Abstract
Formins are a growing class of actin nucleation proteins that promote the polymerization of actin microfilaments, forming long stretches of actin microfilaments to confer actin filament bundling in mammalian cells. As such, microfilament bundles can be formed in specific cellular domains, in particular in motile mammalian cells, such as filopodia. Since ectoplasmic specialization (ES), a testis-specific adherens junction (AJ), at the Sertoli cell-cell and Sertoli-spermatid interface is constituted by arrays of actin microfilament bundles, it is likely that formins are playing a significant physiological role on the homeostasis of ES during the epithelial cycle of spermatogenesis. In this Commentary, we provide a timely discussion on formin 1 which was recently shown to be a crucial regulator of actin microfilaments at the ES in the rat testis (Li N et al. Endocrinology, 2015, in press; DOI: 10.1210/en.2015-1161, PMID:25901598). We also highlight research that is needed to unravel the functional significance of formins in spermatogenesis.
Keywords: actin nucleator, cytoskeleton, F-actin, formins, formin 1, seminiferous epithelial cycle, spermatogenesis, testis
Introduction
During spermatogenesis, preleptotene spermatocytes transformed from type B spermatogonia residing in the basal compartment of the seminiferous epithelium are being transported across the blood-testis barrier (BTB) to enter the adluminal compartment to prepare for meiosis I/II at stage VIII of the epithelial cycle in the rat testis1,2 (Fig. 1). Following meiosis I and II that takes place at stage XIV of the cycle, round spermatids derived from secondary spermatocytes undergo spermiogenesis to transform into spermatozoa via 19 steps, from step 1 through step 19 spermatids in the rat, so that the release of sperm can take place at stage VIII of the epithelial cycle.3,4 Thus, it is conceivable that there are extensive restructuring and/or remodeling at the cell-cell interface between Sertoli cells at the BTB and also between Sertoli and germ cells, most notably spermatids, during the epithelial cycle of spermatogenesis.5,6 It is of interest to note that there is a testis-specific actin-rich ultrastructure known as the ectoplasmic specialization (ES) that is confined to: (i) the Sertoli cell-cell interface at the BTB, and (ii) the Sertoli-spermatid (step 8–19) interface in the rat testis designated basal and apical ES, respectively (Fig. 1) due to their restrictive localization in the corresponding basal and the apical (adluminal) compartment of the seminiferous epithelium6-11 (Fig. 1). The ES is typified by the presence of an array of actin microfilament bundles that lie perpendicular to the Sertoli cell plasma membrane and are sandwiched between the cisternae of endoplasmic reticulum and the opposing Sertoli-Sertoli and Sertoli-spermatid plasma membranes at the basal and apical ES, respectively (Fig. 1). Thus, during the transport of preleptotene spermatocytes across the BTB and also the transport of developing spermatids across the epithelium of the adluminal compartment throughout spermiogenesis, these actin filament bundles must be rapidly re-organized from their “bundled” to “de-bundled” configuration and vice versa. In short, these actin bundles must be continously “de-bundled” and “re-bundled” to facilitate germ cell transport across the seminiferous epithelium (Fig. 1) as well as other cellular events pertinent to spermatogenesis, such as endocytic vesicle-mediated protein trafficking10,12 (Fig. 1). This notion is indeed supported by findings in a recent report using a genetic model. It was reported that Sertoli cell-specific knockout of N-WASP (neuronal Wiskott Aldrich Syndrome protein), an activator of the Arp2/3 (actin-related protein 2/3, a branched actin polymerization protein) that stripped the ability of the Sertoli cell to convert actin filaments from their bundled to de-bundled/branched configuration, the Dhh-Cre; N-WASPflox/N-WASP− males (i.e., N-WASPSC-cKO male mice) were infertile,13 due to the inability of round spermatids to undergo spermiogenesis.13,14 Detailed analysis of these transgenic mice during post-natal development reveals that proper F-actin spatiotemporal localization in the seminiferous epithelium at the Sertoli-Sertoli and Sertoli-germ cell interface versus age-matched wild type (WT) control mice has all been disrupted following specific deletion of N-WASP in Sertoli cells in these N-WASPSC-cKO male mice.14 This is likely the result of an inability of the ES to re-organize actin microfilaments in response to changes of the epithelial cycle due to the lack of a functional Arp2/3-N-WASP complex following Sertoli cell-specific N-WASP KO, leading to defects in the localization of occludin, N-cadherin, coxsackievirus and adenovirus receptor (CAR), and connexin 43 at the BTB, as well as β1-integrin and nectin-3 at the apical ES.14 Thus, round spermatids arise from meiosis fail to enter spermiogenesis to produce functional elongating/elongated spermatids, illustrating the significance of actin microfilaments at the ES.
Figure 1.
