26:978-92; PMID:15351968; http://dx.doi.org/ 10.1002/bies.20099 [DOI] [PubMed] [Google Scholar]
- 56. Tarakanov AO, Goncharova LB. Cell-cell nanotubes: Tunneling through several types of synapses. Commun Integr Biol 2009; 2:359-61; PMID:19721891; http://dx.doi.org/ 10.4161/cib.2.4.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lou E, Fujisawa S, Barlas A, Romin Y, Manova-Todorova K, Moore MA, Subramanian S. Tunneling nanotubes. A new paradigm for studying intercellular communication and therapeutics in cancer. Commun Integr Biol 2012; 5:399-403; PMID:23060969; http://dx.doi.org/ 10.4161/cib.20569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol 2010; 6:380-95; PMID:20571538; http://dx.doi.org/ 10.1038/nrendo.2010.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell 1977; 9:475-98; PMID:929577; http://dx.doi.org/ 10.1016/0040-8166(77)90007-6 [DOI] [PubMed] [Google Scholar]
- 60. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11 [DOI] [PubMed] [Google Scholar]
- 61. Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 2012; 763:237-59; PMID:23397628 [PubMed] [Google Scholar]
- 62. Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 1977; 148:313-28; PMID:857632; http://dx.doi.org/ 10.1002/aja.1001480303 [DOI] [PubMed] [Google Scholar]
- 63. Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat 1979; 155:259-79; PMID:474448; http://dx.doi.org/ 10.1002/aja.1001550208 [DOI] [PubMed] [Google Scholar]
- 64. Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177-211; PMID:3894273; http://dx.doi.org/ 10.1016/S0074-7696(08)60397-6 [DOI] [PubMed] [Google Scholar]
- 65. Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 2008; 105:9657-62; PMID:18621709; http://dx.doi.org/ 10.1073/pnas.0801527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis 2011; 1:137-46; PMID:22319662; http://dx.doi.org/ 10.4161/spmg.1.2.16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mruk DD, Lau ASN. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod 2009; 80:590-601; PMID:19074001; http://dx.doi.org/ 10.1095/biolreprod.108.071647 [DOI] [PubMed] [Google Scholar]
- 68. Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology 2013; 154:1907-20; PMID:23546604; http://dx.doi.org/ 10.1210/en.2012-2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555-67; PMID:19293393; http://dx.doi.org/ 10.1096/fj.06-070573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 2010; 107:11411-6; PMID:20534520; http://dx.doi.org/ 10.1073/pnas.1001823107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp23 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis 2011; 1;291-7; PMID:22332112; http://dx.doi.org/ 10.4161/spmg.1.4.18393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 2012; 109:12562-7; PMID:22797892; http://dx.doi.org/ 10.1073/pnas.1202316109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 2009; 44:245-63; PMID:19622063; http://dx.doi.org/ 10.1080/10409230903061207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 2011; 1:14-35; PMID:21866274; http://dx.doi.org/ 10.4161/spmg.1.1.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec 1979; 194:233-46; PMID:464324; http://dx.doi.org/ 10.1002/ar.1091940205 [DOI] [PubMed] [Google Scholar]
- 76. Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol 2013; 303:319-55; PMID:23445814; http://dx.doi.org/ 10.1016/B978-0-12-407697-6.00008-8 [DOI] [PubMed] [Google Scholar]
- 77. Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec 1979; 194:213-32; PMID:464323; http://dx.doi.org/ 10.1002/ar.1091940204 [DOI] [PubMed] [Google Scholar]
- 78. Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod 2009; 80:153-61; PMID:18799755; http://dx.doi.org/ 10.1095/biolreprod.108.070615 [DOI] [PubMed] [Google Scholar]
- 79. Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod 2009; 80:162-74; PMID:18799754; http://dx.doi.org/ 10.1095/biolreprod.108.070623 [DOI] [PubMed] [Google Scholar]
- 80. Young JS, Takai YK, Kojic KL, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction 2012; 143:347-57; PMID:22157319; http://dx.doi.org/ 10.1530/REP-11-0317 [DOI] [PubMed] [Google Scholar]
