Abstract
Background
Completion lymph node dissection (CLND), although considered a standard approach for patients with melanoma and a positive sentinel lymph node (SLN), is not performed in as many as 50% of indicated cases. This study evaluates the outcome of patients who had a positive SLN but did not undergo CLND at Memorial Sloan-Kettering Cancer Center.
Methods
A prospective database was used to identify all patients with a positive SLN from 1992 to 2008. Patient and tumor characteristics, number of positive SLNs, recurrence pattern, reason for not performing a CLND, and current status were evaluated.
Results
There were 2269 patients who underwent SLN biopsy. Three hundred thirteen had a positive SLN, of whom 271 (87%) had a CLND and 42 (13%) did not. Patients in the no-CLND group were older (median age 70 vs. 56 years, P < .01), and had a trend toward thicker melanomas (3.5 vs. 2.8 mm, P < .06). A significantly higher percentage of no- CLND patients had lower-extremity melanomas (40% vs. 13% CLND; P < .01). The most common reason for not performing a CLND was patient refusal (45%). There were similar rates and patterns of recurrence between the two groups. Recurrence-free survival and disease-specific survival were also similar between the groups.
Conclusions
It remains unclear whether CLND must be performed in all melanoma patients with a positive SLN. For selected informed patients who choose not to participate in the Multicenter Selective Lymphadenectomy Trial II trial, or in centers where the trial is not available, nodal observation may be an acceptable option.
The 2008 National Comprehensive Cancer Network (NCCN) Melanoma Practice Guidelines recommend that patients with a positive sentinel lymph node biopsy (SLNB) undergo a completion lymph node dissection (CLND) or participate in a clinical trial “assessing alternatives to complete lymph node dissection, such as careful observation.”1 Despite these guidelines, a recent study demonstrated great disparity in the percentage of patients who undergo CLND.2 Bilimoria et al. used the National Cancer Data Base, which captures approximately 70% of newly diagnosed cancers annually, to determine the use of CLND.3 Surprisingly, only 50% of patients with a positive SLNB underwent CLND. Factors that were statistically significant independent predictors of patients who had positive SLNB but did not undergo a CLND were age >75 years, lower-extremity melanoma, or thin melanomas. These findings were consistent with those of Cormier et al., who used the Surveillance, Epidemiology, and End Results Program database to determine treatment trends from 1998 to 2001.4 They found that 69% of patients with a positive SLNB underwent CLND. Factors statistically significantly associated with no CLND in the study of Cormier et al. were age of >65 years compared to <35 years, single relationship status, geographic location, and African American race. Given these two large population-based studies, it is clear that even though NCCN guidelines have been in place for >5 years, national practice of CLND after positive SLNB is far from uniform.
Morbidity of CLND, lack of effective adjuvant therapy, and failure to show a survival benefit with CLND have all been described as reasons why surgeons have not performed CLND.5–7 Previously, in a multi-institutional retrospective study, Wong et al. found no statistically significant difference in nodal recurrence-free survival or disease-specific survival between sentinel lymph node (SLN)-positive patients with and without CLND.6 That study was limited by short median follow-up of 20 months. The present study looks at a group of SLNB-positive patients from a single institution with a median follow-up of 41 months and examines characteristics of patients who did not have a CLND versus those who did. To place the striking findings of Bilimoria et al. into clinical context, this study examined why these patients did not undergo CLND, their recurrence pattern, and their survival relative to those who had a CLND.
METHODS
Data were obtained from a prospectively maintained database at Memorial Sloan-Kettering Cancer Center (MSKCC); the database spans the years 1992 to 2008. The institutional review board committee at MSKCC approved the utilization and analysis of these data. Patients who underwent their SLNB at outside facilities but were referred to MSKCC for management were included in this database. SLNB and mapping techniques were performed in a standard fashion, with a combination of lymphoscintigraphy and blue dye localization. Patients with a positive SLNB were divided into two groups, one that underwent CLND after the positive SLNB, and one that did not undergo CLND. Patients in the no-CLND group who developed subsequent clinical nodal recurrence without systemic disease underwent salvage therapeutic lymph node dissection. These patients were included in the no-CLND group, and details of their recurrence were recorded in the database. If no tests were performed before patients come to MSKCC, for patients with melanomas <4 mm, a chest X-ray and basic laboratory work were performed before SLNB and wide excision. Selective use of computed tomographic and/or positron emission tomography scan was used for patients with melanomas of >4 mm thick. For patients with a positive SLNB, computed tomographic scan of the chest/abdomen/pelvis with contrast or positron emission tomography scan and magnetic resonance imaging of the brain were used for the extent-of-disease workup. Patients who do not undergo CLND underwent ultrasound surveillance of the relevant nodal basin every 3 to 6 months for the first 2 years after surgery.
