Abstract
Plant metallothioneins (MTs) are a family of low molecular weight, cysteine-rich, and metal-binding proteins, which play an important role in the detoxification of heavy metal ions, osmotic stresses, and hormone treatment. Sequence analysis revealed that the open-reading frame (ORF) of ZjMT was 225 bp, which encodes a protein composed of 75 amino acid residues with a calculated molecular mass of 7.376 kDa and a predicated isoelectric point (pI) of 4.83. ZjMT belongs to the type I MT, which consists of two highly conserved cysteine-rich terminal domains linked by a cysteine free region. Our studies showed that ZjMT was primarily localized in the cytoplasm and the nucleus of cells and ZjMT expression was up-regulated by NaCl, CdCl2 and polyethylene glycol (PEG) treatments. Constitutive expression of ZjMT in wild type Arabidopsis plants enhanced their tolerance to NaCl stress during the germination stage. Compared with the wild type, transgenic plants accumulate more Cd2+ in root, but less in leaf, suggesting that ZjMT may have a function in Cd2+ retension in roots and, therefore, decrease the toxicity of Cd2+.
Keywords: cadmium, metallothionein, salt tolerance, Ziziphus jujube, ZjMT
1. Introduction
Heavy metals are essential for plant growth and development [1], however, excessive levels of essential as well as non-essential metals, such as Cadmium (Cd), are toxic to plants, causing a wide range of deleterious effects [2]. Cd2+ is a type of non-essential element and is taken up by plant roots and causes growth retardation [3]. Low concentration of Cd2+ in the rhizosphere can cause alterations in many physiological processes, including carbohydrate metabolism [4], nitrogen metabolism [5], photosynthesis [6], and therefore damage the nucleolus and membrane ATPase activity of plant cells [7]. In order to maintain metal homeostasis, plants have evolved numerous ways to mitigate detrimental effects of excessive metals ions, such as metal-chelating proteins metallothionein (MT).
The MTs are a class of low-molecular (6–7 kDa) cysteine (Cys)-rich proteins that bind heavy metals [8,9], and were first reported as a cadmium binding protein in the cortex of horse kidney [10]. This protein not only has effects on detoxification of heavy metals like cadmium and mercury [11], regulation of the homeostasis of essential metals including zinc and copper [12,13], but also has functions like protecting reactive oxygen species [14,15] and DNA damage [16], in animals, plants and microorganisms. A large number of cysteine residues in MTs are able to bind a variety of metals by the formation of mercaptide bonds [17]. Based on the distribution of Cys residues in their N- and C-terminal regions, plant MTs have been classified into four types, MT1, MT2, MT3 and MT4 [18,19]. Each type of MT exhibits a distinct spatial and temporal expression pattern in plant tissues during development and possibly has different functions. Type 1 MT genes are predominantly expressed in both leaves and roots, whereas type 2 MT genes are expressed in primarily in leaves, stems, and developing seed [20,21,22,23]. Type 3 MT genes are expressed in leaves or in ripening fruits [24], and the expression of type 4 MT genes are reported not only in seed, but also detected in reproductive organs and vegetative tissues [25,26]. The genes encoding the MTs have been identified and cloned from many plant species, including Arabidopsis [21], wheat [27], soybean [28], rice [29] and tomato [30], and increasing evidence suggests that plant MTs are also play an important role in physiological processes, including fruit ripening [31], root development, embryo germination [32], suberization [33] and response to multiple abiotic stresses [34]. Previous studies showed that, type 1 MT was required for Cd2+ and Cu2+ tolerance and accumulation [35,36], maintaining Zn2+ homeostasis, confer the adaptability of plant to drought stress and scavenging reactive oxidant species (ROS) [14,37].
Chinese jujube is a unique and economically important fruit tree, and has a long cultivation history in China. Moreover, it is well known for its high tolerance to stresses, such as cold, drought and high salinity, although the mechanisms underlying such stresses are still unknown. In this study, ZjMT, encoding a type I metallothionein, was cloned from Chinese jujube (Ziziphus jujuba Mill) full-length cDNA libraries, and expression pattern of ZjMT was identified in response to NaCl, CdCl2 and PEG treatments. In order to examine the function of ZjMT, an expression vector carrying the ZjMT gene driven by the cauliflower mosaic virus 35S (CaMV 35S) promoter was introduced into Arabidopsis thaliana genomes by the Agrobacterium-mediated transformation method. Transgenic plants showed tolerance to NaCl and CdCl2 stresses, and the Cd2+ was accumulated in roots and showed decreased accumulation in leaves.
