Abstract
Background
Women with previous gestational diabetes mellitus (pGDM) and postpartum normal glucose tolerance (NGT) may carry impaired islet β cell secretion, insulin resistance and subsequent altered glucose homeostasis. And certain normoglycemic groups at risks of diabetes were presented with elevated glycemic variability. The aim of study was to investigate the glycemic variability in NGT women with pGDM.
Methods
Total 48 NGT women with pGDM (pGDM group) and 48 age- and BMI-matched NGT women without pGDM (control group) were recruited in the study. Integrated β cell function was assessed with the Insulin Secretion-Sensitivity Index-2 (ISSI-2) derived from oral glucose tolerance test. All subjects were monitored using the continuous glucose monitoring system for consecutive 72 h. The multiple parameters of glycemic variability included the mean blood glucose (MBG), standard deviation of blood glucose (SDBG), mean of daily differences (MODD), mean amplitude of glycemic excursions (MAGE) and the incremental areas above preprandial glucose values (AUCpp).
Results
The pGDM group had a higher MBG (6.5 ± 0.9 vs. 5.9 ± 0.8 mmol/L, p < 0.05), SDBG (1.3 ± 0.3 vs. 0.9 ± 0.2 mmol/L, p < 0.05), MODD (1.4 ± 0.3 vs. 1.1 ± 0.2 mmol/L, p < 0.05), MAGE (2.7 ± 0.4 vs. 1.8 ± 0.5 mmol/L, p < 0.05), and AUCpp (26.8 ± 3.4 vs. 19.2 ± 3.2 mmol/L·h, p < 0.05), when compared to the control group, and the differences remained significant after adjusting for anthropometric indices and metabolic risk factors. Islet β cell function index ISSI-2 in the pGDM group was lower than in the control group (p < 0.05). MBG, SDBG, MODD, MAGE and AUCpp were all negatively associated with ISSI-2 in the pGDM group (r = −0.31, −0.30, −0.34, −0.48 and −0.54, respectively, p < 0.05), and the correlations remained significant after adjusting for anthropometric indices and metabolic risk factors.
Conclusions
Normal glucose tolerance women with pGDM were presented with elevated glycemic variability, which may be associated with impaired islet β cell function.
Keywords: Glycemic variability, Normal glucose tolerance, Gestational diabetes mellitus
Background
Gestational diabetes mellitus (GDM) was associated with increased risks of developing postpartum pre-diabetes, type 2 diabetes and metabolic syndrome [1, 2]. The relationships reflected the fact that GDM and type 2 diabetes shared a similar pathophysiology, characterized by deficiency in islet β cell secretion and insulin sensitivity [3, 4]. GDM may serve as a window to reveal a predisposition to type 2 diabetes.
It also had been repeatedly demonstrated that women with previous GDM (pGDM) and even with postpartum normal glucose tolerance (NGT) carried deficiency in islet β cell secretion and insulin sensitivity and subsequent altered glucose homeostasis [5–7]. Definition of NGT was based on fasting plasma glucose (FPG) <5.6 mmol/L and 2-h postload plasma glucose (2hPG) <7.8 mmol/L during a 75-g oral glucose tolerance test (OGTT) [8]. Detection of glucose values at 0 and 120 min by OGTT may miss the potential glycemic variability. And previous studies conducted in NGT subjects at risks of developing diabetes showed that glycemic excursions were in the range of prediabetes as well as the diabetes [9, 10]. So we hypothesized that women with pGDM and even with NGT status may have altered glycemic variability.
Efforts to quantify glycemic variability mainly relied on intermittent glucose determinations which acquired from the continuous glucose monitoring (CGM) system (CGMS), and CGM system can detect glycemic variability in more details than the conventional self-monitoring methods of blood glucose [11, 12]. Glycemic variability parameters estimated by the multiple modalities of CGM data [13] may differ in NGT women with and without the pGDM.
The present study was designed to investigate the glycemic variability assessed by CGM in postpartum NGT women with the pGDM.
