Abstract
Granulomatous interstitial nephritis (GIN) is a rare entity detected in ∼0.5–0.9% of all renal biopsies. GIN has been linked to several antibiotics such as cephalosporins, vancomycin, nitrofurantoin and ciprofloxacin. It is also associated with NSAIDs and granulomatous disorders such as sarcoidosis, tuberculosis, fungal infections, and granulomatosis with polyangiitis. Renal biopsy is critical in establishing this diagnosis, and the extent of tubular atrophy and interstitial fibrosis may aid in determining prognosis. Retrospective data and clinical experience suggest that removal of the offending agent in conjunction with corticosteroid therapy often results in improvement in renal function. We describe a patient with a history of multiple spinal surgeries complicated by wound infection who presented with confusion and rash with subsequent development of acute kidney injury. Urinalysis demonstrated pyuria and eosinophiluria, and renal biopsy revealed acute interstitial nephritis with granulomas. These findings were attributed to doxycycline treatment of his wound infection. This review explores the clinical associations, presentation, diagnosis, and treatment of this uncommon cause of acute kidney injury.
Keywords: AIN, AKI, doxycycline, granuloma
Background
Acute interstitial nephritis (AIN) is an important cause of acute kidney injury where antibiotics are the most common offending agents [1, 2]. The presence of granulomas with AIN is rare and antibiotics such as vancomycin, ciprofloxacin, nitrofurantoin, penicillin and cephalosporins have been implicated [3–5]. To our knowledge, doxycycline-induced granulomatous interstitial nephritis (GIN) has not been previously described.
Case report
A 69-year-old Caucasian man with a history of untreated hepatitis C, type 2 diabetes mellitus, chronic obstructive pulmonary disease, depression and chronic back pain for which he underwent four cervical and four lumbar spine surgeries presented with confusion, diffuse rash and leucocytosis from his rehabilitation facility. Two months prior to admission, after his last posterior spinal fusion, he developed a wound infection with coagulase negative Staphylococcus species. He was treated with intravenous vancomycin for 1 month. Sixteen days prior to admission, he was switched from vancomycin to doxycycline 100mg by mouth twice daily. Ten days prior to admission, the patient developed a pruritic, erythematous rash involving his face, arms, torso and back. Doxycycline was discontinued, and he was started on prednisone 40mg daily for 4 days. His rash did not improve; he became delirious and was transferred for further evaluation.
His medications included amitriptyline, pregabalin, methadone, fluoxetine, trazodone, loratadine, ranitidine, docusate senna, polyethylene glycol, bisacodyl suspension, hydrocortisone/aloe topical cream and menthol/camphor topical lotion. He was not taking non-steroidal anti-inflammatory medications. He had no known allergies.
On physical exam, the patient was afebrile, normotensive and not hypoxic. He had a diffuse erythematous maculopapular rash on his face, arm, chest, abdomen and back with excoriations on his face and arms. He had a grossly normal ocular, oral, heart, lung and neurologic exam.
Laboratory results on admission were significant for creatinine of 0.8 mg/dL and white blood cell count of 16.89k/mm3 of which 53% were eosinophils (Table 1). Non-contrast CT imaging of the thoracolumbar spine, chest, abdomen and pelvis showed non-specific perinephric stranding and bladder wall thickening without evidence of obstruction and mild splenomegaly. There was no clear evidence of infection or abscess.
Table 1.
Laboratory data during hospitalization at Johns Hopkins Hospital
| Variable | Hospital Day 1 | Hospital Day 7 (Day 1 prednisone, after biopsy on Day 6) | Hospital Day 8 (peak creatinine) | Hospital Day 21 (partial remission − new baseline) | Reference range |
|---|---|---|---|---|---|
| Sodium | 133 | 134 | 134 | 132 | 135–148 mEq/L |
| Potassium | 4.5 | 4.3 | 5.2 | 4.5 | 3.5–5.1 mEq/L |
| Chloride | 100 | 101 | 102 | 97 | 96–109 mEq/L |
| Carbon dioxide | 22 | 19 | 18 | 22 | 21–31 mEq/L |
| Blood urea nitrogena | 15 | 34 | 47 | 39 | 7–22 mg/dL |
| Creatininea | 0.8 | 2.6 | 3.1 | 1.7 | 0.6–1.3 mg/dL |
| Glucosea | 86 | 134 | 251 | 359 | 60–99 mg/dL |
| White blood cells | 16.89 | 14.99 | 13.64 | 9.18 | 4.5–11k/mm3 |
| Eosinophils (%) | 53 | 22 | 7 | 1 | <1–4 |
| Polymorphonuclear cells (%) | 42 | 63 | 86 | 84 | 31–46 |
| Lymphocytes (%) | 3 | 7 | 2 | 12 | 24–44 |
| Monocytes (%) | 1 | 4 | 2 | 2 | 2–11 |
| Hemoglobin | 15.7 | 13.7 | 13.1 | 13.2 | 13.9–16.3 g/dL |
| Mean corpuscular volume | 84.1 | 83.9 | 84.2 | 85.1 | 80–100 fL |
| Platelet | 21b | 174 | 160 | 117 | 150–300k/mm3 |
| INR | 1.6 | Not measured | Not measured | 1.2 | 0.9–1.1 |
| Albumin | 2.7 | 2.3 | 2.6 | 2.5 | 3.5–5.3 g/dL |
| Total protein | 6.3 | 6.1 | 6.2 | 6.3 | 6.0–8.2 g/dL |
| Total bilirubin | 0.7 | 0.7 | 0.8 | 0.8 | 0–1.2 mg/dL |
| Alkaline phosphatase | 98 | 89 | 92 | 85 | 30–120 U/L |
| Aspartase amino transferase | Hemolyzed, repeat was 40 | 81 | 75 | 35 | 0–37 U/L |
| Alanine amino transferase | 33 | 101 | 100 | 50 | 0–40 U/L |
| C-reactive protein | 1.0 | Not measured | Not measured | <0.5 mg/dL |
aConversion factor for units: Serum creatinine in mg/dL to µmol/L, ×88.4; blood urea nitrogen in mg/dL to mmol/L, ×0.357; glucose mg/dL to mmol/L, 0.05551.
bPlatelet count may not have been accurate. Although no clot was documented to have been found in the tube, repeat platelet count 6 h later was 129 k/cc3.
