Abstract
Fatty acids are precursors of potent lipid signaling molecules. They are stored in membrane phospholipids and released by phospholipase A2 (PLA2). Lysophospholipid acyltransferases (ATs) oppose PLA2 by re-esterifying fatty acids into phospholipids, in a biochemical pathway known as the Lands Cycle. Drosophila Lands Cycle ATs oys and nes, as well as 7 predicted PLA2 genes, are expressed in the male reproductive tract. Oys and Nes are required for spermatid individualization. Individualization, which occurs after terminal differentiation, invests each spermatid in its own plasma membrane and removes the bulk of the cytoplasmic contents. We developed a quantitative assay to measure individualization defects. We demonstrate that individualization is sensitive to temperature and age but not to diet. Mutation of the cyclooxygenase Pxt, which metabolizes fatty acids to prostaglandins, also leads to individualization defects. In contrast, modulating phospholipid levels by mutation of the phosphatidylcholine lipase Swiss cheese (Sws) or the ethanolamine kinase Easily shocked (Eas) does not perturb individualization, nor does Sws overexpression. Our results suggest that fatty acid derived signals such as prostaglandins, whose abundance is regulated by the Lands Cycle, are important regulators of spermatogenesis.
Keywords: actin, drosophila, fatty acids, individualization, lipid metabolism, prostaglandins, spermiogenesis
Abbreviations
- PLA2
phospholipase A2
- AT
acyltransferase
- IC
individualization complex
- GSC
germline stem cell
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
Introduction
In most species, spermatozoa contribute only DNA, centrioles, and select signaling molecules to the developing zygote.1–3 In the testis, sperm are stripped of their cytoplasmic contents following terminal differentiation and acquire their characteristic streamlined shape.4 The sperm of many species develop as a syncytium.5 The mitotic and meiotic divisions of spermatogenesis end with incomplete cytokinesis, leaving intercellular bridges that permit cytoplasmic sharing among sister sperm, which are thought to synchronize differentiation and protect spermatids against the deleterious effects of haploidy.6–8 In both mammals and Drosophila, the removal of excess cytoplasm and the dissolution of intercellular bridges occur concurrently in an actin mediated process.4,9–12 This process is important for fertility, but the mechanisms that regulate it are not well understood.13
The Drosophila male gonad is organized such that the developing sperm cells are arranged chronologically within the testis tubule, permitting identification and observation of all spermatogenic stages simultaneously. The germline stem cells (GSCs) reside at the apical end of the testis, clustered around a stem cell niche called the hub.14 Each GSC divides to produce another stem cell that remains associated with the hub and a gonialblast cell that is displaced from the hub and is destined to differentiate.15 The gonialblast undergoes a series of 4 mitoses with incomplete cytokinesis to produce 16 interconnected spermatocytes that comprise the developing germ cell cyst. The spermatocytes increase their volume 25-fold and undergo 2 meiotic divisions to produce 64 spermatids.85 As differentiation proceeds, each cyst is displaced basally down the testis by newly formed gonialblasts and cysts. After meiosis is complete, the spermatid nuclei condense, and the microtubule-based axonemes elongate. Finally, spermatid individualization occurs. Throughout the course of spermatogenesis, each cyst is enveloped by 2 non-mitotic somatic cells, called cyst cells, which originate from a population of somatic stem cells located at the hub.16
Cytoplasm removal and resolution of individual sperm are mediated by an actin-rich individualization complex (IC) following axoneme elongation.17,85 Each IC is composed of 64 actin cones, which form around the 64 germ nuclei of the cyst.9,11 Once the actin cones have formed, the IC moves away from the nuclei, which are located basally in the testis, and travels along the axonemes back toward the apical end of the testis (Fig. 1A, B). As the IC progresses, the spermatids’ cytoplasmic contents accumulate in a cystic bulge around the IC.9 When the cystic bulge and associated IC reach the end of the axoneme, they form a waste bag and are degraded.17 The ICs are readily visible with fluorophore-conjugated phalloidin.11
Figure 1.
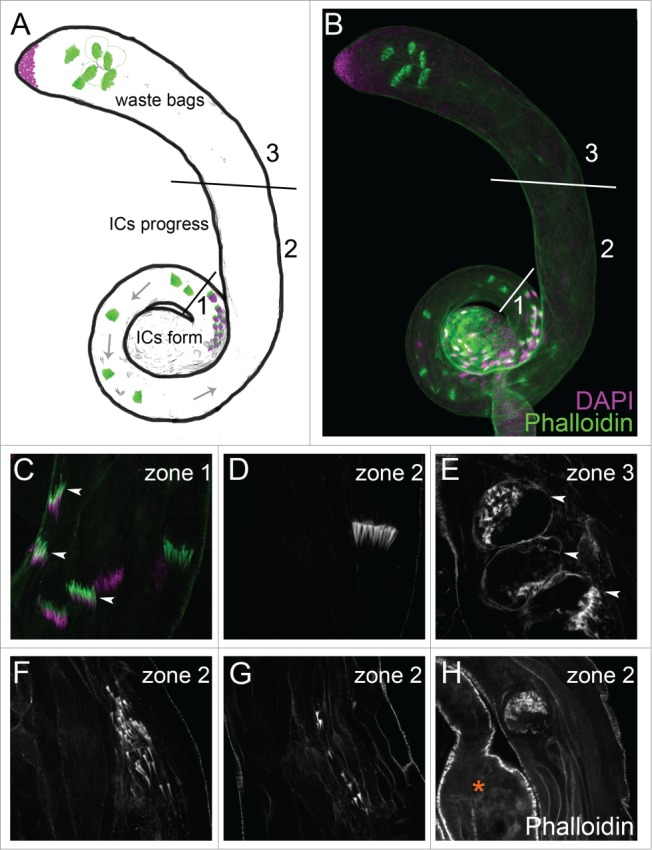
(See previous page). Characterization of normal and abnormal individualization structures. (A, B) Testes can be divided schematically into 3 domains. In zone 1, the ICs (phalloidin, green) form in apposition to the nuclei (DAPI, purple). The ICs migrate away from the nuclei along the spermatid axonemes toward the apical testis (zone 2). In zone 3, the ICs are degraded within the waste bags. (C–E) Testes from Sco/SM6;MKRS/TM6B. (C) Newly formed actin cones in zone 1 are thin and needle-shaped (arrowheads). (D) As the IC progresses, actin cones in zone 2 become conical and migrate synchronously. (E) Waste bags in zone 3 are bounded by cortical actin and contain the degenerating actin cones (arrowheads). (F-H) Testes from Canton S males reared at 29oC. Abnormal ICs contain scattered actin cones (F), unaffiliated actin cones (G), or degenerate before reaching zone 3, as indicated in the photograph by the proximity to the seminal vesicle (asterisk, H).
Many mutants that disrupt individualization have been identified.18,19 However, different mutants have distinct effects, some disrupting both nuclei and actin cones, some disrupting the morphology of the actin cones, and others perturbing the movement of the ICs without affecting the nuclei or actin cones themselves.11,20 We found previously that deletion of the lysophospholipid acyltransferases (ATs) Oysgedart (Oys) and Nessy (Nes) results in male sterility and failure of spermatid individualization.21 In oys nes double mutants, actin cone formation and morphology appear normal, but synchronous translocation of the actin cones in the IC is disrupted.
Oys and Nes regulate the metabolism of membrane phospholipids via a biochemical pathway called the Lands Cycle (Fig. 7A).21,22 Lands Cycle enzymes of the phospholipase A2 (PLA2) family catabolize phospholipids into lysophospholipids and fatty acids, which can be converted into potent short-range signaling molecules.23,24 Oys and Nes catalyze the reverse reaction, re-esterifying lysophospholipids and fatty acids into phospholipids.25,26 Both lysophospholipid and fatty acid derived signaling molecules bind G-protein coupled receptors (GPCRs) on the surface of target cells and activate a variety of downstream signal transduction cascades.27,28 They can also bind and activate nuclear receptors, such as the peroxisome proliferator-activated receptor PPAR-γ.29–31 Fatty acid derived mediators of the prostaglandin and leukotriene families are especially important in the mammalian immune and reproductive systems.32,33 The lysophospholipid derived mediator lysophosphatidic acid is important for wound healing, vascular development, and neuronal development and function.34 PLA2s and ATs are critical for regulating lipid signal availability,26 and disruptions in lipid signaling contribute to infertility, inflammation, atherosclerosis, metabolic syndrome, and cancer.35,36 Lipid signaling molecules are thought to play important roles in invertebrates as well, but their target receptors and signal transduction pathways are uncharacterized.37,38 In addition to regulating the availability of bioactive lipid mediators, the Lands Cycle modulates the molecular species composition of membrane phospholipids through acyl chain exchange.22,39 The Lands Cycle is thought to function in all cells to maintain the membranes’ physical properties (e.g. fluidity and curvature), but its regulation and its influence on cellular physiology and behavior are not well understood.40,41
Figure 7.