Methods
Study subjects
This cross-sectional study was performed at outpatient and inpatient department of the Second Affiliated Hospital of Nantong University in China from January 2010 to May 2014. Total 502 women who had the diagnosis of GDM between 24 and 28th week of pregnancy were revisited and screened by 75-g oral glucose tolerance test (OGTT) after delivery for 1 year. And 48 women, who detected with postpartum NGT and agreed to be performed with CGM, were recruited for the further study (pGDM group). The diagnoses of NGT and GDM were based on the criteria of the ADA 2008 [8]. NGT was defined as FPG <5.6 mmol/L and 2hPG <7.8 mmol/L during the OGTT. GDM was made when two of the following plasma glucose values in the 75-g OGTT are exceeded: fasting, 5.3 mmol/L; 1 h, 10.0 mmol/L; 2 h, 8.6 mmol/L. Meanwhile, 48 healthy women without pGDM, who selected and diagnosed with NGT after delivery for 1 year in the same department, were recruited and set as controls in the study (control group). The two groups were also matched for age and body mass index (BMI). And exclusion criteria for control group were as follows: family history of diabetes, lipid abnormalities, hypertension, hepatic disease, chronic kidney disease, cardiovascular disease, malignancy, or other disorders affecting glucose metabolism such as hyperthyroidism. The study was approved by the institutional review board of the Second Affiliated Hospital of Nantong University, with written informed consent being obtained from all participants.
β cell function determination
Blood samples were taken at 0, 30, 60, 120 and 180 min for the measurement of plasma glucose and insulin concentrations (glucose unit: mmol/L, insulin unit: miu/L) during 75-g oral glucose test. Glut and Inst represent the plasma glucose and insulin concentrations, respectively, at time t during the OGTT. Insulin sensitivity was estimated using the insulin sensitivity index (ISI) of Matsuda and DeFronzo: ISI = 10,000/square root of (Ins0 × Glu0) × (mean glucose × mean insulin during OGTT) [14]. Insulin secretion was defined as the ratio of the area-under-the-insulin-curve to the area-under-the-glucose curve (AUCins/glu) [15, 16]. Integrated β cell function was assessed with the Insulin Secretion-Sensitivity Index-2 (ISSI-2) (AUCins/glu multiplied by ISI) [16, 17].
CGM in all subjects
After OGTT, all subjects were monitored by CGMS (Medtronic MiniMed, Northridge, CA 91325, USA) for 72 h. The CGM system sensor was inserted in all subjects on day 0 and removed on day 3. Data were downloaded and glucose profiles were evaluated based on the data collected on days 1 and 2. The subjects were instructed to input at least four calibration readings per day and the times of key events. During the study, all subjects have standard meals provided by dietary division. The total calorie intake was 30 kcal/kg per day, with 50 % carbohydrates, 15 % proteins, and 35 % fats. The calorie distribution between breakfast, lunch, and dinner was 20, 40, and 40 %, respectively. Three daily meals were required to consume at time of 6:30–7:30, 11:30–12:30, and 18:00–19:00, respectively. All subjects were instructed to avoid strenuous exercise during CGM.
The parameters of glycemic variability included the standard deviation of blood glucose (SDBG), mean of daily continuous 24 h blood glucose (MBG), mean of daily differences (MODD), mean amplitude of glycemic excursions (MAGE) and the incremental areas above preprandial glucose values (AUCpp). MODD was calculated from the absolute difference between paired continuous glucose monitoring values during two successive 24 h periods and was used to assess inter-day glycemic variability [18]. MAGE, designed to quantify major swings of glycemia and to exclude minor ones, was used for assessing intra-day glycemic variability in this study [19]. AUCpp, calculated incremental areas of glucose above the each meal, was performed to evaluate the characteristics of postprandial glucose excursion [20]. It should be noted that MBG was a measure of overall of glycemic level and not specifically variability [21].
Anthropometric indices and laboratory examination
Body mass index (BMI) was calculated (kg/m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) taken three times using a sphygmomanometer and then averaged. Capillary glucose concentrations were measured with Lifescan Surestep blood glucose meter. Plasma glucose levels were measured using the glucose oxidase method. HbA1c was measured by high performance liquid chromatography (HPLC) with D-10 hemoglobin Testing Program (Bio-Rad). The serum insulin assay used magnetic beads-based enzymatic spectrofluorometric immunoassay with automatic enzyme immunoassay apparatus (AIA360, TOSOH). Serum glucose concentrations, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDLC), and low density lipoprotein cholesterol (LDLC) were measured with Hitachi Model 7600 Series Automatic Analyzer.