A review of clinical notes revealed the reason for not undergoing a CLND. The rationale for this decision was categorized as: patient preference, patient/doctor preference, comorbidities, metastases found on further staging workup after the sentinel node biopsy, or unknown. The patient/doctor preference category included cases where observation was agreed on by both the physician and patient. The clinical follow-up schedule was consistent with the NCCN’s stage-specific guidelines. Recurrences were identified from clinical follow-up visits and available radiographic studies. They were categorized as nodal, regional, or systemic. Nodal recurrences were defined as recurrences in the draining nodal basin from the primary lesion, regional recurrences included local and in-transit lesions, and systemic disease included lesions in all other locations.
Histopathologic evaluation of the SLN was performed by standard pathological protocols maintained for melanoma at MSKCC. Initial sections were evaluated by hematoxylin and eosin staining. If these were negative, additional sections were studied with hematoxylin and eosin staining and immunohistochemical evaluation. Immunohistochemical stains included combinations of S-100, SMB-45, A-103, and tyrosinase.
For analysis of data, patients were categorized into CLND and no-CLND groups at the time of the SLNB. Clinicopathologic correlates were analyzed by the χ2 test or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables. A P value of <.05 was considered statistically significant.
Disease-specific survival was defined as the time from the date of SLNB to date of death due to disease or last follow-up; and relapse-free survival was defined as the time from the date of SLNB to the date of relapse, death, or date of last follow-up. Survival distributions were estimated by Kaplan-Meier methodology and comparisons made by the log rank test. A Cox proportional hazard regression was used for an adjusted analysis. All analyses were performed by SAS software (SAS Institute, Cary, NC).
RESULTS
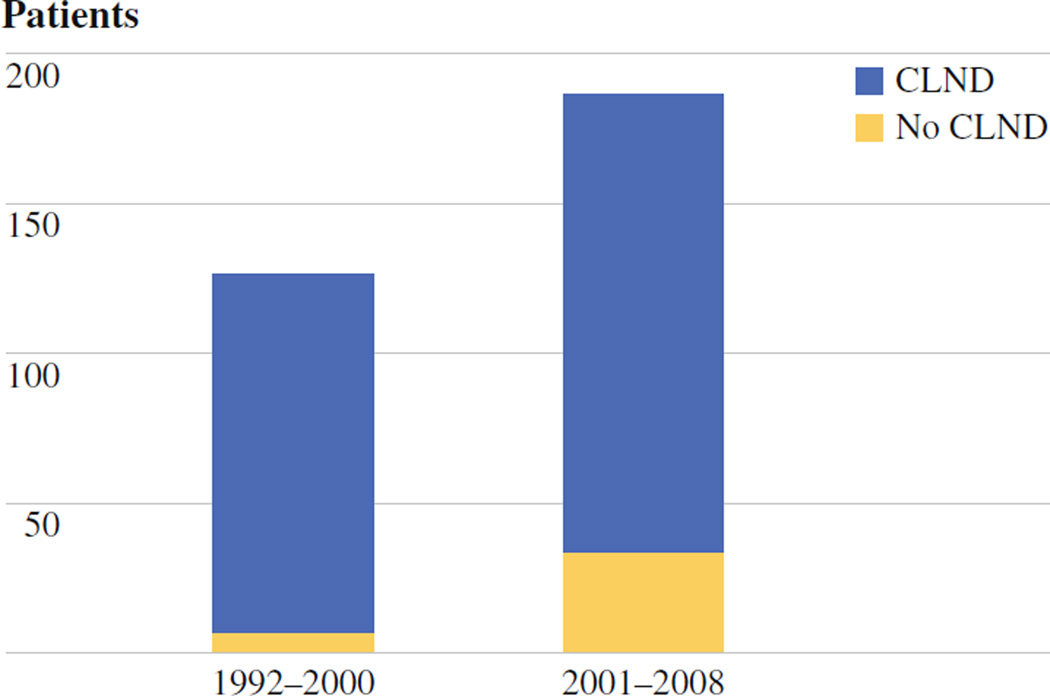
The prospective melanoma database maintained at MSKCC identified 313 patients with positive SLNs from 1992 to 2008, from 2269 patients who underwent SLNB (14%). Two hundred seventy-one patients underwent CLND, while 42 patients (13%) did not. There were similar rates of patients with no CLND between the two principal contributors to the MSKCC database (D.G.C., M.S.B.). Clinical and pathologic data describing patients in the no-CLND and CLND groups are listed in Table 1. Patients who did not undergo CLND had a significantly higher median age compared to those patients who did undergo CLND (70 vs. 56 years, P < .01). Although there was a higher percentage of men in the CLND group (63%) compared to the no-CLND group (55%), this difference was not significant. There was a trend toward thicker melanomas in the group that did not undergo CLND, with median