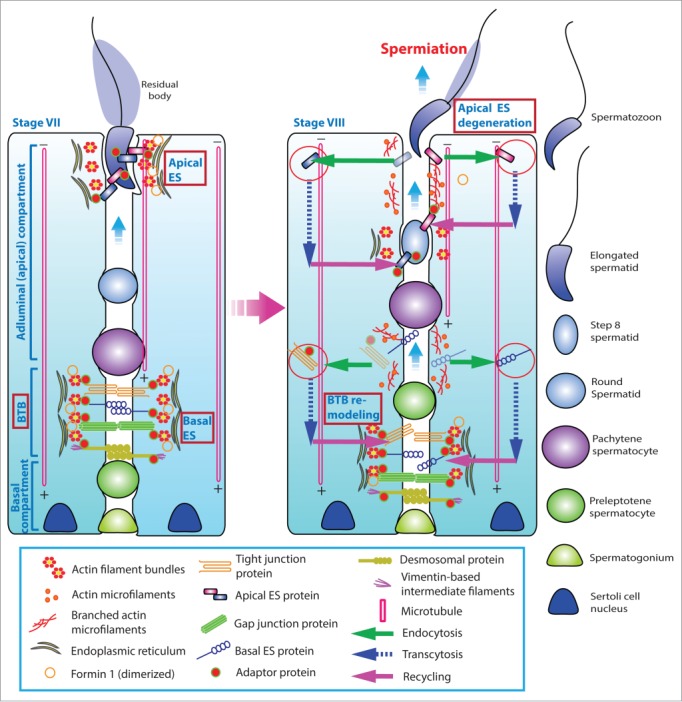
A schematic drawing that illustrates the morphological features of the ectoplasmic specialization (ES) and its relative location in the seminiferous epithelium of adult rat testes. The left panel depicts a stage VII tubule in which the BTB physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartment. In the adluminal compartment, formin 1 is predominantly expressed at the concave (ventral) side of spermatid head but only at stage VII to be used for actin nucleation at the barbed end of an existing microfilament. This is likely being used to facilitate remodeling of the site to allow endocytic vescile-mediated protein trafficking events (see right panel) that begins at late stage VII through VIII of the epithelial cycle, involving protein endocytosis, transcytosis and recycling. At the basal compartment, preleptotene spermatocytes transformed from type B spermatogonia are being transported across the BTB that begin at late stage VII so that formin 1 is also involved in remodeling of the BTB since formin 1 remains considerably expressed at the BTB until late stage VII. Formin 1 is likely working in concert with branched actin polymerization proteins, such as the Arp2/3 complex, to facilitate BTB remodeling.
Studies have shown that F-actin dynamics in the testis are regulated by an array of actin regulatory proteins besides the Arp2/3 complex,15 N-WASP,14,15 and drebrin E,16 which contribute to branched actin polymerization. These include actin barbed end capping and bundling protein Eps8 (epidermal growth factor receptor pathway substrate 8),17 actin cross-linking and bundling protein palladin18 and actin branching protein filamin A.19,20 For instance, it was shown that the restrictive spatiotemporal expression of Arp3 and Eps8 at the apical and basal ES are crucial to facilitate the timely degeneration of these testis-specific anchoring junctions to facilitate spermatid transport and the transit of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle.21 In this Commentary, we critically review some of the latest findings on formins, another family of actin nucleators that are crucial to the formation of long stretches of actin microfilaments necessary to be used to assemble actin filament bundles at the ES during spermatogenesis. We also provide an updated model regarding the role of formins, in particular formin 1 that works in concert with other pertinent actin binding and regulatory proteins to confer ES dynamics in the testis during spermatogenesis. We also highlight areas of research that deserve attention in future investigations.
Formins – Actin nucleators and regulators of actin dynamics
Formins are referred to a large family of morphoregulatory proteins, which initially were shown to modulate vertebrate limb pattern formation, budding and conjugation in yeasts, cell junction assembly, actin- and tubulin-based cytoskeletal organization in multiple epithelia, and Drosophila oocyte polarity.22-27 Initial studies have shown that mutation of murine limb deformity (ld) gene led to limb deformation, and it was shown that the Id gene produced multiple proteins collectively called formins via alternative splicing of mRNA transcripts in the 1990s.22,28 Studies have shown that formins are actin-based cytoskeleton organizing proteins, but subsequent studies also illustrate that they are involved in tubulin-based cytoskeleton organization in both fungi, plants, and animals; and many of formins are effectors of Rho GTPases known to regulate F-actin dynamics.22,29,30 Due to their involvement in actin nucleation, formins and other actin nucleation proteins (such as the N-WASP-Arp2/3 complex) are one of the primary targets of chemotherapy in particular metastasis and cancer invasion31 since tumor cells require extensive re-organization of their F-actin cytoskeleton during tumorigenesis. In fact, studies of cancer genomics have identified missense mutations in several formins, including formin-like 2 (FMNL2) and FMNL3 in patients with glioblastoma or pancreatic cancer.32,33 Also, the expression of FMNL2 is up-regulated in metastatic colorectal cancer.34 All proteins of the formin family (see Table 1) contain the conserved formin homology 2 (FH2) domain, which is the signature domain of the formins protein family that confers and promotes actin assembly35,36 (Fig. 2A). FH2 is preceded by a proline-rich FH1 (formin homology 1) domain, which binds to profilin-actin to accelerate elongation of actin filaments at the plus-end37 (Fig. 2A). FH1 also interacts with a variety proteins, including profilin, SH3 (Src homology 3) domain proteins, and WW (domain with two conserved Trp (W) residues, also known as rsp5-domain38 or WWP repeating motif39,40 that binds to proline-rich peptide motifs) domain proteins. In fact, all formins, including those from Dictyostelium, fungi, Drosophila, rodents and humans, with the exception of formin 1, display a common GBD/FH3-FH1-FH2-DAD (GBD, GTPase binding protein; FH3, formin homology 3; DAD, Diaphanous auto-inhibition domain) architecture in their primary sequence41 (Fig. 2A). Once formins are activated by Rho GTPases, formins form dimers with their FH2 domains, which then interacts with other nucleation cofactors and profilin-actin complexes near the C-terminus to promote the progressive addition of actin monomers onto the plus-end of a growing actin filament42 (Fig. 2B). This process as depicted in Figure 2B thus assembles linear actin filaments that can be bundled to create microfilament bundles unique to the ES in the testis. Since formins remain attached to the growing plus (+) end of actin microfilaments, sometimes for minutes without being dissociated, they can form stretches of microfilaments as long as >50 µm,37,43-45 such as those necessary to maintain the actin filament bundles at the ES. Besides localized at or near the plasma membrane, formins are also found in cell cytosol and also nucleus in particular mDia1 and mDia2 (Table 1). A recent report has shown that formins, such as mDia, that are responsible for the assembly of the nuclear actin network is also involved in transcriptional regulation,46 illustrating the growing list of physiological function regulated by this family of actin nucleators. In this Commentary, we highlight some interesting aspects of formin 1 during spermatogenesis using the rat testis as a study model.