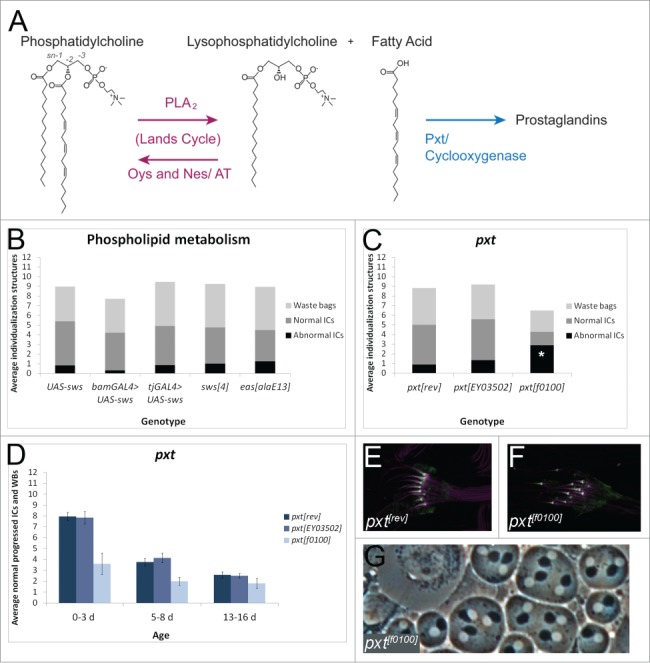
A strong pxt mutant disrupts individualization. (A) Schematic of the Lands Cycle. Long chain unsaturated fatty acids are esterified to single chain lysophospholipids, like lysophosphatidylcholine, by ATs Oys and Nes to form PC.21 When stimulated, PLA2 enzymes catabolize phospholipids to release fatty acids and lysophospholipids.23 Cyclooxygenases such as Pxt metabolize fatty acids to prostaglandin signaling molecules.83 (B) Males overexpressing UAS-Sws in the germline with bam-GAL4-VP16 (n = 18), males overexpressing UAS-Sws in the somatic cyst cells with tj-GAL4 (n = 21), males hemizygous for the strong hypomorphic sws4 allele50 (n = 36), or males hemizygous for the strong hypomorphic easalaE13 allele84 (n = 25) show no individualization defects compared to control males bearing UAS-Sws alone (n = 13). p > 0.1 for each experimental condition. (C) 0–3 d old homozygous pxtf0100 males have significantly more abnormal ICs per testis (average 2.9, n = 10) than revertant males (rev, average 0.9, n = 20), p <0.05. pxtEY03502 homozygotes do not show a significant increase in abnormal ICs (average 1.35, n = 20), p = 0.32. (D) Males homozygous for the strong hypomorphic pxtf0100 mutation55 (light blue bars) have a reduced number of normal individualization structures at 0–3 d old, 5–8 d old, and 13–16 d old, when compared to precise excision controls (rev, dark blue bars). Males homozygous for the weak pxtEY03502 mutation do not show individualization defects (medium blue bars). N ≥ 10 testes each condition. (E, F) Actin cones (purple, phalloidin) from pxtrev (E) and pxtf0100 (F) males both show normal Myosin VI localization to the front of the cones (green). (G) pxtf0100 mutants do not show cytokinesis defects in phase contrast testis squashes. p values were obtained using 2-tailed students’ t-test with unequal variance. Experiments were repeated at least twice; data shown are from a representative trial.
Here we show that oys and nes are expressed in the Drosophila male germline and that 7 PLA2 family members are expressed in the adult male reproductive tract. We introduce a quantitative method to measure defects in spermatid individualization and find that individualization is highly sensitive to temperature. Applying our methodology to other mutants reveals that individualization is sensitive to disruptions in prostaglandin metabolism but not other phospholipid metabolism pathways. Together, our results suggest that fatty acid derived signals in the testis are critical for successful completion of spermatogenesis.
Results
Characterization and quantification of individualization
To understand the individualization process in more detail, we characterized normal and abnormal ICs and waste bags. We conceptually divided the testis into 3 zones (Fig. 1A, B). In zone 1, the ICs formed around the spermatid nuclei, and nascent actin cones were thin and needle-shaped (Fig. 1C). Progressing actin cones in zone 2 accumulated actin and acquired their characteristic conical shape (Fig. 1D).42 In normal progressing ICs, the actin cones of each IC remained tightly assembled (Fig. 1D). Waste bags were distinguishable from ICs by their disorganized appearance, degenerating actin cones, and the distinct cortical actin surrounding them (Fig. 1E). Under normal conditions, waste bags formed only in zone 3, upon reaching the ends of the axonemes (Fig. 1A, B).
Under environmental or genotypic conditions that disrupt individualization (see below), abnormal ICs were observed. Abnormal ICs had loosely associated actin cones (Fig. 1F) or formed waste bag-like structures that degenerated before reaching the apical region of the testis (Fig. 1H). Testes with aberrant individualization also had many stray actin cones not associated with a distinguishable IC (Fig. 1G). In some cases, abnormal ICs appeared basally oriented instead of apically oriented.
To quantify the individualization process, we counted the number of normal progressing ICs (those not affiliated with nuclei) and waste bags in 0–3 day old males from 3 control genotypes at room temperature (23–24oC): w1118, Canton S, and the balancer stock Sco/SM6;MKRS/TM6B to control for non-specific effects from balancer chromosomes. The average number of normal progressing ICs and waste bags was similar among the 3 control genotypes (8–11 per testis, Figure 2D). This average was reproducible and consistent across many control genotypes (see below). We designated ICs having 4 or more separated actin cones as abnormal. Even with this stringent criterion, we did not observe more than 4 abnormal ICs per testis in either w1118 or the balancer stock at room temperature. Canton S showed slightly more variability, with an occasional testis displaying more than 4 abnormal ICs.
Figure 2.
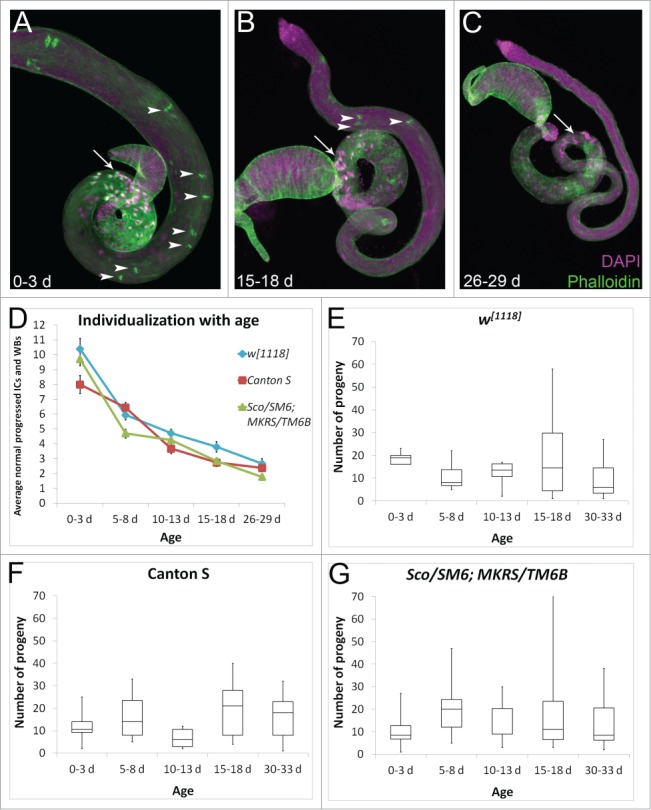
(See previous page). The effects of aging on spermatogenesis. (A) Young testes are large and have many late stage spermatogenic cysts with clustered nuclei in the basal testis (arrow, DAPI, purple) and many progressing ICs (arrowheads, phalloidin, green). (B, C) Older testes are thin and have fewer cysts with basal nuclear clusters (arrows) and fewer ICs (arrowheads). Testes are from w1118 males. (D) Young (0–3 d old) males at room temperature from 3 control genotypes have 8–11 normal progressed ICs and waste bags (WBs) per testis on average. Older males show a decline in the average number of individualization structures per testis, with 5–8 d old males having 4–7 normal individualization structures, 10–13 d old males having 3–5 normal individualization structures, 15–18 d old males having 2–4 normal individualization structures, and 26–29 d old males having fewer than 3 normal individualization structures per testis on average. N ≥ 18 testes each data point. In this an all subsequent figures, error bars represent standard error mean. (E–G) Fertility in 0–3 d old, 5–8 d old, 10–13 d old, 15–18 d old, or 30–33 d old males assessed in single matings (one male and one female each) for each control genotype (w1118, Canton S, or the balancer stock Sco/SM6;MKRS/TM6B). F1 progeny from 10 individual crosses were counted. Box plots show the distribution of progeny numbers for each age group, including maximum, minimum, interquartile range, and median numbers of progeny. ANOVA analysis comparing the mean number of progeny in each age group within each genotype reveals p > 0.1 for all 3 genotypes.