thickness of 3.5 mm compared to 2.8 mm (P = .06). There was a statistically significant difference (P < .01) between the locations of melanomas in patients who did not undergo CLND: 40% of no-CLND patients had lower extremity (vs. 13% CLND), 26% with trunk (vs. 45% CLND), 17% head and neck (vs. 8% CLND), and 12% upper extremity (vs. 32% CLND) melanomas. When the patients were split chronologically into two groups, one from 1992 to 2000 and one from 2001 to 2008, there was a statistically significant increase in the percentage of patients who did not undergo CLND in the more recent period compared to the earlier era (Fig. 1). The use of adjuvant therapy was comparable between the two groups because all patients with stage III disease are offered similar access to several different adjuvant trials.
TABLE 1.
Patient demographics and location of primary tumors
| Characteristic | No CLND n = 42 (13%) |
CLND n = 271 |
P value |
|---|---|---|---|
| Age (year), median (range) | 70 (20–95) | 56 (7–89) | <.01 |
| Sex, n (%) | |||
| Male | 23 (55%) | 170 (63%) | .4 |
| Female | 19 (45%) | 101 (37%) | |
| Tumor thickness (mm), median (range) | 3.5 (1.1–18) | 2.8 (.7–38) | .06 |
| Location, n (%) | |||
| Lower extremity | 17 (40%) | 32 (13%) | <.01 |
| Trunk | 11 (26%) | 122 (45%) | |
| Head/neck | 7 (17%) | 22 (8%) | |
| Upper extremity | 5 (12%) | 86 (32%) | |
| Unknown/other | 2 (5%) | 5 (2%) | |
| Clark level (n) | |||
| II | 0 | 0 | .5 |
| III | 1 | 13 | |
| IV | 27 | 194 | |
| V | 8 | 38 | |
| Unknown | 8 | 14 | |
| Ulceration | |||
| Present, n (%) | 26 (62%) | 119 (44%) | .09 |
| Absent (n) | 11 | 112 | |
| Unknown (n) | 5 | 40 |
CLND completion lymph node dissection
FIG. 1.
Six percent of patients in the first half of this series (1992–2000) did not undergo completion lymph node dissection, compared to 18% in the recent era (2001–2008)
Although there was a statistically significant difference between the location of melanomas in the CLND and no-CLND groups, there was no significant difference between the locations of the draining nodal basins between the two groups (Table 2). For example, the percentage of patients in each group that mapped to the axilla was similar, with 48% of patients in the no-CLND group compared to 41% of the CLND group. Most patients had a single positive SLN (76%). The number of positive sentinel nodes was not a significant factor in predicting whether CLND was performed: 86% of patients in the no-CLND group had a single positive SLNB, compared to 74% of those patients in the CLND group (P = .12). Of the patients who underwent CLND, 84% had negative nonsentinel nodes. In patients with positive nonsentinel nodes, the median number of positive nonsentinel nodes was 1 (range, 1–8). The number of nodes collected with the sentinel node biopsy was not a predictor of CLND, as the median number of sentinel nodes was 2 (range, 1–14) in the CLND group and 2 (range, 1–9) in the no-CLND group.
TABLE 2.