Table 1.
Functions of formins in mammalian cells
| Formin | Localization | Cellular Function | Phenotype(s) following KO or KD |
|---|---|---|---|
| DIAPH1/DIA1(mDia1) | Phagocytic cup, cell cortex59 | Microtubule stabilization,60 phagocytosis, polarized cell migration61 | Mice devoid of mDia1 exhibit an age-dependent myelodysplastic phenotype62; lymphopenia characterized by diminished T cells in lymphoid tissues63; unilateral ventricular enlargement64 |
| DIAPH3 (mDia2) | Endosomes65 | Microtubule stabilization,66 endosome dynamics65 | Mice without mDia2 impairs cytokinesis during fetal erythropoiesis, leading to severe anemia and die in utero by embryonic day 12.567 |
| DIAPH2 (mDia3) | Kinetochore68 | Stabilized microtubules remain attached to kinetochore69 | mDia1/3 double-KO impairs tangential migration of cortical and olfactory inhibitory interneurons, mDia1/3-deficient neuroblasts exhibit reduced separation of the centrosome from the nucleus and retarded nuclear translocation70 |
| DAAM1 | Cell cortex71 | Planar cell polarity72 | DAAM1-deficient mice exhibit embryonic and neonatal lethality due to cardiac abnormalities73 |
| FMNL1 | Cell cortex, microtubule-organizing center74 | Phagocytosis75 | Depletion of FMNL1 in cytotoxic lymphocytes abrogate cell-mediated killing activity since a loss of FMNL1 reduces microtubule-organizing center polarization74 |
| FMN1 | ES, microtubules47 | ES assembly and stabilization47 | FMN1-deficient mice exhibit a reduction of digits from 5 to 4, a deformed posterior metatarsal phalangeal soft tissue fusion as well as the absence of a fibula76; formin 1 KD affects Sertoli cell actin microfilament organization, perturbing Sertoli cell tight junction barrier function, and disrupting the transport of spermatids and phagosomes in the seminiferous epithelium47 |
| INF1/FHDC1 | Microtubules77 | Microtubule stabilization77 | KD of INF1 leads to reduced level of acetylated microtubules (i.e., more stabilized and aged microtbules) and reduced microtubule bundles in multiple mammalian cells77 |
There are 15 known members of formins to date,24,25,31 however, none of these proteins have been studied in the testis functionally except a recent report on formin 1 (FMN1).47 There are 2 other earlier studies, reporting the identification of mDia1, mDia2 and mDia3 and their involvement in the acroplaxome-manchette complex in spermatids during spermiogenesis.78,79 In short, the functional significance of formins in spermatogenesis remains unknown. Thus, not all the members of formins are listed herein, selected members are listed which are likely to be involved in spermatogenesis based on their studies in other epithelia. Abbreviations used: DIAPH1/DIA1, diaphanous-related formin 1, also known as mDia1 which is the mouse version of the diaphanous homolog 1 of Drosophila; DIAPH3, diaphanous-related formin 3 or mDia2 which is also a Rho-regulated actin nucleator; DIAPH2, diaphanous-related formin 2 or mDia3; DAAM1, disheveled-associated activator of morphogenesis 1; ES, ectoplasmic specialization; FMNL1, formin-like protein 1; FMN1, formin 1; INF1/FHDC1, inverted formin1/FH2 domain containing 1, a microtubule-associated formin; KD, knock down; KO, knock out.
Figure 2.
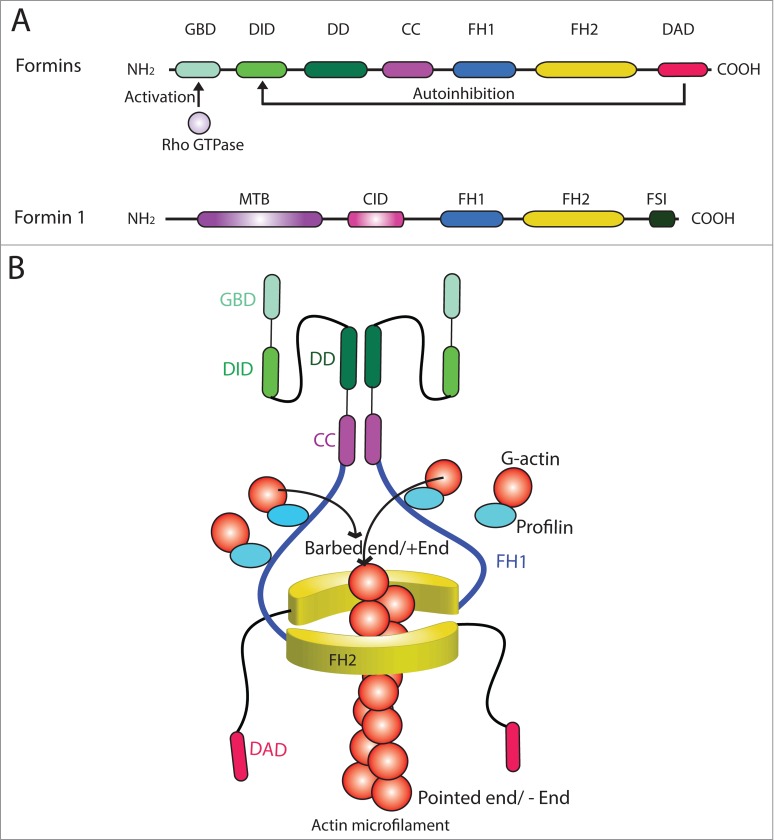
Functional domains of members of formin family. (A) The functional domains of members of the formin family (upper panel). Formins are activated by Rho GTPase following its binding onto the GBD domain, which can also be auto-inhibited by DAD via its action onto DID domain. The functional domains of formin 1 are also shown (lower panel). (B) Two formin polypeptide chains are recruited together at their CC and DD domains, and dimerized via their FH2 domains by creating a functional formin molecule which is capable of nucleating actin microfilament at its barbed end/+ (plus) end by adding actin monomers using the G-actin/profilin complexes. This thus promotes actin polymerization, forming long stretches of actin microfilaments rapidly, necessary to create bundles of actin microfilaments at the ES. Abbreviations used: CC, coiled-coil domain; CID, catenin interacting domain; DAD, diamphanous autoregulatory domain; DD, dimerization domain; DID, diaphanous inhibitory domain; FH, formin homology; FSI, Formin Spir interaction motif; GBD, GTPase-binding domain; MTB, microtubule binding domain.