Aged males have smaller testes, because the GSCs divide less frequently.43 We examined IC progression in testes of our 3 control genotypes in aged flies. Males were collected within 0–3 d of eclosion and allowed to age in the presence of females for 5 d, 10 d, 15 d, or 26 d. The average number of normal progressed ICs and waste bags per testis decreased dramatically with age. While the youngest males had an average of 8–11 normal individualization structures per testis, males aged for 5 d had 4–7 normal individualization structures per testis, and older males had fewer than 5 normal individualization structures per testis on average (Fig. 2D). The average number of abnormal ICs also declined with age, such that the proportion of abnormal ICs versus normal individualization structures stayed similar at all ages (∼1:10; Figure S1). This effect seemed to be due primarily to a smaller proportion of aged testes having any abnormal ICs. At least one abnormal IC was observed in over half of the testes from 0–3 day old males of all 3 genotypes. In contrast, after aging for 26 days, only 38% of testes from w1118, 13% of testes from Canton S, and 16% of testes from Sco/SM6;MKRS/TM6B males had any abnormal ICs. Aged testes also had dramatically fewer nuclear bundles, suggesting that the decline in individualization structures was due to the production of fewer cysts (Fig. 2A–C, arrows).
Due to the decline in cyst number, we expected male fertility to decline with age. We performed single matings with 0–3 day old males or males aged for 5 days, 10 days, 15 days, or 30 d at room temperature. Suprisingly, progeny numbers did not decline significantly even after 30 d (Fig. 2E, G). This is consistent with the observation that aging did not disrupt the ICs, and it suggests that young males produce sperm in large excess, perhaps to foster sperm competition or to ensure reproductive success in the wild.44 Additionally, older males may maintain their fertility by storing sperm.
Individualization is sensitive temperature
To determine whether spermatid individualization was sensitive to environmental conditions, we quantified individualization structures in flies reared at a range of temperatures. Flies raised at 27oC had a slight decrease in the average number of normal individualization structures per testis, whereas flies raised at 29oC showed severe individualization defects. At 29oC, w1118, Canton S, and Sco/SM6;MKRS/TM6B showed averages of 5.5 or fewer normal progressed ICs and waste bags per testis (Fig. 3A). Low temperature also disrupted individualization. At 18oC, w1118 had an average of only 1.7 normal progressed ICs and wastebags per testis (Fig. 3B). Canton S males raised at 18oC showed an average of 8.2 normal individualization structures per testis, and Sco/SM6;MKRS/TM6B at 18oC displayed an average of 6.4 normal individualization structures per testis (Fig. 3B). More abnormal individualization structures were observed in all 3 genotypes at 18oC (Fig. 3C-E). This was in contrast to aged males, in which the presence of fewer cysts led to fewer individualization structures but the number of abnormal structures did not increase. Males also displayed reduced fertility at both 18oC and 29oC (Fig. 3F). Male fertility in all 3 genotypes was strongly reduced at 29oC, with Canton S showing the strongest effect. At 18oC, Canton S and balancer males had slightly reduced fertility, whereas the fertility of w1118 males was dramatically reduced. This is in accord with the effects on individualization, in which Canton S displayed the strongest effect at 29oC and w1118 showed the strongest effect at 18oC. Cytokinesis defects were occasionally observed in w1118 but not the other genotypes when males were reared at 18oC and in all 3 genotypes at 29oC (Figure S2). Thus, rearing flies at extreme temperatures disrupted spermatogenesis. Different genetic backgrounds rendered flies more or less susceptible to temperature-induced defects. Interestingly, the wild-type strain Canton S showed more variability in individualization at room temperature but was less sensitive to cooler temperatures.
Figure 3.
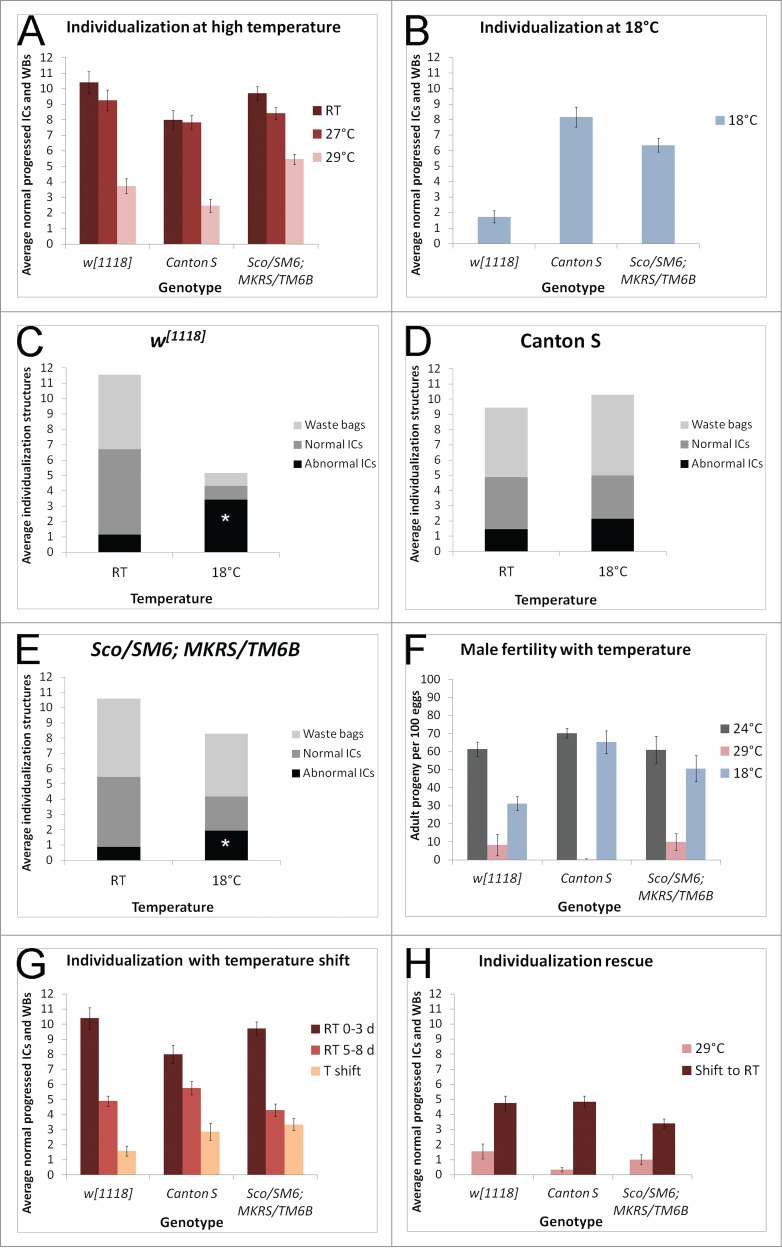
(See previous page). Individualization is sensitive to temperature. (A) Young (0–3 d old) males raised at 27oC have a slight decrease in the average number of normal individualization structures per testis (red bars, n ≥ 18 each genotype) compared to room temperature (RT) males of the same genotypes (maroon bars, n ≥ 18 each genotype). At 29oC, testes average fewer than 6 normal individualization structures each (pink bars, n > 10 each genotype). (B) At 18oC, young w1118 males (0–6 d old) have fewer than 2 normal individualization structures per testis on average (n = 57). In contrast, Canton S males at 18oC have more than 8 normal individualization structures per testis (n = 37), and balancer males at 18oC have an average of more than 6 normal individualization structures per testis (n = 34). (C) Young w1118 males raised at room temperature have an average of 1.15 abnormal ICs per testis, compared to young w1118 males raised at 18oC, which have an average of 3.42 abnormal ICs per testis. p < 0.001. (D) Young Canton S males raised at room temperature have an average of 1.44 abnormal ICs per testis, compared to young Canton S males raised at 18oC, which have an average of 2.14 abnormal ICs per testis. (E) Young Sco/SM6;MKRS/TM6B males raised at room temperature have an average of 0.875 abnormal ICs per testis, compared to young Sco/SM6;MKRS/TM6B males raised at 18oC, which have 1.94 abnormal ICs per testis. p < 0.001. (F) Young males raised and mated at 29oC or 18oC have reduced fertility compared to young males raised at 24oC. Number of adult progeny resulting from 100 eggs collected from each cross was counted and averaged between 3 trials. Error bars represent SEM. (G) Allowing males to develop at room temperature and shifting them to 29oC within 0–3 d of eclosion significantly reduces the average number of normal individualization structures per testis (peach bars, T shift, n ≥ 15 each genotype). Temperature shifted males were kept at 29oC for 5 d. Control males were 0–3 d old room temperature males (maroon bars, n ≥ 18 each genotype) or 5–8 d old room temperature males (light red bars, n ≥ 18 each genotype). (H) Rearing males at 29oC and shifting them to room temperature within 0–3 d of eclosion (maroon bars, n ≥ 16 each genotype) rescues the average number of normal individualization structures per testis. Temperature shifted males were kept at room temperature for 5 d. In contrast, males kept at 29oC for 5 d have fewer than 2 normal individualization structures per testis on average (pink bars, n ≥ 11 each genotype).
We also quantified spermatid individualization in flies that were raised at room temperature and shifted to 29oC as adults. Males were collected 0–3 d after eclosion and allowed to age in the presence of females for 5 d either at room temperature or at 29oC. Temperature shifted males of all 3 control genotypes had significant individualization defects compared to siblings kept at room temperature, showing averages of fewer than 3.5 normal progressed ICs and waste bags per testis (Fig. 3G, peach bars). The temperature shifted males also had more abnormal ICs per testis than the corresponding room temperature controls (Figure S3). In the reverse experiment, males reared at 29oC and shifted to room temperature for 5 d showed rescue of individualization compared to age-matched males kept at 29oC (Fig. 3H). These experiments revealed that the individualization process itself was sensitive to temperature.