Comparison of draining lymph node basins between the no-CLND and CLND groups and characteristics of positive lymph nodes
| Characteristic | No CLND (n = 42) |
CLND (n = 271) |
P value |
|---|---|---|---|
| Nodal basin | |||
| Axilla | 15 (36%) | 127 (47%) | .36 |
| Groin | 20 (48%) | 112 (41%) | |
| Neck | 7 (17%) | 32 (12%) | |
| Median number of nodes examined with SLNB (range) | 2 (1–9) | 2 (1–14) | |
| Patients with single positive SLN | 36 (86%) | 201 (74%) | |
| Patients with positive non-SLN | 43 (16%) | ||
| If non-SLN positive, median number of additional positive nodes (range) | 1 (1–8) |
CLND completion lymph node dissection, SLNB sentinel lymph node biopsy, SLN sentinel lymph node
The most common reason for not undergoing CLND was patient refusal (45%, Table 3). When patients refused to undergo CLND, it was difficult to determine their exact reason for making this decision, given the retrospective nature of this review. A more detailed look, however, at the 14 patients (33%) who did not undergo CLND as a result of physician/patient preference reveals the complexity of this decision. Five of the patients had head and neck melanomas, and surgeons cited the low probability of positive non-SLN as the reason for no CLND. Three patients had thick melanomas (7, 17, and 10 mm), and the concern about a high risk of systemic recurrence outweighed the need for local nodal treatment. In the other six patients who did not undergo CLND as a result of surgeon preference, the reason for no dissection included necessity to treat synchronous lymphoma (n = 1), thin melanomas with micrometastases in the lymph node (n = 4), and satellitosis of the primary tumor (n = 1). The least common reason for patients to forego CLND was that they were not cleared for surgery because of comorbid conditions (n = 2). Five patients (12%) did not undergo CLND because metastatic disease was found on further workup after their sentinel node biopsy procedure was performed. These patients were removed from subsequent survival analyses because these stage IV patients do not represent the natural history of nodal observation.
TABLE 3.
Reason why patients did not undergo CLND was determined by examining the clinic record
| Reason no CLND | n | % |
|---|---|---|
| Patient refusal | 19 | 45 |
| Patient/doctor decision | 14 | 33 |
| Metastases found | 5 | 12 |
| Unknown | 2 | 5 |
| Comorbidities | 2 | 5 |
CLND completion lymph node dissection
As expected, with nodal observation more commonly practiced in the recent era, median follow-up for the no-CLND group was 32 months, compared to 43 months for the CLND group (Table 4). There was no difference in the pattern of first recurrence between patients who had CLND and those who did not. Fifty-four percent (n = 146) of patients who underwent CLND experienced recurrence, which was similar to the 48% (n = 20) of patients whose disease recurred in the group that did not undergo CLND. Although the follow-up time between the two groups is 11 months different, in patients whose disease recurred, the median interval to recurrence was similar (14 vs. 13 months, respectively). There was no significant difference in the location of first recurrences between the two groups. Examination of the type of recurrence revealed that 5% of patients in the no-CLND group (n = 2) and 6% of patients in the CLND group (n = 15; P = NS) had first recurrences isolated to regional lymph nodes alone. Nodal recurrences as a component of first recurrences were found in 7% of the CLND group (n = 3) and 6% of the no-CLND group (n = 17). The three patients in the no-CLND group that had nodal recurrence alone or as a component of their initial recurrence pattern all underwent CLND at a median of 16 months after the SLNB. The two patients with recurrence only in their nodes had no evidence of disease at 62 and 63 months of follow-up, while the third patient with recurrence in nodes and lungs experienced recurrence at 16 months and died of systemic disease with pulmonary metastases at 53 months after SLNB. Systemic recurrences were found in 29% of patients in the CLND group (n = 78) and in 26% of the no-CLND group (n = 11).
TABLE 4.