Statistical analyses
Data analyses were performed using the SPSS16.0 statistical software (SPSS Inc., USA). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) in the case of skewed distributions. Categorical variables were described as frequency (percentage). The Student t test was applied to compare differences of continuous variables between the pGDM and control groups, nonparametric test (Mann–Whitney U test) was applied to compare non-normally distributed variables between the two groups, and Chi squared test was applied to compare categorical variables between the two groups. Relationship between glycemic variability and integrated β cell function was assessed using the Pearson’s correlation test and partial correlation test. p < 0.05 was considered to be statistically significant.
Results
Baseline characteristics in the subjects
As shown in Table 1, age, BMI and DBP were comparable between the pGDM and control groups (p > 0.05). SBP of pGDM group was higher than that in control group (p < 0.05). The prevalence of familial diabetes of pGDM group was higher than in control group (p < 0.05). TC, LDLC of pGDM group were higher than in control group (p < 0.05), HDLC of pGDM group was lower than in control group (p < 0.05), but there was no differences in TG between the two groups (p > 0.05). HbA1c of pGDM group was higher than that in control group (p < 0.05).
Table 1.
Comparisons of clinical variables in pGDM and control groups
| Variables | pGDM group | Control group | t | p |
|---|---|---|---|---|
| n | 48 | 48 | – | – |
| Age (year) | 29.1 ± 4.1 | 29.8 ± 3.6 | 0.941 | 0.349 |
| Familial diabetes, n (%) | 7 (14.6) | 0 (0.0) | – | 0.012* |
| BMI (kg/m2) | 26.8 ± 3.2 | 26.3 ± 2.9 | 0.768 | 0.444 |
| SBP (mmHg) | 130 ± 13 | 119 ± 15 | 3.502 | 0.001 |
| DBP (mmHg) | 76 ± 9 | 75 ± 10 | 0.493 | 0.623 |
| TG (mmol/L) | 1.5 (1.2–2.0) | 1.3 (1.2–1.8) | – | 0.442** |
| TC (mmol/L) | 6.2 ± 1.0 | 4.8 ± 0.8 | 8.320 | 0.000 |
| HDLC (mmol/L) | 1.1 ± 0.3 | 1.3 ± 0.3 | 2.265 | 0.026 |
| LDLC (mmol/L) | 3.3 ± 0.9 | 2.4 ± 0.5 | 6.588 | 0.000 |
| HbA1c (%) | 5.8 ± 0.4 | 5.4 ± 0.3 | 8.617 | 0.000 |
Normally distributed values in the table are given as the mean ± SD, the non-normally distributed values are given as the median (25 and 75 % interquartiles)
pGDM group: NGT women with the previous GDM; Control group: NGT women without the previous GDM
BMI body mass index, SBP/DBP systolic/diastolic blood pressure, TC total cholesterol; TG triglyceride, HDLC: high density lipoprotein cholesterol, LDLC low density lipoprotein cholesterol, HbA1c glycosylated hemoglobin A1c
* Test with Fisher’s Exact test; ** Test with Mann–Whitney U test
Changes of plasma glucose and insulin concentrations during the OGTT, and β cell functions derived from OGTT
The statistical comparisons of measurements characterizing the plasma glucose and insulin concentrations during the OGTT were summarized in Table 2. Plasma glucose levels at baseline, 30, 60 and 120 min after glucose ingestion were higher in pGDM group was higher than that in control group (p < 0.05), plasma glucose level at 180 min had no significantly difference between the two groups (p > 0.05). Insulin concentration at 60 min was significantly higher in pGDM group than in control group (p < 0.05), but there were no differences in insulin concentrations at baseline, 30, 120 and 180 min between the two groups (p > 0.05). The area under the glucose curve (AUCglu) was significantly higher in pGDM group than in control group (p < 0.05), but the area under the insulin curve (AUCins) was comparable between the pGDM and control groups (p > 0.05).
Table 2.
