Successful completion of gametogenesis is critical for perpetuation of the species. In addition to the inherent interest, studies of gamete development, in particular spermatogenesis, have yielded insight into diverse biological processes, including actin and microtubule organization, mitochondrial dynamics, plasma membrane remodeling, lipid signaling, apoptosis, and many others.
Mammalian sperm are formed from germline stem cells that reside near the basal surface of the seminiferous tubules.1 Spermatogonia produced from these stem cells undergo amplifying mitotic divisions with incomplete cytokinesis to eventually produce interconnected chains of spermatocytes that synchronously transition into meiosis.2,99 Cytokinesis of the meiotic divisions also is incomplete, such that cytoplasmic channels remain between sister spermatids after each division.3,4 This allows for the sharing of cytoplasm between sister spematids, which synchronizes their development and protects them from the genetic effects of haploidy.3,5,6 Following meiosis, the haploid spermatids undergo spermiogenesis, the terminal differentiation process wherein acrosomes are formed from Golgi, chromatin compacts, the nuclei are reshaped, and the flagella elongate.7,8 After terminal differentiation, the cytoplasmic contents are removed and the cytoplasmic bridges connecting sister spermatozoa are dissolved.9, 10 This last process is dependent on the actin cytoskeleton and is essential for proper sperm function.11-14 The spermatozoa are released from the testis into the epididymis, where their plasma membranes undergo molecular changes.15,16 Epididymal activation is required for motility and fertilization.17
Spermatogenesis is strikingly similar in the fruit fly, and many molecular players are conserved between mammals and Drosophila.18,19 A single Drosophila gonialblast, formed by division of a germline stem cell, undergoes four mitotic divisions and two meiotic divisions to produce 64 interconnected sister spermatids in a germline cyst.20,97 As in mammals, incomplete cytokinesis leads to cytoplasmic sharing between sister spermatids, via intercellular bridges called ring canals.3 Following nuclear compaction and formation of the flagella, the interspermatid bridges are dissolved concurrently with cytoplasm removal in an actin-dependent process called spermatid individualization.21,22 Much has been discovered about this process in the 21st century.
Individualization is carried out by the individualization complex (IC), which first forms at the rostral end of the cyst, around the spermatid nuclei (Figure 1). The IC is composed of 64 actin cones, one for each germ nucleus of the cyst.21,22 Actin filaments form a meshwork at the leading edge of the cones and are organized into parallel bundles at the rear of the cones.23 The meshwork is formed by the Arp2/3 actin nucleating complex.24,25 The actin motor Myosin VI works with unknown binding partners to localize Arp2/3 and to stabilize the meshwork at the front of the cones.23,24,26 Other factors at the cone fronts include Actin Capping Protein and Cortactin, and the membrane binding protein Amphiphysin.24 At the rear of the cones, the actin bundling proteins Quail/Villin, Chickadee/Profilin, and Singed/Fascin localize.25 As individualization proceeds, the actin cones of the IC move synchronously away from the nuclei toward the caudal end of the cyst, traversing the spermatid flagella at an average speed of 3 μm/minute and finishing the 1.8 mm journey in 10 hours.27 As it travels, the IC removes the cyst cytoplasmic contents and individualizes each spermatozoon in its own plasma membrane (Figure 1).21 The cones accumulate actin during this process, especially at their front edges, and proper accumulation of actin filaments in the leading edge meshwork is required for cytoplasmic extrusion.23,25 Extruded cytoplasmic contents are collected in a cystic bulge that forms around the IC.21 When the IC and cystic bulge reach the end of the flagella, the actin cones and cytoplasmic contents find themselves in a waste bag, the contents of which are degraded.21 It is not yet known what generates the force for IC movement. Although Myosins V and VI are important for this process, motor activity does not seem to power migration of the IC.23,27-29 The observation that actin polymerization is essential for IC progression suggests that the IC moves by incorporating new actin filaments at the cones,21,27 and experimental evidence indicates that the rear bundles specifically are involved.25
Figure 1.
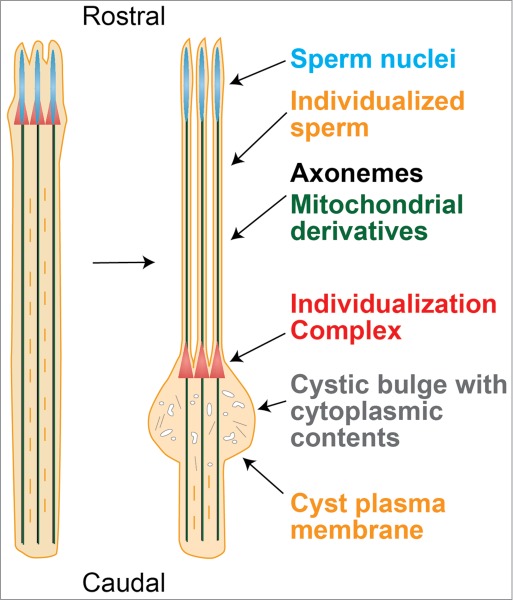
Spermatid individualization in Drosophila. The actin cones of the individualization complex (IC, red) form around the spermatid nuclei, which are located rostrally in the germline cyst. As the IC moves caudally down the spermatid flagella, it removes the cytoplasmic contents and invests each sperm in its own plasma membrane.
Classical genetic studies have identified at least 70 genes that mutate to give individualization phenotypes (Table 1). Grouping these factors according to function underlines several molecular pathways that contribute to the process. As expected, a number of actin binding proteins and regulators are required for individualization, many of which localize to the IC. Myosin V and the focal adhesion protein Lasp localize to the actin cones during their formation and are required for assembly of the cones around the nuclei.29,30 Chickadee/Profilin is required for IC movement and localizes to the rear of the cones. In its absence, the cones are short, lack rear bundles, and do not progress away from the nuclei.25 In contrast, ICs lacking leading edge proteins, such as Myosin VI or Arp3, have thin actin cones that are able to progress caudally, but these cones accumulate less actin, particularly in the front meshwork, do not remain in sync, and fail to successfully individualize the spermatids.23,25 Intriguingly, the actin regulator Rotund, a GTPase activating protein (GAP) for the signaling protein Rac, also plays a role in this process, suggesting that a signal cascade initiates IC formation or movement.31 The identity of such signal has yet to be discovered. Casein kinase might also be involved in transduction of the signal.32
Table 1.