Formin 1 and spermatogenesis
Introduction
Formin 1 (also known as limb deformity protein) is a 1466-amino acid protein in the mouse, which is also known to be involved in adherens junction (AJ) assembly and polymerization of actin filaments, serving as an actin nucleator.48,49 From its N-terminus, formin 1 has a microtubule-binding domain (MTB), to be followed by an α-catenin-interacting domain (CID) which overlaps with a coil-coiled domain, an FH1 and an FH2 domain near its C-terminus (Fig. 2A). FH2 domain is the site where formin 1 promotes nucleation of an actin microfilament that grows rapidly at its barbed end (Fig. 2A). Thus, due to the presence of MTB and CID, formin 1 is known to interact with α-catenin and also tubulin.49,50 Besides localized at the AJ, formin is also found in cell cytosol and nucleus,49,50 such as in Sertoli cells.47 Formin 1 is present in the kidney, testis, limb, ovary, brain, small intestine, and salivary gland.28,49 At least 5 isoforms of formin 1 are known to date – isoforms 1, 2 and 5 are detected in skin and keratinocytes, and isoform 5 is abundant in the embryo.28,49 In the rat testis, formin 1 is a 180 kDa protein, and its expression by Sertoli cells is induced during the assembly of the functional TJ-permeability barrier in vitro,47 illustrating its involvement in the formation of actin microfilament bundles at the basal ES since its knockdown by RNAi leads to truncation and disorganization of actin microfilaments in Sertoli cells.47
Formin 1 is a regulatory component of actin microfilament bundles at the basal and apical ES
Formin 1, due to its intrinsic activity of inducing actin filament polymerization, is localized at the F-actin-rich basal and apical ES in the seminiferous epithelium as anticipated.47 As such, formin 1 can regulate actin microfilament organization at the ES. However, formin 1 displays restrictive spatiotemporal expression at the ES during the epithelial cycle. For instance, formin 1 is highly expressed at the basal ES/BTB during stage III-VI of the cycle, and mildly diminished by VII, and virtually non-detectable by stage VIII.47 This is understandable, since long stretches of actin microfilaments are needed at the basal ES/BTB in stage III-VII tubules to maintain the BTB integrity. However, extended actin microfilaments are no longer needed in stage VIII tubules at the basal ES/BTB when the immunological barrier undergoes extensive remodeling, in which F-actin network at the site assumes an un-bundled and branched conformation induced by the Arp2/3 complex to support the transport of preleptotene spermatocytes at the BTB. This notion is supported by findings that Arp3 is highly expressed at the basal ES/BTB in stage VIII tubules.15 At the apical ES, formin 1 is highly expressed but limited only to stage VII of the epithelial cycle and confined to the concave (ventral) side of spermatid heads.47 This is also the site called apical tubulobulbar complex (TBC)51 which is known to have extensive endocytic vesicle-mediated trafficking events to take place at stage VII,51,52 such that “old” apical ES proteins can be endocytosed, transcytosed, and recycled for the assembly of “new” apical ES when step 8 spermatids arise in stage VIII tubules.12,53 Formin 1 also co-localizes with TJ protein ZO-1 and basal ES protein N-cadherin at the basal ES, as well as Apr3 and Eps8 at the apical ES.47 Furthermore, formin 1 structurally interacts with Arp3, actin and tubulin in the testis as demonstrated by immunoprecipitation,47 illustrating it is working in concert with Arp3 to regulate actin microfilament organization at the ES.
Formin 1 confers Sertoli cell tight junction (TJ)-permeability barrier function by regulating actin microfilament bundling activity
When the expression of formin 1 in Sertoli cell epithelium with an established functional TJ-barrier is silenced by ∼70% using RNAi, a knockdown of formin 1 with specific siRNA duplexes is shown to associate with a disruption of the Sertoli cell TJ-permeability.47 This is likely due to changes in the organization of actin microfilaments at the basal ES as noted by truncation and defragmentation of actin microfilaments in Sertoli cells, mediated by changes in the localization of both Arp3 and Eps8, thereby impeding the localization of basal ES proteins, such as N-cadherin and β-catenin, but not TJ proteins (e.g., occludin, ZO-1), at the Sertoli cell-cell interface.47 This thus destabilizes the TJ-barrier, perturbing the barrier function. Subsequent biochemical analysis has shown that a knockdown of formin 1 also impedes the ability of Sertoli cells to bundle actin microfilaments besides a reduced actin nucleation activity.47 This finding is also consistent with earlier reports that formin1 possesses intrinsic F-actin bundling activity besides promoting barbed end actin nucleation,44,54 illustrating formin 1 is intimately related to ES homeostasis during the epithelial cycle of spermatogenesis.
