Abstract
Previous studies in our laboratory found that the extract from seeds of Amorpha fruticosa in the Leguminosae family had lethal effects against mosquito larvae, and an insecticidal compound amorphigenin was isolated. In this study, the inhibitory effects of amorphigenin against the mitochondrial complex I of Culex pipiens pallens (Diptera: Culicidae) were investigated and compared with that of rotenone. The results showed that amorphigenin and rotenone can decrease the mitochondrial complex I activity both in vivo and in vitro as the in vivo IC50 values (the inhibitor concentrations leading to 50% of the enzyme activity lost) were determined to be 2.4329 and 2.5232 μmol/L, respectively, while the in vitro IC50 values were 2.8592 and 3.1375 μmol/L, respectively. Both amorphigenin and rotenone were shown to be reversible and mixed-I type inhibitors of the mitochondrial complex I of Cx. pipiens pallens, indicating that amorphigenin and rotenone inhibited the enzyme activity not only by binding with the free enzyme but also with the enzyme-substrate complex, and the values of KI and KIS for amorphigenin were determined to be 20.58 and 87.55 μM, respectively, while the values for rotenone were 14.04 and 69.23 μM, respectively.
Keywords: Culex pipiens pallens, amorphigenin, mitochondrial complex I, inhibitory mechanism, kinetics
1. Introduction
Culex pipiens pallens Coquillett, 1998, is the most common mosquito in houses of Northern China, Korea and Japan, and is the primary vector of filariasis, epidemic encephalitis B [1] and a potential vector of the West Nile virus [2]. Control of Cx. pipiens pallens populations in China has been provided principally by the use of various contact and residual insecticides since the 1950s [3]. However, 50 years of sustained struggle against harmful mosquitos using synthetic and oil-derivative molecules has produced pervasive secondary effects such as mammalian toxicity, mosquito population resistance to organochlorine, organophosphate, carbamate and pyrethroid insecticides [4,5,6,7,8], and ecological hazards. Hence, much attention must be taken to develop alternatives to chemical insecticides for mosquito control.
Among the alternative strategies, the use of plants, insecticidal phytochemicals appears to be promising. More than 2000 plant species have been known to produce chemical factors and metabolites of value in pest control programmes [9]. In recent years, many studies on plant extracts and new active molecules to combat mosquito larvae have been conducted around the world, and some novel mosquito larvicidal compounds have been isolated and identified [10]. Those plant materials and isolated compounds are known to possess biological activity such as insecticidal activity, repellency, reproduction retardation, and insect growth regulation for instance against various mosquito species, and have received considerable attention in the search for new biopesticides as potential mosquitocides, as summaried by Kishore et al. [11]. Although numerous reports are available concerning the larvicidal potential of the plant secondary metabolites, the mode of action of botanicals insecticides is still uncertain and most of them are under investigation for their insecticidal mechanisms. Among botanicals tested against mosquitos are the following: the essential oils of Lippia turbinate and Lippia polystachya (Family: Leguminosae) at doses ranging from sublethal to lethal (20, 40 and 80 ppm) modify the temporal pattern of locomotion of Culex quinquefasciatus (Diptera: Culicidae) larvae [12]. Aedes aegypti (Diptera: Culicidae) organophosphate susceptible and resistant larvae were distinctly affected by lectins WSMoL and cMoL from the seeds of Moringa oleifera (Family: Moringaceae). The determination results of digestive (amylase, trypsin, and protease) and detoxifying (superoxide dismutase (SOD), α- and β-esterases) enzymes indicated that the larvicidal mechanism of WSMoL may involve the deregulation of digestive enzymes, while cMoL interfered mainly on SOD activity [13]. Furthermore, the trypsin inhibitor MoFTI from M. oleifera flower extract interfered with the survival and development of A. aegypti larvae and killed bacteria inhabitant of larvae midgut [14]. Four purified flavones, one flavanone and a diterpenoid isolated from Andrographis paniculata Nees (Family: Acanthaceae) exhibited an inhibitory effect on the cytochrome P450 monooxygenases CYP6AA3 and CYP6P7 of Anopheles minimus (Diptera: Culicidae) [15]. The crude extract of Agave sisalana of the Agavaceae family can cause cell lysis and destruction of the peritrophic membrane, reduce the concentration of NO in the hemolymph from A. aegypti larvae [16]. Acetylcholinesterse, β-carboxylesterase and acid phosphatases activity were significantly reduced in A. aegypti larvae exposed to the aqueous kernel extract of soapnut Sapindus emarginatus belongs to the family Sapindaceae [17]. Two constituents of the Alaskan yellow cedar tree, the monoterpenoid carvacrol and the sesquiterpenoid nootkatone, are both toxic against several arthropods. Carvacrol was observed to cause slight inhibition of the acetylcholinesterase enzyme in house flies, ticks and cockroaches, but it did not inhibit the mosquito acetylcholinesterase enzyme. Nootkatone did not inhibit the acetylcholinesterase enzyme in any of the four arthropod models tested [18]. Thus, mode of action and site of effect for larvicidal phytochemicals and extracts has received little attention [10].