Drosophila genes in spermatid individualization
| Gene/protein | IC phenotype in mutant | EMa showing individualization defects? | Caspase staining? | Other spermatogenesis phenotypes | Reference | Molecular function | Cellular function |
|---|---|---|---|---|---|---|---|
| Ark/ Apaf-1 |
Scattered migrating ICs, reduced cystic bulges and waste bags |
+ |
Active caspase staining reduced |
None reported |
58,59 |
Apoptosis effector |
Apoptosis |
| Dcp-1 |
NDb |
+ |
ND |
None reported |
58 |
Caspase |
Apoptosis |
| Dredd |
Scattered migrating ICs |
+ |
No changec |
None reported |
58 |
Caspase |
Apoptosis |
| Drice |
ND |
+ |
No change |
None reported |
61 |
Caspase |
Apoptosis |
| Driceless |
Normal ICs |
+ |
Active caspase staining eliminated |
None reported |
58 |
Apoptosis effector |
Apoptosis |
| Dronc |
Scattered migrating ICs, reduced cystic bulges and waste bags |
+ |
Active caspase staining reduced |
None reported |
58,59 |
Caspase |
Apoptosis |
| Fadd |
Scattered migrating ICs |
+ |
No change |
None reported |
58 |
Apoptosis effector |
Apoptosis |
| Hid |
Scattered migrating ICs |
+ |
No change |
None reported |
58 |
Apoptosis effector |
Apoptosis |
| Tango7 |
Scattered migrating ICs |
ND |
Active caspase staining eliminated |
None reported |
60 |
Apoptosis effector |
Apoptosis |
| Cytochrome-c-d |
No migrating ICs |
+ |
Active caspase staining eliminated |
Axoneme microtubules are not properly polyglycylated, defective mitochondrial derivatives |
58,59,62 |
Apoptosis effector, electron transport chain |
Apoptosis, mitochondria |
| Bruce |
ICs do not form normally |
ND |
No change |
Scattered misshapen nuclei |
62,63 |
Inhibitor of apoptosis (IAP), ubiquitin conjugating enzyme (E2) |
Apoptosis, ubiquitin-proteasome pathway |
| Arp3 |
Thin migrating actin cones, less actin density on ICs, reduced cystic bulges |
+ |
ND |
Defective minor mitochondrial derivative |
25 |
Actin meshwork nucleator |
Cytoskeleton |
| Abnormal spindle |
ND |
+ |
ND |
Meiosis failure, abnormal spindles, defective mitochondrial derivatives |
33 |
Cytoskeleton regulator |
Cytoskeleton |
| Bug22 |
Scattered migrating ICs, reduced cystic bulges |
+ |
No change |
Defective mitochondrial derivatives, abnormal axoneme structure, abnormal centrioles, axoneme microtubules are not properly polyglycylated |
42 |
Ciliary protein |
Cytoskeleton |
| CDIC/ Short wing |
Scattered migrating ICs |
ND |
ND |
Scattered nuclei |
34 |
Microtubule motor |
Cytoskeleton |
| Dynein intermediate chain 61B |
Scattered migrating ICs |
ND |
ND |
Defective mitochondrial derivatives, abnormal axoneme structure |
44 |
Microtubule motor |
Cytoskeleton |
| Dynein light chain 1/ Cut up |
Scattered migrating ICs, less actin density on ICs |
+ |
ND |
Scattered nuclei, spermatid elongation abnormal |
34,35 |
Microtubule motor |
Cytoskeleton |
| Dynein light chain 90F/Tctex |
Scattered migrating ICs |
ND |
ND |
Scattered misshapen nuclei, defective basal body positioning |
36 |
Microtubule motor |
Cytoskeleton |
| Lasp |
ICs do not form normally, less actin density on ICs |
+ |
ND |
Hub is displaced from apical tip, premature sperm coiling, cyst degeneration |
30 |
Actin binding protein |
Cytoskeleton |
| Merlin |
Scattered migrating ICs |
+ |
ND |
Mild cytokinesis defects, cyst polarization defects, scattered misshapen nuclei, defective mitochondrial derivatives |
38 |
Cytoskeleton regulator |
Cytoskeleton |
| Mulet |
Scattered migrating ICs |
ND |
ND |
Cytoplasmic microtubules persist |
22,40,41 |
Tubulin-specific chaperone E-like |
Cytoskeleton |
| Myosin V |
ICs do not form normally, scattered migrating ICs |
ND |
ND |
None reported |
29 |
Actin motor |
Cytoskeleton |
| Myosin VI/ Jaguar |
Scattered migrating ICs, less actin density on ICs, reduced cystic bulges |
+ |
No change |
Mild nuclear scattering |
23,28,62 |
Actin motor |
Cytoskeleton |
| Profilin/ Chickadee |
No migrating ICs, short actin cones |
ND |
ND |
Cytokinesis defects |
25 |
Actin bundling regulator |
Cytoskeleton |
| RotundRacGAP |
No migrating ICs |
+ |
ND |
Spermatid elongation abnormal, reduced interspermatid membranes, randomly oriented axonemes, defective mitochondrial derivatives, disorganized cytoplasmic microtubules |
31 |
GTPase activating protein |
Cytoskeleton |
| Tubulin Tyrosine Ligase-like 3B |
Scattered migrating ICs |
+ |
ND |
Swollen testis tips, axonemes degenerate |
43 |
Glycylase |
Cytoskeleton |
| Yuri gagarin |
ICs do not form normally |
+ |
ND |
Scattered misshapen nuclei, coiling defects, defective mitochondrial derivatives, defective basal body positioning |
37 |
Cytoskeleton regulator |
Cytoskeleton |
| Fan |
Scattered migrating ICs |
ND |
Ectopic caspases behind IC |
None reported |
55 |
ER resident, OSBP binding |
Lipid metabolism |
| Noa |
Scattered migrating ICs |
ND |
ND |
Small testes, scattered nuclei |
90 |
Fatty acid elongase |
Lipid metabolism |
| NPC1 |
Scattered migrating ICs |
+ |
Ectopic caspases behind IC |
Scattered misshapen nuclei, mild cytokinesis defects |
56 |
Sterol transport |
Lipid metabolism |
| Osbp |
Scattered migrating ICs |
+ |
Ectopic caspases behind IC |
