Abstract
Calcific uraemic arteriolopathy (CUA) is a rare disease and continues to be a clinical challenge. The typical course of CUA is characterized by painful skin discolouration and induration evolving to necrotic ulcerations. Medial calcification of cutaneous arterioles and extensive extracellular matrix remodelling are the hallmarks of CUA. The epidemiology and risk factors associated with this disease are still not fully understood. Moreover, CUA treatment strategies vary significantly among centres and expert recommendations are heterogeneous. Registries may provide important insights and information to increase our knowledge about epidemiology and clinical aspects of CUA and may help to optimize its therapeutic management. In 2006, we established an internet-based registry in Germany (www.calciphylaxie.de) to allow online notification of patients with established or suspected CUA. The registry includes a comprehensive database with questions covering >70 parameters and items regarding patient-related and laboratory data, clinical background and presentation as well as therapeutic strategies. The next phase will be to allow international patient registration via www.calciphylaxis.net as part of the multinational EuCalNet (European Calciphylaxis Network) initiative, which is supported by the ERA-EDTA scientific working group ‘CKD-MBD’. Based on the valuable experience with the previous German CUA registry, EuCalNet will be a useful tool to collect data on the rare disease CUA and may become a basis for prospective controlled trials in the near future.
Keywords: calcific uraemic arteriolopathy, calcification, chronic kidney disease, dialysis, vascular disease
Introduction
Calciphylaxis (calcific uraemic arteriolopathy, CUA) is a rare disease (Orphanet number ORPHA280062) at the interface of nephrology, dermatology and cardiovascular medicine. CUA typically occurs in chronic dialysis patients and hence nephrologists are most likely involved in primary diagnosis and patient care [1]. However, anecdotal reports also exist about cases in patients without relevant kidney disease (so-called non-uraemic calciphylaxis) [2]. Clinically, CUA is characterized by the stepwise development of superficial painful sensations and cutaneous lesions similar to livedo reticularis [3, 4]. Skin necrosis and ulceration represent the full-blown, ‘late’ clinical picture. Panniculitis and circumferential calcification of cutaneous arterioles dominate the histological picture together with endothelial detachment and luminal occlusion. The prognosis of CUA is extremely poor. CUA confers a high risk of morbidity and mortality, largely resulting from underlying cardiovascular disease, wound infection and septicaemia [3]. The aetiology of CUA is still not completely understood. In most cases, bone and mineral metabolism is altered and may play a significant role in the pathophysiology. Despite remarkable progress in recent years, evidence-based therapeutic options are unfortunately absent, since controlled randomized treatment trials have not yet been undertaken.
What do we know about calciphylaxis?
As with any rare disease, registry studies such as the German CUA registry (www.calciphylaxie.de) can effectively support central data collecting and analysis upon the entire spectrum of the disease from potential risk factors to good clinical practice. The German calciphylaxis registry has been actively recruiting patients since 2006. More than 230 patients have been registered in the registry up to the beginning of 2015. This impressive number of patients already provides a rough overview on what is generally considered ‘good clinical practice’ in the therapeutic management of these patients (Table 1). In addition, Table 1 summarizes the diversity of the therapeutic actions available. Obviously, superiority in terms of outcome improvement among these therapeutical steps cannot be established based on such purely observational, uncontrolled and sometimes anecdotal data.
Table 1.
Therapeutic management of CUA patients according to data extracting from the literature and the German calciphylaxis registry
| Modifying dialysis therapy |
| Increase frequency and duration |
| Modification of dialysis modality |
| Switch from peritoneal dialysis to haemodialysis/haemodiafiltration |
| Reduction of calcium supply and uptake |
| Switch to calcium-free phosphate binders |
| Reduction in calcium concentration in the dialysis bath |
| Using citrate as the buffer in the dialysis bath |
| Reduction in active vitamin D dosage |
| Replacing vitamin K antagonist treatment by heparin or any other oral anticoagulation if really needed |
| Giving vitamin K (unless contraindications are present for one of these measures) |
| Therapy against uncontrolled hyperparathyroidism |
| Give oral calcimimetic |
| Surgical parathyroidectomy |
| Optimize CKD-MBD therapy |
| Efforts to increase adherence to medical therapies |
| Strengthen anti-calcification properties of serum |
| Apply sodium thiosulfate |
| Apply bisphosphonates |
| Normalizing serum albumin concentration |
| Parental or intra-dialytic nutrition |
| Increase oxygen supply to tissue |
| Treat occluding peripheral arterial disease |
| Hyperbaric O2 therapy |
| Give statins |
| Supportive treatment |
| Wound care |
| Pain relief |
| Antibiotics |
| Amputation |
The efficacy and safety of particular items have not been systematically tested.