Comparisons of glucose and insulin concentrations during OGTT in pGDM and control groups
| Variables | pGDM group | Control group | t | p |
|---|---|---|---|---|
| n | 48 | 48 | – | – |
| Glu0 (mmol/L) | 5.5 ± 0.6 | 5.1 ± 0.5 | 3.845 | 0.000 |
| Glu30 (mmol/L) | 9.0 ± 1.7 | 7.5 ± 1.4 | 4.771 | 0.000 |
| Glu60 (mmol/L) | 8.8 ± 2.8 | 6.7 ± 2.3 | 4.071 | 0.000 |
| Glu120 (mmol/L) | 6.0 ± 1.1 | 5.4 ± 1.4 | 2.722 | 0.008 |
| Glu180 (mmol/L) | 4.9 ± 1.0 | 4.8 ± 1.0 | 0.677 | 0.500 |
| Ins0 (miu/L) | 6.3 (3.9–9.7) | 5.6 (4.0–7.8) | – | 0.391* |
| Ins30 (miu/L) | 54.6 (36.4–88.7) | 56.1 (37.9–77.0) | – | 0.800* |
| Ins60 (miu/L) | 63.1 (40.9–85.0) | 44.1 (28.5–65.4) | – | 0.011* |
| Ins120 (miu/L) | 31.3 (14.9–42.6) | 28.5 (21.6–40.1) | – | 0.823* |
| Ins180 (miu/L) | 9.2 (5.5–13.6) | 8.1 (4.7–17.2) | – | 0.496* |
| AUCglu (mmol/L·h) | 18.4 ± 2.8 | 15.7 ± 2.5 | 4.712 | 0.000 |
| AUCins (miu/L·h) | 114.7 (74.17–163.8) | 99.6 (75.2–132.1) | – | 0.356* |
| AUCins/glu | 5.6 (4.0–7.8) | 6.6 (5.6–7.7) | – | 0.031* |
| ISI | 140.7 (81.9–190.0) | 155.0 (120.3–205.7) | – | 0.034* |
| ISSI-2 | 729.8 (496.9–943.0) | 1027.2 (872.7–1177.5) | – | 0.000* |
Non-normally distributed values are given as the median (25 and 75 % interquartiles)
pGDM group: NGT women with the previous GDM; Control group: NGT women without the previous GDM
Glut plasma glucose concentrations at time t during OGTT, Inst plasma insulin concentrations at time t during OGTT, AUC glu the area under the curve of glucose in 180 min, AUC ins the area under the curve of insulin in 180 min, ISI insulin sensitivity index, AUC ins/glu insulin secretion index, ISSI-2 insulin secretion-sensitivity index-2
* Test with Mann–Whitney U test
After comparison of insulin sensitivity and insulin secretion index derived from OGTT, insulin sensitivity index (Matsuda ISI) and insulin secretion index (AUCins/glu) in pGDM group were lower than in control group (p < 0.05) (Table 2). Integrated β cell function (ISSI-2) was significantly lower in pGDM group than in control group (p < 0.01).
Glycemic variability in the subjects
Glycemic variability detected from CGM system of pGDM and control groups were represented in Fig. 1. Although both pGDM and control groups were presented with NGT, the pGDM group had a greater MBG (6.5 ± 0.9 vs. 5.9 ± 0.8 mmol/L, p = 0.004), SDBG (1.3 ± 0.3 vs. 0.9 ± 0.2 mmol/L, p = 0.000), MODD (1.4 ± 0.3 vs. 1.1 ± 0.2 mmol/L, p = 0.002), MAGE (2.7 ± 0.4 vs. 1.8 ± 0.5 mmol/L, p = 0.000), and AUCpp (26.8 ± 3.4 vs. 19.2 ± 3.2 mmol/L·h, p = 0.000), when compared to the control group (Table 3). And the differences remained significant after adjusting for age, familial diabetes, BMI, SBP, DBP, TG, TC, HDLC and LDLC.
Fig. 1.
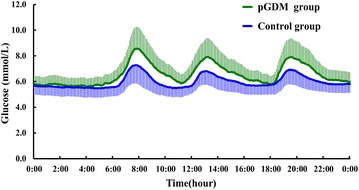
Continuous glucose profiles represented mean data from 24 h in pGDM and control groups
Table 3.