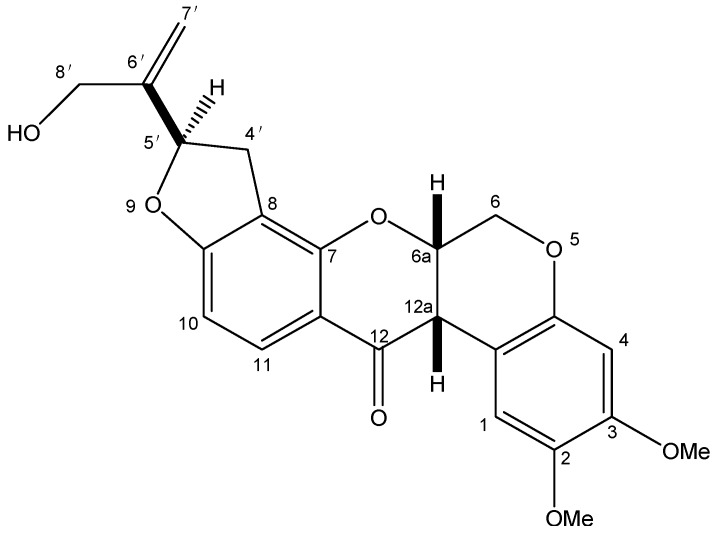
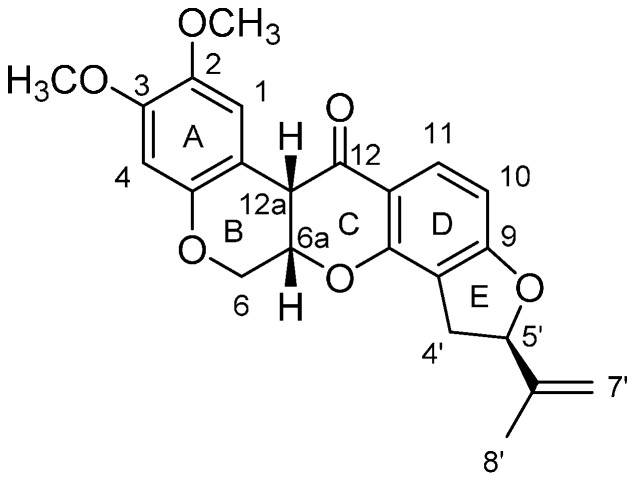
Amorphigenin (Figure 1), an aglycone of the rotenoid glycoside amorphin [19,20], has been isolated from the leaves, seeds and seedlings of Amorpha fruticosa [21,22] and has been shown to have significant anti-proliferative [23], anti-cancer (in many cell types) [24,25], hepatoprotective [26] and neuraminidase inhibition [27] activities. As for insecticidal activity, earlier research of the 1940s showed that extracts of A. fruticosa possessed repellent and insecticidal properties [28,29], and the acetone extract of A. fruticosa seeds to be more toxic against A. aegypti larvae than 1% pure rotenone [29]. Also, amorphigenin 8′-β-glucoside at 10 ppm was shown to lead to an 85% loss of the fourth instar larvae of A. aegypti and without the formation of pupae [30]. Our previous study [31] showed that ethanol extract from seeds of A. fruticosa had good contact effect and antifeedant activity against Schizaphis graminums (Homoptera: Aphididae). Then, amorphigenin, a rotenoid compound which exhibits a strong larvicidal activity with LC50 and LC90 values of 4.29 and 11.27 mg/L, respectively, was isolated from the ethanol extract by column chromatograpy [32]. However, up to now little is known about inhibition effect of amorphigenin on mosquito larvae. Indeed, insecticidal activity and inhibitory effect on mitochondrial respiratory complex I of rotenoids are clearly different. For example, 5′β- epirotenone, the stereoisomer of natural rotenone, was about 1000-times less inhibiting the active of mammalian NADH-ubiquinone reductase than rotenone [33]. The toxicity of 5′β- epirotenone against 3th instar larvae of Bombyx mori (Lepidoptera: Bombycidae) was about 24-times less than rotenone, and the LC50 values of two compounds were 225.70 and 9.33 mg/L, respectively [34]. In consideration of similar the chemical structure of amorphigenin and rotenone [22], we can not simply deduce whether amorphigenin possess similar inhibition mechanism with rotenone. So bioassay and enzyme kinetics study are needed.
Figure 1.
Chemical structure of amorphigenin.
The mitochondrial complex I (NADH-ubiquinone oxidoreductase, EC 1.6.5.3) catalyzes the first step of oxidative phosphorylation and, hence, it is the key for the efficient ATP production in most prokaryotic and eukaryotic cells [35]. The enzyme transfers electrons from NADH to ubiquinone which is the terminal electron acceptor. The inhibition of the complex I results in the termination of ATP production. It is well known that the mitochondrial complex I can be specifically inhibited by natural products such as piericidin, rotenone and rotenoids [36], and annonaceous acetogenins [37]. Photoaffinity labeling research showed that arylazidoamorphigenin can inhibit the bovine heart mitochondrial complex I activity at concentrations comparable with those of rotenone [38].
Although a large number of complex I inhibitors have already been reported, the inhibitory effect of these were rarely investigated. The purpose of the present work is, therefore, to carry out a bioassay and a kinetic study of the mitochondrial complex I activity inhibition of Cx. pipiens pallens mitochondria and to evaluate kinetic parameters and inhibition mechanisms of amorphigenin. In addition, these data can provide the basis for the development of novel and effective mosquito larvae control agents.
2. Results and Discussion
2.1. Results
2.1.1. In Vivo and in Vitro Inhibition of the Mitochondrial Complex I Activity in Cx. pipiens pallens
The in vivo inhibitions of the mitochondrial complex I activities by amorphigenin and rotenone were compared among the fourth-instar larvae of Cx. pipiens pallens exposed to water containing the solvent alcohol (control) and different concentrations of amorphigenin and rotenone for 24 h. Amorphigenin at 1.25, 2.5 and 5 μmol/L decreased the mitochondrial complex I activities by 1.52-, 1.78-, and 2.43-fold, respectively, compared with those of the control when NADH was used as the substrate (Table 1). Similarly, rotenone at 1.25, 2.5 and 5 μmol/L decreased the mitochondrial complex I activities by 1.45-, 1.77- and 2.41-fold, respectively.
Table 1.