2. Results
2.1. ZjMT Encodes a Protein with a Metallothionein (MT) Domain
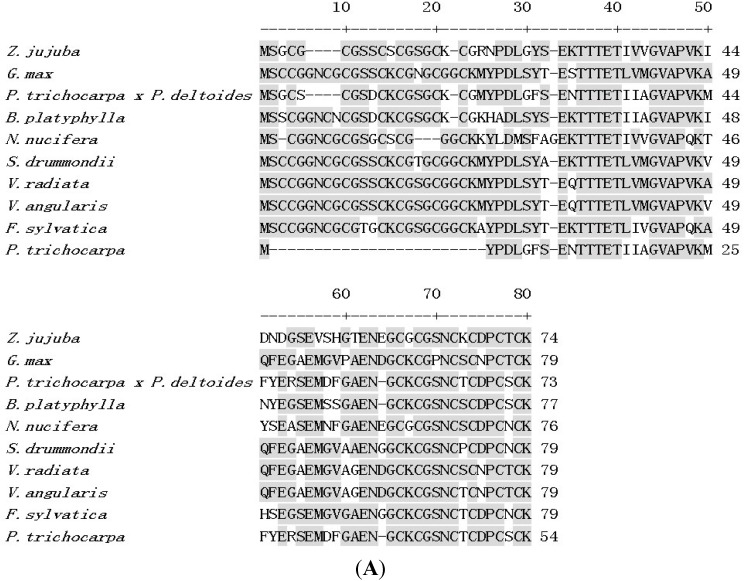
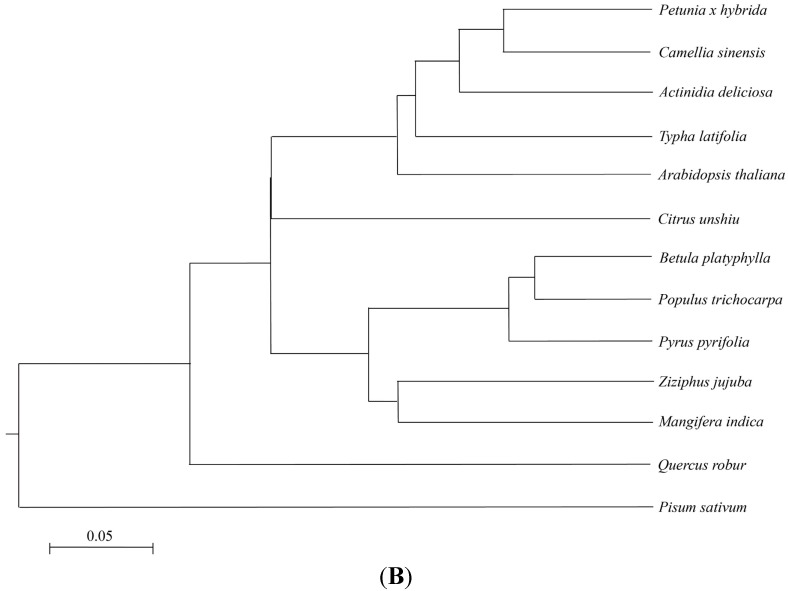
ZjMT (GenBank No. AB513130) was obtained by screening jujube full-length cDNA libraries. The ZjMT cDNA is 225 bp in length and encodes a polypeptide of 74 amino acid residues and with a predicted molecular mass of 7.376 kDa. The deduced amino acid sequence analysis indicated that ZjMT contains highly conserved cysteine-rich domains in its N- and C-terminal respectively and a cysteine-free region between them, which was the common feature of the Type 1 MT proteins reported in other plants. With the BLASTN search from the NCBI database, the deduced amino acid sequence showed homology with counterpart Type I MT family members from other plant species (Figure 1A). Phylogenetic analysis revealed that ZjMT was clustered in the same clade with Mangifera indica, but distinct from Pisum sativum (Figure 1B). The proteins used in the alignment and phylogenetic tree all had an MT domain and were obtained by database searching in NCBI.
Figure 1.
Multiple alignment of ZjMT and phylogenetic analysis. (A) Multiple alignments of MT proteins from selected species. Identical amino acid residues are highlighted in gray; (B) Phylogenetic analysis of MT domains from different species. All of the proteins used in the phylogenetic tree came from database of NCBI. The corresponding accession numbers of the names are as follows: Petunia x hybrida (AAG36945.1), Camellia deliciosa (ABD97257.1), Actinidia deliciosa (P43390.1), Typha latifolia (AAK28022.1), Arabidopsis thaliana (CAA44630.1), Citrus unshiu (BAA31561.1), Betula platyphlla (AAY166439.1), Populus trichocarpa (EEF07605.1), Pyrus pyrifolia (BAA96449.1), Ziziphus jujuba (AB513130), Mangifera indica (ACD69680.1), Quercus robur (CAE12162.1), and Pisum sativum (P20830.1).