He was empirically treated with vancomycin, piperacillin-tazobactam and briefly ciprofloxacin for a urinary tract infection. Cultures were negative, and the antibiotics were discontinued. However, the rash and altered mental status persisted. A skin biopsy was performed and was non-diagnostic.
By Day 5 of his hospitalization, his creatinine had risen to 1.9 mg/dL with a concomitant increase in AST and ALT levels. Urinalysis revealed 1+ proteinuria, 16 RBC/hpf and 7 WBC/hpf with eosinophiluria.
A kidney biopsy was performed. Light microscopy demonstrated relatively uninvolved glomeruli and marked diffuse interstitial inflammation composed of activated lymphocytes, plasma cells and numerous eosinophils (>50/hpf) with frequent foreign body giant cells associated with non-caseating granulomas (Figures 1 and 2). There was moderate acute tubular injury and presumed mild interstitial fibrosis. Special stains for acid-fast bacilli, bacteria (Brown & Hopps) and fungi (GMS) were negative. Immunofluorescence revealed non-specific findings. Electron microscopy showed scattered immune deposits possibly corresponding to IgM on immunofluorescence, suggesting an immune-mediated process; however, the extensive granulomatous inflammation was considered the prevailing pathologic process. He was diagnosed with severe AIN with numerous eosinophils and foreign body giant cell granulomas, secondary to doxycycline.
Fig. 1.
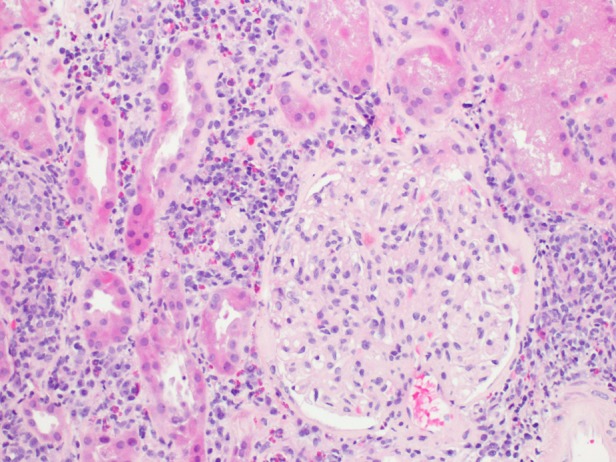
Hematoxylin and eosin stain at ×200 magnification on light microscopy showing an uninvolved glomerulus in a background of interstitial inflammation with numerous eosinophils.
Fig. 2.
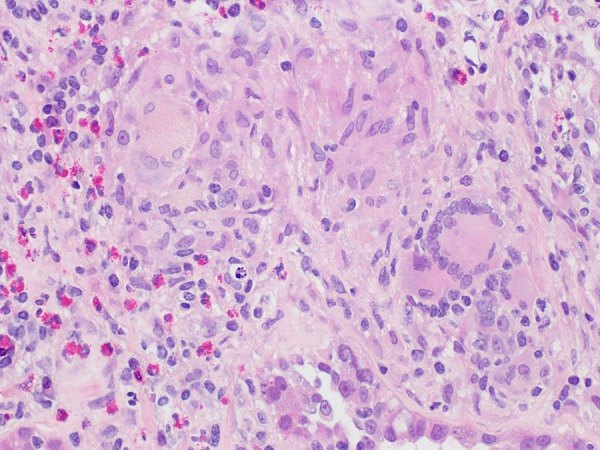
Hematoxylin and eosin stain at ×400 magnification on light microscopy demonstrating a poorly formed granuloma with foreign body-type giant cells.
He underwent another skin biopsy that demonstrated spongiotic and interface dermatitis with eosinophils compatible with drug reaction with eosinophilia and systemic symptoms (DRESS). He was started on prednisone 60mg by mouth daily. Two days later, his creatinine peaked at 3.1 mg/dL, but declined to 1.7–1.9 mg/dL while on prednisone.
On Day 17 of his hospitalization, the patient was transferred to the medical intensive care unit for worsening altered mental status and intermittent apneic episodes. His course was further complicated by Stevens Johnson syndrome/toxic epidermal necrolysis. Ciprofloxacin, which was started empirically a few days prior, was the presumed culprit. A few days later, the patient developed respiratory failure and hypotension necessitating mechanical ventilatory support and vasopressors. Blood cultures grew methicillin-sensitive Staphylococcus aureus. He ultimately succumbed to refractory septic shock and multi-organ failure.
Discussion
This patient's exposure to doxycycline was followed by rash, eosinophilia, eosinophiluria and acute kidney injury resulting in a biopsy-proven diagnosis of GIN. Although the patient was exposed to vancomycin, piperacillin-tazobactam and ciprofloxacin prior to renal biopsy, the patient's rash and laboratory derangements were traced to the initiation of doxycycline. Renal manifestations of AIN typically occur within 3 weeks of starting the offending drug in ∼80% of cases, where the average delay is ∼10 days with antibiotics [5]. The patient's exposure to vancomycin preceded this presentation by months, and generally vancomycin-induced interstitial nephritis appears 7–25 days after drug onset; therefore, it is less likely this drug was responsible [6]. Piperacillin-tazobactam and ciprofloxacin were initiated only a few days prior to the patient's rise in creatinine and urine abnormalities, which would ordinarily be too short to develop interstitial nephritis with granulomas. The patient's concomitant rash and eosinophilia following treatment with doxycycline also suggest this was the most likely offending agent.
