Abstract
Background
When prostate cancer is suspected, the prostate gland is biopsied with the aid of transrectal ultrasound (TRUS). The sensitivity of prostatic biopsy is about 50%. The fusion of magnetic resonance imaging (MRI) data with TRUS enables the targeted biopsy of suspicious areas. We studied whether this improves the detection of prostate cancer.
Methods
168 men with suspected prostate cancer underwent prostate MRI after a previous negative biopsy. Suspicious lesions were assessed with the classification of the Prostate Imaging Reporting and Data System and biopsied in targeted fashion with the aid of fused MRI and TRUS. At the same sitting, a systematic biopsy with at least 12 biopsy cores was performed.
Results
Prostate cancer was detected in 71 patients (42.3%; 95% CI, 35.05–49.82). The detection rate of fusion-assisted targeted biopsy was 19% (95% CI, 13.83–25.65), compared to 37.5% (95% CI, 30.54–45.02) with systematic biopsy. Clinically significant cancer was more commonly revealed by targeted biopsy (84.4%; 95% CI, 68.25–93.14) than by systematic biopsy (65.1%; 95% CI, 52.75–75.67). In 7 patients with normal MRI findings, cancer was detected by systematic biopsy alone. Compared to systematic biopsy, targeted biopsy had a higher overall detection rate (16.5% vs. 6.3%), a higher rate of infiltration per core (30% vs. 10%), and a higher rate of detection of poorly differentiated carcinoma (18.5% vs. 3%). Patients with negative biopsies did not undergo any further observation.
Conclusion
MRI/TRUS fusion–assisted targeted biopsy improves the detection rate of prostate cancer after a previous negative biopsy. Targeted biopsy is more likely to reveal clinically significant cancer than systematic biopsy; nevertheless, systematic biopsy should still be performed, even if the MRI findings are negative.
The current standard for the diagnosis of prostate cancer is the systematic transrectal ultrasound (TRUS)–guided biopsy with 10 to 12 biopsy cores (1). By including an additional targeted biopsy of suspicious hypoechoic areas, the detection rate of prostate cancer can be increased by 3.5% during the initial biopsy (2). However, the sensitivity of the conventional biopsy method is limited: autopsy studies that compare the prostate biopsy with whole-mount sections of the entire prostate gland place it at 53% (3, 4). Because of this diagnostic uncertainty, approximately one-third of patients with ongoing cancer suspicion must undergo a repeat biopsy within five years after the initial biopsy, which in turn gives a cancer diagnosis in 13% to 41% of cases (5, 6). To reduce the rate of false-negative biopsies, the methods recommended for rebiopsies are the extended biopsy approach (such as saturation biopsy) or the modified access path approach (such as transperineal mapping) (7, 8). If prostate cancer is confirmed, the question arises as to whether the biopsy result can correctly identify the histological tumor stage and thus can be used for therapy planning or prognosis estimation. In fact, the risk of misclassification with conventional prostate biopsy ranges between 21% and 54% (9– 11).
The diagnostic accuracy of prostate biopsies depends significantly on which imaging technique is used. Since the prostate can be distinguished from its surrounding structures according to its zonal anatomy, the current default method for prostate biopsies is transrectal ultrasound, which uses modern, high-resolution ultrasound probes to guide the needle. However, the transrectal ultrasound itself is clearly limited in predicting prostate cancer; a recent meta-analysis showed it to have a sensitivity of 73.6% and a specificity of 61.3% (12).
In addition to the conventional grayscale ultrasound, alternative ultrasound techniques have been established over the past years. However, due to inconsistencies in the currently available studies, these methods have not yet not been included in the German S3 guidelines for use in primary diagnosis of prostate cancer (Table 1). At the moment, in addition to grayscale ultrasound, magnetic resonance imaging (MRI) is frequently recommended for repeat biopsies after a negative result (13).