Pattern of first recurrencea
| Characteristic | No CLND (n = 42) | CLND (n = 271) |
|---|---|---|
| Median follow-up (montd) | 32 | 43 |
| Recurrence | 20 (48%) | 146 (54%) |
| Median time to recurrence in patients with recurrent disease (month) (range) | 14 (3–35) | 13 (2–125) |
| Local/in-transit disease only | 7 (17%) | 48 (18%) |
| Nodal disease only | 2 (5%) | 15 (6%) |
| Systemic disease only | 9 (21%) | 73 (27%) |
| Local/in-transit disease as component | 7 (17%) | 45 (17%) |
| Nodal disease as component | 3 (7%) | 17 (6%) |
| Systemic disease as component | 11 (26%) | 78 (29%) |
CLND completion lymph node dissection
The percentage is of total recurrences in each respective group
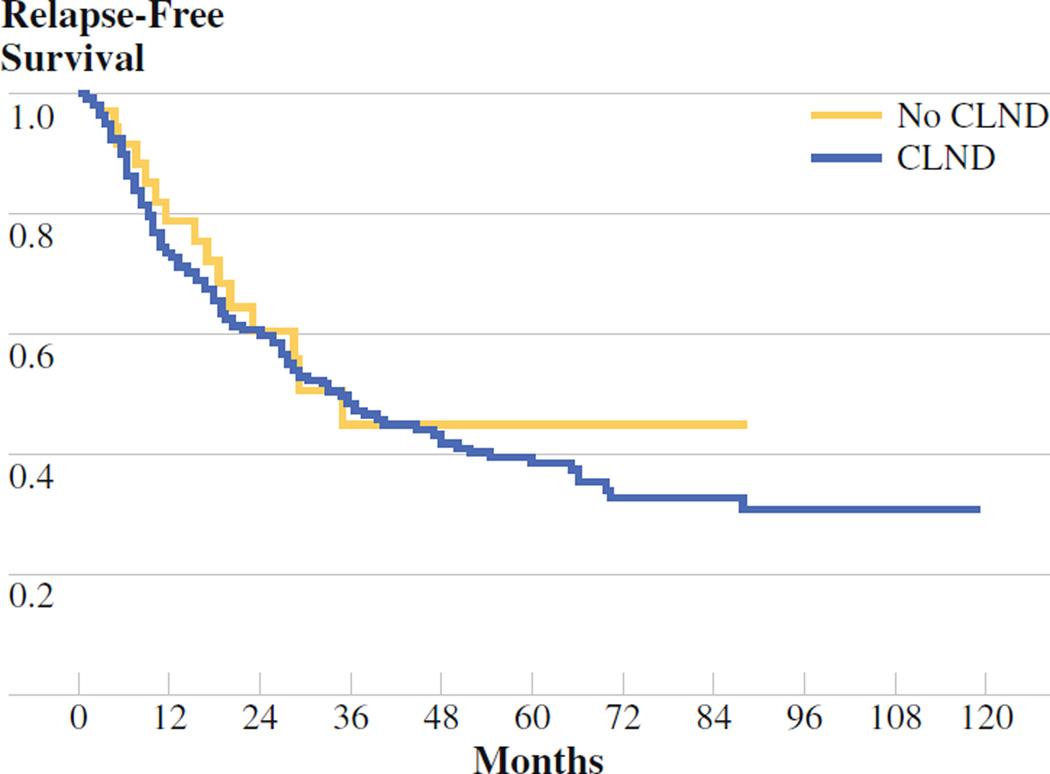
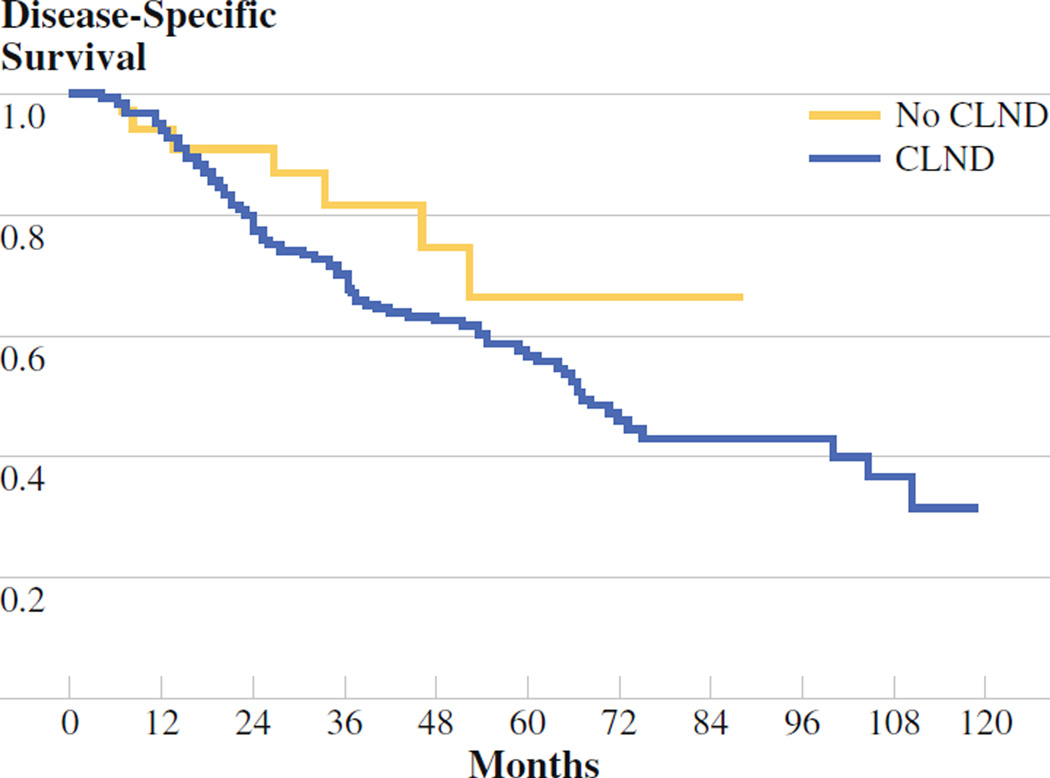
The five patients who did not undergo CLND as a result of the discovery of metastatic disease after the SLNB procedure was completed were removed from the no-CLND group for survival analyses of all patients with stage III disease, leaving a total of 37 patients in the no-CLND group. The median relapse-free survival was 35 months for the no- CLND group and 36 months for the CLND group (P = .63; Fig. 2). Similarly, there was no difference in disease-specific survival between the CLND and no-CLND group (73 months vs. median not reached, respectively, P = .26; Fig. 3). Given the low number of patients who had nodal recurrences, it was not possible to examine differences in nodal recurrence-free survival between the two groups.
FIG. 2.
Recurrence-free survival (RFS) no-completion lymph node dissection (CLND) (n = 37) vs. CLND (n = 271). Median RFS was 35 months for the no-CLND group and 36 months for the CLND group (P = .63)
FIG. 3.
Disease-specific survival no-completion lymph node dissection (CLND) (n = 37) vs. CLND (n = 271). Median disease-specific survival was 73 months for CLND; median was not reached for no CLND (P = .26)
DISCUSSION