Incidentally, serum quantiferon testing for tuberculosis was positive 13 days following renal biopsy. Although special staining for acid-fast bacilli on renal biopsy tissue was negative, this does not exclude tuberculosis-induced GIN. However, other signs of active tuberculosis were lacking. Latent tuberculosis was a possibility, but treatment was deferred in light of active Stevens Johnson syndrome.
The patient also had a history of untreated hepatitis C, which can be associated with renal complications such as cryoglobulinemic glomerulonephritis, membranoproliferative glomerulonephritis, membranous nephropathy, IgA nephropathy, polyarteritis nodosa, fibrillary and immunotactoid glomerulonephritis and interstitial nephritis [7, 8]. In a retrospective report of 68 patients with hepatitis C and renal dysfunction who underwent renal biopsy, 2 had interstitial nephritis, and 1 had GIN attributed to sarcoidosis [9]. A direct association between hepatitis C and GIN has not been established.
The role of renal biopsy was critical in excluding a progressive glomerulonephritis and in delineating the extent of inflammation, fibrosis and eosinophilic infiltration. The findings of mild tubulointerstitial fibrosis are associated with a more favorable response to corticosteroid therapy [10]. Unfortunately, despite a partial recovery in renal function, he ultimately expired from septic shock and multi-system organ failure due to Stevens Johnson syndrome/toxic epidermal necrolysis.
Epidemiology and clinical associations
GIN occurs in 0.5–0.9% of native kidney biopsies [11–16]. The relative contribution of different etiologies to GIN is unknown since our knowledge is based on case series and case reports for the description of this condition. In a report by Mignon et al. [12] of 32 cases, ∼28% were due to drugs, 16% were caused by granulomatosis with polyangiitis (GPA) and 9% were attributed to sarcoidosis and tuberculosis. In the series presented by Viero and Cavallo [15], 25% of cases were due to drugs, sarcoidosis and infections each. Bijol et al. [13] reviewed 9779 biopsies between January 1987 and July 2004 to describe cases of GIN, where a single but definite granuloma was deemed enough for inclusion. They found 46 cases of which 38 had available clinical information. Seventeen patients had drug-induced GIN, 11 patients had sarcoidosis-related GIN and 2 had GPA. Javaud et al. [17] evaluated 40 consecutive renal biopsies between January 1991 and February 2004 with GIN defined as the presence of at least one epithelioid granuloma in the interstitium. The majority of their cases were linked to sarcoidosis (50%), where medications (17.5%) and tuberculosis (7.5%) accounted for fewer cases [17]. The variability in these series can be explained by both sampling and publication bias of these cohorts. Known causes of GIN are listed in Table 2.
Table 2.
| Antimicrobials | Analgesics | Other drugs | Infections | Inflammatory /Rheumatologic | |
|---|---|---|---|---|---|
| Etiology | Penicillin Methicillin Ampicillin Amoxicillin Oxacillin Cephalothin Erythromycin Spiramycin Rifampicin Vancomycin Sulfonamides Ciprofloxacin Levofloxacin Gentamicin Nitrofurantoin Acyclovir Clotrimazole Doxycycline |
Fenoprofen Ketoprofen Indomethacin Diclofenac Clometacine Ibuprofen Diflunisal Benoxaprofen Paracetamol Dihydrocodeine Naprosyn |
Allopurinol Omeprazole Alendronate Furosemide Hydrochlorothiazide Chlorthiazide Triamterene Amiloride Chlorpropamide Tienlinic acid Captopril Carbamazepine Lamotrigine Levetiracetam Phenytoin Phenindione Sulfasalazine |
Mycobacterium tuberculosis Mycobacterium leprae Mycobacterium kansasii Histoplasmosis Candidiasis Toxoplasmosis Tricosporon laibachii Cryptococcus neoformans Escherichia coli Epstein–Barr virus |
Sarcoidosis TINU syndrome Intestinal bypass Heroin Oxalosis Crohn's disease Granulomatosis with polyangiitis (GPA) Eosinophilic granulomatosis with polyangiitis (EGPA) Bacillus Calmette–Guerin therapy |
| Epidemiology | Usually presents few weeks after exposure Often middle-aged patients |
Usually months after exposure Often middle-aged patients |
Diuretics reported onset >4 weeks after exposure Often middle-aged patients |
TB more common in those with Indian or African descent Consider fungal and mycobacterial causes post-transplant TB patients are younger |
TINU in adolescent girls Sarcoidosis often in African Americans GPA and EGPA have bimodal age distribution |
| Presentation | May have hypersensitivity symptoms, variable degree of renal failure, mild proteinuria, ±microscopic hematuria and pyuria | Can present with nephrotic syndrome | May also have hypersensitivity symptoms, varying degrees of renal failure, may necessitate renal replacement therapy | Insidious presentation, diagnosis often is delayed, can have extrarenal symptoms corresponding to infectious etiology | Depends on underlying cause Diagnosis of sarcoidosis can occur at any time relative to renal involvement Uveitis often follows renal failure in TINU |
| Histology | Ill-defined, non-caseating granulomas | Ill-defined, non-caseating granulomas | Ill-defined, non-caseating granulomas | Necrotizing granulomas | Necrotizing, well-formed granulomas in GPA, EGPA Non-caseating, well-formed granulomas in sarcoidosis, TINU, Crohn's disease |
Common causes of GIN
Sarcoidosis
Renal involvement in sarcoidosis is most often due to nephrocalcinosis, hypercalciuria or calculi secondary to hypercalcemia resulting from increased 1,25 dihydroxyvitamin D3 production by activated macrophages in areas of inflammation [26–28]. Granulomatous renal involvement is considered rare and is mostly described in case reports or case series [26]. However, ∼7–27% of patients with sarcoidosis have evidence of granulomatous tubulointerstitial nephritis on post-mortem series, although this may not result in clinically significant renal disease [29, 30].