None reported |
55 |
Sterol transport |
Lipid metabolism |
| Oys and Nes |
Scattered migrating ICs |
ND |
No change |
None reported |
57 |
Lysophospholipid acyltransferase |
Lipid metabolism |
| Pxt |
Scattered migrating ICs |
ND |
ND |
None reported |
98 |
Cyclooxygenase |
Lipid metabolism |
| Hsp60B |
Scattered migrating ICs |
ND |
ND |
None reported |
91 |
Heat shock protein, chaperone |
Mitochondria |
| Mitoferrin |
ICs do not form normally |
+ |
ND |
Scattered nuclei, defective mitochondrial derivatives, spermatid elongation abnormal |
72 |
Mitochondrial carrier protein |
Mitochondria |
| Parkin |
Scattered migrating ICs |
+ |
ND |
Defective mitochondrial derivatives, mild nuclear scattering, disorganized cytoplasmic microtubules |
65,67-69 |
Ubiquitin ligase (E3) |
Mitochondria |
| Pink1 |
ND |
+ |
ND |
Defective mitochondrial derivatives |
66-68 |
Kinase |
Mitochondria |
| Tafazzin |
ND |
+ |
ND |
None reported |
70 |
Cardiolipin transacylase |
Mitochondria |
| Topo IIIα |
Scattered migrating ICs |
ND |
ND |
Scattered nuclei, germline stem cell loss |
71 |
Topoisomerase |
Mitochondria |
| Ago2 |
Scattered migrating ICs |
ND |
ND |
Scattered nuclei |
81 |
RISC complex |
RNAi pathway |
| Blanks/ Lump |
Scattered migrating ICs, arrested ICs |
ND |
ND |
Scattered nuclei |
82,83 |
dsRNA binding |
RNAi pathway |
| Dcr2 |
Scattered migrating ICs |
ND |
ND |
Scattered nuclei |
81 |
dsRNA processing |
RNAi pathway |
| HpRNA1 |
Scattered migrating ICs |
ND |
ND |
Mild nuclear scattering |
81 |
Non-coding hairpin RNA |
RNAi pathway |
| Crossbronx |
Scattered ICs |
ND |
ND |
Scattered nuclei |
22 |
Ubiquitin conjugating enzyme (E2) |
Ubiquitin-proteasome pathway |
| Cullin-3 |
ND |
ND |
Active caspase staining eliminated |
None reported |
64 |
Ubiquitin ligase complex |
Ubiquitin-proteasome pathway |
| Klhl10 |
ND |
ND |
Active caspase staining eliminated |
None reported |
64 |
Ubiquitin ligase complex |
Ubiquitin-proteasome pathway |
| Nutcracker |
ICs do not form normally |
+ |
Active caspase staining eliminated |
None reported |
92 |
Ubiquitin ligase complex |
Ubiquitin-proteasome pathway |
| Prosα6T |
Scattered migrating ICs |
ND |
Active caspase staining reduced |
Scattered misshapen nuclei |
93 |
Proteasome core subunit |
Ubiquitin-proteasome pathway |
| Purity of essence |
Reduced cystic bulges, less actin density on ICs |
ND |
No change |
Mild nuclear scattering |
22,62 |
Ubiquitin ligase (E3) |
Ubiquitin-proteasome pathway |
| Roc1b |
ND |
ND |
Active caspase staining reduced |
None reported |
64 |
Ubiquitin ligase complex |
Ubiquitin-proteasome pathway |
| Scotti |
Scattered migrating ICs, arrested ICs, reduced cystic bulges |
ND |
Elevated active caspase staining |
None reported |
63 |
Ubiquitin ligase inhibitor |
Ubiquitin-proteasome pathway |
| Auxilin |
IC formation delayed, scattered migrating ICs |
+ |
ND |
Mild nuclear scattering, abnormal spermatid plasma membranes, mild cytokinesis defects |
50 |
Clathrin regulator |
Vesicle transport |
| Clathrin heavy chain |
No migrating ICs |
+ |
ND |
Scattered nuclei, abnormal spermatid plasma membranes |
22,50 |
Vesicle coat |
Vesicle transport |
| Dynamin/Shibire |
ICs do not form normally, less actin density on ICs |
ND |
ND |
None reported |
24,34 |
GTPase, endocytosis |
Vesicle transport |
| Rab11 |
Less actin density on ICs,scattered migrating ICs |
ND |
ND |
None reported |
52 |
GTPase |
Vesicle transport |
| Scattered |
Scattered migrating ICs |
ND |
ND |
Scattered misshapen nuclei |
22 |
Vps54-like, Golgi Associated Retrograde Protein (GARP) complex |
Vesicle transport |
| Vps28 |
Scattered migrating ICs |
ND |
ND |
None reported |
51 |
ESCRT-I complex |
Vesicle transport |
| Asunder |
Scattered migrating ICs |
ND |
ND |
Cytokinesis defects, meiosis failure, abnormal spindles, defective basal body positioning, scattered misshapen nuclei |
94 |
Integrator complex |
RNA metabolism |
| eIF4E-3 |
ICs do not form normally |
ND |
ND |
Cytokinesis defects, abnormal meiotic segregation, scattered misshapen nuclei |
86 |
Translation initiation factor |
RNA metabolism |
| GLD2 |
ICs do not form normally |
ND |
ND |
Scattered nuclei, abnormal chromatin compaction, defective basal body positioning |
88 |
Cytoplasmic poly(A) polymerase |
RNA metabolism |
| Novel spermatogenesis regulator |
Scattered migrating ICs, reduced cystic bulges |
+ |
ND |
Coiling defects, abnormal axoneme structure |
87 |
RNA binding protein |
RNA metabolism |
| Orb2 |
ICs do not form normally, scattered migrating ICs |
ND |
ND |
Meiosis failure, scattered misshapen nuclei, defective nebenkerne, spermatid elongation abnormal, swollen testis tips |
85 |
Cytoplasmic Polyadenylation Element Binding protein |
RNA metabolism |
| Polypyrimidine tract-binding protein/ hnRNP1/ Hephaestus |
Scattered migrating ICs |
ND |
No change |
Scattered nuclei, swollen testis tips |
62,89 |
RNA binding protein |
RNA metabolism |
| Dud |
Scattered migrating ICs |
ND |
ND |
Mild nuclear scattering, lacking nebenkerne |
22 |
ND |
|
| Gilgamesh |
Scattered migrating ICs |
+ |
ND |
Scattered nuclei |