The Multicenter Selective Lymphadenectomy Trial (MSLT) I trial confirmed the importance of the prognostic significance of SLNB, as there was a statistically significant difference in 5-year melanoma-specific survival with a positive sentinel node (72%) compared to a negative sentinel node (90%) in the subgroup of patients who underwent SLNB.8 What remains unproven, however, in the group of patients with positive SLNB, is whether subsequent CLND confers a therapeutic benefit for all, or even a subset, of patients with intermediate-thickness melanoma. MSLT I showed no improvement in melanoma-specific survival when all patients were evaluated together, although there was an increase in recurrence-free survival, a secondary end point, in the intermediate-thickness subgroup. The difference was attributed primarily to the increased rate of nodal relapse in the nodal observation group. Rosenberg, in an editorial, concluded that the MSLT I trial showed no difference in survival between the main study groups (not the subgroups) who underwent SLNB and CLND or observation, so SLNB provides prognostic information but confers no survival benefit.7 MSLT I did not, however, address whether there is any therapeutic advantage to CLND, given that because 16% of patients had a positive SLNB in the MSLT I study and approximately 16% of patients with positive SLNB have positive nonsentinel nodes, only 2% of the study population fall into the category of patients who are required for study to determine whether there is a survival benefit to CLND. MSLT II was designed to answer this question by randomizing patients with positive SLNs to CLND or observation with ultrasound surveillance.
Although considered the standard of care by the NCCN, data from the study of Bilimoria et al. revealed that 50% of patients nationwide with positive SLNB do not undergo CLND.2 Despite the NCCN guideline advocating CLND or enrollment onto a clinical trial with an observation arm for patients with a positive SLN, the national controversy on whether all patients with positive SLNB require CLND is evident in the disparity reported by Bilimoria et al.