The in vivo and in vitro inhibition of amorphigenin and rotenone against the mitochondrial complex I of Cx. pipiens pallens.
| Drug Delivery | Compounds | Regression Equation | IC50 (μmol/L) | R Value |
|---|---|---|---|---|
| In vivo | Amorphigenin | y = 4.0495 + 2.4616x | 2.4329 | 0.9046 |
| Rotenone | y = 3.9764 + 2.5466x | 2.5232 | 0.9152 | |
| In vitro | Amorphigenin | y = 3.0076 + 4.3668x | 2.8592 | 0.9884 |
| Rotenone | y = 3.2495 + 3.5250x | 3.1375 | 0.9982 |
Significant inhibitions of the mitochondrial complex I activities by amorphigenin and rotenone were also observed in in vitro assays. When NADH was used as the substrate, Amorphigenin at 2 μmol/L decreased the mitochondrial complex I activity by 27.38% as compared with the control whereas amorphigenin at 3 and 4 μmol/L decreased the enzyme activities by 50.00% and 70.24%, respectively. Furthermore, parallel assays using rotenone, a known the mitochondrial complex I inhibitor [39], were performed. The results showed that rotenone at 3 and 4 μmol/L significantly decreased the mitochondrial complex I activities when NADH was used as a substrate. Table 1 shows the IC50 values and linear regression equations of amorphigenin and rotenone against the mitochondrial complex I. The in vivo IC50 values of amorphigenin and rotenone were determined to be 2.4329 and 2.5232 μmol/L, respectively, while the in vitro IC50 values of amorphigenin and rotenone were 2.8592 and 3.1375 μmol/L, respectively.
2.1.2. The Effect of Amorphigenin and Rotenone on the Mitochondrial Complex I
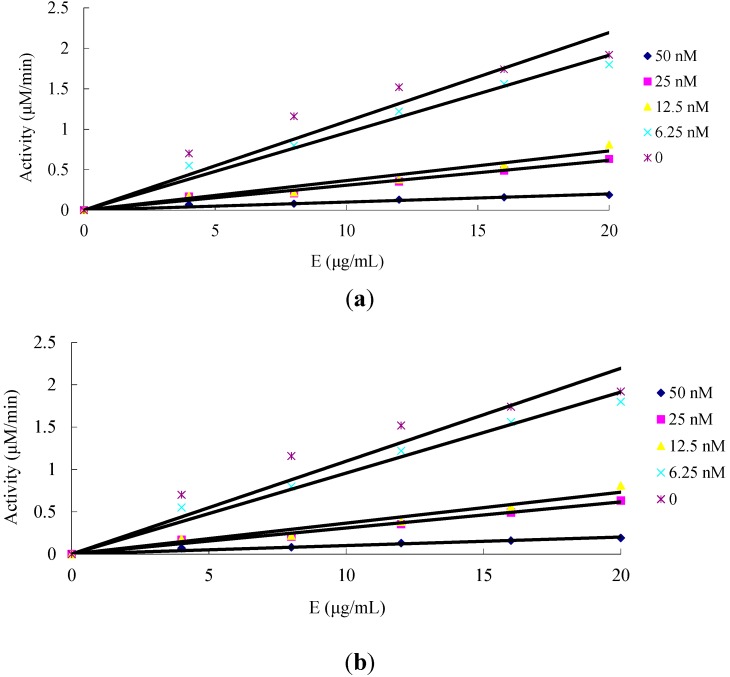
The inhibition effect on the mitochondrial complex I by amorphigenin and rotenone was also studied. Figure 2 shows the relationship between enzyme activity and enzyme concentration in the presence of different concentrations of amorphigenin and rotenone. The plots yielded straight lines passing through the origin. Increasing the inhibitor concentration resulted in decreasing the slope of the lines, thus the presence of amorphigenin and rotenone did not decrease the amount of effective enzyme, but simply inhibited and decreased the enzyme activity, indicating that inhibition of amorphigenin and rotenone on the mitochondrial complex I was reversible.
Figure 2.
The effect of mitochondrial complex I concentration on catalysis activity of β-Nicotinamide adenine dinucleotide, reduced disodium salt hydrate (NADH) at different concentrations of amorphigenin (a) and rotenone (b).
2.1.3. Inhibition Kinetics of Amorphigenin and Rotenone on the Mitochondrial Complex I Activity
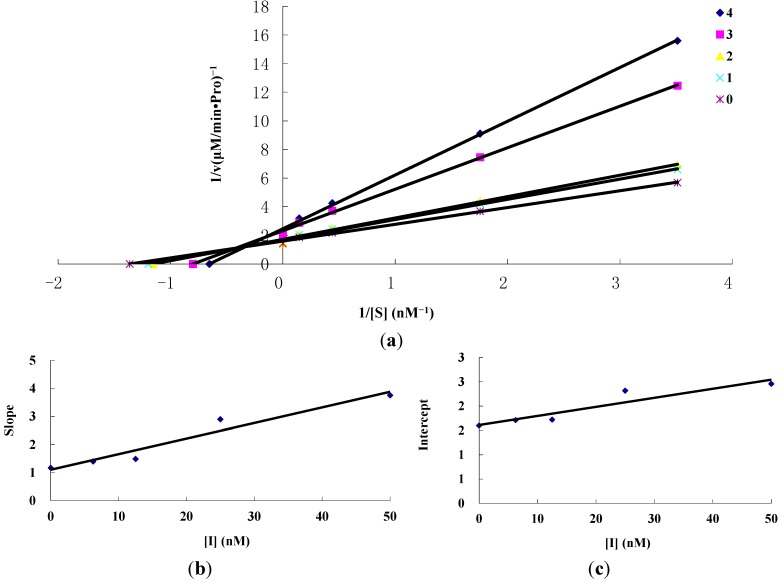
The inhibitory kinetics of the mitochondrial complex I by amorphigenin and rotenone were studied by Lineweaver–Burk plots. Figure 3 shows the double-reciprocal plots of the enzyme inhibited when using amorphigenin as the inhibitor. Under the experimental conditions employed, the oxidative reaction of NADH by the mitochondrial complex I followed Michaelis–Menten kinetics. The double-reciprocal plots yielded a family of straight lines with different slopes and intercepts but they intersected one another in the second quadrant. Thus, it was a mixed type inhibitor, indicating that amorphigenin inhibited the enzyme activity not only by binding with the free enzyme but also with the enzyme-substrate complex. Equilibrium constants for inhibitor binding with the free enzyme (KI) and with the enzyme-substrate complex (KIS) can be calculated according to the Foumulations (1) and (2) based on the slope and the vertical intercept against the concentration of inhibitors in Figure 3, respectively [40].
| (1) |
| (2) |
Figure 3.