Table 1. Diagnostic value of imaging techniques for identifying intraprostatic PCa lesions (reference standard: whole-mount sections after radical prostatectomy).
| Study/reference | Patients n |
Sensitivity % |
Specificity % |
Positive predictive value % |
Negative predictive value % |
S3 Guidelines (strength of recommendation) | |
|---|---|---|---|---|---|---|---|
| Grayscale TRUS | Brock et al. (29) | 229 | 18.3 | 90.4 | 76.7 | 37.2 | positive (0, "may") |
| Real-time elastography | Salomon et al. (30) | 109 | 75.4 | 76.6 | 87.7 | 59 | negative (A, "should") |
| Contrast-enhanced TRUS | Halpern et al. (31) | 12 | 42 | NA | 56 | NA | negativ (A, "should") |
| C-TRUS/ANNA | Walz et al. (32) | 28 | 83.1 | 63.9 | 80.1 | 68.4 | NA |
| HistoScanning™482; | Simmons (33) | 27 | 89.7 | 72.3 | 82.9 | 82.5 | negative (A, "should") |
| Multiparametric 3-Tesla MRI | Turkbey et al. (27) | 45 | 58 | 93 | 98 | 90 | positive (0, "may") |
ANNA, artificial neural network analysis; C-TRUS, computerized transrectal ultrasound; NA, not available; MRI, magnetic resonance imaging; PCa, prostate cancer; TRUS, transrectal ultrasound
MRI is superior to other imaging techniques in visualizing prostate cancer and contributes to both better detection and more precise staging of the tumor (14). However, performing biopsies of suspicious lesions by MRI-guided “in-bore” prostate biopsy requires considerable training and effort for the examiner and is expensive, so that this method is not widely offered (15). A different approach, of MRI/TRUS fusion biopsy, can be used in real-time during ultrasound examination with the pre-acquired image data of MRI, thereby enabling a targeted biopsy of suspicious areas that are not visible in the transrectal ultrasound. MRI/TRUS fusion biopsy can be performed as an outpatient procedure under local anesthesia and, in comparison to the in-bore prostate biopsy method, saves both time and money (the current cost in Germany for MRI/TRUS fusion biopsy is €700, as compared with €1600 for MRI plus „in-bore“ biopsy).
The aim of this study was to critically examine the value of MRI/TRUS fusion biopsy in prostate cancer detection and to compare it with conventional systematic 12-core or 24-core biopsy.
Methods
Study design
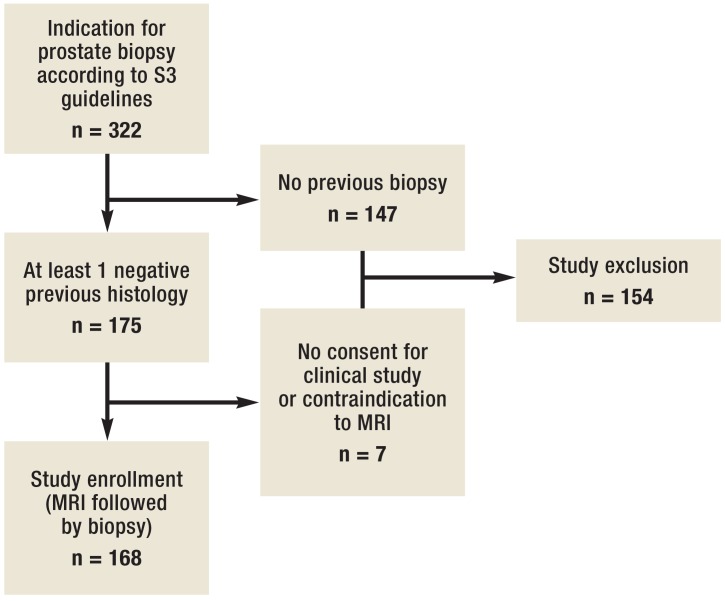
From November 2013 through October 2014, 168 men with suspected prostate cancer (according to S3 guidelines) were included in the study (figure 1), with at least one of the following criteria required (13):
Figure 1.