2.2. ZjMT Is a Potential Stress-Related Gene
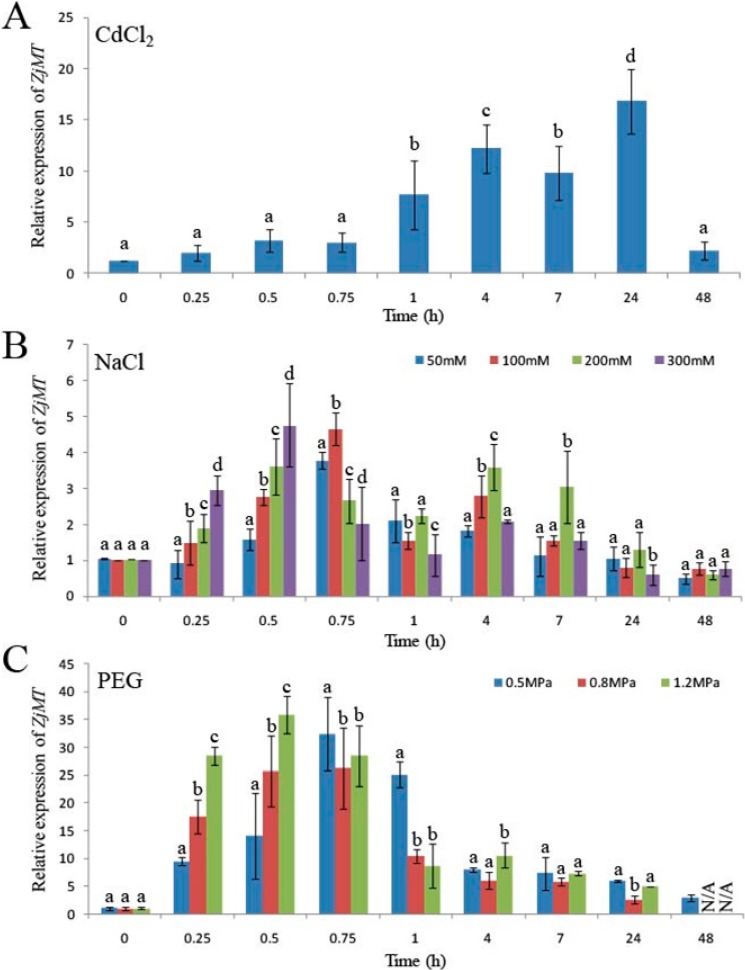
To identify whether ZjMT could be induced by heavy metal or other abiotic stresses, the expression profiles of ZjMT in Z. jujuba young seedlings under CdCl2, NaCl and PEG treatments were investigated using quantitative RT-PCR. ZjMT expression was significantly activated by CdCl2, NaCl and PEG stresses. The transcripts level of ZjMT increased at 0.25 h after CdCl2 treatment, reached a peak at 24 h, and then declined at 48 h (Figure 2A). ZjMT transcript level reached a peak at 0.75 h when the young seedlings were under 50 and 100 mM NaCl treatments, however at 0.5 h, it reached the peak under 200 and 300 mM NaCl treatments (Figure 2B). Similarly, the ZjMT transcript level reached a peak at 0.25 and 0.75 h under 1.2 MPa PEG treatments and 0.5 and 0.8 MPa PEG treatments, respectively (Figure 2C).
Figure 2.
The expression patterns of ZjMT. The relative expression levels of ZjMT gene in leaves under CdCl2 (A), NaCl (B) and PEG (C) stress were measured using qRT-PCR. Six-week-old Z. jujuba young seedlings were treated with 100 mM CdCl2, 50, 100, 200 and 300 mM NaCl, and 10% PEG 6000 under different conditions at indicated time points. Different letters (a–d) indicate statistically significant differences between means at p < 0.05 (Student’s t-test). N/A: Not applicable. Standard errors were calculated from three biological replicates in which ZjH3 (an actin gene, accession number EU916201) transcripts were used as internal controls. The 2−ΔΔCt method was used to measure the relative expression levels of the target gene in stressed and non-stressed leaves. Error bars represent standard error.