Comparisons of glycemic variability parameters in pGDM and control groups
| Variables | pGDM group | Control group | t | p |
|---|---|---|---|---|
| n | 48 | 48 | – | – |
| MBG (mmol/L) | 6.5 ± 0.9 | 5.9 ± 0.8 | 2.967 | 0.004 |
| SDBG (mmol/L) | 1.3 ± 0.3 | 0.9 ± 0.2 | 6.169 | 0.000 |
| MODD (mmol/L) | 1.4 ± 0.3 | 1.1 ± 0.2 | 3.186 | 0.002 |
| MAGE (mmol/L) | 2.7 ± 0.4 | 1.8 ± 0.5 | 8.696 | 0.000 |
| AUCpp (mmol/L·h) | 26.8 ± 3.4 | 19.2 ± 3.2 | 11.267 | 0.000 |
Normally distributed values in the table are given as the mean ± SD
pGDM group: NGT women with the previous GDM; Control group: NGT women without the previous GDM
MBG mean of blood glucose, SDBG standard deviation of blood glucose, MODD mean of daily differences, MAGE mean amplitude of glycemic excursions, AUC pp incremental areas above preprandial glucose values
Relationships between glycemic variability and ISSI-2 in pGDM group
When the relationships between glycemic variability parameters and ISSI-2 were analyzed by Pearson’s correlation test, MBG (r = –0.31, p = 0.028), SDBG (r = −0.30, p = 0.037), MODD (r = −0.34, p = 0.017), MAGE (r = −0.48, p = 0.000) and AUCpp (r = −0.54, p = 0.000) of pGDM group were all negatively associated with ISSI-2 in pGDM group (Fig. 2a–e). And the correlations remained significant after adjusting for age, familial diabetes, BMI, SBP, DBP, TG, TC, HDLC and LDLC.
Fig. 2.
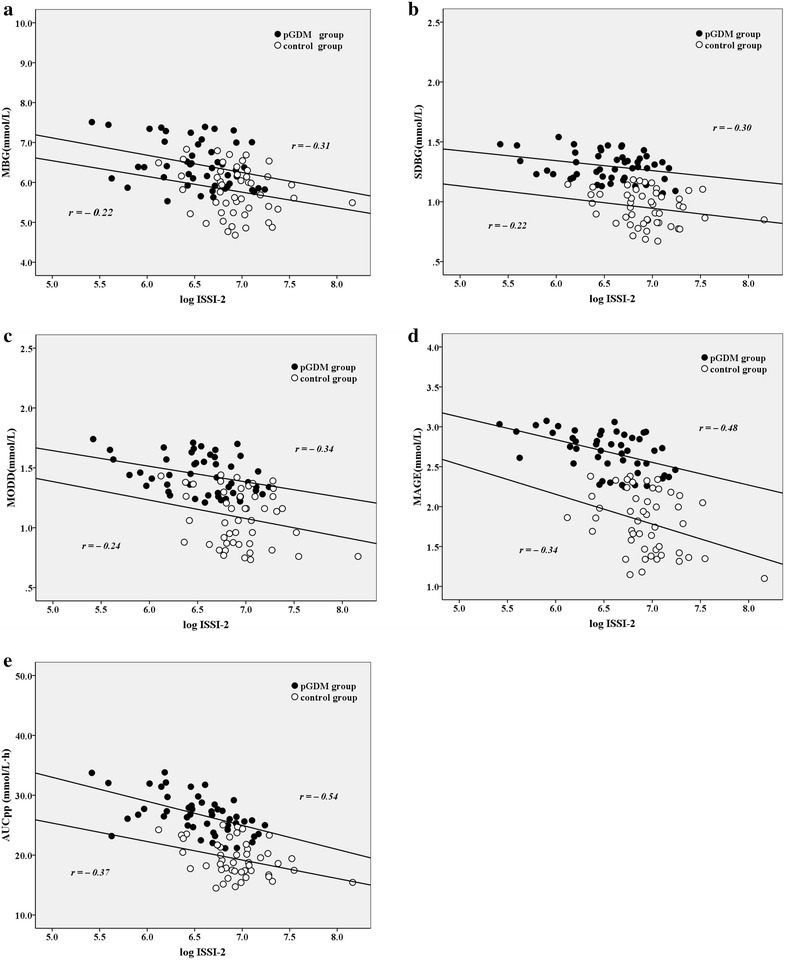
The relationships between glycemic variability parameters (a MBG, b SDBG, c MODD, d MAGE, e AUCpp) and ISSI-2 in pGDM and control groups
Discussion
In the specific group of pGDM women, our present study found that these women even with postpartum NGT were presented with elevated glycemic variability parameters. CGM can provide additional glycemic information compared to OGTT. Detailed 24 h glycemic profiles can be documented by CGM. Certain groups at high risks of diabetes, with so-called NGT status based on the OGTT, were presented with elevated glycemic variability. The study by Ma et al. [22] demonstrated that glycemic variability was increased in abdominally obese men with NGT. A study conducted in cystic fibrosis patients, it had been observed that normoglycemic subjects by OGTT had glucose excursions in the prediabetes as well as the diabetes range [9]. Madhu et al. [10] showed the normoglycemic obese first-degree relatives of type 2 diabetes had excursions into the higher dysglycemic range when studied by CGMS, and 90 % of subjects had excursions into the IGT range, and 15 % had excursions into the diabetes range. A1c-Derived Average Glucose (ADAG) study [23] showed 93 % of non-diabetic subjects exceeded the IGT threshold of 7.8 mmol/L, 7 % of them reached diabetes threshold of 11.1 mmol/L during CGMS, and the mean HbA1c in these subjects was within the normal range according to the ADA recommendations. Our previous study also showed that glycemic variability in normoglycemic subjects with elevated 1-h postload plasma glucose levels (NGT 1 h ≥ 8.6 mmol/L group) was higher than those in NGT 1 h < 8.6 mmol/L group [24]. Women with pGDM, and with postpartum NGT classified by the OGTT, had increased glycemic variability compared to NGT ones without pGDM in the present study. Glycemic variability parameters SDBG, MBG, MODD, MAGE and AUCpp were elevated in women with pGDM. Our finding demonstrated the characteristics of glycemic variability in NGT women with pGDM.