32 |
Casein kinase γ1 |
Signaling |
| Gudu |
Scattered migrating ICs |
ND |
ND |
None reported |
95 |
ND |
|
| Long island expressway |
Scattered migrating ICs |
ND |
ND |
Scattered nuclei |
22 |
ND |
|
| Mozzarella |
ICs do not form normally |
ND |
ND |
Scattered misshapen nuclei |
22 |
ND |
|
| Nanking |
ICs do not form normally |
ND |
ND |
Mild nuclear scattering, defective nebenkerne |
22 |
ND |
|
| Rae1 |
ICs do not form normally |
ND |
ND |
Nuclear herniation, abnormal chromatin compaction, abnormal spindles, meiosis failure, defective nebenkerne, scattered misshapen nuclei |
96 |
WD40 protein |
|
| Thousand points of light | Scattered migrating ICs | ND | ND | Scattered misshapen nuclei | 22 | ND |
aEM, electron micrograph.
bND, no data.
cNo change indicates that active caspases are still seen, but in many of these mutants, cystic bulges are not normal.
The microtubule cytoskeleton, as well as the actin cytoskeleton, seems to be important for individualization. Loss of the microtubule binding protein Abnormal Spindle (Asp) results in many spermatogenesis defects, including failed individualization.33 Mutations in components of the Dynein-Dynactin complex, including Cytoplasmic Dynein Intermediate Chain (CDIC) and two Drosophila Dynein Light Chains, DDLC1 and DLC90F, perturb synchronous movement of the actin cones, but they also perturb nuclear shaping and positioning.34-36 Mutations in two other genes implicated in cytoskeletal dynamics, yuri gagarin and merlin, disrupt both nuclei and ICs as well.37,38 Cytoplasmic microtubules adjacent to the nuclei are important for nuclear shaping,39 which in turn may be required for the IC to assemble properly. Alternatively, microtubules in the vicinity of the nuclei might play independent roles in nuclear shaping and in aligning the actin cones during their formation. Recently, the individualization mutant mulet was mapped to a tubulin-specific chaperone E-like protein (TBCEL), again pointing to a role for microtubules.40,41 Unlike the Dynein-Dynactin complex mutations, the mulet mutation disrupts IC translocation without affecting the nuclei.22,40 The TBCEL protein can block microtubule assembly by disrupting tubulin heterodimers, and in the mulet mutant, cytoplasmic microtubules persist aberrantly in individualizing cysts, suggesting that these microtubules interfere with IC progression.40 Altogether, these observations indicate that cytoplasmic microtubules are important for assembly of the IC around the nuclei but must be cleared in order for the IC to translocate. Experiments with microtubule depolymerizing or stabilizing drugs in cultured cysts suggest that cytoplasmic microtubules are not involved in IC movement per se, but it is not clear whether the progressing IC interacts with axonemal microtubules.27 When axonemal microtubules are not properly post-translationally modified, individualization is affected.42,43 Furthermore, the putative axonemal Dynein Intermediate Chain Dic61B is required for individualization.44 However, other studies suggest that individualization can occur normally in the absence of certain axonemal components.45 Finally, DDLC1 plays a role in actin accumulation on the cones, which is independent of the Dynein-Dynactin motor and could result from its association with Myosin V.34,46
The individualization process may require deposition of new membrane between the spermatids. Other processes that involve new plasma membrane deposition, such as cytokinesis and spermatid elongation, use vesicles to shuttle phospholipids from the Golgi.47-49 However, visualization of membranes with fluorescent dye shows little vesicle trafficking at the cystic bulge during IC progression.27 Despite this observation, a number of vesicle trafficking factors are required for individualization, including Auxilin, Clathrin Heavy Chain, Rab11, Shibire/Dynamin, Vps28, and the Vps54-like protein Scattered.22,24,34,50-52 The cystic bulge contains numerous membraneous structures, and puncta within the cystic bulge stain positively for the endocytic adaptor α-adaptin.21,24,27 Because most of the cytoplasmic contents are removed by the individualization process, perhaps vesicle trafficking prior to IC progression segregates cellular components destined for degradation from those that will remain in the mature spermatozoa. Vesicles within the cystic bulge could provide specific lipids for incorporation into sperm membranes as well (see below). shibire mutants show additional defects in actin accumulation on the ICs, suggesting that Shibire/Dynamin plays a role in IC assembly as well as translocation.24,34 Dynamin could anchor the plasma membrane to the IC, possibly in concert (or in parallel) with Amphiphysin, Cortactin, and Myosin VI.24,26,53 Alternatively, Dynamin could play a role in actin deposition independent of the membrane, perhaps with DDLC1.34,54 Some of the other vesicle trafficking mutants show nuclear defects, suggesting that they may also be required for IC assembly.22,50 Thus, it is not clear whether vesicle trafficking plays a direct role in IC movement.