2.3. Subcelluar Localization of ZjMT
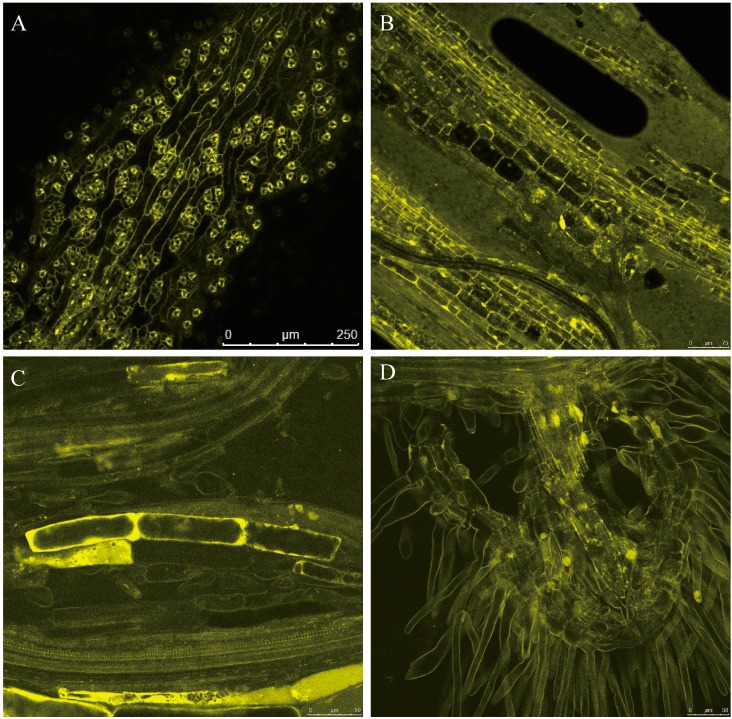
To investigate the localization of ZjMT, the 35S:ZjMT-YFP plasmid was constructed and transformed into Arabidopsis by the floral dipping method. Homozygous transgenic lines were used for localization analysis. Firstly, we analyzed the localization of the ZjMT-YFP fusion protein in epidermal cells; it was primarily localized in the cytoplasm of the stomata guard cells (Figure 3A). In addition, the fluorescence could also be detected in the cytoplasm and nucleus in stem and roots, respectively (Figure 3B–D).
Figure 3.
ZjMT is localized to cytoplasm and nucleus. ZjMT-YFP fusion proteins were constitutively expressed under control of the CaMV 35S promoter in Arabidopsis and observed with a laser scanning confocal microscope. Subcellular localization of ZjMT in Arabidopsis leaf epidermal cells (A); stem (B); roots (C) and root hairs (D). Scale bar = 250 μm (A), 75 μm (B), 50 μm (C,D).
2.4. Constitutive Expression of ZjMT in Arabidopsis Enhances Their High Salinity Salt Tolerance
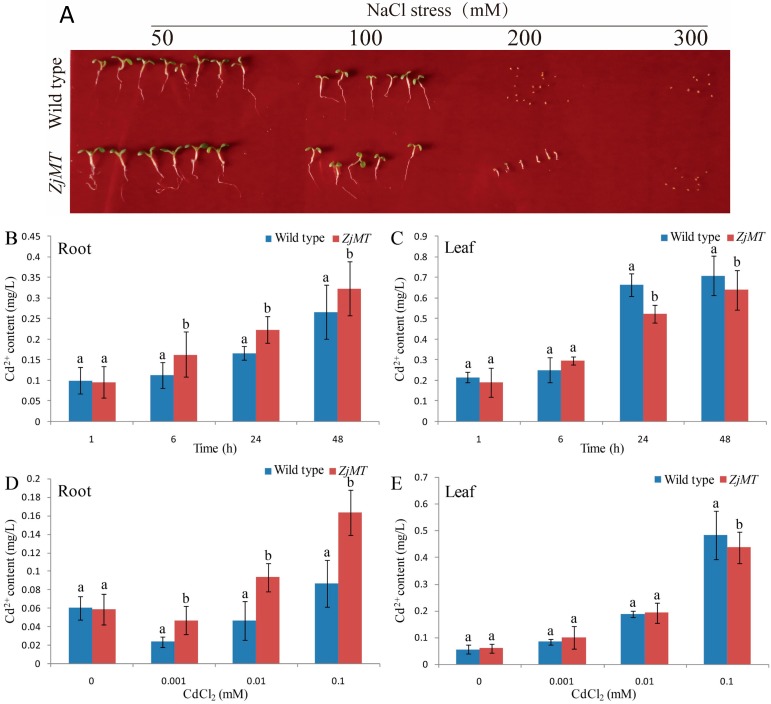
We examined the role of ZjMT in NaCl stress responses, under normal conditions, no significant difference was observed between transgenic and wild type plants (data not shown). Although the cotyledon greening rate was similar between transgenic and wild type plants, the radicle emergence of transgenic seedlings was slightly higher than wild type in the presence of 50 mM NaCl medium. Furthermore, the radicle emergence of transgenic seedlings increased significantly compared to those of the wild type plants on the medium containing 100 mM NaCl, and the wild type seedlings were failed to develop radicle on the medium containing 200 mM NaCl. The wild type and transgenic seeds were both failed to germinate when the NaCl concentration reach to 300 mM (Figure 4). These results indicate that constitutive expression of ZjMT leads to enhanced tolerance in transgenic seedlings under salt stress and that ZjMT might act as a positive regulator to salt stress.
Figure 4.
High salinity assays of ZjMT transgenic plants and the concentrations of Cd2+ in roots and leaves of wild type and transgenic Arabidopsis plants. (A) Phenotypic comparison of root lengths. Wild type and transgenic seeds were germinated and grown on MS medium with 50, 100, 200 or 300 mM NaCl for 7 days. The concentrations of Cd2+ in the roots (B) and leaves (C) of wild type and transgenic plants exposed to 0.1 mM CdCl2 with indicated time points. Cd2+ content in the roots (D) and leaves (E) of wild type and transgenic plants treated with various concentrations of CdCl2 for 24 h. Different letters in (B–E) indicate statistically significant differences between means at p < 0.05 (Student’s t-test).