Flowchart depicting the composition of the study cohort according to inclusion criteria. MRI, magnetic resonance imaging
a serum prostate-specific antigen (PSA) level of ≥4 ng/mL, taking into account influencing factors and age
suspicious results from digital rectal examination
a significant increase in PSA levels
suspicious results from a previous prostate biopsy (e.g. extensive high-grade PIN or atypical small acinar proliferation).
The project was approved by the Ethics Committee of the Ruhr-Universität Bochum (registration no. 4514–12), and written informed consent was obtained from participants. Patients were only included if they had had at least one prior negative systematic biopsy result and were excluded if they had contraindications to performing a MRI (e.g., allergic to gadolinium contrast agents or electronic implants).
Multiparametric magnetic resonance imaging (MRI)
All patients underwent multiparametric MRI at 3 Tesla (Siemens Skyra) of the prostate with surface coil but without endorectal coil (the examination protocol is available in the eBox). In addition, lesions were divided into three prostate risk groups (not evidence-based). A PI-RADS score of <8 was considered as low risk, of between 8 and 12, as intermediate risk, and of >12, as high risk for prostate cancer. The average period between MRI and the consecutive biopsy was 16 days (range, 1 to 21 days).
eBox.
The MRI examination protocol consisted of a T2-weighted sequence, a diffusion-weighted sequence with ADC analysis, and a contrast-enhanced sequence. As a contrast agent, a bolus of 0.1 mmol/kg gadolinium (Gadovist Bayer Vital, Leverkusen, Germany) was used. For subsequent fusion of MRI and ultrasound, a 3D data set with 0.8 mm isotropic voxels was produced in addition to the T2-weighted images. MRI scans were assessed by two experienced uroradiologists according to the current guidelines of the European Society of Radiology (ESUR) (34). Suspicious lesions were classified using the Prostate Imaging Reporting and Data System (PI-RADS) and assigned to a 12-sector prostate scheme. The probability of the presence of a clinically significant tumor was specified for each MRI sequence separately using a 5-point scale, giving a summed score for each lesion (3 to 15 points).
Image fusion and prostate biopsy
If at least one suspicious target lesion was identified, MRI data were uploaded into the ultrasound system (Hi-Vision Preirus, Hitachi, Tokyo, Japan) in DICOM format and the suspicious lesions were highlighted in the 3D T2-weighted MRI data set. Patients were examined with an endocavity end-fire probe (V53W, Hitachi, Tokyo, Japan) in the left lateral position. Each lesion was sampled with 2 cores using the MRI/TRUS fusion method (Figure 1), followed by a systematic 12-core biopsy. If no target lesion was defined by MRI, a 24-core saturation biopsy was performed. Biopsies were taken by a urologist with extensive experience in prostate cancer diagnosis, and biopsy cores were examined by an experienced uropathologist. Tumors were staged according to the Epstein criteria as clinically significant (Gleason score >6 and/or prostate carcinoma infiltration depth per core of ≥50%) or as insignificant (16).
Statistics
The detection rate of prostate cancer with targeted and systematic biopsy was compared and evaluated according to START criteria (17). Differences between medians and ratios of descriptive statistics were analyzed using the Mann–Whitney U-test and the chi-square test. Statistical analysis was performed in SPSS. Levels were considered statistically significant at α= 0.05.
Results
Demographics of patients in the study and the results of multiparametric MRI are listed in Table 2. All patients had at least one previous negative histological result (1 to 7 sessions). A total of 2530 biopsy cores were analyzed as part of the study protocol. Of these, 2136 were taken by systematic biopsy and 394 were targeted by MRI/TRUS fusion biopsy. MRI was used to define 197 target lesions based on the PI-RADS classification, with 23, 97, and 77 targeted lesions classified as low risk, intermediate risk, and high risk for prostate cancer, respectively. An average of 1.2 lesions per patient (range, 0 to 3) were analyzed by biopsy.