- 81. Young JS, Vogl AW. Focal adhesion proteins zyxin and vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis 2012; 2:63-8; PMID:22553491; http://dx.doi.org/ 10.4161/spmg.19391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Du M, Young J, De Asis M, Cipollone J, Roskelley C, Takai Y, Nicholls PK, Stanton PG, Deng W, Finlay BB, et al. A novel subcellular machine contributes to basal junction remodeling in the seminiferous epithelium. Biol Reprod 2013; 88:60; PMID:23303684; http://dx.doi.org/ 10.1095/biolreprod.112.104851 [DOI] [PubMed] [Google Scholar]
- 83. Xiao X, Mruk DD, Wong EW, Lee WM, Han D, Wong CK, Cheng CY. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier. Am J Physiol Endocrinol Metab 2014; 307:E553-62; PMID:25117412; http://dx.doi.org/ 10.1152/ajpendo.00176.2014; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell 1989; 21:361-79; PMID:2479117; http://dx.doi.org/ 10.1016/0040-8166(89)90051-7 [DOI] [PubMed] [Google Scholar]
- 85. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747-806; PMID:15466940; http://dx.doi.org/ 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- 86. Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays 2007; 29:36-48; PMID:17187371; http://dx.doi.org/ 10.1002/bies.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tang EI, Mruk DD, Cheng CY. MAPmicrotubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol 2013; 217:R13-23; PMID:23449618; http://dx.doi.org/ 10.1530/JOE-12-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis 2013; 3:e23473; PMID:23687615; http://dx.doi.org/ 10.4161/spmg.23473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wan HT, Mruk DD, Tang EI, Xiao X, Cheng YH, Wong EW, Wong CK, Cheng CY, et al. Role of non-receptor protein kinases in spermatid transport during spermatogenesis. Sem Cell Dev Biol 2014; 30:65-74; PMID:24727349; http://dx.doi.org/ 10.1016/j.semcdb.2014.04.013; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations). J Cell Sci 2000; 113:2167-76; PMID:10825290 [DOI] [PubMed] [Google Scholar]
- 91. Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update 2004; 10:349-69; PMID:15192055; http://dx.doi.org/ 10.1093/humupd/dmh026 [DOI] [PubMed] [Google Scholar]
- 92. O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol 2014; 30:45-54; PMID:24440897; http://dx.doi.org/ 10.1016/j.semcdb.2014.01.003; (in press) [DOI] [PubMed] [Google Scholar]
- 93. Boekelheide K, Neely MD, Sioussat TM. The Sertoli cell cytoskeleton: a target for toxicant-induced germ cell loss. Toxicol Appl Pharmacol 1989; 101:373-89; PMID:2690397; http://dx.doi.org/ 10.1016/0041-008X(89)90188-9 [DOI] [PubMed] [Google Scholar]
- 94. Allard EK, Johnson KJ, Boekelheide K. Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biol Reprod 1993; 48:143-53; PMID:8418902; http://dx.doi.org/ 10.1095/biolreprod48.1.143 [DOI] [PubMed] [Google Scholar]
- 95. Nakai M, Hess RA. Morphological changes in the rat Sertoli cell induced by the microtubule poison carbendazim. Tissue Cell 1994; 26:917-27; PMID:7886678; http://dx.doi.org/ 10.1016/0040-8166(94)90041-8 [DOI] [PubMed] [Google Scholar]
- 96. Lim JP, Miller MG. The role of the benomyl metabolite carbendazim in benomyl-induced testicular toxicity. Toxicol Appl Pharmacol 1997; 142:401-10; PMID:9070363; http://dx.doi.org/ 10.1006/taap.1996.8042 [DOI] [PubMed] [Google Scholar]
- 97. Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, Linder RE. Acute and long-term effects of a single dose of the fungicide carbendazim (methyl 2-benzimidazole carbamate) on the male reproductive system in the rat. J Androl 1992; 13:507-18; PMID:1293130 [PubMed] [Google Scholar]
- 98. Winder BS, Strandgaard CS, Miller MG. The role of GTP binding and microtubule-associated proteins in the inhibition of microtubule assembly by carbendazim. Toxicol Sci 2001; 59:138-46; PMID:11134553; http://dx.doi.org/ 10.1093/toxsci/59.1.138 [DOI] [PubMed] [Google Scholar]
- 99. Nakai M, Miller MG, Carnes K, Hess RA. Stage-specific effects of the fungicide carbendazim on Sertoli cell microtubules in rat testis. Tissue Cell 200; 34:73-80; PMID:12165241; http://dx.doi.org/ 10.1016/S0040-8166(02)00006-X [DOI] [PubMed] [Google Scholar]