Individualization is not sensitive to diet
Because individualization was sensitive to environmental perturbations, we asked whether diet can affect the individualization process. We reared animals on regular medium and shifted them following eclosion to glucose medium for 4 d or 8 d at room temperature. This medium contained only glucose and cholesterol. Individualization appeared normal in these flies (Fig. 4A, B). Flies reared throughout their development on glucose medium did not survive to adulthood. However, some flies did survive to adulthood when reared on lipid depleted food supplemented with cholesterol.45 These flies had normal individualization (data not shown). Thus, the individualization process was not sensitive to reduced dietary lipid intake.
Figure 4.
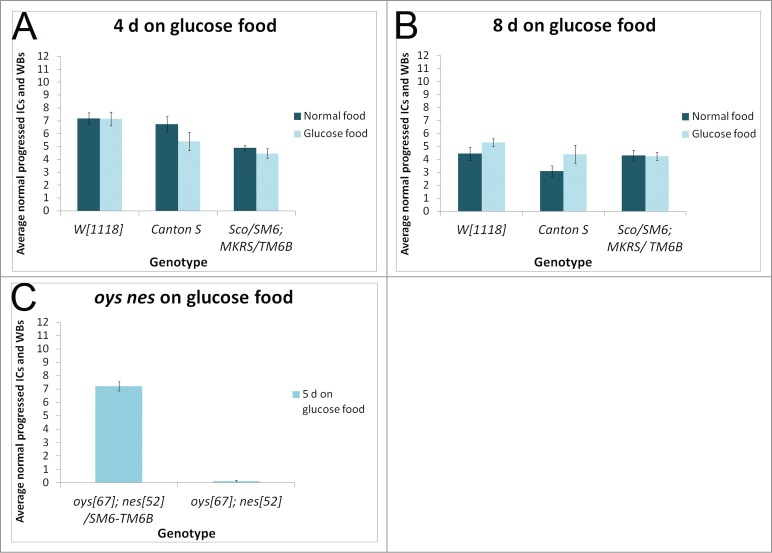
Individualization is not sensitive to diet. Males reared on normal food and shifted within 3 d of eclosion to glucose food (light bars) for either 4 d (A) or 8 d (B) show no individualization defects compared to age matched siblings kept on normal food (dark bars). N ≥ 10 testes each condition. (C) oysΔ67 nesΔ52 double mutant males collected within 5 d of eclosion and cultured on glucose food for 5 d show highly abnormal individualization, with an average of fewer than one normal IC per testis. Controls are balancer siblings cultured in parallel. N ≥ 17 testes each condition.
Oys and Nes are expressed in the germline
Testes from 0–5 day old oys nes double mutant males raised at room temperature showed an average of ∼2 normal individualization structures per testis (Fig. 5A). Many abnormal individualization structures were observed in the mutant (Fig. 5C). Actin cones from mutant ICs were almost always scattered (Fig. 5B, D), and waste bags appeared devoid of actin cones (Fig. 5E). Isogenic control males had 8.5 normal individualization structures per testis on average, like the control genotypes above. Males homozygous for either oys or nes and heterozygous for the other also showed normal individualization (Fig. 5A). Thus, individualization was not sensitive to the dosage of oys and nes. Endogenous oys and nes transcripts were detectable in the germline using in situ hybridization (Fig. 5F, G). Cytokinesis was normal in oys nes homozygotes and heterozygotes (Fig. 5H, I).21
Figure 5.
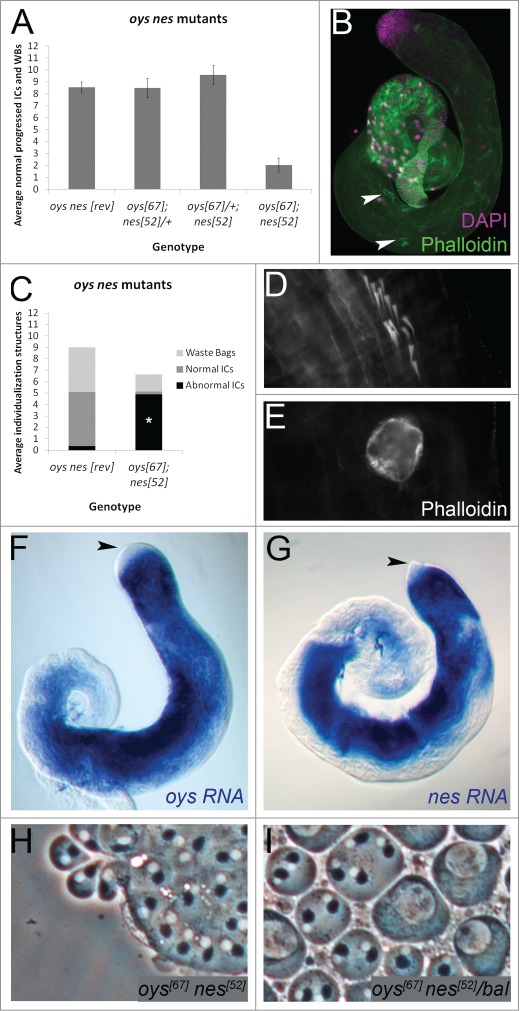
Oys and Nes are required for individualization and are expressed in the germline. (A) oysΔ6 nesΔ52 mutant males (0–5 d old) have an average of 2 normal individualization structures per testis (n = 20), while precise excision controls (revertant, “rev”) have more than 8 (n = 31). Males homozygous for either oysΔ67 or nesΔ52 and heterozygous for the other also have more than 8 normal individualization structures per testis on average (n ≥ 24 each). (B–E) oys nes mutant testes have scattered ICs (B, arrowheads, phalloidin, green, close-up in D) and waste bags devoid of actin cones (E). The average number of abnormal ICs in the oys nes mutant (4.9, n = 22) is significantly increased compared to the revertant control (0.36, n = 11), p <0.001 (C). (F–G) RNA in situ hybridization to endogenous oys (F) or nes (G) in w1118 testes shows expression in the germline beginning in premeiotic spermatocytes (blue). Endogenous transcripts are not detected in the germinal proliferation center (black arrowheads). (H-I) Phase contrast microscopy of testis squashes reveals no cytokinesis defects in either oysΔ67nesΔ52 homozygotes (H) or sibling heterozygotes (I, “bal” = SM6-TM6B balancer). Each phase dark Nebenkern has one associated phase light nucleus.
Because Oys and Nes esterify fatty acids into phospholipids,21 thereby reducing free fatty acid concentrations, we expected fatty acid levels to increase in the oys nes double mutant. Therefore, we investigated whether reducing dietary intake of fatty acids could rescue the oys nes mutant phenotype. However, culturing oys nes mutant males on glucose food lacking fatty acids did not rescue the individualization defect (Fig. 4C).
PLA2 genes are expressed in the male reproductive tract
PLA2 enzymes act in opposition to ATs, hydrolyzing the sn-2 ester bond in phospholipids and releasing lysophospholipids and fatty acids (Fig. 7A).23 Mammals possess more than 20 PLA2 homologs, which can be broadly grouped into 4 categories: cytoplasmic PLA2s (cPLA2s), secreted PLA2s (sPLA2s), calcium independent PLA2s (iPLA2s), and platelet-activating factor acetylhydrolases (PAFAHs) (Fig. 6A).23,24 cPLA2s reside in the cytosol and play key roles in fatty acid signaling.24 sPLA2s are secreted and also have been implicated in fatty acid signaling.46,47 The iPLA2s do not show strong specificity for the sn-2 position, and they degrade phospholipids by deacylating at both sn-1 and sn-2 positions.24 PAFAHs hydrolyze lipids like platelet-activating factor, which carry an acetyl group at the sn-2 position.24,48 We and others have identified 10 putative PLA2 genes in the Drosophila genome.49 Drosophila does not possess members of the cPLA2 or PAFAH subfamilies (Fig. 6A).24 Using RT-PCR, we found that the iPLA2s encoded by sws, CG6718, and CG10133 were expressed endogenously in the male reproductive tract, as were the sPLA2s encoded by CG14507, CG17035, and CG11124 (Fig. 6B). The sPLA2 CG1583 was weakly expressed as well (Fig. 6B), consistent with ModENCODE and FlyAtlas expression data (flybase.org). Thus, like Oys and Nes, PLA2s may be important for Drosophila male fertility.
Figure 6.