Table 2. Patient characteristics.
| Number of patients (%) | Median (IQR) | |||
|---|---|---|---|---|
| Age (years) | 64 | (59–70) | ||
| PSA, ng/mL | 9.2 | (6.7–13.4) | ||
| Prostate volume, mL | 55.4 | (42–80) | ||
| Suspicious digital rectal exam findings | 38 | (22.6) | ||
| Suspicious MRI results | 144 | (85.7) | ||
| Number target lesions | 197 | – | 1 | (1–2) |
| Prior biopsies | 2 | (1–2) | ||
| 1 | 71 | (42.3) | ||
| 2 | 56 | (33.3) | ||
| 3 | 23 | (13.7) | ||
| 4–7 | 10 | (5.9) | ||
PSA, prostate-specific antigen; MRI, magnetic resonance imaging; IQR, interquartile range
Overall, the detection rate for prostate cancer was 42.3% (71 of 168 patients; 95% confidence interval [CI], 35.05 to 49.82). The detection rate per patient using targeted biopsy was 19% (32 of 168 patients; 95% CI, 13.83 to 25.65) and using systematic biopsy, 37.5% (63 of 168 patients; 95% CI, 30.54 to 45.02) (p = 0.003; chi-square test). Clinically significant cancers were found in 84.4% of the targeted biopsies (27 of 32 tumors; 95% CI, 68.25 to 93.14) and in 65.1% of the systematic biopsies (41 of 63 tumors; 95% CI, 52.75 to 75.67). MRI gave suspicious results for 144 patients. Of the 77 targeted lesions classified as high risk for prostate cancer by MRI PI-RADS, targeted fusion biopsy detected cancer in 35.1% (95% CI, 25.35 to 46.2; n = 27). Of the 97 lesions classified as intermediate risk, targeted biopsy detected cancer in 10.3% (10 of 97). The proportion of clinically significant tumors with high risk was 85.2% (23 of 27 lesions; 95% CI, 67.52 to 84.08) and of intermediate risk, 60% (6 of 10 lesions; 95% CI, 31.27 to 83.18). No cancers were detected by targeted biopsy from lesions classified as low risk.
MRI did not reveal any target lesions for 24 patients. Nevertheless, systematic biopsies detected prostate cancer for 7 of these patients, with clinically relevant tumors identified for 3 patients. A detailed comparison between targeted and systematic biopsies is shown in Table 3. Without the combined use of both biopsy methods, 8 tumors (11.3%) would have been overlooked by systematic biopsy. These cancers were detected only by the targeted fusion biopsy method. About half of the target lesions were located in the ventral prostate sector, with an average diameter of 13.4 mm and an average PI-RADS score of 13.7 points, as determined by MRI. Histological analysis revealed that all of these tumors were clinically significant. Conversely, 39 cancers (54.9%) would have been overlooked if only the targeted fusion biopsy method had been used. The proportion of insignificant tumors according to the Epstein criteria was 44%.
Table 3. Prostate cancer detection rates per patient as a function of biopsy technique and clinical significance*.