2.5. Cd2+ Accumulation and Distribution in Transgenic Plants
To explore whether constitutive expression of ZjMT influences endogenous Cd2+ content, the concentrations of Cd2+ in transgenic plants and wild type plants were measured. Although the concentration of Cd2+ in leaves of transgenic plants and wild type plants gradually increased after CdCl2 treatment, the transgenic plant accumulated less Cd2+ in leaves compared to wild type (Figure 4C). However, the root Cd2+ concentrations for transgenic plants were higher than those of WT plants (Figure 4B). In addition, similar results were observed after various concentrations of CdCl2 treatment (Figure 4D,E).
3. Discussion
Heavy-metal contamination is a great environmental concern globally, and the risk posed to humans is increasing. Metallothioneins (MTs) are Cys-rich proteins, which are involved in the metal tolerance of diverse living organisms. Although many studies have revealed the roles of MTs in plants in response to diverse metal stresses, the function of plant MTs remain poorly understood [38].
In this study, the ZjMT cDNA was cloned from Ziziphus jujube full-length cDNA libraries and determined as type-I MT based on the protein sequence alignment. Phylogenetic analysis also revealed that ZjMT shared high similarity of cysteine residue levels with other species (Figure 1). Previous research results of MT subcellular location showed that BjMT2 was localized in the cytoplasm of tobacco leaf cells, and AtMT4a and AtMT4b were both localized in cytoplasm, nucleus and membrane of Arabidopsis hypocotyls cells [39,40]. In our work, we found that the ZjMT was located in cytoplasm and nucleus (Figure 3). Microarray analysis indicated that MT transcripts were significantly up-regulated under salt and drought conditions in rice and barley [41,42]. In our study, the expression of ZjMT was generally induced after CdCl2 stress (Figure 2A), and the transcripts levels were also influenced by NaCl and PEG treatments (Figure 2B,C). To gain additional insight into the function of ZjMT in these stress responses, we evaluated the effect of salt stress on the growth of transgenic seedlings. On MS medium supplemented with NaCl, the wild type seedlings grew slowly, and failed to germination on the medium containing 200 mM NaCl, while the transgenic seedlings still develop radicles (Figure 4A). In addition, under CdCl2 stress, the transgenic plants exhibit increased accumulation of Cd2+ in roots and decreased the accumulation in leaves, whereas the accumulation of Cd2+ were increased both in roots and leaves in wild type plants (Figure 4B–E). In previous study, Cd2+ is taken up by plant roots and caused growth retardation [4], however further studies are needed to answer the underlying mechanisms of Cd2+ accumulation in different tissues. Furthermore, overexpression of plant MT genes increased Cd2+, Cu2+ and Zn2+ accumulation in transgenic plants [43], and type-I MT genes were more abundantly expressed in roots [20], according to these results we propose that ZjMT likely has a function in retention of Cd2+ in roots and decreased the Cd2+ toxic to leaves.
4. Experimental Section
4.1. Stress Treatments and Real-Time Polymerase Chain Reaction (PCR) Analyses
Z. jujuba Mill “Hupingzao” was used in this study and its seeds were germinated and grown in a greenhouse under controlled conditions: temperature at 25 ± 1 °C, a relative humidity of 65%–70%, and light density of ~2500 Lux at 12:12 h dark/light circle. For CdCl2 treatment, the six-week-old seedlings were transferred to ½ MS liquid medium (pH = 6.0) containing 100 mM CdCl2. For NaCl treatment, the seedlings were transferred to ½ MS liquid medium containing 50, 100, 200 or 300 mM NaCl. For PEG treatment, seedlings were transferred to ½ MS liquid medium containing 20% PEG (molecular weight 6000) under 0.5, 0.8, or 1.2 MPa [44]. The leaves of seedlings were harvested at indicated time points, and were snap-frozen in liquid nitrogen and stored at −80 °C before RNA isolation. ZjH3, an actin gene (accession number EU916201) transcripts were used as internal controls. The relative level of gene expression was detected using the 2−ΔΔCt method.
4.2. Sequence Analysis of ZjMT
The conserved domains of MT from Z. jujube, G. max, P. trichocarpa x P. deltoids, B. platyphylla, N. nucifera, Sdrummondii, V. radiate, V. angularis, F. sylvatica and P. trichocarpa were aligned using the ClustalX program (version 1.83) with default parameters. The phylogenetic tree was constructed using the neighbor-joining (NJ) method in MEGA (version 5.05) [45]. Bootstrap analysis was performed using 1000 replicates in MEGA to evaluate the reliability of different phylogenetic groups.