Several lipid metabolism factors are required for individualization. In the absence of the sterol trafficking proteins OSBP, Fan, and NPC1, the actin cones do not migrate synchronously.55,56 Using filipin dye, sterols can be visualized in puncta within the cystic bulge, suggesting that trafficking of specific lipids occurs during this process.55 In mammals, the molecular composition of the sperm plasma membrane changes during maturation, and proper composition is required for fertility.15,16 Furthermore, failure to remove the cytoplasm can lead to peroxidation of membrane lipids and infertility.13,14 Perhaps a similar process occurs during Drosophila individualization, wherein the molecular composition of the sperm membranes is determined during migration of the IC. In this case, membranes and vesicles within the cystic bulge may act as a depot for the lipids. Specific lipids might also tether the IC to the membrane. In addition to cholesterol, phospholipid metabolism pathways contribute to individualization. In the absence of the lysophospholipid acyltransferases Oys and Nes or the cyclooxygenase Pxt, the actin cones do not migrate properly, suggesting that prostaglandin-like lipids generated from membrane phospholipids are important for this process.57,98 When phospholipid levels are genetically manipulated, no effect is seen, indicating that specific molecules, rather than bulk phospholipids, are critical.98 Whether these lipids play structural or signaling roles remains to be determined.
The discarded cytoplasm undergoes an apoptosis-like program during the process of individualization. Numerous apoptotic proteins are required for individualization to proceed correctly, including the apoptosis effectors Tango7, Fadd, and Hid and the apoptosome component Ark/Apaf-1.58-60 These proteins activate the pathway via initiator caspases Dronc and Dredd and effector caspases Drice and Dcp-1.58,59,61 The spermatid apoptosis program seems to be limited by the inhibitor of apoptosis (IAP) Bruce, and Bruce in turn is localized by the ubiquitin-proteasome system.62-64 Ubiquitylation of Bruce by the Klhl-10/Cullin-3 ubiquitin ligase complex at the rostral end of the cyst reduces Bruce levels, either by degradation or redistribution, which permits apoptosis initiation there.63,64 At the caudal end of the cyst, the ubiquitin ligase inhibitor Scotti protects Bruce by preventing its ubiquitylation, thereby preventing apoptosis initiation.63 Thus, by this mechanism, the apoptosis pathway is limited to the region of the cystic bulge, which begins at the rostral end. However, the spermatid nuclei also reside at the rostral end, and it is not known how they are protected from apoptotic degradation. Several mutants that disrupt movement of the IC have no effect on apoptosis initiation, suggesting that activation of this program is independent of other individualization events.62 However, mutation of apoptosis components disrupts migration of the IC, indicating that faulty apoptosis can disturb the entire process.
Many other ubiquitin-proteasome pathway components have been identified that participate in individualization (see Table 1). Their targets are not currently known. There seems to be large-scale degradation of cellular components following cytoplasm extrusion.21 Therefore, it is not clear if the individualization defects observed in these mutants are due to the persistence of specific targets or to a general failure of protein degradation.
Bruce removal alone may not be sufficient to initiate apoptosis. Similarly to mammalian apoptosis pathways, the mitochondria also play a role in apoptosis initiation in spermatids, via Cytochrome c-d.58,59,62 Intriguingly, mutations that disrupt the mitochondria prevent proper individualization, including those in the genes pink1, parkin, mitoferrin, mitochondrially-targeted topoisomerase IIIα, and the cardiolipin transacylase gene tafazzin.65-72 It has yet to be determined if this effect is mediated by Cytochrome c-d.
Spermiogenesis, in particular spermatid individualization, appears to be easily disrupted. Mutagenesis screens have discovered many genes that block spermatogenesis at this late step.73-75,97 This may be because the process is complex, requiring many factors, as detailed above. Another hypothesis, not mutually exclusive, is that this step represents a checkpoint for the removal of improperly differentiated spermatids.76 Support for this idea is found in flies experiencing meiotic drive, e.g. heterozygotes for a Segregation Distorter (SD) second chromosome that prevents formation of viable sperm carrying the other, normal second chromosome by interfering with proper chromatin condensation.77-79 In heterozygous cysts, in which half of the 64 sister spermatids carry the SD chromosome and half carry the normal homolog, the spermatids carrying the normal homolog are blocked at the individualization step, while their sisters are properly individualized and released from the testis.21,78 Individualization also is very sensitive to temperature, suggesting that cellular stress can halt the process.98 Other cell stressors have not been tested, but Wolbachia infection has been seen to induce mild individualization defects in some cases.80 Recently, it was found that genetic perturbation of the RNAi pathway causes individualization phenotypes.81-83 RNAi pathway mutations also perturb cytoskeletal reorganization of the oocyte in a checkpoint-mediated process.84 This seems to be a way for the oocyte to abort development when chromosomal integrity is disturbed by unregulated transposon activity. Perhaps a similar mechanism operates in spermatogenesis. Some, but not all, mutants that disrupt individualization show other spermatogenesis phenotypes; thus their effects on individualization may be indirect.
In conclusion, genetic studies have identified numerous genes required for individualization of the differentiated spermatozoa, the final step of spermatogenesis. Many of these genes have been characterized molecularly, and they have highlighted important mechanisms at play during this process, including actin and microtubule dynamics, plasma membrane reorganization, and apoptotic elimination of the cytoplasmic contents. Many questions still persist, including: What are the signals that initiate individualization? How is the membrane reorganized, structurally and molecularly? How is membrane reorganization coordinated with IC movement? What propels IC movement? How are cytoplasmic components correctly partitioned into the cystic bulge? What protects the nucleus from the apoptosis pathway? Do all mutations that perturb individualization do so directly? How is gene expression coordinated at this developmental stage? It is likely that many factors are regulated post-transcriptionally, as the spermatid nuclei are highly condensed by this time, and indeed, RNA metabolism proteins play important roles in this process.85-89 Furthermore, several genes necessary for individualization have not been characterized molecularly yet, and more genes acting in the process likely will be discovered. Future studies will elucidate a more coherent model that will undoubtedly reveal interesting molecular mechanisms and shed light on human fertility and infertility as well.