Formin 1 regulates spermatid transport and protein distribution at the BTB
Actin microfilaments, similar to microtubules, are polarized ultrastructures, which are known to be involved in preleptotene spermatocyte transport at the BTB, and also the transport of developing spermatids during spermiogenesis at the adluminal compartment.55-57 Thus, it is not unexpected that the knockdown of formin 1 by RNAi in the testis in vivo would impede spermatid transport, such as at stage VII of the epithelial cycle when formin 1 expression at the apical ES is high.47 For instance, some step 19 spermatids were found to be entrapped deep inside the seminiferous epithelium in late stage VIII tubules, and a few were even detected in stage XI tubules when all elongated spermatids should have undergone spermiation.47 Furthermore, the transport of residual bodies that have already been integrated into phagosomes was also found to be disrupted since some phagosomes remained in the adluminal compartment in stage IX tubules47 when they should have been transported to the base of Sertoli cells near the basement membrane for lysosomal degradation.58 This is likely due to the mis-localization of F-actin at the ES, perturbing proper cell adhesion and intracellular transport function at the ES, thereby impeding spermatogenesis, causing defects in spermiation as well as spermatid polarity.47
Formin 1 regulates endocytic vesicle-mediated trafficking and spermatid adhesion/transport at the ES during spermatogenesis
Since formin 1 is predominantly expressed at the concave side of spermatid heads at stage VII of the epithelial cycle – the site of apical TBC where extensive endocytic vesicle-mediated trafficking takes place. We speculate that formin 1 is heavily involved in F-actin organization at this site to facilitate the events of endocytosis, transcytosis and recycling as depicted in Figure 3A. Formin 1 is also involved in conferring integrity of the BTB at stage III-VII of the epithelial cycle to maintain the network of actin microfilament bundles at the basal ES/BTB, perhaps in collaboration with other actin cross-linking and bundling proteins such as Eps8, palladin, ezrin and fascin 1 (Fig. 3B). For instance, it is likely that the timely up-regulation of formin 1 at the concave (ventral) side of spermatid head known as the apical TBC at stage VII of the cycle47 that is concomitant with expression of Arp3 at the same site15 are being used to facilitate the extensive endocytic vesicle-mediated protein trafficking events – which are also the core function of the TBC51,52 (Fig. 3A). On the other hand, formin 1 is also being used to generate the long stretches of actin microfilaments at the rear end of the preleptotene spermatocytes that are being transported across the BTB to facilitate the assembly of “new” BTB, which take place before the Arp2/3 complex-mediated “old” BTB is degenerated47 (Fig. 3B).
Figure 3.
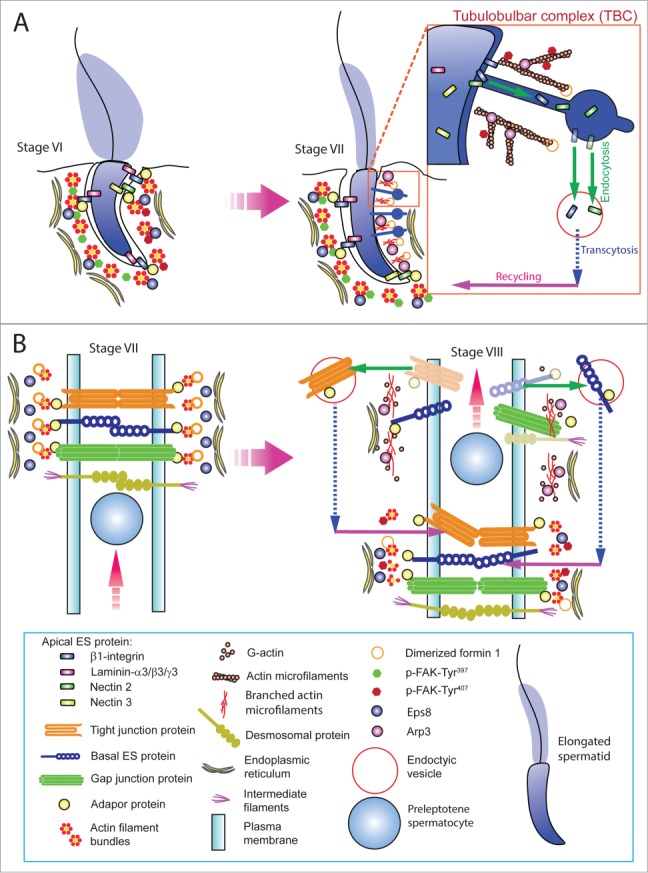
For figure legend, see next page. Figure 3 (See previous page). A hypothetical model that illustrates the involvement of formin 1 in regulating actin microfilament organization at the ES. (A) At the apical ES (left panel), formin 1 is not expressed in any stage of the epithelial cycle such as at stage VI in which bundles of actin microfilaments serve as the attachment sites for adhesion protein complexes, such as laminina-333/α6β1-integrin and nectin 2/nectin 3 adhesion protein complexes. However, formin 1 is robustly expressed at the concave side of spermatid heads at stage VII (right panel) in an ultrastructure known as the apical TBC, representing giant endocytic vesicles (see enlagred view in the boxed area). This endocytic vesicle-mediated protein trafficking event is facilitated by changes in the organization of actin microfilaments via the concerted efforts of the Arp2/3 complex and formin 1 (note: both Arp3 and formin 1 are highly expressed and localized to the same site in stage VII tubules) which, in turn, facilitate the events of endocytosis, transcytosis and recycling so that apical ES proteins can be recycled to assemble “new” apical ES for the newly derived step 8 spermatids that appear in stage VIII of the cycle. (B) Formin 1 is also robustly expressed at the BTB in stages V-VII, likely being used to maintain the actin microfilaments and to facilitate their organization into a bundled configuration to serve as attachment sites for adhesion protein complexes (left panel). At stage VIII, formin 1 expression at the basal/BTB is considerably diminished, and the Arp3 expression is up-regulated at the site. The intrinsic activity of Arp2/3 complex thus induces branched actin polymerization, converting the microfilament bundles into a branched/unbundled configuration to facilitate the events of endocytosis, transcytosis and recycling so that the “old” BTB adhesion proteins can be recycled to assemble a “new” BTB behind the preleptotene spermatocyte being transported across the immunological barrier (right panel). In short, actin microfilaments at the apical ES and the basal ES/BTB can be rapidly re-organized, through the unique and stage-specific spatiotemporal expression of formin 1, Arp3, and possibly other actin bundling proteins (e.g., Eps8, fascin 1, palladin) known to be expressed at the site.