Inhibition kinetics of amorphigenin on mitochondrial complex I by Lineweaver–Burk plots. (a) Concentrations for curves 0–4 were 0, 6.25, 12.5, 25 and 50 nM, respectively; and (b,c) represent the secondary plots of the slope an d intercept of straight line versus concentration of inhibitor, respectively.
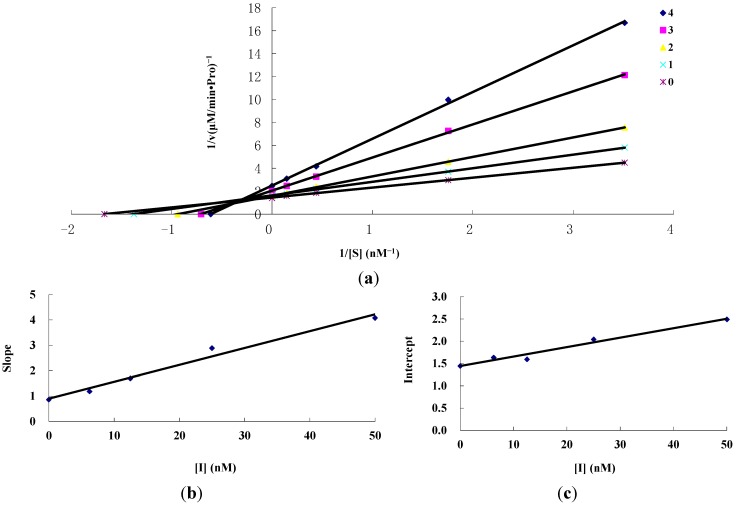
From Figure 3b,c, the values of KI and KIS for amorphigenin were determined to be 20.58 and 87.55 μM, respectively. Figure 4 shows the results for rotenone, where the inhibition behavior was found to be the same as amorphigenin. The values of KI and KIS for rotenone were determined to be 14.04 and 69.23 μM, respectively. The value of KIS was approximately four times as high as KI, indicating that the affinity of the inhibitor for the free enzyme was stronger than that for the enzyme–substrate complex.
Figure 4.
Inhibition kinetics of rotenone on mitochondrial complex I by Lineweaver–Burk plots. Concentrations of (a) for curves 0–4 were 0, 6.25, 12.5, 25 and 50 nM, respectively; Figure (b) and (c) represent the secondary plot of the slope and the intercept of the straight lines versus concentration of inhibitor, respectively.
2.2. Discussion
The mitochondrial complex I catalyzes the transfer of two electrons from NADH to ubiquinone, coupled to the translocation of four protons across the inner mitochondrial membrane. The generated electrochemical proton gradient drives energy-consuming processes such as ATP synthesis [41]. The mitochondrial complex I can be specifically inhibited by many natural products [36]. It has been reported that rotenone and rotenone reduced with NaBH4 were the most potent inhibitors of the mitochondrial complex I prepared from a pig’s heart [39] with in vitro IC50 values of 1.8 and 2.0 × 10−5 μmol/mg protein, respectively. An in vitro study by Earley et al. showed that amorphigenin and arylazidoamorphigenin were potent inhibitors of the mitochondrial complex I, comparable with rotenone [38].
Our study showed inhibition of the mitochondrial complex I of Cx. pipiens pallens larvae both in vivo and in vitro in the presence of amorphigenin and rotenone. These results showed that amorphigenin is a potent inhibitor of the mitochondrial complex I of Cx. pipiens pallens larvae with similar IC50 values to rotenone. The IC50 values obtained from the in vivo and in vitro assays can be attributed to similar chemical structures in the two compounds. Natural rotenone (Figure 5) consists of a five-ring structure (A- to E-rings) and has three chiral centers (6a-C, 12a-C and 5′a-C) [42]. Structure-function relationship studies of rotenone analogues suggest that the A-B cycles of rotenone mimic the quinone ring of ubiquinone [43], the C-D-E cycles must functionally correspond to the hydrophobic isoprenyl tail of ubiquinone [44]. The rotenone-binding site recognizes the whole molecular structure (or shape) of rotenone in a strict sense [45]. Structure-activity study of a structurally systematic set of rotenone analogues showed that the stereochemical configuration of B and C rings is a very important factor for the activity [39], the modification of the E-ring moiety can affect both the inhibitory potency and the pattern of inhibition, and the inhibitory potency increased with an increase of the hydrophobicity of the portion corresponding to the E-ring moiety [33]. Furthermore, the configuration of the isopropenyl group attached to 5′a-C atom of the E-ring is also important for the activity [43], but the π-electron system of the isopropenyl group attached to the E-ring does not contribute to the inhibitory action [33]. However, the substituents in the A-ring results in almost complete retention of activity [43]. Spectroscopy research showed that amorphigenin differed from rotenone by the presence of a hydroxy group in the substituent on the isopropenyl group [22], and further research indicated that modifications to this region of the rotenoid structure did not seem to affect the inhibitory potency of the mitochondrial respiratory chain complex [38]. Thus, the amorphigenin exhibit the same resistance risk as rotenone. Until to now, as an insecticide which has been in use for more than 150 years, only two rotenone resistant strains of Spodoptera eridania (Lepidoptera: Noctuidae) [46] and Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) [47] were occasionally found in the field. Taking into account the chemical instability, easy degradation of rotenone, which all its toxicity will lost in 2–3 days of summer sunlight [48], the use of rotenone will give little selection pressure on pests. So the potential resistance risk of rotenone [49] and analog amorphigenin was optimistic. However, further investigation is necessary to fully assess the resistance risk.
Figure 5.
Structure of natural rotenone.