Arguments for CLND include the need to ascertain whether additional positive non-SLNs are present and to maintain nodal basin control. The overall incidence of positive nonsentinel nodes varies between 15% and 17% in the literature. Predictors of positive nonsentinel nodes include sentinel node tumor burden, primary tumor thickness, and the number of sentinel nodes collected.9 It is possible that the nonsentinel node positivity rate is even higher than normally reported, as Wrightson et al. reported 15% of histologically negative nonsentinel nodes were positive when evaluated by reverse transcriptase–polymerase chain reaction.10 The therapeutic benefit of CLND to identify and remove nodes that may be positive implies that nodal recurrences are a source of subsequent systemic disease. In addition, there is the concern of nodal basin failure leading to uncontrolled disease in the nodal basin, which can act as a nidus for subsequent distant metastases. The reported nodal failure rate after nodal dissection in the literature is 9% to 15%.11–13 Wong et al. reported nodal relapse as first site of recurrence in 14% of patients in a cohort that underwent CLND and in 29% of patients who did not undergo CLND. Despite these differences, survival was similar between the two groups. In addition, it is unlikely for nodal disease to present in a manner that is unmanageable if close surveillance is implemented.
The present study showed no difference in nodal recurrence rates between the no-CLND and the CLND groups, which hints at the aggressive biology of nodal failure, regardless of nodal dissection. The variability of nodal-only recurrence is demonstrated by two of the three patients in the no-CLND group, who exhibited long disease-free survival after being rendered disease-free with therapeutic lymph node dissection. Finally, only 5% of patients experienced recurrent disease in the nodal basin, not the expected 15% to 20% that is anticipated from the rate of positive non-SLN in MSLT I. This is likely because this is a group at very high risk for systemic recurrence, unlike the MSLT I patients, where most patients did not have positive SLN.
There are several arguments against performing CLND after a positive SLNB. The first is the morbidity of the procedure, especially with inguinal lymphadenectomy. The second is that CLND may be prognostic, not therapeutic. The Sunbelt Melanoma Trial examined 2120 patients who underwent SLNB.5 They reported a rate of 4.6% major or minor complications with SLNB compared to 23.2% major or minor complication rate associated with SLNB and CLND. Complications were highest in the groin and included lymphedema, hematoma, and infection. Another study looked at results from 100 patients who underwent CLND and reported 47.6% morbidity rates in the groin and 46.8% in the axilla.14 De Vries et al. compared inguinal sentinel node biopsy to inguinal CLND and reported 50% of patients with CLND had complications, compared to 6% of patients who underwent SLNB (P < .001).15 Arguments that CLND is only a prognostic procedure rely on data that patients with positive SLNs are at increased risk for systemic failure, limiting the value of additional nodal dissections.16 In one retrospective review of patients who underwent CLND, positive non-SLN were found to be the most important predictor of survival.17 The poor prognosis of these patients, after CLND, raises the question of the therapeutic benefit of the nodal dissection. Most recurrences in this study were systemic, demonstrating that in patients with a positive SLNB, distant relapse is the most important factor affecting survival. This is consistent with the study of Chao et al. that examined 233 patients who had a positive SLNB.18 Sixty-seven percent of patients with a positive SLNB had distant metastases compared to 46% of patients with a negative SLNB (P < .05). Similarly, Pidhorecky et al. also reported that 54% of patients after elective lymph node dissections had systemic recurrences without evidence of nodal disease.13
This current study is a single-institution study with longer follow-up than the previous multi-institutional study that looked at a similar topic. This study reports on the natural history of a large number of patients who did not undergo CLND. A limitation of this study is that it was not possible to quantify the burden of tumor in the SLN of enough patients in each group to determine whether any differences existed between the groups. It is possible that a difference in tumor burden could affect recurrence rates in the two groups. As a retrospective review of a prospectively maintained database, there is also limited information about why patients chose to not have a CLND. In the group that did not undergo CLND, it is difficult to determine why 45% of patients chose this course despite physician recommendation. In the 33% of the no-CLND cases where physicians and patients agreed to follow a course of observation, there were several factors, including morbidity, perception of limited utility of CLND in head and neck melanoma, and use in thick or thin melanomas.