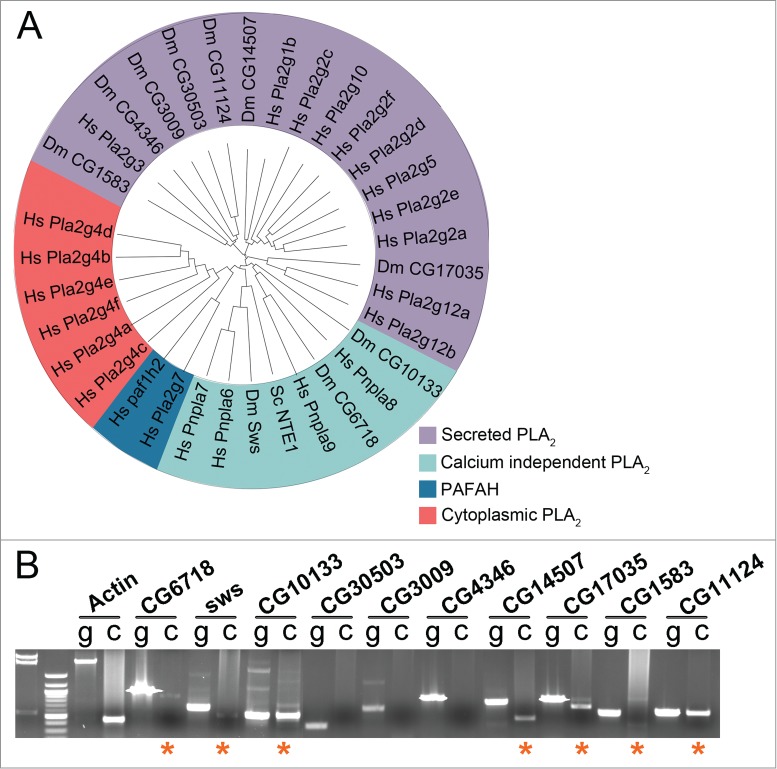
Drosophila PLA2 homologs are expressed in the male reproductive tract. (A) Phylogenetic tree of PLA2 family members from Saccharomyces cerevisiae (Sc), Drosophila melanogaster (Dm), and Homo sapiens (Hs). Tree was compiled using software provided by Interactive Tree of Life (itol.embl.de)82 and following classifications in ref. 24 (B) RT-PCR on cDNA (“c”) prepared from w1118 reproductive tracts showing expression of CG6718, sws, CG10133, CG14507, CG17035, CG1583 and CG11124 (asterisks). Control PCR was run for each set of primers on genomic DNA (“g”). Actin-5c PCR was used as a control for cDNA integrity.
Perturbation of prostaglandin synthesis affects individualization
Phosphatidylcholine (PC) molecular species composition is abnormal in oys nes mutants compared to controls.21 However, altering PC levels in the testis by overexpression or mutation of the iPLA2/PC lipase Swiss cheese (Sws) did not affect individualization (Fig. 7B).50 Inhibiting de novo phosphatidylethanolamine (PE) synthesis by mutation of the ethanolamine kinase Easily shocked (Eas) also had no effect on individualization (Fig. 7B).51 Thus, altered phospholipid composition did not inhibit individualization.
In mammals, fatty acids liberated from membrane phospholipids by the Lands Cycle are oxygenated and further metabolized into eicosanoid signaling molecules, such as prostaglandins.27 The most upstream enzymes in the prostaglandin production cascade are the cyclooxygenases.36 There is evidence for prostaglandin activity in invertebrates,37,38,52 and the peroxidase Pxt was identified recently as having cyclooxygenase activity.53 pxt mutant females have reduced fertility and actin-based oogenesis defects, and the severity of the defects increases with age. Similarly, males homozygous for the strong pxtf0100 mutation showed individualization defects at all ages (Fig. 7C, D). Despite the individualization defects, Myosin VI localized normally to pxt mutant actin cones (Fig. 7E, F). Cytokinesis appeared normal in pxt mutants (Fig. 7G).
Discussion
Fatty acid mediators as regulators of spermatogenesis
Oys and Nes are ATs that esterify fatty acids to lysophospholipids to make phospholipids. They prefer long (C16-C20) unsaturated fatty acid substrates. Although Oys can act on a range of lysophospholipid substrates, Nes shows a strong preference for lysophosphatidylcholine.21 Because Oys and Nes are genetically redundant, we expect that together they regulate levels of long chain fatty acids and lysophosphatidylcholine, as well as the molecular species composition of PC. Indeed, we have shown previously that PC acyl chain composition is altered in the oys nes mutant.21 Which lipid is responsible for the mutant phenotype? Our data suggest that individualization is not sensitive to alterations in PC, since genetic perturbation of the PC lipase Sws does not affect individualization. Likewise, individualization is not disrupted by mutations in Eas, the ethanolamine kinase that initiates de novo PE synthesis.54 Thus, we infer that phospholipid composition is not critical for individualization. Instead, our data suggest that altered levels of fatty acids and/or their metabolites disrupt individualization, as mutation of the cyclooxygenase Pxt leads to individualization defects.
In the absence of Oys and Nes, we expect fatty acid levels to be elevated. In the presence of wild-type Pxt, these excess fatty acids may be converted into prostaglandins. Prostaglandins regulate the actin cytoskeleton via various molecular pathways.55 pxt mutant females show premature actin bundling in the female germline during stage 9 of oogenesis, as well as a failure of normal actin bundling during later stages of oogenesis.55 Therefore, precise temporal regulation of prostaglandin signals organizes the actin cytoskeleton during oogenesis. A similar process may be occurring in spermatogenesis. Excess prostaglandins, formed from excess fatty acids in the oys nes mutant, may prevent timely completion of spermatid individualization. However, loss of prostaglandins in the pxt mutant also disrupts individualization. Thus, we hypothesize that individualization requires a precisely calibrated prostaglandin pulse. In the Drosophila oocyte, the actin regulatory proteins Fascin (Singed) and Enabled are downstream of prostaglandin signaling.55,56 Interestingly, Singed localizes to the ICs, suggesting that molecular players downstream of prostaglandin signals may be conserved in gametogenesis.20 Singed still localizes to pxt mutant ICs (data not shown), but its regulation might be altered. Endogenous Oys and Nes are expressed in the male germline. Similarly, Pxt acts in the germline during oogenesis.53
In mammals, altered dietary fatty acid intake affects phospholipid composition, lipid mediator availability, and fertility.57,58 A recent study found that Drosophila phospholipid composition also is sensitive to dietary lipid alterations.59 We were surprised to find that removal of fatty acids from the adult diet does not disrupt individualization in wild-type flies. This may indicate that sufficient fatty acids are stored during development, which may be facilitated by the relatively short life span of Drosophila. Alternatively, flies may be capable of synthesising necessary fatty acids from carbohydrate sources.37,60,61 Dietary fatty acid depletion also does not rescue the individualization defect of oys nes mutant flies. Thus, individualization is not sensitive to global changes in fatty acid levels. Consistently, Sws overexpression is expected to increase fatty acid levels as a byproduct of PC catabolism,62 yet it has no effect on individualization. Together, our results support the idea that spermatid individualization relies on a specific fatty acid signaling molecule.
Sperm cell development before individualization is normal in oys nes mutants,21 again implicating a specific regulatory pathway, as opposed to a general perturbation of cell function, in the mutant phenotype. Furthermore, other unrelated lipid metabolic pathways affect different aspects of spermatogenesis. Phosphatidylinositol lipds are required for spermatocyte cytokinesis, cyst polarity, and axoneme growth.63–69 A fatty acid elongase, encoded by the bond gene, is required for cytokinesis as well,60,70 while Oys, Nes, and Pxt are dispensible for cytokinesis. These observations indicate that different lipids play distinct roles during sperm development.
The Lands Cycle has been found to influence Golgi dynamics,71 and alterations in membrane phospholipid composition have been seen to induce ER stress and apoptosis.72,73 It is unlikely that these effects underlie the oys nes phenotype, because mutations that perturb trafficking through the Golgi apparatus, such as 4 way stop and syntaxin 5, disrupt spermatocyte cytokinesis and axoneme elongation, and such defects are not observed in the oys nes mutant.74,75 Additionally, immunofluorescent staining of the Golgi appears normal in oys nes mutant cells, and we do not observe ectopic germ cell apoptosis in the oys nes mutant.21
Temperature sensitivity of individualization
Individualization in Drosophila melanogaster is extremely temperature sensitive. Our temperature shift experiments demonstrate that individualization itself is the sensitive process, as opposed to gonad formation during larval development. Cytokinesis defects also are apparent at extreme temperatures, but at lower penetrance than the individualization defects. It has been shown previously that spermatogenesis is sensitive to temperatures above 28oC.76 Here we show that low temperature, i.e. 18oC, also disrupts spermatogenesis. Individualization defects may underlie the observed effects on male fertility, because these 2 parameters are well correlated at both high and low temperatures. A practical implication of this observation relates to the temperature at which GAL4 experiments are conducted in lab strains. Although GAL4 activity is maximized at 29oC,77 we recommend using 27oC when assessing spermatogenesis phenotypes, as few defects are observed at this temperature. A conceptual implication relates to how different populations and species cope with seasonal or daily climatic variations.76,78
In conclusion, we have developed a quantitative assay to assess defects in Drosophila spermatid individualization, using fluorescent detection of the actin-rich ICs. We have characterized the individualization process as it occurs over a range of environmental parameters. Many mutations have been discovered that perturb individualization, and we hope that our system will be of use in analyzing these mutations in more detail. Individualization is sensitive to genetic disruption of the Lands Cycle and prostaglandin metabolism, and AT and PLA2 homologs are expressed in the Drosophila male reproductive tract. Fatty acid mediators are important for male fertility in mammals as well as Drosophila.32,36,58 PLA2 knockout mice exhibit spermatogenesis defects24 and mammalian ATs are highly expressed in testis,79 suggesting that the roles of the Lands Cycle in spermatogenesis are evolutionarily conserved throughout metazoa.