Uraemia and uraemia-associated conditions such as disturbances in bone and mineral metabolism and inflammation certainly play a key role as risk factors. We have previously analysed and reviewed the available literature regarding the clinical picture, risk factors and outcome in CUA patients [5, 6]. Previous treatment with vitamin K antagonists (VKA) for oral anticoagulation therapy has been considered as a prominent risk factor [5], which makes the community speculate about iatrogenic aspects as part of the pathophysiology cascade [7]. This deserves particular attention, since previous VKA might represent a potentially avoidable and modifiable component [5].
What do we not yet know? Why EuCalNet?
Despite the fact that substantial progress in our understanding about the clinical picture of the disease has been made, significant gaps in our knowledge still exist. Systematic personal transfer of CUA patients to specialized research and clinical expert facilities is virtually impossible facing the overwhelming disease burden of these patients. Moreover, registry initiatives may facilitate exchange of expertise and stimulate networking between scientists and clinicians. Accordingly, the consortium members from ‘EuCalNet’, an international consortium involving clinically active research partners, have carefully identified a range of burning questions, the answers of which are not understood or are still not fully understood (Table 2). In order to overcome these unmet issues, EuCalNet was founded, which is fully supported by the ERA-EDTA scientific working group ‘CKD-MBD’. Those issues (outlined in Table 2) represent major (clinical) research targets and can partly be answered after reaching a critical mass of patients with EuCalNet. So EuCalNet is intended to target unmet medical needs in terms of calciphylaxis.
Table 2.
Burning questions and unsolved issues regarding CUA
| Epidemiology |
| Changes in incidence over time? |
| Difference in prevalence/incidence in different countries? |
| Female predominance? |
| Incidence in different dialysis settings? |
| Confirmation of diagnosis |
| Early warning signs and diagnosis of abortive stages? |
| Procedures to diagnose and optimal diagnostic steps? |
| Systematic exclusion of differential diagnosis? |
| How to differentiate from ischaemic lesions? |
| Risk factors: iatrogenic factors in terms of ‘over-treatment’? |
| Therapy |
| Data about therapeutical strategies or most promising treatment strategies? |
| Outcome |
| Change in outcome over time? |
| Overall mortality? |
| Different outcome with central versus accrual CUA (predilection site in the body)? |
How is EuCalNet going to be undertaken?
We plan to initiate an internet-based multilingual registry in which treating physicians will provide anonymous patient data on:
demographics and comorbidities
the clinical picture including photo documentation and pain scale reporting
laboratory data of patients at the time of CUA diagnosis
concomitant medications
All the above-mentioned data can be repetitively entered, based on serial follow-up visits. Additionally, a biobank for storage of serum, plasma and full blood is an integral part of EuCalNet. A corresponding ethical committee voting will be part of the application process. The project partners include the RWTH Aachen University Hospital as sponsor of the project. Scientific collaborators will be acting on behalf of ERA-EDTA scientific working CKD-MBD (www.era-edta.org/ckdmbd/index.html), which defined calciphylaxis as one of the major future working group-specific research targets. The sponsor and the collaborators will coordinate EuCalNet activities in a total of seven European countries:
Belgium
France
Germany
Italy
Portugal
Spain
The Netherlands
What is the aim of EuCalNet?
The international registry will allow identification of more patients with calciphylaxis than on a national basis alone. Hence, EuCalNet can contribute to the following issues:
increase awareness of the disease via educational programmes associated with registry activities
help establish diagnostic algorithms
more detailed identification of risk factors
estimation of overall prognosis and prognosis in subgroups of patients as well as identification of biomarkers for estimation of prognosis
establishing a biobank for serum, plasma and tissue (skin) samples as well as autopsy findings
investigations regarding subgroups of patients
genotype/phenotype correlations
description of international state-of-the-art treatment
regular exchange between experts and treating physicians about treatment strategies and support treating physicians with feed back
Methodology of the registry and data management
Patients diagnosed with calciphylaxis irrespective of clinical conditions or comorbidities and time of diagnosis (all-comer registry), who are able to provide written and informed consent, can be included.