Most patients with GIN in sarcoidosis present with extrarenal manifestations such as pulmonary, skin or eye involvement [14, 27, 31–33]. However, there are a few series reporting sarcoid GIN without extrarenal involvement [32, 34]. In addition, there are several cases where the diagnosis of GIN then leads to a subsequent diagnosis of sarcoidosis [14, 28, 35]. It is therefore possible that some cases of idiopathic GIN may represent unrecognized renal limited sarcoidosis [32]. Thus, patients with sarcoidosis can develop GIN at any time during the course of their disease, and it may even precede the diagnosis in some cases [17].
Membranous nephropathy can be detected in conjunction with GIN in patients with sarcoidosis. Three case reports document patients with nephrotic syndrome and sarcoidosis who undergo renal biopsy and are found to have both pathologic lesions [28, 36, 37]. These patients responded well to glucocorticoid therapy.
Drugs
Antibiotics
Antibiotics are a well-established etiology of GIN, and the number of implicated agents has grown over time. Sulfonamide therapy was described by More et al. [38] in 1946 to cause granulomatous renal lesions in 8/22 autopsies reviewed. Typically, granulomas exhibited more widespread interstitial infiltration and involved the cortex more than the medulla in these cases. Penicillins (ampicillin, oxacillin and methicillin) and cephalosporins have been linked to GIN in several case reports [12, 17, 39–42]. Fluoroquinolones, which have been associated with AIN, ATN and crystalluria, have also been described to cause GIN. Ramalakshmi et al. [43] reported levofloxacin-induced GIN in a patient treated for a urinary tract infection who subsequently developed fever, rise in liver enzymes and acute kidney injury. Ciprofloxacin has also been reported in association with GIN, where a patient was treated for cellulitis [4]. Nitrofurantoin has been reported as the cause of GIN in two cases, and in both cases, patients improved with drug withdrawal [3, 18]. Vancomycin was added to this list of offenders in 2007 when a patient developed DRESS syndrome and GIN, similar to our case presentation with doxycycline [6]. Of note, the latency period between antibiotic use and the diagnosis of GIN is shorter than that seen with other medications.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) have been reported to cause GIN in several case reports and notably in at least three large case series. Schwarz et al. [44] described two patients who presented with renal insufficiency of whom, one developed end-stage renal disease after months of treatment with NSAIDs (ketoprofen/indomethacin and diclofenac/indomethacin). Viero and Cavallo [15] found two instances of GIN out of 12 where naprosyn and aspirin were implicated. Javaud et al. [17] found 7 out of 40 consecutive renal biopsies between January 1991 and February 2004 to have GIN that were considered drug induced. Two of these patients were exposed to ibuprofen and tenoxicam. Finally, Joss et al. [14] described two patients between 1990 and 2004 who were found to have GIN after exposure to omeprazole/diclofenac and sulfasalazine/indomethacin, respectively. Both patients presented with eGFR of ∼17 mL/min but increased to ∼56 mL/min after prednisone treatment.
NSAID-induced AIN with or without granulomas often occur after months of exposure, with the mean duration of 6 months [5]. Although proteinuria is usually subnephrotic in patients with GIN, patients with NSAID-related disease can develop nephrotic syndrome [5].
Diuretics
Some of the earliest reports of diuretic-induced GIN were in 1983. Ebert described a patient treated with hydrochlorothiazide and triamterene who developed fevers, flank pain, eosinophilia, pyuria and acute kidney injury [45]. Magil et al. reported nine patients with drug-induced GIN between 1977 and 1981 [46]. These patients were treated with hydrocholorothiazide, triamterene, fenoprofen and/or furosemide. None of these patients had pre-existing renal disease, tuberculosis or sarcoidosis. Renal failure typically occurred 4–10 weeks after introduction of the drug, which is longer than reported with antibiotics. Amiloride and hydrochlorothiazide were implicated in a later case report in 1995 [19]. These patients improved with drug withdrawal.
Other medications
Allopurinol and anti-epileptics have been associated with GIN. Allopurinol is also associated with granulomatous hepatitis. In the first description of allopurinol-induced GIN, the patient improved with drug withdrawal and prednisone therapy [47]. GIN has also been traced to carbamazepine, phenytoin and levetiracetam [16, 48, 49].
Infection
Tuberculosis is the most common infectious etiology of GIN [50]. Most cases are reported in patients of Asian Indian or African descent, which may reflect the higher incidence of disease in these populations [16, 50–53]. Renal involvement is insidious and can remain undetected for up to 20 years [54]. The disease is easily overlooked, such that diagnoses are made during an operation or post-mortem [55]. The first description of GIN as a sole manifestation of renal tuberculosis was made by Mallinson et al. [53] in 1981. They describe three patients with pulmonary tuberculosis and advanced renal disease secondary to chronic tubulointerstitial nephritis with granulomas. One patient developed end-stage renal disease despite treatment, and the other two patients had progressive decline in renal function with treatment, which was attributed to drug toxicity.
A case series from India assessed 2798 renal biopsies performed between January 2000 and October 2012 and found that 14 patients were diagnosed with GIN during that period [16]. Tuberculosis was considered the culprit in 9 out of the 14 cases. Mean age in this cohort was 35 years, which is younger than what is usually seen in patients with GIN. Pulmonary involvement and/or mediastinal lymphadenopathy was evident in four cases. Mean serum concentration at presentation was 6.7 mg/dL and six patients required dialysis initially, which reflects the delay in diagnosing this condition. Two patients were able to discontinue dialysis with treatment, but the majority of patients ultimately progressed to CKD or ESRD.
Fungal and atypical bacterial infections have also been implicated in GIN. Disseminated Mycobacterium kansasii infection has been reported in association with GIN and liver granulomas [20]. Ogura et al. [21] reported two cases of fungal GIN related to Trichosporon laibachii in one patient treated with chemotherapy for pharyngeal cancer and the second related to Candida albicans in a patient treated with steroids for asthma. Another report describes a patient with untreated systemic lupus erythematosis with CD4 lymphopenia who developed cryptococcal GIN [22]. These cases highlight the consideration of fungal interstitial nephritis in immunocompromised patients.