4.3. Subcellular Localization
The open reading frame (ORF) of ZjMT was cloned into pEarleyGate-103 vector [46], which contained the yellow fluorescent protein (YFP) reporter gene, to generate a ZjMT-YFP fusion construct under the control of the CaMV 35S promoter. Arabidopsis plants were transformed by Agrobacterium-mediated floral dip. YFP fluorescence was observed under a confocal laser scanning system (Nikon, Tokyo, Japan), and examined at 514 nm (excitation) using an argon laser with an emission band of 515–530 nm.
4.4. Generation of Transgenic Plants
ZjMT cDNA was cloned into the vector under the control of CaMV 35S promoter. The construct was introduced into Agrobacterium tumefaciens GV3101 strain cells and then transferred into wild type Arabidopsis (ecotype Columbia) plants by floral infiltration [47]. The seeds of T0 generation were harvested and sown in soil, and 10-day-old seedlings of T1 plants were screened by spraying with 0.05% (v/v) phosphinothricin (ppt) solution. The survival transformants (T1) were confirmed by PCR amplification of ZjMT. The T2 seeds were planted on MS [48] agar medium containing 10 mg/L ppt and the transgenic lines with a 3:1 (resistant:sensitive) segregation ratio were selected to produce T3 seeds. The T3 lines displaying 100% ppt resistance were considered homozygous and used for further experiments.
4.5. Stress Treatments
For salt assays on plates, wild type and transgenic lines were planted on MS agar medium with various concentrations of NaCl for three days before being placed at a controlled environment. After 10 days, the phenotypes of the plants were examined and pictures were taken.
For CdCl2 experiments, two-week-old transgenic and wild type plants were planted in MS medium with various concentrations of CdCl2 for 24 h and then measured the Cd2+ content of roots and leaves.
4.6. Measurement of Cd2+ Content
For measurement of Cd2+ content in plant tissues, the seedlings of Arabidopsis wild type and transgenic plants were planted. After 14 days, seedlings were treated with 0.1 mM CdCl2 for 1, 6, 24, and 48 h. Roots and rosette leaves were excised carefully to determine their Cd2+ content. After 24 h at 105 °C, the dry weight was measured. The resulting dry matter was dissolved in nitric and perchloric acid (4:1) on a muffle furnace at 175 °C for 3 h. When the liquid became limpid, the Cd2+ content of the samples was determined with an atomic absorption spectrophotometer.
5. Conclusions
In conclusion, overexpression of ZjMT in Arabidopsis positively has a function in retention of Cd2+ in roots and decreased the Cd2+ toxic to leaves and enhances the salt tolerance of Arabidopsis. Therefore, ZjMT can be used as a candidate gene to improve stress tolerance by genetic transformation in crops.
Acknowledgments
The study was funded by a part of the Basic Key Projects of National Science and Technology Program (2012 FY110100-5), the International Science and Technology Collaborative Project of Shanxi Province (2012081010) and the National Natural Science Foundation of China (41271531) and Research project supported by Shanxi Scholarship Council of China (2009-9-84).
Author Contributions
Qiufen Cao and Mingchang Chen conceived and designed the experiments; Mingxia Yang and Fan Wang performed the experiments; Fan Zhang and Zhigang Dong analyzed the data; Fan Zhang and Zhigang Dong wrote the manuscript. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hall J. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama M. Role of cellular antioxidants in metal-induced damage. Cell Biol. Toxicol. 1994;10:1–22. doi: 10.1007/BF00757183. [DOI] [PubMed] [Google Scholar]
- 3.Benavides M.P., Gallego S.M., Tomaro M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005;17:21–34. doi: 10.1590/S1677-04202005000100003. [DOI] [Google Scholar]
- 4.Trvedi S., Erdei L. Effects of cadmium and lead on the accumulation of Ca2+ and K+ and on the influx and translocation of K+ in wheat of low and high K+ status. Physiol. Plant. 1992;84:94–100. doi: 10.1111/j.1399-3054.1992.tb08770.x. [DOI] [Google Scholar]
- 5.Hernandez L., Garate A., Carpena-Ruiz R. Effects of cadmium on the uptake, distribution and assimilation of nitrate in Pisum sativum. Plant Soil. 1997;189:97–106. doi: 10.1023/A:1004252816355. [DOI] [Google Scholar]
- 6.Krupa Z., Öquist G., Huner N. The effects of cadmium on photosynthesis of Phaseolus vulgaris—A fluorescence analysis. Physiol. Plant. 1993;88:626–630. doi: 10.1111/j.1399-3054.1993.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 7.Fodor E., Szabó-Nagy A., Erdei L. The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J. Plant Physiol. 1995;147:87–92. doi: 10.1016/S0176-1617(11)81418-5. [DOI] [Google Scholar]