The pGDM identified a population of young women predisposed for type 2 diabetes and related cardiovascular disease. Retnakaran et al. [25, 26] showed any degree of abnormal glucose homeostasis detected on antepartum screening for GDM should be associated with an increased risk of postpartum pre-diabetes, diabetes and latent metabolic syndrome. Elevated circulating markers of endothelial dysfunction in young women with a history of GDM could reflect an early stage on the pathway to the manifestation of future cardiometabolic disorders [27]. And Bo et al. [28] showed women with previous GDM have been shown to express early markers of vascular dysfunction such as increased intima-media thickness of carotid arteries(C-IMT). Zajdenverg et al. [29] showed microcirculation abnormality, evaluated by papillae rectification, was carried in young non-diabetic women with pGDM. Our study showed the normoglycemic women with pGDM were presented with elevated glycemic variability parameters, when compared with the women without pGDM. And elevated glycemic variability could be considered as a marker of metabolic abnormality of pGDM.
Glycemic variability could be an independent risk factor for vascular complications in addition to average glucose [30, 31]. Glucose variability parameters could be calculated with complex formulas designed specifically for the CGM data. In the previous studies, the SDBG around the mean glucose value was considered as a classical index to assess the glycemic variability [32]. MODD and MAGE are objective and valid indices to measure inter-day and intra-day glucose variability, respectively [18, 19]. AUCpp was performed to evaluate the characteristics of postprandial glucose excursion [20]. And several studies had demonstrated that glycemic variability, assessed by MAGE, was closely associated with micro- and macro-vascular complications [33, 34]. Our presented study demonstrated that glycemic variability parameters SDBG, MBG, MODD, MAGE and AUCpp in pGDM group were higher than in the control group. Women with pGDM carried with early markers of vascular dysfunction [28, 29]. Hence, it implies that glycemic variability may be related to early markers of vascular dysfunction in women with pGDM. But it needs further study to document whether glycemic variability remission can improve the markers of vascular dysfunction in pGDM group.
GDM and type 2 diabetes shared a similar pathophysiology, characterized by deficiency in islet β cell secretion and insulin sensitivity, and GDM was a stress situation that may reveal predisposition to type 2 diabetes. Previous studies had shown that women with previous GDM even with postpartum normal oral glucose tolerance test had both insulin secretion and action defects [5, 6]. And our study showed insulin secretion and insulin sensitivity indices derived from OGTT were decreased in pGDM group than in control group. ISSI-2 proposed by Retnakaran et al. [16, 17] was a composite measure and may be a better index than either AUCins/glu or ISI alone to reflect the notion of declining β cell function and account for glycemic disorders. After correlation analyzing, SDBG, MBG, MODD, MAGE, and AUCpp all negatively associated with the ISSI-2 in pGDM group. The decreased ISSI-2 of pGDM may be responsible for elevated glycemic variability.