Acknowledgements
I am very grateful to Sarah Weil, Rebecca Burgess, and James Fabrizio for critical comments on the manuscript.
References
- 1. Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harbor perspectives in biology 2011; 3:a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Advances in experimental medicine and biology 2008; 636:1-15. [DOI] [PubMed] [Google Scholar]
- 3. Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM. Germ cell intercellular bridges. Cold Spring Harbor perspectives in biology 2011; 3:a005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren HP, Russell LD. Clonal development of interconnected germ cells in the rat and its relationship to the segmental and subsegmental organization of spermatogenesis. The American journal of anatomy 1991; 192:121-8. [DOI] [PubMed] [Google Scholar]
- 5. Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature 1989; 337:373-6. [DOI] [PubMed] [Google Scholar]
- 6. Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. The Journal of biophysical and biochemical cytology 1959; 5:453-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 3: developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Microscopy research and technique 2010; 73:320-63. [DOI] [PubMed] [Google Scholar]
- 8. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microscopy research and technique 2010; 73:279-319. [DOI] [PubMed] [Google Scholar]
- 9. O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 2011; 1:14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breucker H, Schafer E, Holstein AF. Morphogenesis and fate of the residual body in human spermiogenesis. Cell and tissue research 1985; 240:303-9. [DOI] [PubMed] [Google Scholar]
- 11. Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue & cell 1989; 21:361-79. [DOI] [PubMed] [Google Scholar]
- 12. Akbarsha MA, Latha PN, Murugaian P. Retention of cytoplasmic droplet by rat cauda epididymal spermatozoa after treatment with cytotoxic and xenobiotic agents. Journal of reproduction and fertility 2000; 120:385-90. [DOI] [PubMed] [Google Scholar]
- 13. Keating J, Grundy CE, Fivey PS, Elliott M, Robinson J. Investigation of the association between the presence of cytoplasmic residues on the human sperm midpiece and defective sperm function. Journal of reproduction and fertility 1997; 110:71-7. [DOI] [PubMed] [Google Scholar]
- 14. Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reproductive biology and endocrinology : RB&E 2012; 10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall JC, Hadley J, Doman T. Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. Journal of andrology 1991; 12:76-87. [PubMed] [Google Scholar]
- 16. Rejraji H, Sion B, Prensier G, Carreras M, Motta C, Frenoux JM, Vericel E, Grizard G, Vernet P, Drevet JR. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biology of reproduction 2006; 74:1104-13. [DOI] [PubMed] [Google Scholar]
- 17. Jones RC. To store or mature spermatozoa? The primary role of the epididymis. International journal of andrology 1999; 22:57-67. [DOI] [PubMed] [Google Scholar]
- 18. White-Cooper H. Studying how flies make sperm–investigating gene function in Drosophila testes. Molecular and cellular endocrinology 2009; 306:66-74. [DOI] [PubMed] [Google Scholar]
- 19. White-Cooper H, Bausek N. Evolution and spermatogenesis. Philosophical transactions of the Royal Society of London Series B, Biological sciences 2010; 365:1465-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. Journal of ultrastructure research 1979; 69:180-90. [DOI] [PubMed] [Google Scholar]
- 21. Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z Zellforsch Mikrosk Anat 1972; 124:479-506. [DOI] [PubMed] [Google Scholar]
- 22. Fabrizio JJ, Hime G, Lemmon SK, Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster. Development 1998; 125:1833-43. [DOI] [PubMed] [Google Scholar]
- 23. Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Molecular biology of the cell 2006; 17:2559-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogat AD, Miller KG. A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. Journal of cell science 2002; 115:4855-65. [DOI] [PubMed] [Google Scholar]
- 25. Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Molecular biology of the cell 2008; 19:2363-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isaji M, Lenartowska M, Noguchi T, Frank DJ, Miller KG. Myosin VI regulates actin structure specialization through conserved cargo-binding domain sites. PloS one 2011; 6:e22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noguchi T, Miller KG. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development 2003; 130:1805-16. [DOI] [PubMed] [Google Scholar]
- 28. Hicks JL, Deng WM, Rogat AD, Miller KG, Bownes M. Class VI unconventional myosin is required for spermatogenesis in Drosophila. Molecular biology of the cell 1999; 10:4341-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mermall V, Bonafe N, Jones L, Sellers JR, Cooley L, Mooseker MS. Drosophila myosin V is required for larval development and spermatid individualization. Developmental biology 2005; 286:238-55. [DOI] [PubMed] [Google Scholar]
- 30. Lee S, Zhou L, Kim J, Kalbfleisch S, Schock F. Lasp anchors the Drosophila male stem cell niche and mediates spermatid individualization. Mechanisms of development 2008; 125:768-76. [DOI] [PubMed] [Google Scholar]
- 31. Bergeret E, Pignot-Paintrand I, Guichard A, Raymond K, Fauvarque MO, Cazemajor M, Griffin-Shea R. RotundRacGAP functions with Ras during spermatogenesis and retinal differentiation in Drosophila melanogaster. Molecular and cellular biology 2001; 21:6280-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nerusheva OO, Dorogova NV, Gubanova NV, Yudina OS, Omelyanchuk LV. A GFP trap study uncovers the functions of Gilgamesh protein kinase in Drosophila melanogaster spermatogenesis. Cell biology international 2009; 33:586-93. [DOI] [PubMed] [Google Scholar]
- 33. Casal J, Gonzalez C, Wandosell F, Avila J, Ripoll P. Abnormal meiotic spindles cause a cascade of defects during spermatogenesis in asp males of Drosophila. Development 1990; 108:251-60. [DOI] [PubMed] [Google Scholar]