To investigate the mechanism of the inhibition of the mitochondrial complex I of Cx. pipiens pallens larvae by amorphigenin in this article, we used NADH and endogenous ubiquinone as substrates to determine the complex I activity, inhibitory type and inhibition constant. The results indicated that amorphigenin and rotenone were reversible and mixed-I type inhibitors of the mitochondrial complex I of Cx. pipiens pallens. This conclusion however, conflicted with the observations of others. Indeed, several studies about the inhibitory mechanism of rotenone have provided contradictory results. The research of Crombie and Charalambous showed that rotenone was a reversible competitive inhibitor of the mitochondrial complex I from submitochondrial particles obtained from blow fly (Calliphora erythrocephala (Diptera: Calliphoridae)) flight-muscle [50]. Meanwhile, the research of Ueno [33] indicated that rotenone inhibited NADH-ubiquinone reductase of bovine heart submitochondrial particles in a noncompetitive manner against exogenous quinine DB and DPB. Alternatively, these contradictory results may have been due to the origin of the mitochondria, the membrane bounded enzyme or partially purified complex I substrate, and/or the experimental conditions [51]. In our study, amorphigenin and rotenone inhibited the enzyme activity not only by binding with the free enzyme but also with the enzyme-substrate complex, and the affinity of the inhibitor for the free enzyme was stronger than that for the enzyme-substrate complex.
3. Experimental Section
3.1. Chemicals
Amorphigenin, derived from the seeds of Amorpha fruticosa by the authors, was identified by 1H nuclear magnetic resonance. Rotenone was purchased from Guangxi Shile Agrochemical Co., Ltd. (Nanning, China). Bicinchoninic acid disodium salt hydrate (BCA), β-nicotinamide adenine dinucleotide, reduced disodium salt hydrate (NADH) and bovine serum albumin (BSA) were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). The other chemicals used in this study were of analytical grade. The deionized water used was from a Milli-Q reagent water system (Millipore, Bedford, MA, USA).
3.2. Mosquito Culture
Culex pipiens pallens was maintained in our laboratory without exposure to any insecticide, at 27 ± 2 °C with 75%–85% relative humidity and a 12:12 L/D photoperiod. The larvae of Cx. pipiens pallens were reared in a plastic basin containing a sterilized diet (40 mesh goat liver powder/yeast (2:1) in water). Adult mosquitoes were maintained on a 10% sucrose solution and blood from a mouse.
All procedures performed on animals within this study were conducted following guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
3.3. Isolation of Mitochondria
The mitochondria of the fourth instar larvae of Cx. pipiens pallens were isolated according to the method of Akbar et al. [52] with suitable modifications. The larvae were washed in a cold extraction medium (0.25 M sucrose solution containing 0.25% defatted BSA), then homogenized in a Potter–Elvehjem homogenizer under cold conditions. The homogenate was filtered through a moist muslin cloth and the filtrate centrifuged at 1000× g for 15 min at 4 °C. The residue was re-suspended in the extraction medium and centrifuged at 1000× g for 15 min at 4 °C. The supernatants from both centrifugations were combined and centrifuged at 12,000× g for 15 min at 4 °C. The mitochondrial pellet was re-suspended in the extraction medium, and stored at −80 °C until used. The protein concentration was determined by Smith’s method [53] using BSA as the standard.
3.4. Assay of the Mitochondrial Complex I Activity and in Vitro Inhibition
The mitochondrial complex I activity of Cx. pipiens pallens was determined as described by Birch-Machin et al. [54] with adaptations for a 96-well format. It is well known that the mitochondrial complex I has two substrates, NADH and ubiquinone. Rotenone and its analogue do not compete with ubiquinone [55,56,57], therefore in this study we used NADH and endogenous ubiquinone of isolated mitochondria as substrates to determine the complex I activity. The activity was measured by following the decrease in absorbance due to the oxidation of NADH at 340 nm with 425 nm as the reference wavelength. In brief, 20 μL of mitochondria and 160 μL of assay buffer containing 25 mM phosphate buffer (pH 7.2), 5 mM MgCl2, 2 mM NaN3 and 2.5 mg/mL BSA were added to each well of the 96-well plate, then the microplate was incubated for 20 min at 37 °C in a microplate reader (Spectramax 190 plate reader; Molecular Devices, Sunnyvale, CA, USA). Finally, the reaction was initiated by the addition of 10 μL of 0.13 mM NADH and measured for 5 min.
For the assay of in vivo inhibition of the mitochondrial complex I activities, first, the fourth-instar larvae of Cx. pipiens pallens were exposed to sub-lethal concentrations of amorphigenin and rotenone [32] for 24 h, then isolated the mitochondria from the survival tested mosquito larvae and determined the mitochondrial complex I activity as described above. For the assay of in vitro inhibition of the mitochondrial complex I activities, 10 μL of different concentrations each of amorphigenin and rotenone were prepared in alcohol and diluted in 25 mM phosphate buffer (pH 7.2), then mixed with 10 μL of the mitochondria. The enzyme activity was immediately determined by the method as described above. For negative controls, 10 μL of 25 mM phosphate buffer (pH 7.2) instead of amorphigenin or rotenone were used in the assays. The inhibitory extent of the compounds was expressed as the inhibitor concentrations leading to 50% of the enzyme activity reduction (IC50).
3.5. Determination of the Type and Constant of Amorphigenin and Rotenone Inhibition of the Mitochondrial Complex I Activity
Different concentrations of NADH were incubated with the mitochondria and assay buffer to examine the enzyme kinetics of NADH with and without the addition of various concentrations of rotenoids. The inhibition type of the compounds on the enzyme was assayed by Lineweaver-Burk plots. The inhibition constant was determined by plots of the apparent 1/Vm or Km/Vm versus concentration of the inhibitor [40,58].
3.6. Statistical Analysis
Applying Microsoft Excel 2003 software (Microsoft, Redmond, WA, USA), the average enzyme activitie results, adjusted by Abbott [59], were subjected to probit analysis to calculate IC50 and correlation coefficient.