Concluding remarks and future perspectives
As briefly discussed herein, formins are a growing class of barbed end nucleation proteins that are capable of forming relatively long stretches of actin microfilaments used for the assembly of filopodia and stress fibers in motile mammalian cells, as well as actin microfilaments at the ES in Sertoli cells in the testis. While Sertoli cells are not motile cells in vivo, such as macrophages, fibroblasts or neutrophils, Sertoli cells need to generate many cytoplasmic processes which are inter-digitally linked to developing germ cells to provide the structural, nutritional and paracrine supports across the entire seminiferous epithelium. In order for this to occur, long stretches of microfilaments are continuously formed and re-organized so that germ cells can be transported across the epithelium in response to the stages of the epithelial cycle. Based on its intrinsic actin nucleation and bundling activities, formins, such as formin 1, confer actin microfilaments their bundled configuration at the ES to maintain adhesion function during spermatogenesis. However, formins also are working in concert with other branched actin inducing proteins, such as the Arp3/3 complex, and possibly actin bundling proteins such as palladin, plastins, fascins, and Eps8 to modulate F-actin organization at the ES in responses to the stages of the epithelial cycle. There are several outstanding questions remain to be addressed. For instance, what is the mechanism(s) and/or involving molecule(s) that regulate the spatiotemporal expression of formin 1 at the apical ES (limited to stage VII) and the basal ES/BTB (limited to I-VII)? Does other formin family members are involved in apical and basal ES function in other stages of the epithelial cycle when formin 1 is not expressed? Does the mechanism and/or molecule that regulate formin 1 spatiotemporal expression also plays a role in coordinating the expression of other actin bundling proteins, such as palladin and Eps8? Does formin 1 regulates Sertoli cell microtubulin-based cytoskeletal function? What are the roles of testosterone and cytokines in these events since these are known regulators of cell adhesion in the testis? The answers to these outstanding questions will help us to better understand the regulation of actin- and microtubule-based cytoskeletons in the seminiferous epithelium during spermatogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5 to CYC; R01 HD056034 to CYC).
References
- 1.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 2008; 636:1-15; PMID:19856159; http://dx.doi.org/ 10.1007/978-0-387-09597-4_1 [DOI] [PubMed] [Google Scholar]
- 2.Clermont Y, Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972; 52:198-235; PMID:4621362 [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 2011; 1:14-35; PMID:21866274; http://dx.doi.org/ 10.4161/spmg.1.1.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev 2008; 60:146-80; PMID:18483144; http://dx.doi.org/ 10.1124/pr.107.07105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82, 825-74; PMID:12270945; http://dx.doi.org/ 10.1152/physrev.00009.2002 [DOI] [PubMed] [Google Scholar]
- 6.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747-806; PMID:15466940; http://dx.doi.org/ 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- 7.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 1977; 148:313-28; PMID:857632; http://dx.doi.org/ 10.1002/aja.1001480303 [DOI] [PubMed] [Google Scholar]
- 8.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell 1977; 9:475-98; PMID:929577; http://dx.doi.org/ 10.1016/0040-8166(77)90007-6 [DOI] [PubMed] [Google Scholar]
- 9.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11 [DOI] [PubMed] [Google Scholar]
- 10.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 2012; 64:16-64; PMID:22039149; http://dx.doi.org/ 10.1124/pr.110.002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta 2008; 1778:692-708; PMID:18068662; http://dx.doi.org/ 10.1016/j.bbamem.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol 2010; 6:380-395; PMID:20571538; http://dx.doi.org/ 10.1038/nrendo.2010.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Schejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development 2011; 138:2729-39; PMID:21652648; http://dx.doi.org/ 10.1242/dev.059865 [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Mruk DD, Tang EI, Massarwa R, Mok KW, Li N, Wong CK, Lee WM, Snapper SB, Shilo BZ, et al.. N-WASP is required for structural integrity of the blood-testis barrier. Proc Natl Acad Sci USA 2013; 10:e1004447; PMID:24967734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 2010; 107:11411-6; PMID:20534520; http://dx.doi.org/ 10.1073/pnas.1001823107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY, et al.. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 2011; 1:123-36; PMID:22319661; http://dx.doi.org/ 10.4161/spmg.1.2.16393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555-67; PMID:19293393; http://dx.doi.org/ 10.1096/fj.06-070573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis 2013; 3:e23473; PMID:23687615; http://dx.doi.org/ 10.4161/spmg.23473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology 2012; 153:5023-35; PMID:22872576; http://dx.doi.org/ 10.1210/en.2012-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su WH, Mruk DD Cheng CY. Filamin A: a regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis 2012; 2:73-8; PMID:22670216; http://dx.doi.org/ 10.4161/spmg.20223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng CY, Lie PPY, Wong EWP, Mruk DD. Focal adhesion kinase and actin regulatory/binding proteins that modulate F-actin organization at the tissue barrier. Lession from the testis. Tissue Barriers 2013; 1:e24252.; PMID:24665388; http://dx.doi.org/ 10.4161/tisb.24252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeller R, Haramis AG, Zuniga A, McGuigan C, Dono R, Davidson G, Chabanis S, Gibson T. Formin defines a large family of morphorgulatory genes and functions in establishment of the polarising region. Cell Tissue Res 1999; 296:85-93; PMID:10199968; http://dx.doi.org/ 10.1007/s004410051269 [DOI] [PubMed] [Google Scholar]