- 100. Wiebe J, Barr K. The control of male fertility by 1,2,3-trihydroxypropane (THP; glycerol): rapid arrest of spermatogenesis without altering libido, accessory organs, gonadal steroidogenesis, and serum testosterone, LH, and FSH. Contraception 1984; 29:291-302; PMID:6428807; http://dx.doi.org/ 10.1016/S0010-7824(84)80009-8 [DOI] [PubMed] [Google Scholar]
- 101. Wiebe J, Barr K, Buckingham K. Sustained azoospermia in squirrel monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception 1989; 39:447-57; PMID:2721196; http://dx.doi.org/ 10.1016/0010-7824(89)90122-4 [DOI] [PubMed] [Google Scholar]
- 102. Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, Kwon EJ, Patel SR, Rasoulpour RJ, Schoenfeld HA, et al. 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol 2003; 43:125-47; PMID:12471174; http://dx.doi.org/ 10.1146/annurev.pharmtox.43.100901.135930 [DOI] [PubMed] [Google Scholar]
- 103. Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis 2014; (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulated kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis 2012; 2:117-26; PMID:22670221; http://dx.doi.org/ 10.4161/spmg.20724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J 2010; 429:419-33; PMID:20626351; http://dx.doi.org/ 10.1042/BJ20100567 [DOI] [PubMed] [Google Scholar]
- 106. Green K, Gaudry C. Are desmosomes more than tethers for intermediate filaments? Nat Rev Cell Biol 2000; 1:208-16; PMID:11252896; http://dx.doi.org/ 10.1038/35043032 [DOI] [PubMed] [Google Scholar]
- 107. Green KJ, Simpson CL. Desmosomes: New perspectives on a classic. J Invest Dermatol 2007; 127:2499-515; PMID:17934502; http://dx.doi.org/ 10.1038/sj.jid.5701015 [DOI] [PubMed] [Google Scholar]
- 108. Cheng CY, Mruk DD. Cell junction dynamics in the testis: sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825-74; PMID:12270945 [DOI] [PubMed] [Google Scholar]
- 109. Mruk DD, Cheng CY. Desmosomes in the testis. Moving into an unchartered territory. Spermatogenesis 2011; 1:47-51; PMID:21866275; http://dx.doi.org/ 10.4161/spmg.1.1.15443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: A versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol 2011; 286:223-69; PMID:21199783 [http://dx.doi.org/ 10.1016/B978-0-12-385859-7.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat 1977; 148:301-12; PMID:857631; http://dx.doi.org/ 10.1002/aja.1001480302 [DOI] [PubMed] [Google Scholar]
- 112. Franke W, Grund C, Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol 1979; 19:269-75; PMID:385322 [PubMed] [Google Scholar]
- 113. Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2010; 2:a000125; PMID:20182611; http://dx.doi.org/ 10.1101/cshperspect.a000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 1994; 79:679-94; PMID:7954832; http://dx.doi.org/ 10.1016/0092-8674(94)90553-3 [DOI] [PubMed] [Google Scholar]
- 115. Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol 2012; 763:260-80; PMID:23397629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1607-20; PMID:20403873; http://dx.doi.org/ 10.1098/rstb.2009.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: The Sertoli Cell. eds. Russell LD, Griswold MD. Clearwater: Cache River Press, 1993, 365-90. [Google Scholar]
- 118. Hall ES, Eveleth J, Boekelheide K. 2,5-Hexanedione exposure alters the rat Sertoli cell cytoskeleton. II. Intermediate filaments and actin. Toxicol Appl Pharmacol 1991; 111:443-53; PMID:1746025; http://dx.doi.org/ 10.1016/0041-008X(91)90249-E [DOI] [PubMed] [Google Scholar]
- 119. Chapin RE, Morgan KT, Bus JS. The morphogenesis of testicular degeneration induced in rats by orally administered 2,5-hexanedione. Exp Mol Pathol 1983; 38:149-69; PMID:6832342; http://dx.doi.org/ 10.1016/0014-4800(83)90082-5 [DOI] [PubMed] [Google Scholar]
- 120. Corsi G, Palazzo G, Germani C, Scorza Barcellona P, Silvestrini B. 1-Halobenzyl-1H-indazole-3-carboxylic acids. A new class of antispermatogenic agents. J Med Chem 1976; 19:778-83; PMID:950645; http://dx.doi.org/ 10.1021/jm00228a008 [DOI] [PubMed] [Google Scholar]
- 121. Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod 2001; 65:449-61; PMID:11466213; http://dx.doi.org/ 10.1095/biolreprod65.2.449 [DOI] [PubMed] [Google Scholar]