4. Conclusions
Continuous and excessive application of insecticides has resulted in the rapid development of insecticide resistance in several mosquito species, including Culex pipiens pallens. Therefore, it is urgent to find alternative compounds to conquer the vector of periodic filariasis and deadly encephalitides. Many extracts and isolated compounds from plant materials are known to possess biological activity such as insecticidal activity, repellency, reproduction retardation, and insect growth regulation for instance against various mosquito species. However, the mode of action of botanicals insecticides is still uncertain and most of the plant secondary metabolites are under investigation for their insecticidal mechanisms. The present study represents a systematic research about inhibition effect, the mode of action of bioactive compounds having mosquito larvicidal activity from the seeds of A. fruticosa. This study improves knowledge of the potent effect of amorphigenin on mitochondrial complex I activity of Cx. pipiens pallens larvae.
Investigation of inhibitory effect against Cx. pipiens pallens demonstrates that amorphigenin and rotenone can decrease the mitochondrial complex I activity both in vivo and in vitro. Both amorphigenin and rotenone were shown to be reversible and mixed-I type inhibitors of the mitochondrial complex I of Cx. pipiens pallens. Our results indicate that amorphigenin is strong candidate for a natural, safe and effective phyto-larvicide to be used in population control of Cx. pipiens pallens.
Acknowledgments
The authors would like to acknowledge the anonymous reviewer for their helpful comments and suggestions, and Zhi-Guo Yu for reviewing this manuscript. This work was supported by research fund for young teacher from Shenyang agricultural university (No. 20101012).
Author Contributions
Xiuwei Li and Mingshan Ji conceived and designed the research; Xiuwei Li, Yaping Liang and Zumin Gu performed the experiments and analyzed the data; Xiuwei Li and Mingshan Ji wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interests
The authors declare no conflict of interest.
References
- 1.Li X.L., Ma L., Sun L.X., Zhu C.L. Biotic characteristics in the deltamethrin-susceptible and resistant strains of Culex pipiens pallens (Diptera: Culicidae) in China. Appl. Entomol. Zool. 2002;37:305–308. doi: 10.1303/aez.2002.305. [DOI] [Google Scholar]
- 2.Jiang S.F., Zhao T.Y., Zhang Y.M., Dong Y.D., Guo X.X. RT-PCR detection of West Nile virus in mosquitoes and leghorn chicken infected experimentally. Acta Parasitol. Med. Entomol. Sin. 2006;13:21–24. [Google Scholar]
- 3.Scott J.G., Yoshimizu M.H., Kasai S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 2015;120:68–76. doi: 10.1016/j.pestbp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Cui F., Lin L.F., Qiao C.L., Xu Y., Marquine M., Weill M., Raymond M. Insecticide resistance in Chinese populations of the Culex pipiens complex through esterase overproduction. Entomol. Exp. Appl. 2006;120:211–220. doi: 10.1111/j.1570-7458.2006.00453.x. [DOI] [Google Scholar]
- 5.Cui F., Raymond M., Berthomieu A., Alout H., Weill M., Qiao C.-L. Recent emergence of insensitive acetylcholinesterase in Chinese populations of the mosquito Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2006;43:878–883. doi: 10.1093/jmedent/43.5.878. [DOI] [PubMed] [Google Scholar]
- 6.Cui F., Tan Y., Qiao C.-L. Filariasis vector in China: Insecticide resistance and population structure of mosquito Culex pipiens complex. Pest Manag. Sci. 2007;63:453–458. doi: 10.1002/ps.1356. [DOI] [PubMed] [Google Scholar]
- 7.Kim N.J., Chang K.S., Lee W.J., Ahn Y.J. Monitoring of insecticide resistance in field-collected populations of Culex pipiens pallens (Diptera: Culicidae) J. Asia Pac. Entomol. 2007;10:257–261. doi: 10.1016/S1226-8615(08)60360-X. [DOI] [Google Scholar]
- 8.Shin E.H., Kim N.J., Kim H.K., Park C., Lee D.K., Ahn Y.J., Chang K.S. Resistance of field-collected populations of Culex pipiens pallens (Diptera: Culicidae) to insecticides in the Republic of Korea. J. Asia Pac. Entomol. 2012;15:1–4. doi: 10.1016/j.aspen.2011.07.009. [DOI] [Google Scholar]
- 9.Ghosh A., Chowdhury N., Chandra G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012;135:581–598. [PMC free article] [PubMed] [Google Scholar]
- 10.Shaalan E.A.-S., Canyon D., Younes M.W.F., Abdel-Wahab H., Mansour A.-H. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int. 2005;31:1149–1166. doi: 10.1016/j.envint.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Kishore N., Mishra B., Tiwari V., Tripathi V., Lall N. Natural products as leads to potential mosquitocides. Phytochem. Rev. 2014;13:587–627. doi: 10.1007/s11101-013-9316-2. [DOI] [Google Scholar]
- 12.Kembro J., Marin R., Zygadlo J., Gleiser R. Effects of the essential oils of Lippia turbinata and lippia polystachya (Verbenaceae) on the temporal pattern of locomotion of the mosquito Culex quinquefasciatus (Diptera: Culicidae) larvae. Parasitol. Res. 2009;104:1119–1127. doi: 10.1007/s00436-008-1296-6. [DOI] [PubMed] [Google Scholar]
- 13.Agra-Neto A., Napoleão T., Pontual E., de Lima Santos N., de Andrade Luz L., de Oliveira C., de Melo-Santos M., Coelho L., do Amaral Ferraz Navarro D., Paiva P. Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol. Res. 2014;113:175–184. doi: 10.1007/s00436-013-3640-8. [DOI] [PubMed] [Google Scholar]