Although unusual, immunocompetent patients can also develop fungal GIN as reported by Nasr et al. [56]. In this case, a male patient was found to have dialysis-dependent acute kidney injury, his renal biopsy revealed GIN and he was diagnosed with disseminated Histoplasma capsulatum after careful inspection of renal biopsy tissue for yeast forms and serum antigen measurement.
Others
Tubulointerstitial nephritis with uveitis (TINU) can also cause GIN. This rare condition, which generally affects adolescent girls, has no clear underlying cause but has been linked with rheumatoid arthritis, infections, NSAID administration, antibiotics and Chinese herbs [57–62]. Patients may be misdiagnosed as having drug-induced interstitial nephritis, because uveitis often occurs after the onset of kidney disease [57]. In Joss et al., two patients were diagnosed with TINU out of 18 cases of GIN [14]. One patient presented with simultaneous renal failure and uveitis and the other developed uveitis following a diagnosis of idiopathic GIN. Granulomatous TINU has also been associated with a salt wasting nephropathy [63]. Treatment with corticosteroids generally leads to an improvement in creatinine clearance [13, 14, 57, 59, 63].
GIN can be a histologic finding in patients with GPA. Although Bijol et al. cites the frequency of GIN in GPA between 5 and 67% depending on the series, our review of the literature demonstrates a frequency on the order of 5–16% [12, 13, 15–17]. Patients often present with pulmonary symptoms, renal failure, microscopic hematuria and moderate but usually subnephrotic range proteinuria.
Post-renal transplant GIN has also been described. The reported incidence of GIN in renal allografts is similar to that of native kidneys: 0.6–1% [23–25]. Although acute rejection is a common cause of interstitial nephritis, it is not associated with granuloma formation [23]. Rather, infections such as Mycobacterium tuberculosis, C. albicans, Escherichia coli and viruses are more commonly responsible [23–25, 64, 65]. This reflects an increased predisposition to GIN secondary to increased infection risk from immunosuppression for allograft maintenance.
Crohn's disease, oxalosis and intravesicular bacillus Calmette–Guerin have also been responsible for causing GIN in a few cases [13, 15, 17]. The etiology for GIN remains obscure in ∼10% of cases [13, 17].
Pathophysiology of medication-induced GIN
The putative mechanisms by which medications induce AIN have been described using experimental models and through renal biopsy findings. Drug-induced AIN is most likely related to immune reactions as few patients exposed to a particular drug develop AIN. The response is not dose dependent, and it can be associated with extrarenal signs of hypersensitivity [5]. Furthermore, AIN can recur when patients are re-introduced to the inciting agent or a very similar agent. In experimental models, AIN can occur due to an immune response against an antigen that originates within the kidney or an extrarenal antigen that is deposited in the kidney [66–68]. Potentially, a drug can bind to part of the tubular basement membrane and act as a hapten. Alternatively, the drug can mimic an antigen in the tubular basement membrane or intersitium where via molecular mimicry an immune response targets both the drug and the similar antigen [5]. The response is most likely cell mediated in nature given the number of lymphocytes and macrophages seen on light microscopy specimens and the paucity of immune deposits seen on immunofluorescence. GIN has been attributed to a delayed type hypersensitivity reaction and cell-mediated response type 1 helper T cells [5].
Clinical manifestations and histologic findings
Clinical manifestations, laboratory signs and histologic findings vary in patients with GIN depending on the underlying cause. Generally, there is no predilection for either gender [13–15, 17]. GIN can manifest at any age; however, the mean and median ages of presentation in most series were in the fifth and sixth decade of life [13–15, 17]. Patients with tuberculosis-induced GIN tend to be younger [16]. GIN can result in varying degrees of renal insufficiency and necessitate the initiation of dialysis. The mean creatinine in two series was 4.1–5.1 mg/dL, and the median creatinine clearance in three series ranged between 21 and 27 mL/min [13–15, 17, 28]. Approximately one-third of patients with drug-induced GIN in one series had signs of hypersensitivity such as arthralgia, fevers and eosinophilia [17]. Patients with NSAID-induced GIN often have higher levels of proteinuria (nephrotic range at times) and less eosinophilia [5, 10]. Otherwise, patients usually demonstrate mild proteinuria, normal blood pressures and less frequently have sterile pyuria or microscopic hematuria [12, 13, 17].
On renal biopsy, granulomas with non-necrotizing features are associated with drug-induced GIN and sarcoidosis, whereas, necrotizing granulomas are common in patients with GPA, fungal or tuberculosis-induced GIN [13, 17]. Drug-induced GIN leads to more loose appearing aggregates of epithelioid macrophages, but granulomas in sarcoidosis tend to be rather well defined [13, 45]. Although these findings are suggestive of certain diagnoses, they are not absolute.
Joss et al. [14] found no correlation between the degree of inflammation or fibrosis and the underlying etiology. The concentration of eosinophils cannot direct one to a particular diagnosis either. Bijol and coworkers [13] demonstrated that drug-induced GIN had more diffuse interstitial involvement with a higher concentration of eosinophils and neutrophils. In contrast, Javaud et al. [17] noted that drug-induced GIN should be considered when granulomas are present and when eosinophils are not seen in the inflamed interstitium. This difference surrounding the presence of eosinophils may reflect the timing of when biopsies are performed, the various medications implicated or the sampling bias that is present in patients with presumed AIN.