- 23.Bartolini F, Gundersen GG. Formins and microtubules. Biochem Biophys Acta 2010; 1803:164-73; PMID:19631698; http://dx.doi.org/ 10.1016/j.bbamcr.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baarlink C, Brandt D, Grosse R. SnapShot: Formins. Cell 2010; 142:172; PMID:20603022; http://dx.doi.org/ 10.1016/j.cell.2010.06.030 [DOI] [PubMed] [Google Scholar]
- 25.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell 2005; 16:1-13; PMID:15509653; http://dx.doi.org/ 10.1091/mbc.E04-07-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerkhoff E. Actin dynamics at intracellular membranes: the Spir/formin nucleator complex. Eur J Cell Biol 2011; 90:922-5; PMID:21129813; http://dx.doi.org/ 10.1016/j.ejcb.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 27.Yang C, Svitkina T. Filopoldia initiation. Focus on the Arp2/3 complex and formins. Cell Adh Migr 2011; 5:402-8; PMID:21975549; http://dx.doi.org/ 10.4161/cam.5.5.16971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woychik RP, Maas RL, Zeller R, Vogt TF, Leder P. ‘Formins’: proteins deduced from the alternative transcripts of the limb deformity gene. Nature 1990; 346:850-3; PMID:2392150; http://dx.doi.org/ 10.1038/346850a0 [DOI] [PubMed] [Google Scholar]
- 29.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 2010; 11:62-74; PMID:19997130; http://dx.doi.org/ 10.1038/nrm2816 [DOI] [PubMed] [Google Scholar]
- 30.Chesarone MA, Goode BL. Actin nucleation and elgonation factors: mechanisms and interplay. Curr Opin Cell Biol 2009; 21:28-37; PMID:19168341; http://dx.doi.org/ 10.1016/j.ceb.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer 2011; 11:177-87; PMID:21326322; http://dx.doi.org/ 10.1038/nrc3003 [DOI] [PubMed] [Google Scholar]
- 32.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al.. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321:1807-12; PMID:18772396; http://dx.doi.org/ 10.1126/science.1164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al.. Core signaling pathways in human pancreatic cancers revealed by global genomic analysis. Science 2008; 321:1801-6; PMID:18772397; http://dx.doi.org/ 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu XL, Liang L, Ding YQ. Overexpression of FMN2 is closely related to metastasis of colorectal cancer. Intern J Colorectal Dis 2008; 23:1041-7; PMID:18665374; http://dx.doi.org/ 10.1007/s00384-008-0520-2 [DOI] [PubMed] [Google Scholar]
- 35.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science 2002; 297:612-5; PMID:12052901; http://dx.doi.org/ 10.1126/science.1072309 [DOI] [PubMed] [Google Scholar]
- 36.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and Profilin. Nat Cell Biol 2002; 4:626-31; PMID:12134165 [DOI] [PubMed] [Google Scholar]
- 37.Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol 2006; 18, 11-7; PMID:16364624; http://dx.doi.org/ 10.1016/j.ceb.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 38.Hofmann K, Bucher P. The rsp5-domain is shared by proteins of diverse functions. FEBS Lett 1995; 358:153-7; PMID:7828727; http://dx.doi.org/ 10.1016/0014-5793(94)01415-W [DOI] [PubMed] [Google Scholar]
- 39.Andre B, Springael JY. WWP, a new amino oacid motif present in single or multiple coplies in various proteins including dystrophin and the SH3-binding Yes-associated protein YAP65. Biochem Biophys Res Commun 1994; 205:1201-5; PMID:7802651; http://dx.doi.org/ 10.1006/bbrc.1994.2793 [DOI] [PubMed] [Google Scholar]
- 40.Rotin D. WW (WWP) domains: from structure to function. Curr Top Microbiol Immunol 1998; 228:115-33; PMID:9401204 [DOI] [PubMed] [Google Scholar]
- 41.Rivero F, Muramoto T, Meyer AK, Urushihara H, Uyeda TQ, Kitayama C. A comparative sequence analysis reveals a common GBD/FH3-FH1-FH2-DAD architecture in formins from Dictyostelium, fungi and metazoa. BMC Genomics 2005; 6:28; PMID:15740615; http://dx.doi.org/ 10.1186/1471-2164-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem 2007; 76:593-627; PMID:17373907; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- 43.Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL. Rocket launcher mechanism of collaborative actin assemboly defined by single-molecule imaging. Science 2012; 336:1164-8; PMID:22654058; http://dx.doi.org/ 10.1126/science.1218062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci 2013; 126:1-7; PMID:23516326; http://dx.doi.org/ 10.1242/jcs.107250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neidt EM, Skau CT, Kovar DR. The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J Biol Chem 2008; 283:23872-83; PMID:18577519; http://dx.doi.org/ 10.1074/jbc.M803734200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 2013; 340:864-7; PMID:23558171; http://dx.doi.org/ 10.1126/science.1235038 [DOI] [PubMed] [Google Scholar]
- 47.Li N, Mruk DD, Wong CK, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplamic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology 2015; in press; PMID:25901598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuniga A, Michos O, Spitz F, Haramis AP, Panman L, Galli A, Vintersten K, Klasen C, Mansfield W, Kuc S, et al.. Mouse limb deformity mutations disrupt a global control region within the large regulatory landscape required for Gremlin expression. Genes Dev 2004; 8:1553-64; PMID:15198975; http://dx.doi.org/ 10.1101/gad.299904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 2004; 6:21-30; PMID:14647292; http://dx.doi.org/ 10.1038/ncb1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F, Leder P, Martin SS. Formin-1 protein associates with microtubules through a peptide domain encoded by exon-2. Exp Cell Res 2006; 312:1119-26; PMID:16480715; http://dx.doi.org/ 10.1016/j.yexcr.2005.12.035 [DOI] [PubMed] [Google Scholar]