| Systematic biopsy | |||||
|---|---|---|---|---|---|
| Number | Benign | Insignificant PCa | Significant PCa | Total | |
| MRI/TRUS fusion targeted biopsy | Unsuspicious MRI results | 17 | 4 | 3 | 24 |
| Benign | 80 | 13 | 19 | 112 | |
| Insignificant PCa | 1 | 3 | 1 | 5 | |
| Significant PCa | 7 | 2 | 18 | 27 | |
| Total | 114 | 22 | 41 | ||
Epstein criteria (Gleason ≥3 + 4 and/or infiltration depth per core ≥50%). MRI, magnetic resonance imaging; PCa, prostate cancer; TRUS, transrectal ultrasound
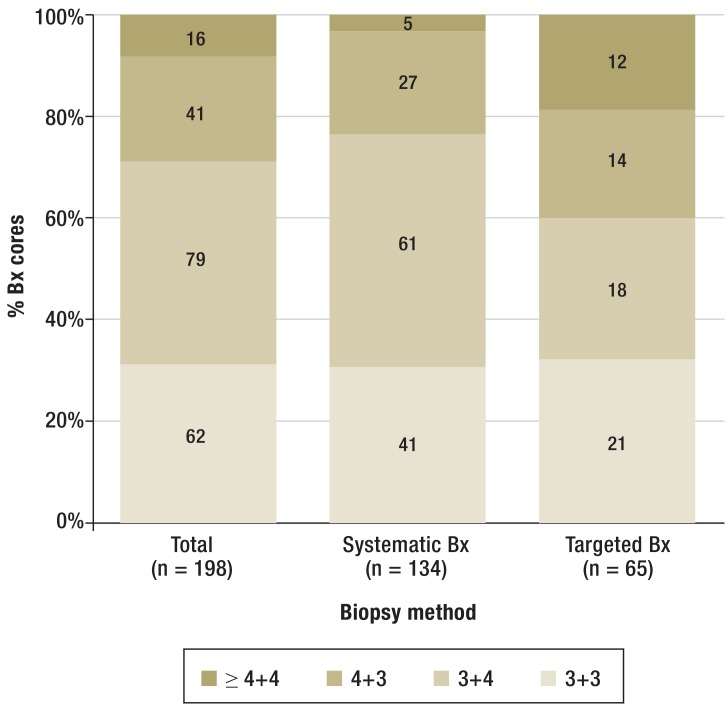
Analyzing each biopsy core showed prostate cancer in 199 (7.9%) of 2530 cores. An average of 15.1 cores were taken per patient (with 2.3 cores from the targeted method, and 12.7 cores from the systematic method). The detection rate for prostate cancer by the targeted biopsy approach was 16.5% (65 out of 394 cores), as compared to 6.3% for the systematic biopsy approach (134 of 2136 cores). The median infiltration rate of cancer-positive cores from targeted biopsies (30%) was significantly higher than that of cores from systematic biopsies (10%) (p<0.001; Mann–Whitney U test). The distribution of the Gleason-scores (Figure 3) showed significantly more poorly differentiated carcinomas with a Gleason score ≥ 4 + 4 in the targeted biopsy method (p<0.001, chi-square test).
Figure 3.
Distribution of Gleason patterns per biopsy core. The Gleason scores in prostate cancer–positive biopsies are compared between targeted MRI/TRUS fusion biopsy and systematic biopsy. Bx, biopsy
Discussion
This study describes a new approach to detecting prostate cancer by combining the targeted MRI/TRUS fusion biopsy with systematic biopsy following previous negative histological results. While the fusion biopsy method detected prostate cancer in 19% of patients, the overall detection rate increased to 42.3% when this was used in combination with systematic biopsy. The majority of cancers detected by targeted fusion biopsy were clinically significant (84.4%). The detection rate of prostate cancer by targeted fusion biopsy correlated directly with the PI-RADS classification by MRI of suspicious lesions.
We can compare the fusion biopsy method in this study with the detection rate of a study with 2526 patients, which likewise studied a cohort with a previous negative biopsy results (18). In the latter case, the detection rate following serial biopsies was 17% after the first 6 rounds of rebiopsy, and this decreased with each subsequent biopsy session to 14%, 11%, and finally 9%. The diagnosis of prostate cancer can thus be ensured by close surveillance. Nonetheless, undergoing repeated biopsy sessions places a high psychological and physical burden on the patient. The detection rate in our study was 19%, with an average removal of 2.3 targeted biopsy cores and of up to 7 pre-biopsies. Other studies on fusion biopsy have not demonstrated that the detection rate is reduced according to the number of pre-biopsies (19, 20).