Although there is shorter follow-up in the no-CLND group, studies have shown the median time to recurrence in this high-risk cohort of patients with positive SLNs is 13 months, so most recurrences would have been identified in this study.19 Despite the limitations of this study, there are several intriguing findings, such as the similar recurrence pattern and survival data between the two groups. This adds to the body of literature raising the question of what the role of CLND is in patients with melanoma. The data described in this study do not support routine CLND in all patients with positive SLN. Additional follow-up may change the recurrence patterns and survival data, but there seems to be at least a subgroup of patients with positive SLN who do not require CLND. Selection criteria for this subgroup are not firmly established. For example, should it be patients with thin melanomas with micrometastases, at low risk for regional failure, or should it be patients with thick melanomas and macrometastases, at high risk for systemic failure? With appropriate prerandomization stratification, MSLT II should shed light on these questions. Until those results are available, nodal observation may be a reasonable alternative to the informed patient who does not want to participate in MSLT II or who does not have access to participation in MSLT II.
REFERENCES
- 1.NCCN Practice Guidelines in Oncology: Melanoma. 2009;v.2. 2009 www.nccn.org/index.asp. [Google Scholar]
- 2.Bilimoria KY, Balch CM, Bentrem DJ, et al. Complete lymph node dissection for sentinel node–positive melanoma: assessment of practice patterns in the United States. Ann Surg Oncol. 2008;15:1566–1576. doi: 10.1245/s10434-008-9885-2. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol. 2005;23:6054–6062. doi: 10.1200/JCO.2005.21.360. [DOI] [PubMed] [Google Scholar]
- 5.Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13:809–816. doi: 10.1245/ASO.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA. Why perform sentinel-lymph-node biopsy in patients with melanoma? (editorial) Nat Clin Pract Oncol. 2008;5:1. doi: 10.1038/ncponc1022. [DOI] [PubMed] [Google Scholar]
- 8.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 9.Gershenwald JE, Andtbacka RH, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26:4296–4303. doi: 10.1200/JCO.2007.15.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrightson WR, Wong SL, Edwards MJ, et al. Reverse transcriptase- polymerase chain reaction (RT-PCR) analysis of nonsentinel nodes following completion lymphadenectomy for melanoma. J Surg Res. 2001;98:47–51. doi: 10.1006/jsre.2001.6160. [DOI] [PubMed] [Google Scholar]
- 11.Gershenwald JE, Berman RS, Porter G, Mansfield PF, Lee JE, Ross MI. Regional nodal basin control is not compromised by previous sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol. 2000;7:226–231. doi: 10.1007/BF02523658. [DOI] [PubMed] [Google Scholar]
- 12.Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989;124:1051–1055. doi: 10.1001/archsurg.1989.01410090061014. [DOI] [PubMed] [Google Scholar]
- 13.Pidhorecky I, Lee RJ, Proulx G, et al. Risk factors for nodal recurrence after lymphadenectomy for melanoma. Ann Surg Oncol. 2001;8:109–115. doi: 10.1007/s10434-001-0109-2. [DOI] [PubMed] [Google Scholar]
- 14.Guggenheim MM, Hug U, Jung FJ, et al. Morbidity and recurrence after completion lymph node dissection following sentinel lymph node biopsy in cutaneous malignant melanoma. Ann Surg. 2008;247:687–693. doi: 10.1097/SLA.0b013e318161312a. [DOI] [PubMed] [Google Scholar]
- 15.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32:785–789. doi: 10.1016/j.ejso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Cady B. Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann Surg Oncol. 2007;14:1790–1800. doi: 10.1245/s10434-006-9234-2. [DOI] [PubMed] [Google Scholar]
- 17.Ariyan C, Brady MS, Gonen M, Busam K, Coit D. Positive nonsentinel node status predicts mortality in patients with cutaneous melanoma. Ann Surg Oncol. 2009;16:186–190. doi: 10.1245/s10434-008-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao C, Wong SL, Ross MI, et al. Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg. 2002;184:520–524. doi: 10.1016/s0002-9610(02)01102-9. [DOI] [PubMed] [Google Scholar]
- 19.Moore Dalal K, Zhou Q, Panageas KS, Brady MS, Jaques DP, Coit DG. Methods of detection of first recurrence in patients with stage I/II primary cutaneous melanoma after sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:2206–2214. doi: 10.1245/s10434-008-9985-z. [DOI] [PubMed] [Google Scholar]