It should be pointed out that our study had some limitations. First, the family history of diabetes could exaggerate the difference of glycemic variability between NGT women with and without previous GDM, but the comparison of glycemic variability between the two groups was adjusted for the familial diabetes. The pGDM group should be theoretically divided into subgroups with and without familial diabetes for further comparison, but the small sample size of subgroups might make some differences insignificant. Second, although we provided standard meals for subjects during the CGM system monitoring period, some factors, such as physical activity and emotional stress, etc., which may affect levels of glycemic variability, could not all be prevented. Third, we could not assess glycemic variability in relation to oxidative stress, inflammation and other markers of vascular dysfunction. The fourth limitation related to ISSI-2 was that circulating insulin levels during the OGTT may be affected by other factors apart from β cell function, such as incretin hormones and hepatic extraction. The two factors may limit the degree to which insulin levels during the OGTT can reflect β cell function.
Conclusions
In summary, the glycemic variability parameters in NGT women with pGDM were higher than those in the NGT ones without pGDM, and elevated glycemic variability parameters may be associated with impaired β cell function.
Authors’ contributions
YW and LZ participated in the design of the study, data collection, analysis of the data, drafting of the manuscript. JS and XW (Xue-qin) conceived of the study, participated in its design and revised the manuscript. QH and TC participated in analysis of the data and revised the manuscript. XW (Xiao-hua), FX, JC and GW participated in data collection. All authors read and approved the final manuscript.
Acknowledgements
The study was funded by the Scientific Research Program of Nantong (No. HS2012028, HS2013006, HS149010).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviations
- NGT
normal glucose tolerance
- Pgdm
previous gestational diabetes mellitus
- BMI
body mass index
- SBP/DBP
systolic/diastolic blood pressure
- TC
total cholesterol
- TG
triglyceride
- HDLC
high density lipoprotein cholesterol
- LDLC
low density lipoprotein cholesterol
- HbA1c
glycosylated hemoglobin a1c
- ISI
insulin sensitivity index
- AUCins/glu
insulin secretion index
- ISSI-2
insulin secretion-sensitivity index-2
- SDBG
standard deviation of blood glucose
- MODD
mean of daily differences
- MAGE
mean amplitude of glycemic excursions
- AUCpp
incremental areas above preprandial glucose values
Footnotes
Yong-mei Wang and Li-hua Zhao contributed equally to this work
Contributor Information
Yong-mei Wang, Email: wangymntyy@163.com.
Li-hua Zhao, Email: ntzlhmm@126.com.
Jian-bin Su, Email: sujbzjx@163.com.
Hai-feng Qiao, Email: ntyyqhf@126.com.
Xiao-hua Wang, Email: drwangxiaohua@163.com.
Feng Xu, Email: xufeng5205529@163.com.
Tong Chen, Email: chentongdr@163.com.
Jin-feng Chen, Email: chenjinfengdr@163.com.
Gang Wu, Email: wugangnfm@163.com.
Xue-qin Wang, Email: wangxueqin@medmail.com.cn.
References
- 1.Fernández-Morera JL, Rodríguez-Rodero S, Menéndez-Torre E, Fraga MF. The possible role of epigenetics in gestational diabetes: cause, consequence, or both. Obstet Gynecol Int. 2010;2010:605163. doi: 10.1155/2010/605163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauenborg J, Mathiesen E, Hansen T, Glümer C, Jørgensen T, Borch-Johnsen K, Hornnes P, Pedersen O, Damm P. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI200524531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kousta E, Lawrence NJ, Godsland IF, Penny A, Anyaoku V, Millauer BA, Cela E, Johnston DG, Robinson S, McCarthy MI. Insulin resistance and beta-cell dysfunction in normoglycaemic European women with a history of gestational diabetes. Clin Endocrinol (Oxf) 2003;59:289–297. doi: 10.1046/j.1365-2265.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Imes S, Liu D, McManus R, Finegood DT, Polonsky KS, Sturis J. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44:506–512. doi: 10.2337/diab.44.5.506. [DOI] [PubMed] [Google Scholar]
- 7.Seghieri G, Tesi F, Anichini R, De Bellis A, Barsotti E, Mari A, Ferrannini E. Influence of gestational diabetes on the long-term control of glucose tolerance. Diabetologia. 2007;50:2234–2238. doi: 10.1007/s00125-007-0802-1. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 9.Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, Kessler R, Kessler L. Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Horm Metab Res. 2008;40:502–506. doi: 10.1055/s-2008-1062723. [DOI] [PubMed] [Google Scholar]