- 8.Kägi J.H., Vallee B.L. Metallothionein: A cadmium-and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960;235:3460–3465. [PubMed] [Google Scholar]
- 9.Kojima Y., Berger C., Vallee B.L., Kägi J. Amino-acid sequence of equine renal metallothionein-1B. Proc. Natl. Acad. Sci. USA. 1976;73:3413–3417. doi: 10.1073/pnas.73.10.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margoshes M., Vallee B.L. A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 1957;79:4813–4814. doi: 10.1021/ja01574a064. [DOI] [Google Scholar]
- 11.Loebus J., Leitenmaier B., Meissner D., Braha B., Krauss G.J., Dobritzsch D., Freisinger E. The major function of a metallothionein from the aquatic fungus Heliscus lugdunensis is cadmium detoxification. J. Inorg. Biochem. 2013;127:253–260. doi: 10.1016/j.jinorgbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Dąbrowska G. Plant metallothioneins: Putative functions identified by promoter analysis in silico. Acta Biol. Cracov. Bot. 2012;54:109–120. doi: 10.2478/v10182-012-0031-x. [DOI] [Google Scholar]
- 13.Zhou B., Yao W., Wang S., Wang X., Jiang T. The metallothionein gene, TaMT3, from Tamarix androssowii confers Cd2+ tolerance in tobacco. Int. J. Mol. Sci. 2014;15:10398–10409. doi: 10.3390/ijms150610398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv Y., Deng X., Quan L., Xia Y., Shen Z. Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to cadmium and copper and decrease production of reactive oxygen species in Arabidopsis thaliana. Plant Soil. 2013;367:507–519. doi: 10.1007/s11104-012-1486-y. [DOI] [Google Scholar]
- 15.Hassinen V., Tervahauta A., Schat H., Kärenlampi S. Plant metallothioneins-metal chelators with ROS scavenging activity? Plant Biol. 2011;13:225–232. doi: 10.1111/j.1438-8677.2010.00398.x. [DOI] [PubMed] [Google Scholar]
- 16.Higashimoto M., Isoyama N., Ishibashi S., Inoue M., Takiguchi M., Suzuki S., Ohnishi Y., Sato M. Tissue-dependent preventive effect of metallothionein against DNA damage in dyslipidemic mice under repeated stresses of fasting or restraint. Life Sci. 2009;84:569–575. doi: 10.1016/j.lfs.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 18.Robinson N.J., Tommey A.M., Kuske C., Jackson P.J. Plant metallothioneins. Biochem. J. 1993;295:1. doi: 10.1042/bj2950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capdevila M., Atrian S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011;16:977–989. doi: 10.1007/s00775-011-0798-3. [DOI] [PubMed] [Google Scholar]
- 20.Guo W.J., Bundithya W., Goldsbrough P.B. Characterization of the Arabidopsis metallothionein gene family: Tissue-specific expression and induction during senescence and in response to copper. New Phytol. 2003;159:369–381. doi: 10.1046/j.1469-8137.2003.00813.x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy A., Zhou J., Goldsbrough P.B., Taiz L. Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiol. 1997;113:1293–1301. doi: 10.1104/pp.113.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J., Goldsbrough P.B. Structure, organization and expression of the metallothionein gene family in Arabidopsis. Mol. Gen. Genet. 1995;248:318–328. doi: 10.1007/BF02191599. [DOI] [PubMed] [Google Scholar]
- 23.Waters D.L., Holton T.A., Ablett E.M., Lee L.S., Henry R.J. cDNA microarray analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin. Funct. Integr. Genomic. 2005;5:40–58. doi: 10.1007/s10142-004-0124-z. [DOI] [PubMed] [Google Scholar]
- 24.Ledger S.E., Gardner R.C. Cloning and characterization of five cDNAs for genes differentially expressed during fruit development of kiwifruit (Actinidia deliciosa var. deliciosa) Plant Mol. Biol. 1994;25:877–886. doi: 10.1007/BF00028882. [DOI] [PubMed] [Google Scholar]
- 25.Chyan C.L., Lee T.T., Liu C.P., Yang Y.C., Tzen J.T., Chou W.M. Cloning and expression of a seed-specific metallothionein-like protein from sesame. Biosci. Biotechnol. Biochem. 2005;69:2319–2325. doi: 10.1271/bbb.69.2319. [DOI] [PubMed] [Google Scholar]
- 26.Leszczyszyn O.I., Imam H.T., Blindauer C.A. Diversity and distribution of plant metallothioneins: A review of structure, properties and functions. Metallomics. 2013;5:1146–1169. doi: 10.1039/c3mt00072a. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima I., Kennedy T.D., Chino M., Lane B.G. Wheat Ec metallothionein genes: Like mammalian Zn2+ metallothionein genes are conspicuously expressed during embryogenesis. Eur. J. Biochem. 1992;209:971–976. doi: 10.1111/j.1432-1033.1992.tb17370.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima I., Inokuchi Y., Chino M., Kimura M., Shimizu N. Isolation of a gene for a metallothionein-like protein from soybean. Plant Cell Physiol. 1991;32:913–916. [Google Scholar]