Systematic online data collection will be performed similar to the system already applied at www.calciphylaxie.de. The registry will be located at www.calciphylaxis.net. Written informed consent will be obtained from each patient in order to allow data and sample collection for storage in a central databank and biobank; additionally photo documentation as well as skin and other specimen will be stored when available. Local ethical approval and data safety certificates will be obtained separately in each participating country.
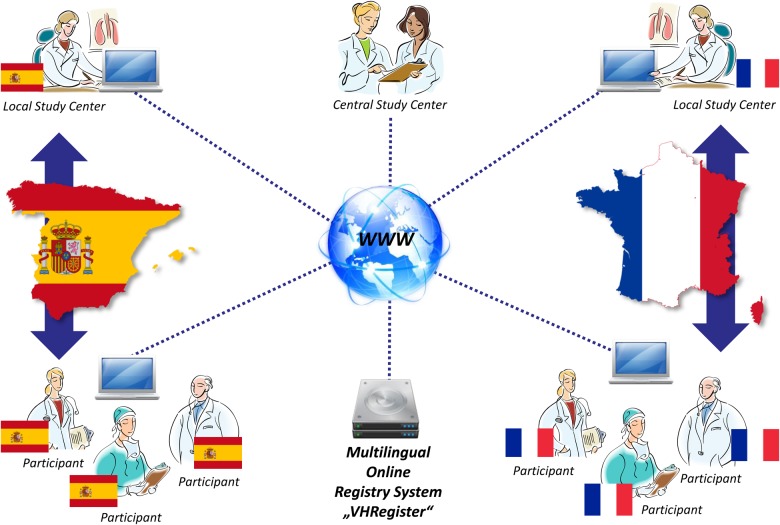
After online notification via www.calciphylaxis.net, a central processing of data will be undertaken on local country-specific levels (first level). Depending on country-specific conditions, the study centre University Hospital Aachen (second level) will assist first-level institutions regarding control of completeness and plausibility of data and also organize expert exchange regarding confirmation of diagnosis CUA. The primary contact between treating physician and peripheral centre will be established on a country level to the local principal investigator (PI). Second-level data management and final analysis is planned to be performed based on the central databank (see Figure 1). Details regarding data management and storage conditions within the biobank may vary according to local data safety restrictions and ethical committee voting. In terms of obtaining positive ethical committee voting, the EuCalNet partners will face divergent country-specific prerequisites regarding authorization of trial sites and physicians. For example, some countries will require such trial site authorization prior to notification of a patient to the registry.
Fig. 1.
Local study centres collect and administrate cases from their country. A central study centre reviews and consolidates all collected data.
Interfacing with the ERA-EDTA scientific working group, the coordinator (PI) of the project will be responsible for overall project management to ensure the correct and timely implementation and overall quality of organization steps and project maintenance. The PI will serve as the contact point for all partners regarding administrative and financial matters for the duration of the project. The PI will be responsible for the correct execution and continuity of the project, online registry data base set-up and maintenance, preparation of documents (e.g. registry protocol, patient information and informed consent and data collection manual) and preparation of documents for notification to ethic committees in addition to statistical (interim) analyses. The role of the investigators and the nature of the registry are non-interventional (i.e. observational). A systematic testing of medications, treatment strategies in general or randomization of patients is not part of the present protocol. In that respect, it is important to mention again that skin biopsies will not be performed on a systematic basis but only upon clinical indication at the discretion of the treating physician. Outcome (mortality) will be assessed by telephone interview with referring physician at 6 months after online notification. These interviews will be performed by local (country-specific) investigators.
Biobank set-up for patients with calciphylaxis
For each patient, the registry initiative aims to collect full blood, plasma, serum and tissue specimens (the latter where available via biopsy or surgical resection).
Tissue samples may allow immunohistochemistry and molecular analysis (e.g. polymerase chain reaction) with special focus on factors indicative for extracellular matrix remodelling and soft tissue calcification [8].
Definition of end points and objectives
The primary objective of the EuCalNet registry is to describe the prognosis of patients with calciphylaxis. Therefore, the primary end point is survival time defined as (i) time between diagnosis of calciphylaxis and death (or end of follow-up) or (ii) in cases where time of diagnosis is not recorded, time between registry recording and death (or end of follow-up). Secondary objectives of the EuCalNet initiative are summarized in Table 3.
Table 3.