Earlier investigation protocols for rebiopsy were not based on targeted tissue sampling, due to insufficient visualization of prostate cancer. Rather, it was postulated that the sensitivity of rebiopsy increased when the number of biopsies was doubled (saturation biopsy) or when the route of access (transperineal mapping) was modified. Thus, a large study of 1056 patients showed that the detection rate significantly increased, from 24.9% to 32.7%, when saturation biopsies were performed with 20-core to 24-core rather than a 12-core to 14-core during rebiopsy, with 40.1% of the cancers detected by saturation biopsy categorized as clinically insignificant (21). The proportion of serious complications increased with increasing number of biopsies, rising to 6.1% in the saturation biopsy scheme (22). The most common complication (81% of hospital admissions) after prostate biopsy is acute bacterial prostatitis (23). The infection rate for transperineal biopsy (1.2%) appears to be lower than that for transrectal biopsy (24). Using transperineal mapping biopsy (with up to 54 cores), Taira et al. achieved a detection rate of prostate cancer of 41.7% and 34.4% from the first and second rebiopsies, respectively. Tumors with a Gleason score ≤6 were detected in 45% of patients. However, the transperineal mapping biopsy regimen is limited by the need for general anesthesia and the high risk (of up to 11.1%) of acute urinary retention (24). Using significantly fewer biopsy cores (with an average of 15.1), the total detection rate in our study was 42.3%. Similarly, the total proportion of clinically insignificant cancers (29.6%) was lower than that for the saturation biopsy or mapping biopsy regimens.
The aim of fusion biopsy is to detect prostate cancer with as few biopsies as possible while delivering a correct histological grading and staging for prognosis assessment and treatment planning (Figure 2). A variety of studies for targeted fusion biopsy have reported prostate cancer detection rates of between 23.7% and 82.1% (25). Vourganti et al. examined 195 patients with suspicious lesions revealed by MRI but with a prior negative histology result using the PercNav system and, using a combination of targeted and systematic transrectal biopsies, detected prostate cancer in 37.4%. The detection of cancers with a Gleason score ≥8 in 21 patients can be mainly attributed to the targeted biopsy scheme (20). These results were confirmed by Sonn et al., even though they used a different fusion system. In a cohort of 105 patients with up to 3 negative pre-biopsies, the detection rate for prostate cancer was 34%, and more clinically significant tumors were detected by targeted biopsy (91%) than by systematic biopsy (54%) (19). This study study confirms the findings of other research groups that using the real-time virtual sonography (RVS)–fusion system improves the overall detection rate and results in a higher proportion of clinically significant tumors among those detected.
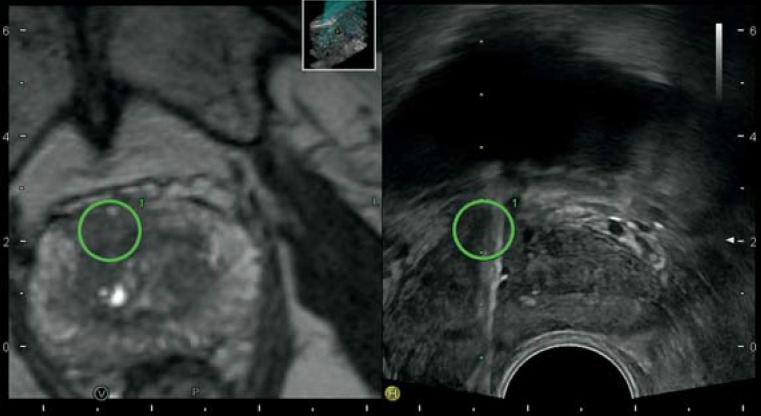
Figure 2.
MRI/TRUS fusion biopsy. MRI (T2-weighted) shows a suspicious hypodense lesion in the ventral prostatic stroma (left panel). By adjusting the image data with the real-time TRUS (right panel), the labeled target lesion (green circle) is visible in real-time TRUS and can be biopsied as a target (hyperechoic artifact)
It is important to note that systematic biopsies still play an important role in our biopsy protocol. Even when preliminary results showed inconspicuous images from MRI or lesions with a low risk for prostate cancer according PI-RADS criteria, systematic 12-core or 24-core biopsy still detected cancers in 39 patients (with 17 scored as insignificant, and 22, as significant). With a transperineal fusion biopsy approach, Kuru et al. detected prostate cancers in 14.9% of patients with unremarkable MRI results using systematic biopsies (26). On the one hand, the rate of false-negative results from fusion biopsies could be explained by the limitations of MRI in visualizing clinically insignificant cancers (27). On the other hand, errors made in collecting and transmitting MRI results could result in suspicious lesions being excluded from targeted biopsies. Results from fusion biopsies directly depend on the expertise of the radiologist and the quality of images from MRI. Additional errors could also occur during the MRI/TRUS fusion protocol. For instance, differences in bladder fullness during MRI, or excessive compression of the prostate with the probe, could easily lead to an inaccurate adjustment of MRI and real-time TRUS, and should be avoided.