- 122. Cheng CY, Mo My, Grima J, Saso L, Tita B, Mruk D, Silvestrini B. Indazole carboxylic acids in male contraception. Contraception 2002; 65:265-8; PMID:12020774; http://dx.doi.org/ 10.1016/S0010-7824(01)00318-3 [DOI] [PubMed] [Google Scholar]
- 123. Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod 2001; 64:1500-8; PMID:11319158; http://dx.doi.org/ 10.1095/biolreprod64.5.1500 [DOI] [PubMed] [Google Scholar]
- 124. Boekelheide K. Sertoli cell toxicants. In: The Sertoli Cell. eds. Russell L, Griswold M. Clearwater: Cache River Press, 1993, 551-75. [Google Scholar]
- 125. Boekelheide K, Hall SJ. 2,5-Hexanedione exposure in the rat results in long-term testicular atrophy despite the presence of residual spermatogonia. J Androl 1991; 12:18-26; PMID:2010348 [PubMed] [Google Scholar]
- 126. Boekelheide K, Johnson KJ, Richburg JH. Sertoli cell toxicants. In: Sertoli Cell Biology. Eds. Skinner MK, Griswold MD. New York: Elsevier Science, 2005, pp. 345-82. [Google Scholar]
- 127. Cheng CY, Wong EWP, Yan HHN, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol Cell Endocrinol 2010; 315:49-56; PMID:19682538 [http://dx.doi.org/ 10.1016/j.mce.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod 1988; 39:105-18; PMID:3207792; http://dx.doi.org/ 10.1095/biolreprod39.1.105 [DOI] [PubMed] [Google Scholar]
- 129. Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of b1 integrin subunit in rat seminiferous epithelium. Biol Reprod 1992; 47:1173-82; PMID:1283530; http://dx.doi.org/ 10.1095/biolreprod47.6.1173 [DOI] [PubMed] [Google Scholar]
- 130. Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain a6b1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod 1998; 58:371-8; PMID:9475391; http://dx.doi.org/ 10.1095/biolreprod58.2.371 [DOI] [PubMed] [Google Scholar]
- 131. Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the b1 integrinlaminin g3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod 2004; 70:945-64; PMID:14645107; http://dx.doi.org/ 10.1095/biolreprod.103.023606 [DOI] [PubMed] [Google Scholar]
- 132. Yan HHN, Cheng CY. Laminin name=a3 forms a complex with b3 and g3 chains that serves as the ligand for a6b1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 2006; 281:17286-303; PMID:16608848; http://dx.doi.org/ 10.1074/jbc.M513218200 [DOI] [PubMed] [Google Scholar]
- 133. Mok KW, Lie PP, Mruk DD, Mannu J, Mathur PP, Silvestrini B, Cheng CY. The apical ectoplasmic specialization-blood-testis barrier functional axis is a novel target for male contraception. Adv Exp Med Biol 2012; 763:334-55; PMID:23397633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nature Med 2006; 12:1323-8; PMID:17072312; http://dx.doi.org/ 10.1038/nm1420 [DOI] [PubMed] [Google Scholar]
- 135. Aoki A, Hoffer A. Re-examination of the lesions in rat testis caused by cadmium. Biol Reprod 1978; 18:579-91; PMID:207367; http://dx.doi.org/ 10.1095/biolreprod18.4.579 [DOI] [PubMed] [Google Scholar]
- 136. Taurog JD, Rival C, van Duivenvoorde LM, Satumtira N, Dorris ML, Sun M, Shelton JM, Richardson JA, Hamra FK, Hammer RE, et al. Autoimmune epididymo-porchitis is esential to the pathogenesis of male-specific spondyoarthritis in HLA-B27-transgenic rats. Arthritis Rheum 2012; 64:2518-28; PMID:22488218; http://dx.doi.org/ 10.1002/art.34480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tung KSK, Unanue ER, Dixon FJ. The immunopathology of experimental allergic orchitis. Am J Pathol 1970; 60:313-28; PMID:4918181 [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang Y, Li N, Chen Q, Yan K, Liu Z, Zhang X, Liu P, Chen Y, Han D. Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. Immunol Cell Biol 2013; 91:416-26; PMID:23689306; http://dx.doi.org/ 10.1038/icb.2013.22 [DOI] [PubMed] [Google Scholar]
- 139. Wheeler KM, Tardif S, Rival C, Luu B, Bui E, Del Rio R, Teuscher C, Sparwasser T, Hardy D, Tung KS. Regulatory T cells control tolerance versus autoimmunity to sperm in vasectomy. Proc Nat Acad Sci U S A 2011; 108:7511-6; PMID:21502500; http://dx.doi.org/ 10.1073/pnas.1017615108 [DOI] [PMC free article] [PubMed] [Google Scholar]