- 34. Ghosh-Roy A, Desai BS, Ray K. Dynein light chain 1 regulates dynamin-mediated F-actin assembly during sperm individualization in Drosophila. Molecular biology of the cell 2005; 16:3107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghosh-Roy A, Kulkarni M, Kumar V, Shirolikar S, Ray K. Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila. Molecular biology of the cell 2004; 15:2470-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li MG, Serr M, Newman EA, Hays TS. The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Molecular biology of the cell 2004; 15:3005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Texada MJ, Simonette RA, Johnson CB, Deery WJ, Beckingham KM. Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. Journal of cell science 2008; 121:1926-36. [DOI] [PubMed] [Google Scholar]
- 38. Dorogova NV, Akhmametyeva EM, Kopyl SA, Gubanova NV, Yudina OS, Omelyanchuk LV, Chang LS. The role of Drosophila Merlin in spermatogenesis. BMC cell biology 2008; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. IV. Nuclear transformation. Journal of ultrastructure research 1974; 48:284-303. [DOI] [PubMed] [Google Scholar]
- 40. Fabrizio JJ, Aqeel N, Cote J, Estevez J, Jongoy M, Mangal V, Tema W, Rivera A, Wnukowski J, Bencosme Y. Mulet (mlt) encodes a tubulin-binding cofactor E-like homolog required for spermatid individualization in Drosophila melanogaster. Fly 2012; 6:261-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nuwal T, Kropp M, Wegener S, Racic S, Montalban I, Buchner E. The Drosophila homologue of tubulin-specific chaperone E-like protein is required for synchronous sperm individualization and normal male fertility. Journal of neurogenetics 2012; 26:374-81. [DOI] [PubMed] [Google Scholar]
- 42. Mendes Maia T, Gogendeau D, Pennetier C, Janke C, Basto R. Bug22 influences cilium morphology and the post-translational modification of ciliary microtubules. Biology open 2014; 3:138-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rogowski K, Juge F, van Dijk J, Wloga D, Strub JM, Levilliers N, Thomas D, Bre MH, Van Dorsselaer A, Gaertig J, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 2009; 137:1076-87. [DOI] [PubMed] [Google Scholar]
- 44. Fatima R. Drosophila Dynein intermediate chain gene, Dic61B, is required for spermatogenesis. PloS one 2011; 6:e27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Timakov B, Zhang P. Genetic analysis of a Y-chromosome region that induces triplosterile phenotypes and is essential for spermatid individualization in Drosophila melanogaster. Genetics 2000; 155:179-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Espindola FS, Suter DM, Partata LB, Cao T, Wolenski JS, Cheney RE, King SM, Mooseker MS. The light chain composition of chicken brain myosin-Va: calmodulin, myosin-II essential light chains, and 8-kDa dynein light chain/PIN. Cell motility and the cytoskeleton 2000; 47:269-81. [DOI] [PubMed] [Google Scholar]
- 47. Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends in cell biology 2005; 15:92-101. [DOI] [PubMed] [Google Scholar]
- 48. Farkas RM, Giansanti MG, Gatti M, Fuller MT. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Molecular biology of the cell 2003; 14:190-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, Trimble WS. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Developmental biology 2002; 251:294-306. [DOI] [PubMed] [Google Scholar]
- 50. Zhou X, Fabian L, Bayraktar JL, Ding HM, Brill JA, Chang HC. Auxilin is required for formation of Golgi-derived clathrin-coated vesicles during Drosophila spermatogenesis. Development 2011; 138:1111-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sevrioukov EA, Moghrabi N, Kuhn M, Kramer H. A mutation in dVps28 reveals a link between a subunit of the endosomal sorting complex required for transport-I complex and the actin cytoskeleton in Drosophila. Molecular biology of the cell 2005; 16:2301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tiwari AK, Alone DP, Roy JK. Rab11 is essential for fertility in Drosophila. Cell biology international 2008; 32:1158-68. [DOI] [PubMed] [Google Scholar]
- 53. Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nature cell biology 2007; 9:176-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menon M, Schafer DA. Dynamin: expanding its scope to the cytoskeleton. International review of cell and molecular biology 2013; 302:187-219. [DOI] [PubMed] [Google Scholar]
- 55. Ma Z, Liu Z, Huang X. OSBP- and FAN-mediated sterol requirement for spermatogenesis in Drosophila. Development 2010; 137:3775-84. [DOI] [PubMed] [Google Scholar]
- 56. Wang C, Ma Z, Scott MP, Huang X. The cholesterol trafficking protein NPC1 is required for Drosophila spermatogenesis. Developmental biology 2011; 351:146-55. [DOI] [PubMed] [Google Scholar]
- 57. Steinhauer J, Gijon MA, Riekhof WR, Voelker DR, Murphy RC, Treisman JE. Drosophila lysophospholipid acyltransferases are specifically required for germ cell development. Molecular biology of the cell 2009; 20:5224-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huh JR, Vernooy SY, Yu H, Yan N, Shi Y, Guo M, Hay BA. Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS biology 2004; 2:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arama E, Bader M, Srivastava M, Bergmann A, Steller H. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. The EMBO journal 2006; 25:232-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. D’Brot A, Chen P, Vaishnav M, Yuan S, Akey CW, Abrams JM. Tango7 directs cellular remodeling by the Drosophila apoptosome. Genes & development 2013; 27:1650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ, Guo M, Baehrecke EH, Hay BA. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development 2006; 133:3305-15. [DOI] [PubMed] [Google Scholar]
- 62. Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Developmental cell 2003; 4:687-97. [DOI] [PubMed] [Google Scholar]
- 63. Kaplan Y, Gibbs-Bar L, Kalifa Y, Feinstein-Rotkopf Y, Arama E. Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Developmental cell 2010; 19:160-73. [DOI] [PubMed] [Google Scholar]
- 64. Arama E, Bader M, Rieckhof GE, Steller H. A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS biology 2007; 5:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proceedings of the National Academy of Sciences of the United States of America 2003; 100:4078-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006; 441:1162-6. [DOI] [PubMed] [Google Scholar]
- 67. Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 2008; 105:14503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006; 441:1157-61. [DOI] [PubMed] [Google Scholar]
- 69. Riparbelli MG, Callaini G. The Drosophila parkin homologue is required for normal mitochondrial dynamics during spermiogenesis. Developmental biology 2007; 303:108-20. [DOI] [PubMed] [Google Scholar]