Materials and Methods
Fly stocks
Stocks used were w1118, Canton S, Sco/SM6;MKRS/TM6B, tj-GAL4, sws4, easalaE1 (from Bloomington Stock Center), UAS-Sws (gift from D. Kretzschmar),50 bam-GAL4-VP16 (gift from Y. Yamashita), pxtEY0350, pxtf0100, and pxtrevTT274 (gifts from T. Tootle).53 The oysΔ6 nesΔ52 mutant stock and oysΔ8 nesΔ19 precise excision revertant control were as described.21
Aging and temperature shift individualization experiments
For aging experiments, males were collected within 3 d of eclosion and aged for 5 days, 10 days, 15 days, or 26 d at room temperature in the presence of sibling females. Flies were transferred to new vials every few days. For aging of pxt mutants, males were collected within 3 d of eclosion and aged for 5 d or 13 d at room temperature in the presence of sibling females.
For temperature experiments, flies were reared at room temperature (23–24oC), 29oC, 27oC, or 18oC. Males at room temperature, 29oC, or 27oC were collected and dissected within 3 d of eclosion. Males at 18oC were collected and dissected within 6 d of eclosion.
For the temperature upshift experiment, stocks were reared at room temperature. Males were collected within 3 d of eclosion and shifted to 29oC for 5 d in the presence of sibling females. Control flies were collected within 3 d of eclosion and kept at room temperature for 5 d. For the temperature downshift experiment, stocks were reared at 29oC. Males were collected within 3 d of eclosion and shifted to room temperature for 5 d in the presence of sibling females. Control flies were collected within 3 d of eclosion and kept at 29oC for 5 d.
Drosophila food
Normal food contained 3.83% molasses, 1.58% yeast, and 3.83% corn meal supplemented with 0.11% methyl paraben and 0.38% propionic acid as mold inhibitors. Glucose food recipe was adapted from ref. 45 and contained 10% glucose, 0.5% agar (MoorAgar Inc., 41004), 0.11% methyl paraben, 0.38% propionic acid, and 6.2 μg/mL cholesterol.45 Cholesterol was dissolved in 100% ethanol before addition to food. Males from control genotypes were collected within 3 d of eclosion and kept on glucose food for 4 d or 8 d at room temperature in the presence of sibling females. Control sibling males were collected within 3 d of eclosion and kept on normal food. oysΔ67 nesΔ52 mutant males or balancer sibling males were collected within 5 d of eclosion and kept on glucose food for 5 d at room temperature in the presence of sibling females.
Lipid depleted food recipe was adapted from ref. 45 and contained 10% chloroform-extracted yeast autolysate (Sigma-Aldrich, 73145–500G-F), 10% glucose, 0.5% agar, 0.11% methyl paraben, 0.38% propionic acid, and 6.2 μg/mL cholesterol.45
Fertility tests
For aging fertility tests, males of each genotype reared at room temperature were collected within 3 d of eclosion and mating singly to one virgin w1118 female each or allowed to age for 5 days, 10 days, 15 days, or 30 d in the presence of sibling females at room temperature and thereafter mated singly to one virgin w1118 female each (virgin females were also reared at room temperature). Matings were performed in standard food vials supplemented with granular dry active yeast (Lab Scientific, FLY-8040–10F). Each mating was allowed to proceed for 3 d at room temperature. After 3 days, P0 adults were discarded, and larvae were allowed to develop at room temperature. F1 adults were scored every 3–4 d until the 20th day after establishment of the vial. Ten matings were performed for each age group. Crosses that yielded no progeny were discarded.
For temperature fertility tests, 10 males of each genotype reared at the experimental temperature were mated to 15–20 virgin w1118 females and transferred to egg collecting cages. Both mating and egg laying were allowed to proceed at the experimental temperature. Within a 24 hour egg laying period, 100 eggs from each cross were collected and placed into food vials, in order to control for reduced egg laying at extreme temperatures. The progeny were allowed to develop in food vials at 24oC, in order to control for larval developmental defects at extreme temperatures. Males reared at 24oC or 29oC were collected within 3 d of eclosion. Males reared at 18oC were collected within 6 d of eclosion. Adult progeny were counted until the 19th day after establishment of the vial.
Fluorescence staining
Adult testes were dissected in phosphate-buffered saline (PBS) and fixed in 5% formaldehyde for 20 min at room temperature, washed in PBX (PBS + 0.1% Triton X-100) for 15–20 min, and stained with rhodamine-phalloidin (1:200, Sigma-Aldrich, P1951) and DAPI (1:4000, Roche, 10236276001) for 20 min at room temperature. Following three washes in PBX, testes were mounted in Fluoromount G (Southern Biotechnology, 0100–01). For antibody staining, the apical ends of the testes were removed before fixation to expose the spermatids. Following fixation in 5% formaldehyde for 20 min, testes were blocked in PBS + 5% normal donkey serum + 1% Triton X-100. Testes were incubated in primary antibodies overnight (+ 5% normal donkey serum) at 4oC, washed 3 times in PBX, and incubated with secondary antibodies (Alexa Fluor 488-conjugated donkey anti-mouse secondary antibody from Molecular Probes, 1:1000, A-21202), DAPI, and rhodamine-phalloidin for 2 hours at room temperature. Mouse anti-myosin VI was kindly provided by K. Miller and used at 1:20.80 Mouse anti-Singed (Fascin) was used undiluted (DSHB, sn 7C). Images were captured using a Zeiss LSM510 Confocal or an Olympus IX-81 motorized inverted microscope with XM-10 monochrome camera. ICs were scored using a 20x objective.
RT-PCR
Testis cDNA was prepared following ref. 81 using SuperScript III first-strand synthesis kit (Life Technologies, 18080051).81 PCR primer pairs were as follows:
| Gene | Forward primer | Reverse primer |
| CG1583 | 5’-CGAACGCCATCGGAAAGCTC | 5´-CATAGACGTGGTGTCTCTGG |
| CG3009 | 5´-CATCTGGTTCGGCGGTGC | 5´-ACCTCTGGTGTGCCGAGC |
| CG11124 | 5´-CGACGAGGCTATCTTTGAGG | 5´-ACGCTGGGATTGTGAAGAAC |
| CG30503 | 5´-GCAGCCAACTACGATGACCTG | 5´-CACTCGGTAGCTGAGACATCG |
| CG4346 | 5´-CTTCAGCTGCTGGCCTCG | 5´-GGAGTCGTTGCTCACACGC |
| CG14507 | 5´-GTGGCGACGACTTCGAGCTG | 5´-CATGGAGTAACCGAAGGGATC |
| CG6718 | 5´-CGACCCACCTCGGATTCC | 5´-GACCACCAGACATTGGACG |
| CG10133 | 5´-GAAGCGGAGGAGCTGTGC | 5´-GTGCGGCGGGATGAGAAC |
| CG17035 | 5´-CTAACCTACGCCTATTCAGG | 5´-CGTTGCGATCCTTCCAGC |
| Sws | 5´-CTAAGCGGTTGGTGGCTGC | 5´-CAGGTCAAGCTCCACGTCC |
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Vienna Drosophila RNAi Center, the Bloomington Drosophila Stock Center, the Kyoto Drosophila Genetic Resource Center, and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We thank EMBL-EBI (European Bioinformatics Institute) for sequence alignment programs and Interactive Tree of Life (itol.embl.de) for phylogenetic tree software. We are grateful to Doris Kretzschmar, Yukiko Yamashita, Tina Tootle, and Jessica Treisman for fly stocks; Kathryn Miller for Myosin VI antibody; Tina Tootle, Hyung Don Ryoo, Jennifer Kennell, and Jessica Treisman for discussions; Ruth Lehmann for use of her confocal; Benjamin Statman for help with temperature fertility experiments. The manuscript was improved by the critical comments of Jessica Treisman and Sergio Astigarraga.
Funding
This work was supported by the Provost's Office of Yeshiva University start-up funding to J.S and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R15HD080511–01 to J.S.).