Treatment
Treatment for GIN depends on the underlying etiology. In drug-induced GIN, treatment involves withdrawal of the offending agent and usually a course of corticosteroids. The use of corticosteroids has been supported by retrospective studies, but no prospective randomized controlled trials demonstrating its efficacy exist. Gonzalez et al. [10] performed a multicenter retrospective study of 61 patients with drug-induced interstitial nephritis. The most common drug offenders were antibiotics and NSAIDs, and the peak mean creatinine was 5.5 ± 3.3 mg/dL. Fifty-two patients were treated with corticosteroids 23 ± 17 days after drug withdrawal and nine did not receive corticosteroids. Steroid regimens varied between institutions, but the most common regimen involved pulsed methylprednisolone 250–500 mg intravenously for 3 to 4 days followed by prednisone 1 mg/kg/day tapered over 8 to 12 weeks. Treated patients had a significantly lower serum creatinine after follow-up, and a significantly lower proportion required hemodialysis compared with those who were not treated. Patients who did not achieve complete return to baseline creatinine with steroids tended to be older, have a higher baseline creatinine and had a delay in steroid administration of >2 weeks following discontinuation of the culprit drug. Although this study excluded patients with GIN, it is plausible that the findings could be relevant to this population, and further investigation is warranted to confirm this.
In some retrospective cases of GIN, withdrawal of the causative drug resulted in rapid improvement in renal function and thus eliminated the need for corticosteroid therapy, but this was more often the case when the inciting drug was a diuretic [3, 46, 47, 69]. Usually, patients have been treated with corticosteroids and receive 0.5–1 mg/kg/day for a mean or median duration of 1–3 months with improvement in renal function in most instances. Patients who even require dialysis may be able to ultimately discontinue dialytic support with corticosteroid therapy. In a case of vancomycin-induced GIN which was refractory to steroid therapy, cyclosporine and mycophenylate mofetil were initiated with partial recovery of renal function [6]. Thus, other immunosuppressive agents may be useful in treating this condition, but further investigation is necessary to support their use.
Patients with sarcoidosis and TINU benefit immensely from treatment with corticosteroids. Unfortunately, these patients are at higher risk of relapse after steroid withdrawal and often require a longer course of corticosteroid therapy than patients with drug-induced GIN [14]. Steroid-sparing agents such as azathioprine and infliximab have also been used in sarcoidosis [14, 70]. Patients with infection-induced GIN are treated for their infection and corticosteroids are generally not used.
Prognosis
A higher degree of tubular atrophy and interstitial fibrosis portends a poorer long-term renal prognosis [5, 34, 44]. Additional risk factors for worse outcome include a higher extent and severity of interstitial cell inflammation, oliguria or anuria, chronic NSAID use and duration of renal failure [5, 44]. The presence of granulomas confers a worse prognosis in patients with AIN as well.
Conclusions
GIN is a rare cause of acute kidney injury that is often the result of medications, infections, sarcoidosis and other rheumatologic conditions. The presence of granulomas on renal biopsy is of diagnostic significance as it can heighten suspicion for these particular entities, although histologic findings are not diagnostic of any single underlying etiology. Nevertheless, renal biopsy should be considered in patients with suspected AIN, because it can facilitate treatment decisions surrounding the use of corticosteroids and allow for grounded prognostication.
We present a case of GIN following exposure to doxycycline. To our knowledge, this is the first association between GIN and doxycycline reported in the literature. As the use of antibiotics and other drugs continue to rise, we will probably find an increase in the incidence of GIN in our practice and in the literature. As such, an awareness of this condition is critical.
Conflicts of interest statement
None declared. The results presented in this paper have not been published previously in whole or part, including in abstract format.
(See related articles by Aleckovic-Halilovic et al. Granulomatous interstitial nephritis: a chameleon in a globalized world. Clin Kidney J (2015) 8: 511–515 and by Agrawal et al. Etiological diagnosis of granulomatous tubulointerstitial nephritis in the tropics. Clin Kidney J (2015) 8: 524–530.)
References
- 1.Michel DM, Kelly CJ. Acute interstitial nephritis. J Am Soc Nephrol 1998; 9: 506–515 [DOI] [PubMed] [Google Scholar]
- 2.Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis 2014; 64: 558–566 [DOI] [PubMed] [Google Scholar]
- 3.Korzets Z, Elis A, Bernheim J, et al. Acute granulomatous interstitial nephritis due to nitrofurantoin. Nephrol Dial Transplant 1994; 9: 713–715 [DOI] [PubMed] [Google Scholar]
- 4.Lien YH, Hansen R, Kern WF, et al. Ciprofloxacin-induced granulomatous interstitial nephritis and localized elastolysis. Am J Kidney Dis 1993; 22: 598–602 [DOI] [PubMed] [Google Scholar]
- 5.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int 2001; 60: 804–817 [DOI] [PubMed] [Google Scholar]
- 6.Hong S, Valderrama E, Mattana J, et al. Vancomycin-induced acute granulomatous interstitial nephritis: therapeutic options. Am J Med Sci 2007; 334: 296–300 [DOI] [PubMed] [Google Scholar]