- 10.Madhu SV, Muduli SK, Avasthi R. Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol Ther. 2013;15:461–465. doi: 10.1089/dia.2012.0333. [DOI] [PubMed] [Google Scholar]
- 11.Klonoff DC, Buckingham B, Christiansen JS, Montori VM, Tamborlane WV, Vigersky RA, Wolpert H, Endocrine Society Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:2968–2979. doi: 10.1210/jc.2010-2756. [DOI] [PubMed] [Google Scholar]
- 12.Chinese Diabetes Society Chinese clinical guideline for continuous glucose monitoring (2012) Chin Med J (Engl) 2012;125:4167–4174. [PubMed] [Google Scholar]
- 13.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, Hanley AJ, Raif N, Hirning CR, Connelly PW, Sermer M, Kahn SE, Zinman B. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48:993–1001. doi: 10.1007/s00125-005-1710-x. [DOI] [PubMed] [Google Scholar]
- 16.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 17.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26:1198–1203. doi: 10.1111/j.1464-5491.2009.02841.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y, Li Y, Gao X, Luan X, Wang W, Jia W. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2011;17:CR9–CR13. doi: 10.12659/MSM.881318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Service FJ Glucose variability. Diabetes. 2013;62:1398–1404. doi: 10.2337/db12-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Jia W, Bao Y, Ma X, Lu W, Li H, Hu C, Xiang K. Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med Sci Monit. 2008;14:CR552–CR558. [PubMed] [Google Scholar]
- 21.Chen T, Xu F, Su JB, Wang XQ, Chen JF, Wu G, Jin Y, Wang XH. Glycemic variability in relation to oral disposition index in the subjects with different stages of glucose tolerance. Diabetol Metab Syndr. 2013;5:38. doi: 10.1186/1758-5996-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma CM, Yin FZ, Wang R, Qin CM, Liu B, Lou DH, Lu Q. Glycemic variability in abdominally obese men with normal glucose tolerance as assessed by continuous glucose monitoring system. Obesity (Silver Spring) 2011;19:1616–1622. doi: 10.1038/oby.2011.5. [DOI] [PubMed] [Google Scholar]
- 23.Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR, ADAG Study Group Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2010;53:1608–1611. doi: 10.1007/s00125-010-1741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su JB, Chen T, Xu F, Wang XQ, Chen JF, Wu G, Jin Y, Wang XH. Glycemic variability in normal glucose regulation subjects with elevated 1-h postload plasma glucose levels. Endocrine. 2014;46:241–248. doi: 10.1007/s12020-013-0047-3. [DOI] [PubMed] [Google Scholar]
- 25.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–677. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo S, Valpreda S, Menato G, Bardelli C, Botto C, Gambino R, Rabbia C, Durazzo M, Cassader M, Massobrio M, Pagano G. Should we consider gestational diabetes a vascular risk factor? Atherosclerosis. 2007;194:e72–e79. doi: 10.1016/j.atherosclerosis.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Zajdenverg L, Rodacki M, Faria JP, Pires ML, Oliveira JE, Halfoun VL. Precocious markers of cardiovascular risk and vascular damage in apparently healthy women with previous gestational diabetes. Diabetol Metab Syndr. 2014;6:63. doi: 10.1186/1758-5996-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göbl CS, Bozkurt L, Yarragudi R, Prikoszovich T, Tura A, Pacini G, Koppensteiner R, Kautzky-Willer A. Biomarkers of endothelial dysfunction in relation to impaired carbohydrate metabolism following pregnancy with gestational diabetes mellitus. Cardiovasc Diabetol. 2014;13:138. doi: 10.1186/s12933-014-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaccardi F, Pitocco D, Ghirlanda G. Glycemic risk factors of diabetic vascular complications: the role of glycemic variability. Diabetes Metab Res Rev. 2009;25:199–207. doi: 10.1002/dmrr.938. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 32.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Techno Ther. 2009;11:551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 33.Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, Wu G, Jin Y, Wang XH. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6:139. doi: 10.1186/1758-5996-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, Zhou Y, Tian L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36:1026–1032. doi: 10.2337/dc12-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]