References
- 1. Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int Rev Cytol 2000; 195:1-65; PMID:10603574; http://dx.doi.org/ 10.1016/S0074-7696(08)62703-5 [DOI] [PubMed] [Google Scholar]
- 2. Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol 2013; 757:321-50; PMID:22872482; http://dx.doi.org/ 10.1007/978-1-4614-4015-4_11 [DOI] [PubMed] [Google Scholar]
- 3. Fitch KR, Yasuda GK, Owens KN, Wakimoto BT. Paternal effects in drosophila: implications for mechanisms of early development. Curr Topic Dev Biol 1998; 38:1-34; PMID:9399075; http://dx.doi.org/ 10.1016/S0070-2153(08)60243-4 [DOI] [PubMed] [Google Scholar]
- 4. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 2011; 1:14-35; PMID:21866274; http://dx.doi.org/ 10.4161/spmg.1.1.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophysical Biochemical Cytol 1959; 5:453-60; PMID:13664686; http://dx.doi.org/ 10.1083/jcb.5.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM. Germ cell intercellular bridges. Cold Spring Harbor Perspect Biol 2011; 3:a005850; PMID:21669984; http://dx.doi.org/ 10.1101/cshperspect.a005850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren HP, Russell LD. Clonal development of interconnected germ cells in the rat and its relationship to the segmental and subsegmental organization of spermatogenesis. Am J Anatomy 1991; 192:121-8; PMID:1759679; http://dx.doi.org/ 10.1002/aja.1001920203 [DOI] [PubMed] [Google Scholar]
- 8. Weber JE, Russell LD. A study of intercellular bridges during spermatogenesis in the rat. Am J Anatomy 1987; 180:1-24; PMID:3661461; http://dx.doi.org/ 10.1002/aja.1001800102 [DOI] [PubMed] [Google Scholar]
- 9. Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in drosophila melanogaster. I. individualization process. Z Zellforsch Mikrosk Anat 1972; 124:479-506; PMID:4622067; http://dx.doi.org/ 10.1007/BF00335253 [DOI] [PubMed] [Google Scholar]
- 10. Xiao X, Yang WX. Actin-based dynamics during spermatogenesis and its significance. J Zhejiang Univ Sci B 2007; 8:498-506; PMID:17610330; http://dx.doi.org/ 10.1631/jzus.2007.B0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabrizio JJ, Hime G, Lemmon SK, Bazinet C. Genetic dissection of sperm individualization in drosophila melanogaster. Development 1998; 125:1833-43; PMID:9550716 [DOI] [PubMed] [Google Scholar]
- 12. Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell 1989; 21:361-79; PMID:2479117; http://dx.doi.org/ 10.1016/0040-8166(89)90051-7 [DOI] [PubMed] [Google Scholar]
- 13. Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Rep Biol Endocrinol 2012; 10:92; PMID:23159014; http://dx.doi.org/ 10.1186/1477-7827-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matunis EL, Stine RR, de Cuevas M. Recent advances in drosophila male germline stem cell biology. Spermatogenesis 2012; 2:137-44; PMID:23087833; http://dx.doi.org/ 10.4161/spmg.21763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harbor Perspectives Biol 2011; 3:a002642; PMID:21791699; http://dx.doi.org/ 10.1101/cshperspect.a002642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zoller R, Schulz C. The drosophila cyst stem cell lineage: partners behind the scenes? Spermatogenesis 2012; 2:145-57; PMID:23087834; http://dx.doi.org/ 10.4161/spmg.21380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noguchi T, Miller KG. A role for actin dynamics in individualization during spermatogenesis in drosophila melanogaster. Development 2003; 130:1805-16; PMID:12642486; http://dx.doi.org/ 10.1242/dev.00406 [DOI] [PubMed] [Google Scholar]
- 18. Fabian L, Brill JA. Drosophila spermiogenesis: big things come from little packages. Spermatogenesis 2012; 2:197-212; PMID:23087837; http://dx.doi.org/ 10.4161/spmg.21798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakimoto BT, Lindsley DL, Herrera C. Toward a comprehensive genetic analysis of male fertility in drosophila melanogaster. Genetics 2004; 167:207-16; PMID:15166148; http://dx.doi.org/ 10.1534/genetics.167.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol Biol Cell 2008; 19:2363-72; PMID:18353976; http://dx.doi.org/ 10.1091/mbc.E07-08-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steinhauer J, Gijon MA, Riekhof WR, Voelker DR, Murphy RC, Treisman JE. Drosophila lysophospholipid acyltransferases are specifically required for germ cell development. Mol Biol Cell 2009; 20:5224-35; PMID:19864461; http://dx.doi.org/ 10.1091/mbc.E09-05-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lands WE. Lipid Metabolism. Annu Rev Biochem 1965; 34:313-46; PMID:14321173; http://dx.doi.org/ 10.1146/annurev.bi.34.070165.001525 [DOI] [PubMed] [Google Scholar]
- 23. Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol 1999; 39:175-89; PMID:10331081; http://dx.doi.org/ 10.1146/annurev.pharmtox.39.1.175 [DOI] [PubMed] [Google Scholar]
- 24. Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A(2) research: from cells to animals to humans. Progress Lipid Res 2011; 50:152-92; PMID:21185866; http://dx.doi.org/ 10.1016/j.plipres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 25. Shindou H, Hishikawa D, Harayama T, Yuki K, Shimizu T. Recent progress on acyl CoA: lysophospholipid acyltransferase research. J Lipid Res 2009; 50:S46-51; PMID:18931347; http://dx.doi.org/ 10.1194/jlr.R800035-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irvine RF. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J 1982; 204:3-16; PMID:6810878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001; 294:1871-5; PMID:11729303; http://dx.doi.org/ 10.1126/science.294.5548.1871 [DOI] [PubMed] [Google Scholar]
- 28. Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids–receptor revelations. Science 2001; 294:1875-8; PMID:11729304; http://dx.doi.org/ 10.1126/science.1065323 [DOI] [PubMed] [Google Scholar]
- 29. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. Fifteen-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995; 83:803-12; PMID:8521497; http://dx.doi.org/ 10.1016/0092-8674(95)90193-0 [DOI] [PubMed] [Google Scholar]
- 30. Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995; 83:813-9; PMID:8521498; http://dx.doi.org/ 10.1016/0092-8674(95)90194-9 [DOI] [PubMed] [Google Scholar]
- 31. Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 2007; 1768:923-40; PMID:17078925; http://dx.doi.org/ 10.1016/j.bbamem.2006.09.026 [DOI] [PubMed] [Google Scholar]
- 32. Narumiya S, Furuyashiki T. Fever, inflammation, pain and beyond: prostanoid receptor research during these 25 years. FASEB J 2011; 25:813-8; PMID:21357250; http://dx.doi.org/ 10.1096/fj.11-0302ufm [DOI] [PubMed] [Google Scholar]
- 33. Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 1987; 237:1171-6; PMID:2820055; http://dx.doi.org/ 10.1126/science.2820055 [DOI] [PubMed] [Google Scholar]
- 34. Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. BioEssays 2004; 26:870-81; PMID:15273989; http://dx.doi.org/ 10.1002/bies.20081 [DOI] [PubMed] [Google Scholar]
- 35. Murakami M. Lipid mediators in life science. Exp AnimalJapanese Association Lab Animal Sci 2011; 60:7-20; PMID:21325748; http://dx.doi.org/ 10.1538/expanim.60.7 [DOI] [PubMed] [Google Scholar]
- 36. Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 2004; 18:790-804; PMID:15117884; http://dx.doi.org/ 10.1096/fj.03-0645rev [DOI] [PubMed] [Google Scholar]
- 37. Vrablik TL, Watts JL. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from caenorhabditis elegans and drosophila melanogaster. Mol Rep Dev 2013; 80:244-59; PMID:23440886; http://dx.doi.org/ 10.1002/mrd.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanley D. Prostaglandins and other eicosanoids in insects: biological significance. Ann Rev Entomol 2006; 51:25-44; PMID:16332202; http://dx.doi.org/ 10.1146/annurev.ento.51.110104.151021 [DOI] [PubMed] [Google Scholar]
- 39. Yamashita A, Sugiura T, Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J Biochem 1997; 122:1-16; PMID:9276665; http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a021715 [DOI] [PubMed] [Google Scholar]
- 40. Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophysic Acta 1984; 779:89-137; PMID:6229284; http://dx.doi.org/ 10.1016/0304-4157(84)90005-4 [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Diaz-Diaz N, Zarringhalam K, Hermansson M, Somerharju P, Chuang J. Dynamics of the ethanolamine glycerophospholipid remodeling network. PloS One 2012; 7:e50858; PMID:23251394; http://dx.doi.org/ 10.1371/journal.pone.0050858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during drosophila spermatid individualization. Mol Biol Cell 2006; 17:2559-71; PMID:16571671; http://dx.doi.org/ 10.1091/mbc.E06-01-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature 2008; 456:599-604; PMID:18923395; http://dx.doi.org/ 10.1038/nature07386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karr TL, Pitnick S. Sperm competition: defining the rules of engagement. Curr Biol 1999; 9:R787-90; PMID:10531020; http://dx.doi.org/ 10.1016/S0960-9822(00)80014-7 [DOI] [PubMed] [Google Scholar]
- 45. Carvalho M, Schwudke D, Sampaio JL, Palm W, Riezman I, Dey G, Gupta GD, Mayor S, Riezman H, Shevchenko A, et al. Survival strategies of a sterol auxotroph. Dev 2010; 137:3675-85; PMID:20940226; http://dx.doi.org/ 10.1242/dev.044560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem 2011; 150:233-55; PMID:21746768; http://dx.doi.org/ 10.1093/jb/mvr088 [DOI] [PubMed] [Google Scholar]