- 51.Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol 2013; 303:319-55; PMID:23445814; http://dx.doi.org/ 10.1016/B978-0-12-407697-6.00008-8 [DOI] [PubMed] [Google Scholar]
- 52.Vogl AW, Du M, Wang XY, Young JS. Novel clathrin/actin-based endocytic machinery associated with junction turnover in the seminiferous epithelium. Semin Cell Dev Biol 2014; 30:55-64; PMID:24280271; http://dx.doi.org/ 10.1016/j.semcdb.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 53.Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology 2014; 29:286-98; PMID:24985332; http://dx.doi.org/ 10.1152/physiol.00001.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohnert KA, Willet AH, Kovar DR, Gould KL. Formin-based control of the actin cytoskeleton during cytokinesis. Biochem Soc Trans 2013; 41:1750-4; PMID:24256286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Donnell L, O'Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol 2014; 30:45-54; PMID:24440897; http://dx.doi.org/ 10.1016/j.semcdb.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 56.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1581-92; PMID:20403871; http://dx.doi.org/ 10.1098/rstb.2009.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol 2013; 217:R13-23; PMID:23449618; http://dx.doi.org/ 10.1530/JOE-12-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci 1987; 513:1-15; PMID:3328532; http://dx.doi.org/ 10.1111/j.1749-6632.1987.tb24994.x [DOI] [PubMed] [Google Scholar]
- 59.Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol 2006; 174:701-13; PMID:16943183; http://dx.doi.org/ 10.1083/jcb.200605006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 2012; 23:4032-40; PMID:22918941; http://dx.doi.org/ 10.1091/mbc.E12-05-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol 2009; 182:3837-45; PMID:19265163; http://dx.doi.org/ 10.4049/jimmunol.0803838 [DOI] [PubMed] [Google Scholar]
- 62.DeWard AD, Leali K, West RA, Prendergast GC, Alberts AS. Loss of RhoB expression enhances the myelodysplastic phenotype of mammalian diaphanous-related Formin mDia1 knockout mice. PLoS One 2009; 4:e7102; PMID:19768111; http://dx.doi.org/ 10.1371/journal.pone.0007102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem 2007; 282:25152-8; PMID:17595162; http://dx.doi.org/ 10.1074/jbc.M703243200 [DOI] [PubMed] [Google Scholar]
- 64.Ercan-Sencicek AG, Jambi S, Franjic D, Nishimura S, Li M, El-Fishawy P, Morgan TM, Sanders SJ, Bilguvar K, Suri M, et al.. Homozygous loss of DIAPH1 is a novel cause of microcephaly in humans. Eur J Hum Genet 2015; 23:165-172; PMID:24781755; http://dx.doi.org/ 10.1038/ejhg.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res 2007; 313:560-71; PMID:17198702; http://dx.doi.org/ 10.1016/j.yexcr.2006.10.033 [DOI] [PubMed] [Google Scholar]
- 66.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol 2008; 181:523-36; PMID:18458159; http://dx.doi.org/ 10.1083/jcb.200709029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe S, De Zan T, Ishizaki T, Yasuda S, Kamijo H, Yamada D, Aoki T, Kiyonari H, Kaneko H, Shimizu R, Yamamoto M, Goshima G, Narumiya S. Loss of a Rho-regulated actin nucleator, mDia2, impairs cytokinesis during mouse fetal erythropoiesis. Cell Rep 2013; 5:926-32; PMID:18815276; http://dx.doi.org/ 10.1016/j.celrep.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 68.Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 2004; 428:767-71; PMID:15085137; http://dx.doi.org/ 10.1038/nature02452 [DOI] [PubMed] [Google Scholar]
- 69.Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, Mao Y. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell 2011; 20:342-52; PMID:21397845; http://dx.doi.org/ 10.1016/j.devcel.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinohara R, Thumkeo D, Kamijo H, Kaneko N, Sawamoto K, Watanabe K, Takebayashi H, Kiyonari H, Ishizaki T, Furuyashiki T, et al.. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci 2012; 15:373-80, S371-372; PMID:22246438; http://dx.doi.org/ 10.1038/nn.3020 [DOI] [PubMed] [Google Scholar]
- 71.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci U S A 2007; 104:6708-13; PMID:17412835; http://dx.doi.org/ 10.1073/pnas.0608946104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol 2011; 22:1654-64; PMID:21804089; http://dx.doi.org/ 10.1681/ASN.2010101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, et al.. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 2011; 138:303-15; PMID:21177343; http://dx.doi.org/ 10.1242/dev.055566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity 2007; 26:177-90; PMID:17306570; http://dx.doi.org/ 10.1016/j.immuni.2007.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naj X, Hoffmann AK, Himmel M, Linder S. The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages. Infect Immun 2013; 81:1683-95; PMID:23460512; http://dx.doi.org/ 10.1128/IAI.01411-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou F, Leder P, Zuniga A, Dettenhofer M. Formin1 disruption confers oligodactylism and alters Bmp signaling. Hum Mol Genet 2009; 18:2472-82; PMID:19383632; http://dx.doi.org/ 10.1093/hmg/ddp185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young KG, Thurston SF, Copeland S, Smallwood C, Copeland JW. INF1 is a novel microtubule-associated formin. Mol Biol Cell 2008; 19:5168-80; PMID:18815276; http://dx.doi.org/ 10.1091/mbc.E08-05-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mironova E, Millette CF. Expression of the diaphanous-related formin proteins mDia1 and mDia2 in the rat testis. Dev Dyn 2008; 237:2170-6; PMID:18651670 http://dx.doi.org/ 10.1002/dvdy.21622 [DOI] [PubMed] [Google Scholar]
- 79.Behnen M, Murk K, Kursula P, Cappallo-Obermann H, Rothkegel M, Kierszenbaum AL, Kirchhoff C. Testis-expressed profilins 3 and 4 show distinct functional characteristics and localize in the acroplaxome-manchette complex in spermatids. BMC Cell Biol 2009; 10:34; PMID:19419568; http://dx.doi.org/ 10.1186/1471-2121-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]