- 7.Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol 2014; 20: 7544–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galossi A, Guarisco R, Bellis L, et al. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis 2007; 16: 65–73 [PubMed] [Google Scholar]
- 9.Sumida K, Ubara Y, Hoshino J, et al. Hepatitis C virus-related kidney disease: various histological patterns. Clin Nephrol 2010; 74: 446–456 [PubMed] [Google Scholar]
- 10.Gonzalez E, Gutierrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008; 73: 940–946 [DOI] [PubMed] [Google Scholar]
- 11.O'Riordan E, Willert RP, Reeve R, et al. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol 2001; 55: 297–302 [PubMed] [Google Scholar]
- 12.Mignon F, Mery JP, Mougenot B, et al. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp 1984; 13: 219–245 [PubMed] [Google Scholar]
- 13.Bijol V, Mendez GP, Nose V, et al. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol 2006; 14: 57–63 [DOI] [PubMed] [Google Scholar]
- 14.Joss N, Morris S, Young B, et al. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007; 2: 222–230 [DOI] [PubMed] [Google Scholar]
- 15.Viero RM, Cavallo T. Granulomatous interstitial nephritis. Hum Pathol 1995; 26: 1347–1353 [DOI] [PubMed] [Google Scholar]
- 16.Naidu GD, Ram R, Swarnalatha G, et al. Granulomatous interstitial nephritis: our experience of 14 patients. Indian J Nephrol 2013; 23: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javaud N, Belenfant X, Stirnemann J, et al. Renal granulomatoses: a retrospective study of 40 cases and review of the literature. Medicine (Baltimore) 2007; 86: 170–180 [DOI] [PubMed] [Google Scholar]
- 18.Namagondlu G, Low SE, Seneviratne R, et al. Acute renal failure from nitrofurantoin-induced acute granulomatous interstitial nephritis. QJM 2010; 103: 49–52 [DOI] [PubMed] [Google Scholar]
- 19.Enriquez R, Cabezuelo JB, Gonzalez C, et al. Granulomatous interstitial nephritis associated with hydrochlorothiazide/amiloride. Am J Nephrol 1995; 15: 270–273 [DOI] [PubMed] [Google Scholar]
- 20.Listwan WJ, Roth DA, Tsung SH, et al. Disseminated Mycobacterium kansasii infection with pancytopenia and interstitial nephritis. Ann Intern Med 1975; 83: 70–73 [DOI] [PubMed] [Google Scholar]
- 21.Ogura M, Kagami S, Nakao M, et al. Fungal granulomatous interstitial nephritis presenting as acute kidney injury diagnosed by renal histology including PCR assay. Clin Kidney J 2012; 5: 459–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David VG, Korula A, Choudhrie L, et al. Cryptococcal granulomatous interstitial nephritis and dissemination in a patient with untreated lupus nephritis. Nephrol Dial Transplant 2009; 24: 3243–3245 [DOI] [PubMed] [Google Scholar]
- 23.Hotta K, Fukasawa Y, Sasaki H, et al. Granulomatous tubulointerstitial nephritis in a renal allograft: three cases report and review of literature. Clin Transplant 2012; 26 (Suppl. 24): 70–75 [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir BH, Sar A, Uyar P, et al. Posttransplant tubulointerstitial nephritis: clinicopathological correlation. Transplant Proc 2006; 38: 466–469 [DOI] [PubMed] [Google Scholar]
- 25.Meehan SM, Josephson MA, Haas M. Granulomatous tubulointerstitial nephritis in the renal allograft. Am J Kidney Dis 2000; 36: E27. [DOI] [PubMed] [Google Scholar]
- 26.Utas C, Dogukan A, Patiroglu TE, et al. Granulomatous interstitial nephritis in extrapulmonary sarcoidosis. Clin Nephrol 1999; 51: 252–254 [PubMed] [Google Scholar]
- 27.Ikeda A, Nagai S, Kitaichi M, et al. Sarcoidosis with granulomatous interstitial nephritis: report of three cases. Intern Med 2001; 40: 241–245 [DOI] [PubMed] [Google Scholar]
- 28.Rajakariar R, Sharples EJ, Raftery MJ, et al. Sarcoid tubulo-interstitial nephritis: long-term outcome and response to corticosteroid therapy. Kidney Int 2006; 70: 165–169 [DOI] [PubMed] [Google Scholar]
- 29.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from the johns hopkins hospital and massachusetts general hospital. Medicine (Baltimore) 1952; 31: 1–132 [PubMed] [Google Scholar]
- 30.Ricker W, Clark M. Sarcoidosis; a clinicopathologic review of 300 cases, including 22 autopsies. Am J Clin Pathol 1949; 19: 725–749 [DOI] [PubMed] [Google Scholar]
- 31.Gobel U, Kettritz R, Schneider W, et al. The protean face of renal sarcoidosis. J Am Soc Nephrol 2001; 12: 616–623 [DOI] [PubMed] [Google Scholar]
- 32.Robson MG, Banerjee D, Hopster D, et al. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003; 18: 280–284 [DOI] [PubMed] [Google Scholar]
- 33.van Dorp WT, Jie K, Lobatto S, et al. Renal failure due to granulomatous interstitial nephritis after pulmonary sarcoidosis. Nephrol Dial Transplant 1987; 2: 573–575 [PubMed] [Google Scholar]
- 34.Hannedouche T, Grateau G, Noel LH, et al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990; 5: 18–24 [DOI] [PubMed] [Google Scholar]
- 35.Nagaraja P, Davies MR. Granulomatous interstitial nephritis causing acute renal failure: a rare presenting feature of sarcoidosis. QJM 2014; 107: 467–469 [DOI] [PubMed] [Google Scholar]
- 36.Toda T, Kimoto S, Nishio Y, et al. Sarcoidosis with membranous nephropathy and granulomatous interstitial nephritis. Intern Med 1999; 38: 882–886 [DOI] [PubMed] [Google Scholar]
- 37.Khan IH, Simpson JG, Catto GR, et al. Membranous nephropathy and granulomatous interstitial nephritis in sarcoidosis. Nephron 1994; 66: 459–461 [DOI] [PubMed] [Google Scholar]
- 38.More RH, McMillan GC, Duff GL. The pathology of sulfonamide allergy in man. Am J Pathol 1946; 22: 703–735 [PubMed] [Google Scholar]
- 39.Olsen S, Asklund M. Interstitial nephritis with acute renal failure following cardiac surgery and treatment with methicillin. Acta Med Scand 1976; 199: 305–310 [DOI] [PubMed] [Google Scholar]