- 47. Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 2008; 77:495-520; PMID:18405237; http://dx.doi.org/ 10.1146/annurev.biochem.76.062405.154007 [DOI] [PubMed] [Google Scholar]
- 48. Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical Rev 2011; 111:6130-85; PMID:21910409; http://dx.doi.org/ 10.1021/cr200085w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Renault AD, Starz-Gaiano M, Lehmann R. Metabolism of sphingosine 1-phosphate and lysophosphatidic acid: a genome wide analysis of gene expression in drosophila. Mech Dev 2002; 119 1:S293-301; PMID:14516700; http://dx.doi.org/ 10.1016/S0925-4773(03)00131-X [DOI] [PubMed] [Google Scholar]
- 50. Muhlig-Versen M, da Cruz AB, Tschape JA, Moser M, Buttner R, Athenstaedt K, Glynn P, Kretzschmar D. Loss of Swiss cheese/neuropathy target esterase activity causes disruption of phosphatidylcholine homeostasis and neuronal and glial death in adult drosophila. J Neuroscience 2005; 25:2865-73; PMID:15772346; http://dx.doi.org/ 10.1523/JNEUROSCI.5097-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nyako M, Marks C, Sherma J, Reynolds ER. Tissue-specific and developmental effects of the easily shocked mutation on ethanolamine kinase activity and phospholipid composition in drosophila melanogaster. Biochem Genet 2001; 39:339-49; PMID:11758729; http://dx.doi.org/ 10.1023/A:1012209030803 [DOI] [PubMed] [Google Scholar]
- 52. Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, Kubagawa HM, Miller MA. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev Cell 2010; 19:858-71; PMID:21145501; http://dx.doi.org/ 10.1016/j.devcel.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tootle TL, Spradling AC. Drosophila Pxt: a cyclooxygenase-like facilitator of follicle maturation. Dev 2008; 135:839-47; PMID:18216169; http://dx.doi.org/ 10.1242/dev.017590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gibellini F, Smith TK. The kennedy pathway–de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010; 62:414-28; PMID:20503434; http://dx.doi.org/ 10.1002/iub.354 [DOI] [PubMed] [Google Scholar]
- 55. Spracklen AJ, Kelpsch DJ, Chen X, Spracklen CN, Tootle TL. Prostaglandins temporally regulate cytoplasmic actin bundle formation during drosophila oogenesis. Mol Biol Cell 2014; 25:397-411; PMID:24284900; http://dx.doi.org/ 10.1091/mbc.E13-07-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Groen CM, Spracklen AJ, Fagan TN, Tootle TL. Drosophila fascin is a novel downstream target of prostaglandin signaling during actin remodeling. Mol Biol Cell 2012; 23:4567-78; PMID:23051736; http://dx.doi.org/ 10.1091/mbc.E12-05-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol 2005; 21:633-57; PMID:16212510; http://dx.doi.org/ 10.1146/annurev.cellbio.21.122303.120624 [DOI] [PubMed] [Google Scholar]
- 58. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Rep 2007; 77:190-201; PMID:17442851; http://dx.doi.org/ 10.1095/biolreprod.107.060558 [DOI] [PubMed] [Google Scholar]
- 59. Carvalho M, Sampaio JL, Palm W, Brankatschk M, Eaton S, Shevchenko A. Effects of diet and development on the drosophila lipidome. Mol Syst Biol 2012; 8:600; PMID:22864382; http://dx.doi.org/ 10.1038/msb.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog Lipid Res 2010; 49:186-99; PMID:20018209; http://dx.doi.org/ 10.1016/j.plipres.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 61. Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res 2003; 42:289-317; PMID:12689621; http://dx.doi.org/ 10.1016/S0163-7827(02)00067-X [DOI] [PubMed] [Google Scholar]
- 62. Zaccheo O, Dinsdale D, Meacock PA, Glynn P. Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J Biol Chem 2004; 279:24024-33; PMID:15044461; http://dx.doi.org/ 10.1074/jbc.M400830200 [DOI] [PubMed] [Google Scholar]
- 63. Fabian L, Wei HC, Rollins J, Noguchi T, Blankenship JT, Bellamkonda K, Polevoy G, Gervais L, Guichet A, Fuller MT, et al. Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in drosophila. Mol Biol Cell 2010; 21:1546-55; PMID:20237161; http://dx.doi.org/ 10.1091/mbc.E09-07-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei HC, Rollins J, Fabian L, Hayes M, Polevoy G, Bazinet C, Brill JA. Depletion of plasma membrane PtdIns(4,5)P2 reveals essential roles for phosphoinositides in flagellar biogenesis. J Cell Sci 2008; 121:1076-84; PMID:18334551; http://dx.doi.org/ 10.1242/jcs.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, Sem KP, Brill JA. PtdIns(4,5)P2 hydrolysis and calcium release are required for cytokinesis in drosophila spermatocytes. Curr Biol 2005; 15:1401-6; PMID:16085493; http://dx.doi.org/ 10.1016/j.cub.2005.06.060 [DOI] [PubMed] [Google Scholar]
- 66. Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Devel 2000; 127:3855-64; PMID:10934029 [DOI] [PubMed] [Google Scholar]
- 67. Giansanti MG, Bonaccorsi S, Kurek R, Farkas RM, Dimitri P, Fuller MT, Gatti M. The class I PITP giotto is required for drosophila cytokinesis. Curr Biol 2006; 16:195-201; PMID:16431372; http://dx.doi.org/ 10.1016/j.cub.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 68. Gatt MK, Glover DM. The drosophila phosphatidylinositol transfer protein encoded by vibrator is essential to maintain cleavage-furrow ingression in cytokinesis. J Cell Sci 2006; 119:2225-35; PMID:16684816; http://dx.doi.org/ 10.1242/jcs.02933 [DOI] [PubMed] [Google Scholar]
- 69. Polevoy G, Wei HC, Wong R, Szentpetery Z, Kim YJ, Goldbach P, Steinbach SK, Balla T, Brill JA. Dual roles for the drosophila PI 4-kinase four wheel drive in localizing rab11 during cytokinesis. J Cell Biol 2009; 187:847-58; PMID:19995935; http://dx.doi.org/ 10.1083/jcb.200908107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Szafer-Glusman E, Giansanti MG, Nishihama R, Bolival B, Pringle J, Gatti M, Fuller MT. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in drosophila spermatocytes. Curr Biol 2008; 18:1426-31; PMID:18804373; http://dx.doi.org/ 10.1016/j.cub.2008.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ha KD, Clarke BA, Brown WJ. Regulation of the golgi complex by phospholipid remodeling enzymes. Biochim Biophysic Acta 2012; 1821:1078-88; PMID:22562055; http://dx.doi.org/ 10.1016/j.bbalip.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Nat Acad Sci U S A 2013; 110:4628-33; PMID:23487760; http://dx.doi.org/ 10.1073/pnas.1217611110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J Biol Chem 2010; 285:22027-35; PMID:20489212; http://dx.doi.org/ 10.1074/jbc.M110.126870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Farkas RM, Giansanti MG, Gatti M, Fuller MT. The drosophila cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized golgi architecture during spermatogenesis. Mol Biol Cell 2003; 14:190-200; PMID:12529436; http://dx.doi.org/ 10.1091/mbc.E02-06-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, Trimble WS. Syntaxin 5 is required for cytokinesis and spermatid differentiation in drosophila. Dev Biol 2002; 251:294-306; PMID:12435359; http://dx.doi.org/ 10.1006/dbio.2002.0830 [DOI] [PubMed] [Google Scholar]
- 76. Rohmer C, David JR, Moreteau B, Joly D. Heat induced male sterility in drosophila melanogaster: adaptive genetic variations among geographic populations and role of the Y chromosome. J Exp Biol 2004; 207:2735-43; PMID:15235002; http://dx.doi.org/ 10.1242/jeb.01087 [DOI] [PubMed] [Google Scholar]
- 77. Brand AH, Manoukian AS, Perrimon N. Ectopic expression in drosophila. Meth Cell Biol 1994; 44:635-54; PMID:7707973; http://dx.doi.org/ 10.1016/S0091-679X(08)60936-X [DOI] [PubMed] [Google Scholar]
- 78. Trotta V, Calboli FC, Ziosi M, Guerra D, Pezzoli MC, David JR, Cavicchi S. Thermal plasticity in drosophila melanogaster: a comparison of geographic populations. BMC Evol Biol 2006; 6:67; PMID:16942614; http://dx.doi.org/ 10.1186/1471-2148-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem 2009; 284:1-5; PMID:18718904; http://dx.doi.org/ 10.1074/jbc.R800046200 [DOI] [PubMed] [Google Scholar]
- 80. Rogat AD, Miller KG. A role for myosin VI in actin dynamics at sites of membrane remodeling during drosophila spermatogenesis. J Cell Sci 2002; 115:4855-65; PMID:12432073; http://dx.doi.org/ 10.1242/jcs.00149 [DOI] [PubMed] [Google Scholar]
- 81. Morris CA, Benson E, White-Cooper H. Determination of gene expression patterns using in situ hybridization to drosophila testes. Nat Protocol 2009; 4:1807-19; PMID:20010932; http://dx.doi.org/ 10.1038/nprot.2009.192 [DOI] [PubMed] [Google Scholar]
- 82. Letunic I, Bork P. Interactive tree of life v2: online annotation and display of phylogenetic trees made easy. Nucl Acid Res 2011; 39:W475-8; PMID:21470960; http://dx.doi.org/ 10.1093/nar/gkr201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol 2004; 36:1187-205; PMID:15109566; http://dx.doi.org/ 10.1016/j.biocel.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 84. Pascual A, Chaminade M, Preat T. Ethanolamine kinase controls neuroblast divisions in drosophila mushroom bodies. Dev Biol 2005; 280:177-86; PMID:15766757; http://dx.doi.org/ 10.1016/j.ydbio.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 85. Fuller MT. Spermatogenesis. The Development of Drosophila Melanogaster, ed. Bate M. and Martinez Arias A., Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993; 71-148 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