- 70. Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M, Ren M. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proceedings of the National Academy of Sciences of the United States of America 2009; 106:2337-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu J, Feng L, Hsieh TS. Drosophila topo IIIalpha is required for the maintenance of mitochondrial genome and male germ-line stem cells. Proceedings of the National Academy of Sciences of the United States of America 2010; 107:6228-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Metzendorf C, Lind MI. Drosophila mitoferrin is essential for male fertility: evidence for a role of mitochondrial iron metabolism during spermatogenesis. BMC developmental biology 2010; 10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 1993; 135:489-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wakimoto BT, Lindsley DL, Herrera C. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics 2004; 167:207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hackstein JH. Spermatogenesis in Drosophila. A genetic approach to cellular and subcellular differentiation. European journal of cell biology 1991; 56:151-69. [PubMed] [Google Scholar]
- 76. McKee BD, Wilhelm K, Merrill C, Ren X. Male sterility and meiotic drive associated with sex chromosome rearrangements in Drosophila. Role of X-Y pairing. Genetics 1998; 149:143-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Larracuente AM, Presgraves DC. The Selfish Segregation Distorter Gene Complex of Drosophila melanogaster. Genetics 2012; 192:33-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in Drosophila melanogaster. VII. Effects of segregation distorter (SD) chromosome. Journal of ultrastructure research 1977; 58:96-107. [DOI] [PubMed] [Google Scholar]
- 79. Peacock WJ, Miklos GL, Goodchild DJ. Sex chromosome meiotic drive systems in Drosophila melanogaster I. Abnormal spermatid development in males with a heterochromatin-deficient X chromosome (sc-4sc-8). Genetics 1975; 79:613-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Riparbelli MG, Giordano R, Callaini G. Effects of Wolbachia on sperm maturation and architecture in Drosophila simulans Riverside. Mechanisms of development 2007; 124:699-714. [DOI] [PubMed] [Google Scholar]
- 81. Wen J, Duan H, Bejarano F, Okamura K, Fabian L, Brill JA, Bortolamiol-Becet D, Martin R, Ruby JG, Lai EC. Adaptive regulation of testis gene expression and control of male fertility by the Drosophila harpin RNA pathway. Molecular cell 2015; 57:165-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sanders C, Smith DP. LUMP is a putative double-stranded RNA binding protein required for male fertility in Drosophila melanogaster. PloS one 2011; 6:e24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gerbasi VR, Preall JB, Golden DE, Powell DW, Cummins TD, Sontheimer EJ. Blanks, a nuclear siRNA/dsRNA-binding complex component, is required for Drosophila spermiogenesis. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:3204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khurana JS, Theurkauf W. piRNAs, transposon silencing, and Drosophila germline development. The Journal of cell biology 2010; 191:905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xu S, Hafer N, Agunwamba B, Schedl P. The CPEB protein Orb2 has multiple functions during spermatogenesis in Drosophila melanogaster. PLoS genetics 2012; 8:e1003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hernandez G, Han H, Gandin V, Fabian L, Ferreira T, Zuberek J, Sonenberg N, Brill JA, Lasko P. Eukaryotic initiation factor 4E-3 is essential for meiotic chromosome segregation, cytokinesis and male fertility in Drosophila. Development 2012; 139:3211-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ding Y, Zhao L, Yang S, Jiang Y, Chen Y, Zhao R, Zhang Y, Zhang G, Dong Y, Yu H, et al. A young Drosophila duplicate gene plays essential roles in spermatogenesis by regulating several Y-linked male fertility genes. PLoS genetics 2010; 6:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sartain CV, Cui J, Meisel RP, Wolfner MF. The poly(A) polymerase GLD2 is required for spermatogenesis in Drosophila melanogaster. Development 2011; 138:1619-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Robida M, Sridharan V, Morgan S, Rao T, Singh R. Drosophila polypyrimidine tract-binding protein is necessary for spermatid individualization. Proceedings of the National Academy of Sciences of the United States of America 2010; 107:12570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jung A, Hollmann M, Schafer MA. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm development. Journal of cell science 2007; 120:2924-34. [DOI] [PubMed] [Google Scholar]
- 91. Timakov B, Zhang P. The hsp60B gene of Drosophila melanogaster is essential for the spermatid individualization process. Cell stress & chaperones 2001; 6:71-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bader M, Arama E, Steller H. A novel F-box protein is required for caspase activation during cellular remodeling in Drosophila. Development 2010; 137:1679-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhong L, Belote JM. The testis-specific proteasome subunit Prosalpha6T of D. melanogaster is required for individualization and nuclear maturation during spermatogenesis. Development 2007; 134:3517-25. [DOI] [PubMed] [Google Scholar]
- 94. Anderson MA, Jodoin JN, Lee E, Hales KG, Hays TS, Lee LA. Asunder is a critical regulator of dynein-dynactin localization during Drosophila spermatogenesis. Molecular biology of the cell 2009; 20:2709-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cheng W, Ip YT, Xu Z. Gudu, an Armadillo repeat-containing protein, is required for spermatogenesis in Drosophila. Gene 2013; 531:294-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Volpi S, Bongiorni S, Fabbretti F, Wakimoto BT, Prantera G. Drosophila rae1 is required for male meiosis and spermatogenesis. Journal of cell science 2013; 126:3541-51. [DOI] [PubMed] [Google Scholar]
- 97. Fuller M. T. Spermatogenesis. In: The Development of Drosophila melanogaster, ed. Bate M. and Martinez Arias A., Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993; 71-148. [Google Scholar]
- 98. Ben-David G, Miller E, Steinhauer J. Drosophila spermatid individualization is sensitive to temperature and fatty acid metabolism. Spermatogenesis 2015; 5(1):e1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wistuba J, Stukenborg JB, Luetjens CM. Mammalian Spermatogenesis. Functional Development and Embryology 2007; 99-117. [Google Scholar]