- 14.Pontual E., de Lima Santos N., de Moura M., Coelho L., do Amaral Ferraz Navarro D., Napoleão T., Paiva P. Trypsin inhibitor from Moringa oleifera flowers interferes with survival and development of Aedes aegypti larvae and kills bacteria inhabitant of larvae midgut. Parasitol. Res. 2014;113:727–733. doi: 10.1007/s00436-013-3702-y. [DOI] [PubMed] [Google Scholar]
- 15.Kotewong R., Duangkaew P., Srisook E., Sarapusit S., Rongnoparut P. Structure–function relationships of inhibition of mosquito cytochrome p450 enzymes by flavonoids of Andrographis paniculata. Parasitol. Res. 2014;113:3381–3392. doi: 10.1007/s00436-014-4003-9. [DOI] [PubMed] [Google Scholar]
- 16.Nunes F., Leite J., Oliveira L.G., Sousa P.P.S., Menezes M., Moraes J.S., Mascarenhas S., Braga V. The larvicidal activity of Agave sisalana against l4 larvae of Aedes aegypti is mediated by internal necrosis and inhibition of nitric oxide production. Parasitol. Res. 2015;114:543–549. doi: 10.1007/s00436-014-4216-y. [DOI] [PubMed] [Google Scholar]
- 17.Koodalingam A., Mullainadhan P., Arumugam M. Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae) Acta Trop. 2011;118:27–36. doi: 10.1016/j.actatropica.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Anderson J.A., Coats J.R. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol. 2012;102:124–128. doi: 10.1016/j.pestbp.2011.12.002. [DOI] [Google Scholar]
- 19.Kondratenko E.S., Kasymov A.U., Abubakirov N.K. Structure of amorphigenin. Chem. Nat. Compd. 1967;3:260–262. doi: 10.1007/BF00574629. [DOI] [Google Scholar]
- 20.Kasymov A.U., Kondratenko E.S., Abubakirov N.K. Stepwise hydrolysis of amorphin. Chem. Nat. Compd. 1970;6:482–482. doi: 10.1007/BF00564261. [DOI] [Google Scholar]
- 21.Crombie L., Dewick P.M., Whiting D.A. Biosynthesis of rotenoids. Chalcone, isoflavone, and rotenoid stages in the formation of amorphigenin by Amorpha fruticosa seedlings. J. Chem. Soc. Perkin Trans. 1973;1:1285–1294. doi: 10.1039/p19730001285. [DOI] [Google Scholar]
- 22.Kadyrova F.R., Shamsutdinov M.R.I., Shakirov T.T. The isolation of fruticin from the seeds of Amorpha fruticosa. Chem. Nat. Compd. 1973;9:107–107. doi: 10.1007/BF00580910. [DOI] [Google Scholar]
- 23.Li L., Wang H.K., Chang J.J., McPhail A.T., McPhail D.R., Terada H., Konoshima T., Kokumai M., Kozuka M., Estes J.R., et al. Antitumor agents, 138. Rotenoids and isoflavones as cytotoxic constitutents from Amorpha fruticosa. J. Nat. Prod. 1993;56:690–698. doi: 10.1021/np50095a005. [DOI] [PubMed] [Google Scholar]
- 24.Konoshima T., Terada H., Kokumai M., Kozuka M., Tokuda H., Estes J.R., Li L., Wang H.K., Lee K.H. Studies on inhibitors of skin tumor promotion, XII. Rotenoids from Amorpha fruticosa. J. Nat. Prod. 1993;56:843–848. doi: 10.1021/np50096a006. [DOI] [PubMed] [Google Scholar]
- 25.Kim B.G., Kwak H.B., Choi E.Y., Kim H.S., Kim M.H., Kim S.H., Choi M.K., Chun C.H., Oh J., Kim J.J. Amorphigenin inhibits Osteoclast differentiation by suppressing c-Fos and nuclear factor of activated T cells. Anat. Cell Biol. 2010;43:310–316. doi: 10.5115/acb.2010.43.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloutek E., Popov A., Drenska D., Uzunov P. Experimental research on the hepatoprotective activity of flavonoids isolated from Amorpha fructiosa. Eksp. Med. Morfol. 1985;24:50–54. [PubMed] [Google Scholar]
- 27.Kim Y.S., Ryu Y.B., Curtis-Long M.J., Yuk H.J., Cho J.K., Kim J.Y., Kim K.D., Lee W.S., Park K.H. Flavanones and rotenoids from the roots of Amorpha fruticosa L. That inhibit bacterial neuraminidase. Food Chem. Toxicol. 2011;49:1849–1856. doi: 10.1016/j.fct.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Brett C.H. Repellent properties of extract of Amorpha fruticosa. J. Econ. Entomol. 1946;39:810. doi: 10.1093/jee/39.6.810. [DOI] [PubMed] [Google Scholar]
- 29.Brett C.H. Insecticidal properties of the indigobush (Amorpha fruticosa) J. Agric. Res. 1946;73:81–96. [PubMed] [Google Scholar]
- 30.Abe F., Donnelly D.M.X., Moretti C., Polonsky J. Isoflavanoid constituents from Dalbergia monetaria. Phytochemistry. 1985;24:1071–1076. doi: 10.1016/S0031-9422(00)83185-4. [DOI] [Google Scholar]
- 31.Ji M., Liu C., Li X., Liu D., Wu D., Wang Y. Insecticidal and antifeeding activity of seeds of Amorpha fruticosa against Schizaphis graminum. Jiangsu Agric. Sci. 2011;39:208–210. [Google Scholar]
- 32.Liang Y., Li X., Gu Z., Qin P., Ji M. Toxicity of amorphigenin from the seeds of Amorpha fruticosa against the larvae of Culex pipiens pallens (Diptera: Culicidae) Molecules. 2015;20:3238–3254. doi: 10.3390/molecules20023238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno H., Miyoshi H., Ebisui K., Iwamura H. Comparison of the inhibitory action of natural rotenone and its stereoisomers with various NADH-ubiquinone reductases. Eur. J. Biochem. 1994;225:411–417. doi: 10.1111/j.1432-1033.1994.00411.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S., Zeng X.-N., Ma L.-M., Ru H.-K. Mechanism of epirotenone enantiomorphs as insecticides. Sci. Agric. Sin. 2007;40:2747–2275. [Google Scholar]