- 29.Hsieh H.-M., Liu W.-K., Huang P. A novel stress-inducible metallothionein-like gene from rice. Plant Mol. Biol. 1995;28:381–389. doi: 10.1007/BF00020388. [DOI] [PubMed] [Google Scholar]
- 30.Giritch A., Ganal M., Stephan U.W., Bäumlein H. Structure, expression and chromosomal localisation of the metallothionein-like gene family of tomato. Plant Mol. Biol. 1998;37:701–714. doi: 10.1023/A:1006001701919. [DOI] [PubMed] [Google Scholar]
- 31.Moyle R., Fairbairn D.J., Ripi J., Crowe M., Botella J.R. Developing pineapple fruit has a small transcriptome dominated by metallothionein. J. Exp. Bot. 2005;56:101–112. doi: 10.1093/jxb/eri015. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J., Chen D., Ren Y., Zhang X., Zhao J. Characteristic and expression analysis of a metallothionein gene, OsMT2b, down-regulated by cytokinin suggests functions in root development and seed embryo germination of rice. Plant Physiol. 2008;146:1637–1650. doi: 10.1104/pp.107.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mir G., Domènech J., Huguet G., Guo W.J., Goldsbrough P., Atrian S., Molinas M. A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. J. Exp. Bot. 2004;55:2483–2493. doi: 10.1093/jxb/erh254. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Chen Y.Y., Yang S.G., Tian W.M. Cloning and characterization of HbMT2a, a metallothionein gene from Hevea brasiliensis Muell. Arg differently responds to abiotic stress and heavy metals. Biochem. Biophys. Res. Commun. 2015;461:95–101. doi: 10.1016/j.bbrc.2015.03.175. [DOI] [PubMed] [Google Scholar]
- 35.Zimeri A.M., Dhankher O.P., McCaig B., Meagher R.B. The plant MT1 metallothioneins are stabilized by binding cadmiums and are required for cadmium tolerance and accumulation. Plant Mol. Biol. 2005;58:839–855. doi: 10.1007/s11103-005-8268-3. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y., Lv Y., Yuan Y., Wang G., Chen Y., Zhang H., Shen Z. Cloning and characterization of a type 1 metallothionein gene from the copper-tolerant plant Elsholtzia haichowensis. Acta Physiol. Plant. 2012;34:1819–1826. doi: 10.1007/s11738-012-0980-4. [DOI] [Google Scholar]
- 37.Yang Z., Wu Y., Li Y., Ling H.Q., Chu C. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol. Biol. 2009;70:219–229. doi: 10.1007/s11103-009-9466-1. [DOI] [PubMed] [Google Scholar]
- 38.Grennan A.K. Metallothioneins, a diverse protein family. Plant Physiol. 2011;155:1750–1751. doi: 10.1104/pp.111.900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An Z., Cuijie L., Yuangang Z., Yejie D., Wachter A., Gromes R., Rausch T. Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J. Exp. Bot. 2006;57:3575–3582. doi: 10.1093/jxb/erl102. [DOI] [PubMed] [Google Scholar]
- 40.Ren Y., Liu Y., Chen H., Li G., Zhang X., Zhao J. Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in Arabidopsis. Plant Cell Environ. 2012;35:770–789. doi: 10.1111/j.1365-3040.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki S., Borchert C., Deyholos M., Wang H., Brazille S., Kawai K., Galbraith D., Bohnert H.J. Gene expression profiles during the initial phase of salt stress in rice. Plant Cell. 2001;13:889–905. doi: 10.1105/tpc.13.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozturk Z.N., Talamé V., Deyholos M., Michalowski C.B., Galbraith D.W., Gozukirmizi N., Tuberosa R., Bohnert H.J. Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol. Biol. 2002;48:551–573. doi: 10.1023/A:1014875215580. [DOI] [PubMed] [Google Scholar]
- 43.Sekhar K., Priyanka B., Reddy V., Rao K. Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan, L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana. Environ. Exp. Bot. 2011;72:131–139. doi: 10.1016/j.envexpbot.2011.02.017. [DOI] [Google Scholar]
- 44.Zhao J., Sun Z., Zheng J., Guo X., Dong Z., Huai J., Gou M., He J., Jin Y., Wang J. Cloning and characterization of a novel CBL-interacting protein kinase from maize. Plant Mol. Biol. 2009;69:661–674. doi: 10.1007/s11103-008-9445-y. [DOI] [PubMed] [Google Scholar]
- 45.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 46.Earley K.W., Haag J.R., Pontes O., Opper K., Juehne T., Song K., Pikaard C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 47.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 48.Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]