Limitations
The significance of this study is limited by the lack of follow-up of patients after a negative biopsy result, as the number of tumor findings in any subsequent biopsy sessions could not be taken into account. In addition, comparing the findings of images taken by MRI with the whole-mount sections after prostatectomy would probably reveal more lesions that had been overlooked by MRI. Therefore, the exact rate of false-negative results from prostate biopsy remains unclear. We also did not take into consideration any influence of the learning curve for applying fusion technology. The examiner in this study has had extensive experience over years in using different imaging methods to detect prostate cancer, and has published a previous pilot study on the MRI/TRUS fusion technology, yet he might have missed some of the target lesions (28). It also remains unclear whether the reduced number of biopsy cores in the cohort used for this study led to fewer complications than for extended biopsy protocols, such as the saturation biopsy regimen. This question could be addressed by a prospective randomized study using two different biopsy protocols.
Conclusion
The MRI/TRUS fusion biopsy approach is a promising method for detecting prostate cancer after a prior negative histological result. Combining MRI-guided targeted biopsies of suspicious lesions with a systematic 12-core biopsy protocol increases the overall cancer detection rate and leads to more clinically relevant cancers being found. Our data suggests that taking additional fusion-guided biopsies from lesions with a low risk score, based on MRI analysis, is not necessary.
Systematic biopsy remains necessary even when MRI results in inconspicuous images. The strategy of a single targeted biopsy without a systematic biopsy is not to be recommended. MRI-guided fusion biopsy holds great promise for the future but must still undergo careful verification. As no unified technical standardization or systematic training exist for this method to date, a high amount of examiner experience is necessary. Finally, because the MRI/TRUS fusion strategy has not yet been considered in the current S3 guidelines for primary diagnosis of prostate cancer, the value of this method should be analyzed in large multicenter studies.
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
Dr. Brock has received consultancy fees and reimbursement of travel and accommodation costs from Hitachi Medical Systems.
The remaining authors declare that no conflict of interests exists.
References
- 1.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. European urology. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Gosselaar C, Roobol MJ, Roemeling S, Wolters T, van Leenders GJ, Schroder FH. The value of an additional hypoechoic lesion-directed biopsy core for detecting prostate cancer. BJU international. 2008;101:685–690. doi: 10.1111/j.1464-410X.2007.07309.x. [DOI] [PubMed] [Google Scholar]
- 3.Nevoux P, Ouzzane A, Ahmed HU, et al. Quantitative tissue analyses of prostate cancer foci in an unselected cystoprostatectomy series. BJU international. 2012;110:517–523. doi: 10.1111/j.1464-410X.2011.10776.x. [DOI] [PubMed] [Google Scholar]
- 4.Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–1489. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 5.Walz J, Graefen M, Chun FK, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. European urology. 2006;50:498–505. doi: 10.1016/j.eururo.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395–1400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 7.Abdollah F, Scattoni V, Raber M, et al. The role of transrectal saturation biopsy in tumour localization: pathological correlation after retropubic radical prostatectomy and implication for focal ablative therapy. BJU international. 2011;108:366–371. doi: 10.1111/j.1464-410X.2010.09876.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed HU, Emberton M, Kepner G, Kepner J. A biomedical engineering approach to mitigate the errors of prostate biopsy. Nature reviews Urology. 2012;9:227–231. doi: 10.1038/nrurol.2012.3. [DOI] [PubMed] [Google Scholar]