- 35.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 36.Hollingworth R.M., Ahammadsahib K.I., Gadelhak G., McLaughlin J.L. New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem. Soc. Trans. 1994;22:230–233. doi: 10.1042/bst0220230. [DOI] [PubMed] [Google Scholar]
- 37.Tormo J.R., González M.C., Cortes D., Estornell E. Kinetic characterization of mitochondrial complex I inhibitors using annonaceous acetogenins. Arch. Biochem. Biophys. 1999;369:119–126. doi: 10.1006/abbi.1999.1343. [DOI] [PubMed] [Google Scholar]
- 38.Earley F.G., Ragan C.I. Photoaffinity labelling of mitochondrial NADH dehydrogenase with arylazidoamorphigenin, an analogue of rotenone. Biochem. J. 1984;224:525–534. doi: 10.1042/bj2240525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgos J., Redfearn E.R. The inhibition of mitochondrial reduced nicotinamide-adenine dinucleotide oxidation by rotenoids. Biochim. Biophys. Acta. 1965;110:475–483. doi: 10.1016/S0926-6593(65)80060-1. [DOI] [Google Scholar]
- 40.Chen Q. Enzymology and Research Technology. Xiamen University Press; Xiamen, China: 2010. [Google Scholar]
- 41.Walker J.E. The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q. Rev. Biophys. 1992;25:253–324. doi: 10.1017/S003358350000425X. [DOI] [PubMed] [Google Scholar]
- 42.Arora S.K., Bates R.B., Grady R.A., Delfel N.E. Crystal and molecular structure of the one to one complex of rotenone and carbon tetrachloride. J. Am. Chem. Soc. 1975;97:5752–5755. doi: 10.1021/ja00853a020. [DOI] [Google Scholar]
- 43.Ueno H., Miyoshi H., Inoue M., Niidome Y., Iwamura H. Structural factors of rotenone required for inhibition of various NADH-ubiquinone oxidoreductases. Biochim. Biophys. Acta. 1996;1276:195–202. doi: 10.1016/0005-2728(96)00078-3. [DOI] [PubMed] [Google Scholar]
- 44.Darrouzet E., Issartel J.-P., Lunardi J., Dupuis A. The 49-kDa subunit of NADH-ubiquinone oxidoreductase (complex I) is involved in the binding of piericidin and rotenone, two quinone-related inhibitors. FEBS Lett. 1998;431:34–38. doi: 10.1016/S0014-5793(98)00719-4. [DOI] [PubMed] [Google Scholar]
- 45.Miyoshi H. Structure–activity relationships of some complex I inhibitors. Biochim. Biophys. Acta. 1998;1364:236–244. doi: 10.1016/S0005-2728(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 46.Valles S.M., Capinera J.L. Response of larvae of the southern armyworm, Spodoptera eridania cramer lepidoptera: Noctuidae, to selected botanical insecticides and soap. J. Agric. Entomol. 1993;10:145–153. [Google Scholar]
- 47.Committee on Strategies for the Management of Pesticide Resistant Pest Populations, B.o.A., National Research Council . Pesticide Resistance: Strategies and Tactics for Management. National Academy Press; Washington, DC, USA: 1986. [Google Scholar]
- 48.Fukami H., Nakajima M. Rotenone and the Rotenoids. Marcel Dekker Inc.; New York, NY, USA: 1971. [Google Scholar]
- 49.Rattanapan A. Biochemical and Molecular Detection of Cypermethrin and Rotenone Resistance in the Tropical Armywrom, Spodoptera litura (Fabricius) Kasetsart University; Bangkok, Thailand: 2007. [Google Scholar]
- 50.Crombie L., Josephs J.L., Cayley J., Larkin J., Weston J.B. The rotenoid core structure: Modifications to define the requirements of the toxophore. Bioorg. Med. Chem. Lett. 1992;2:13–16. doi: 10.1016/S0960-894X(00)80645-9. [DOI] [Google Scholar]
- 51.Lümmen P. Complex I inhibitors as insecticides and acaricides. Biochim. Biophys. Acta. 1998;1364:287–296. doi: 10.1016/S0005-2728(98)00034-6. [DOI] [PubMed] [Google Scholar]
- 52.Akbar S.M., Sharma H.C., Jayalakshmi S.K., Sreeramulu K. Methylparathion- and carbofuran-induced mitochondrial dysfunction and oxidative stress in Helicoverpa armigera (Noctuidae: Lepidoptera) Pestic. Biochem. Physiol. 2012;103:31–37. doi: 10.1016/j.pestbp.2012.02.005. [DOI] [Google Scholar]
- 53.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 54.Birch-Machin M.A., Briggs H.L., Saborido A.A., Bindoff L.A., Turnbull D.M. An evaluation of the measurement of the activities of complexes I–IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol. 1994;51:35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed I., Krishnamoorthy G. The non-equivalence of binding sites of coenzyme quinone and rotenone in mitochondrial NADH-CoQ reductase. FEBS Lett. 1992;300:275–278. doi: 10.1016/0014-5793(92)80862-B. [DOI] [PubMed] [Google Scholar]
- 56.Heinrich H., Werner S. Identification of the ubiquinone-binding site of NADH: Ubiquinone oxidoreductase (complex I) from Neurospora crassa. Biochemistry. 1992;31:11413–11419. doi: 10.1021/bi00161a020. [DOI] [PubMed] [Google Scholar]
- 57.Higgins D.S., Jr., Greenamyre J.T. [3H]Dihydrorotenone binding to NADH: Ubiquinone reductase (complex I) of the electron transport chain: An autoradiographic study. J. Neurosci. 1996;16:3807–3816. doi: 10.1523/JNEUROSCI.16-12-03807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J.-P., Chen Q.-X., Song K.-K., Xie J.-J. Inhibitory effects of salicylic acid family compounds on the diphenolase activity of mushroom tyrosinase. Food Chem. 2006;95:579–584. doi: 10.1016/j.foodchem.2005.01.042. [DOI] [Google Scholar]
- 59.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control. Assoc. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [PubMed] [Google Scholar]