- 40.Burton JR, Lichtenstein NS, Colvin RB, et al. Acute interstitial nephritis from oxacillin. Johns Hopkins Med J 1974; 134: 58–61 [PubMed] [Google Scholar]
- 41.Milman N. Acute interstitial nephritis during treatment with penicillin and cephalothin. Acta Med Scand 1978; 203: 227–230 [DOI] [PubMed] [Google Scholar]
- 42.Linton AL, Clark WF, Driedger AA, et al. Acute interstitial nephritis due to drugs: review of the literature with a report of nine cases. Ann Intern Med 1980; 93: 735–741 [DOI] [PubMed] [Google Scholar]
- 43.Ramalakshmi S, Bastacky S, Johnson JP. Levofloxacin-induced granulomatous interstitial nephritis. Am J Kidney Dis 2003; 41: E7. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz A, Krause PH, Keller F, et al. Granulomatous interstitial nephritis after nonsteroidal anti-inflammatory drugs. Am J Nephrol 1988; 8: 410–416 [DOI] [PubMed] [Google Scholar]
- 45.Case 42-1983—Progressive azotemia in an elderly hypertensive man. N Engl J Med 1983; 309: 970–978 [DOI] [PubMed] [Google Scholar]
- 46.Magil AB. Drug-induced acute interstitial nephritis with granulomas. Hum Pathol 1983; 14: 36–41 [DOI] [PubMed] [Google Scholar]
- 47.Magner P, Sweet J, Bear RA. Granulomatous interstitial nephritis associated with allopurinol therapy. CMAJ 1986; 135: 496–497 [PMC free article] [PubMed] [Google Scholar]
- 48.Hegarty J, Picton M, Agarwal G, et al. Carbamazepine-induced acute granulomatous interstitial nephritis. Clin Nephrol 2002; 57: 310–313 [DOI] [PubMed] [Google Scholar]
- 49.Chau K, Yong J, Ismail K, et al. Levetiracetam-induced severe acute granulomatous interstitial nephritis. Clin Kidney J 2012; 5: 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ram R, Swarnalatha G, Desai M, et al. Membranous nephropathy and granulomatous interstitial nephritis due to tuberculosis. Clin Nephrol 2011; 76: 487–491 [DOI] [PubMed] [Google Scholar]
- 51.Sampathkumar K, Sooraj YS, Mahaldar AR, et al. Granulomatous interstitial nephritis due to tuberculosis-a rare presentation. Saudi J Kidney Dis Transpl 2009; 20: 842–845 [PubMed] [Google Scholar]
- 52.Kaul A, Sharma RK, Krishnasamy J, et al. Rapidly progressive renal failure—a rare presentation of granulomatous interstitial nephritis due to tuberculosis—case report and review of literature. NDT Plus 2011; 4: 383–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallinson WJW, Fuller RW, Levison DA, et al. Diffuse interstitial renal tuberculosis—an unusual cause of renal failure. QJM 1981; 50: 137–148 [PubMed] [Google Scholar]
- 54.Simon HB, Weinstein AJ, Pasternak MS, et al. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med 1977; 63: 410–420 [DOI] [PubMed] [Google Scholar]
- 55.Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol 2001; 12: 1307–1314 [DOI] [PubMed] [Google Scholar]
- 56.Nasr SH, Koscica J, Markowitz GS, et al. Granulomatous interstitial nephritis. Am J Kidney Dis 2003; 41: 714–719 [DOI] [PubMed] [Google Scholar]
- 57.Li C, Su T, Chu R, et al. Tubulointerstitial nephritis with uveitis in Chinese adults. Clin J Am Soc Nephrol 2014; 9: 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki H, Yoshioka K, Miyano M, et al. Tubulointerstitial nephritis and uveitis (TINU) syndrome caused by the chinese herb "goreisan". Clin Exp Nephrol 2009; 13: 73–76 [DOI] [PubMed] [Google Scholar]
- 59.Koeppen-Hagemann I, Binkele-Uihlein U, Waldherr R, et al. Acute granulomatous interstitial nephritis with iritis. possible induction by non-steroidal antiphlogistics. Dtsch Med Wochenschr 1987; 112: 259–261 [DOI] [PubMed] [Google Scholar]
- 60.Iida H, Terada Y, Nishino A, et al. Acute interstitial nephritis with bone marrow granulomas and uveitis. Nephron 1985; 40: 108–110 [DOI] [PubMed] [Google Scholar]
- 61.Stupp R, Mihatsch MJ, Matter L, et al. Acute tubulo-interstitial nephritis with uveitis (TINU syndrome) in a patient with serologic evidence for chlamydia infection. Klin Wochenschr 1990; 68: 971–975 [DOI] [PubMed] [Google Scholar]
- 62.Grefer J, Santer R, Ankermann T, et al. Tubulointerstitial nephritis and uveitis in association with epstein-barr virus infection. Pediatr Nephrol 1999; 13: 336–339 [DOI] [PubMed] [Google Scholar]
- 63.Nzerue C, Schlanger L, Jena M, et al. Granulomatous interstitial nephritis and uveitis presenting as salt-losing nephropathy. Am J Nephrol 1997; 17: 462–465 [DOI] [PubMed] [Google Scholar]
- 64.Goncalves AR, Caetano MA, Paula FJ, et al. Tuberculous interstitial granulomatous nephritis in renal transplants: report of three cases. Transplant Proc 1992; 24: 1911. [PubMed] [Google Scholar]
- 65.al-Sulaiman MH, Dhar JM, al-Hasani MK, et al. Tuberculous interstitial nephritis after kidney transplantation. Transplantation 1990; 50: 162–164 [DOI] [PubMed] [Google Scholar]
- 66.Wilson CB. Study of the immunopathogenesis of tubulointerstitial nephritis using model systems. Kidney Int 1989; 35: 938–953 [DOI] [PubMed] [Google Scholar]
- 67.Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int 1989; 35: 1257–1270 [DOI] [PubMed] [Google Scholar]
- 68.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int 2010; 77: 956–961 [DOI] [PubMed] [Google Scholar]
- 69.Magil AB, Ballon HS, Cameron EC, et al. Acute interstitial nephritis associated with thiazide diuretics. Clinical and pathologic observations in three cases. Am J Med 1980; 69: 939–943 [DOI] [PubMed] [Google Scholar]
- 70.Thumfart J, Muller D, Rudolph B, et al. Isolated sarcoid granulomatous interstitial nephritis responding to infliximab therapy. Am J Kidney Dis 2005; 45: 411–414 [DOI] [PubMed] [Google Scholar]