- 9.Carlson GD, Calvanese CB, Kahane H, Epstein JI. Accuracy of biopsy Gleason scores from a large uropathology laboratory: use of a diagnostic protocol to minimize observer variability. Urology. 1998;51:525–529. doi: 10.1016/s0090-4295(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes ET, Sundaram CP, Long R, Soltani M, Ercole CJ. Biopsy Gleason score: how does it correlate with the final pathological diagnosis in prostate cancer? British journal of urology. 1997;79:615–617. doi: 10.1046/j.1464-410x.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 11.Chun FK, Steuber T, Erbersdobler A, et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. European urology. 2006;49:820–826. doi: 10.1016/j.eururo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Song JM, Kim CB, Chung HC, Kane RL. Prostate-specific antigen, digital rectal examination and transrectal ultrasonography: a meta-analysis for this diagnostic triad of prostate cancer in symptomatic korean men. Yonsei medical journal. 2005;46:414–424. doi: 10.3349/ymj.2005.46.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 3.1, 2014 AWMF Registernummer: 034/022OL. http://leitlinienprogrammonkologie.de/Leitlinien.7.0.html. (Last accessed on: 3 June 2015)
- 14.Sciarra A, Barentsz J, Bjartell A, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. European urology. 2011;59:962–977. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urologic oncology. 2014;32:903–911. doi: 10.1016/j.urolonc.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 17.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. European urology. 2013;64:544–552. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. The Journal of urology. 2002;167:2435–2439. [PubMed] [Google Scholar]
- 19.Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. European urology. 2013 doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. The Journal of urology. 2012;188:2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. The Journal of urology. 2011;186:850–854. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 22.Turk Z, Hollberg H, Dill T, Kaspers I, Isbarn H. [Prostate cancer detection rates : Comparison of standard biopsy with prompt rebiopsy and a one-time extended biopsy] Der Urologe Ausg. 2014 doi: 10.1007/s00120-014-3648-4. [DOI] [PubMed] [Google Scholar]
- 23.Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schroder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. European urology. 2012;61:1110–1114. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 24.Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81:1142–1146. doi: 10.1016/j.urology.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. European urology. 2014 doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. The Journal of urology. 2013;190:1380–1386. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 27.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. The Journal of urology. 2011;186:1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brock M, Roghmann F, Sonntag C, et al. Fusion of Magnetic Resonance Imaging and Real-Time Elastography to Visualize Prostate Cancer: A Prospective Analysis using Whole Mount Sections after Radical Prostatectomy. Ultraschall Med. 2014 doi: 10.1055/s-0034-1366563. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Brock M, von Bodman C, Sommerer F, et al. Comparison of real-time elastography with grey-scale ultrasonography for detection of organ-confined prostate cancer and extra capsular extension: a prospective analysis using whole mount sections after radical prostatectomy. BJU international. 2011;108:E217–E222. doi: 10.1111/j.1464-410X.2011.10209.x. [DOI] [PubMed] [Google Scholar]
- 30.Salomon G, Kollerman J, Thederan I, et al. Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. European urology. 2008;54:1354–1362. doi: 10.1016/j.eururo.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Halpern EJ, McCue PA, Aksnes AK, Hagen EK, Frauscher F, Gomella LG. Contrast-enhanced US of the prostate with Sonazoid: comparison with whole-mount prostatectomy specimens in 12 patients. Radiology. 2002;222:361–366. doi: 10.1148/radiol.2222010582. [DOI] [PubMed] [Google Scholar]
- 32.Walz J, Thomassin-Piana J, Poizat F. External validation of the anna/c-trus system regarding the correct identification of prostate cancer lesions in the diagnosis of prostate cancer. The Journal of urology. 2012;187(Suppl 820) [Google Scholar]
- 33.Simmons LA, Autier P, Zat’ura F, et al. Detection, localisation and characterisation of prostate cancer by prostate HistoScanning. BJU international. 2012;110:28–35. doi: 10.1111/j.1464-410X.2011.10734.x. [DOI] [PubMed] [Google Scholar]
- 34.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. European radiology. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]