Secondary objectives of EuCalNet
| Secondary objectives |
| Increase awareness and facilitate diagnosis of calciphylaxis |
| Description of state-of-the-art treatment strategies |
| The identification of subgroups of patients with potentially distinct patterns of risk factors, clinical picture and prognosis |
| Annual symposia and publications about calciphylaxis based on the EuCalNet registry work |
| Networking among experts |
Financial support for EuCalNet
The EuCalNet project is an integral part of the work package of the ERA-EDTA scientific working group CKD-MBD and this close collaboration has led to a strong overlap between EuCalNet consortium partners and working group board members. Financial support is provided by Amgen and Sanofi.
Potential health impact of a European registry initiative
The above-mentioned multicentre and multinational approach may be particularly valuable due to the following reasons:
The prognosis of patients with CUA is devastating and data collection within a transnational project helps establishing data upon good clinical practice that in the future may be the basis for randomized prospective trials.
A multinational approach may help elaborating geographical differences in terms of incidence, clinical picture, therapy and outcome.
Creation of a central large-scale biobank permits future analysis of novel potentially interesting pathophysiological pathways.
CUA may serve as a high-speed template for other forms of extra-osseous calcification in CKD-MBD. Therefore, data regarding the pathogenesis of CUA may add additional insights into (non-uraemic) calcifying arteriosclerosis and atherosclerosis.
The EuCalNet registry and international cooperations
The EuCalNet initiative will continue with previously established cooperations between the German calciphylaxis registry and international partners. Prospective calciphylaxis registries are recruiting patients also in the UK (UK Calciphylaxis Study) (http://www.gmann.co.uk/website/trials/iccn/home.cfm) and in Australia (Australian Calciphylaxis Registry) (http://www.calciphylaxis.org.au). Those cooperations have been particularly fruitful in the past in terms of expert exchange about, for example, IT solutions, marketing strategies and clinical data collection. Prerequisites in terms of, for example, successful ethical approval regarding data collection and handling in such registries vary substantially between countries and in this respect, EuCalNet initiation will continuously benefit from exchange of knowledge between currently recruiting CUA registries. Merging clinical expertise between these cooperating registry initiatives is a fruitful stimulus for future scientific publications [5].
Summary
Understanding the aetiology of CUA remains to be an important challenge. The initiation of a multinational registry EuCalNet is an important first step. Unfortunately, evidence-based therapeutic options are absent, since controlled treatment trials have not yet been undertaken. The undertaking of such a trial is currently being extensively discussed among experts.
Acknowledgements
The authors would like to thank Colin Gerard Egan, PhD, for his assistance in preparing the manuscript and Mrs Joanna Korbiel for ongoing assistance as project manager at the Clinical Trials Center, University Hospital RWTH Aachen (CTC-A). We also thank Prof. Jordi Bover, Barcelona, for the valuable support of the EuCalNet project. The project received productive support from all members of the ERA-EDTA scientific working group CKD-MBD (http://www.era-edtaworkinggroups.org/en-US/group/ckd-mbd#sthash.bWA5EWIt.dpbs). The EuCalNet team is particularly thankful to Smeeta Sinha (UK registry) and Grahame Elder (Australian registry) for their ongoing and stimulating input into the project.
Conflict of interest statement
The EuCalNet project is supported by grants from Amgen and Sanofi. All authors declare that there is no conflict of interest that influenced the content or the presentation of the present manuscript.
References
- 1.Hafner J, Keusch G, Wahl C, et al. Uremic small artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol 1995; 33: 954–962 [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3: 1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007; 56: 569–579 [DOI] [PubMed] [Google Scholar]
- 4.Franks AG., Jr Skin manifestations of internal disease. Med Clin North Am 2009; 93: 1265–1282 [DOI] [PubMed] [Google Scholar]
- 5.Brandenburg VM, Sinha S, Specht P, et al. Calcific uraemic arteriolopathy: a rare disease with a potentially high impact on chronic kidney disease-mineral and bone disorder. Pediatr Nephrol 2014; 29: 2289–2298 [DOI] [PubMed] [Google Scholar]
- 6.Brandenburg VM, Cozzolino M, Mazzaferro S. Calcific uremic arteriolopathy: a call for action. Semin Nephrol 2014; 34: 641–647 [DOI] [PubMed] [Google Scholar]
- 7.Brandenburg VM, Kramann R, Specht P, et al. Calciphylaxis in CKD and beyond. Nephrol Dial Transplant 2012; 27: 1314–1318 [DOI] [PubMed] [Google Scholar]
- 8.Kramann R, Brandenburg VM, Schurgers LJ, et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 2013; 28: 856–868 [DOI] [PubMed] [Google Scholar]