Abstract
Neuregulin-1 (NRG1) ligand and its epidermal growth factor receptor (EGFR)/ERBB family regulate normal cellular proliferation and differentiation in many tissues including the cochlea. Aberrant NRG1 and ERBB signaling cause significant hearing impairment in mice. Dysregulation of the same signaling pathway in humans is involved in certain types of cancers such as breast cancer or non-small cell lung cancer (NSCLC). A new irreversible pan-ERBB inhibitor, canertinib, has been tested in clinical trials for the treatment of refractory NSCLC. Its possible ototoxicity was unknown. In this study, a significant dose-dependent canertinib ototoxicity was observed in a zebrafish model. Canertinib ototoxicity was further confirmed in two mouse models with different genetic backgrounds. The data strongly suggested an evolutionally preserved ERBB molecular mechanism underlying canertinib ototoxicity. Thus, these results imply that clinical monitoring of hearing loss should be considered for clinical testing of canertinib or other pan-ERBB inhibitors.
Keywords: Ototoxicity, outer hair cell, non-small cell lung cancer, ERBB, NRG1, canertinib
1. Introduction
Lung cancer is the number one cause of cancer mortality globally with few screening and treatment options available (Jemal et al., 2011). Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancer cases (Jemal et al., 2010). Advanced NSCLCs (Stage IIIb and IV) are treated with cisplatin or combination therapies; however, the five-year survival rate is under 5% (de Castria et al., 2013). Cisplatin, as first-line treatment of many solid tumors, has been extensively studied for its ototoxicity (Lobarinas et al., 2013, Harrison et al., 2014), however, its effectiveness against NSCLC is often subverted (Lipp and Hartmann, 2005, Yorgason et al., 2006, Schacht et al., 2012). Recent research into novel anti-NSCLC drugs has focused on inhibition of the epidermal growth factor receptors (EGFRs/ERBB), which belong to the receptor family of tyrosine kinases (RTKs).
RTK inhibitors, which block ERBB receptors, present novel and exciting therapies for NSCLC (Cowan-Jacob, 2006, Pytel et al., 2009, Awada et al., 2012, Ou, 2012, de Castria et al., 2013, Majem and Pallares, 2013). Most ERBB family members have three domains: an extracellular N-terminus responsible for ligand binding, a transmembrane hydrophobic region, and a conserved intracellular domain with a ligand-activated tyrosine kinase on the C-terminal end (Allen et al., 2003). The ERBB family of receptors includes ERBB1 (EGFR), ERBB2 (Her-2/neu), ERBB3 (Her-3), and ERBB4 (Her-4) (Janne et al., 2007). Aberrant signaling of ERBB family members (Hynes and Stern, 1994, Leung et al., 1997) plays an important role in many cancers including breast (Sainsbury et al., 1985), ovarian (Meden and Kuhn, 1997), prostate (Schwartz et al., 1999), gastric (Hirono et al., 1995), laryngeal (Almadori et al., 1999), and indicate a more aggressive cancer phenotype (Klijn et al., 1994, Zinner et al., 2007). ERBB1 is overexpressed in the majority of NSCLC and is found frequently in lung squamous cell carcinoma (57–100%) whereas ERBB2 is found in lung adenocarcinomas (33%).
Gefitinib and erlotinib are first generation EGFR RTK inhibitors that result in 10–20% lung tumor suppression but do not inhibit other ERBB family members (Wakeling et al., 2002, Barbacci et al., 2003). After treatment with first generation RTK inhibitors, 50% of patients have a secondary missense T790 mutation resulting in acquired resistance due to steric hindrance with a bulkier methionine (Pao et al., 2005, Shepherd et al., 2005, Engelman and Janne, 2008, Sequist et al., 2011, Majem and Pallares, 2013). Although no preclinical studies of their ototoxicity, one clinical case was reported with erlotinib ototoxicity (Koutras et al., 2008). The success of this first generation inhibitors coupled with the known acquired resistance has resulted in a need for new RTK inhibitor therapies with more sustained effects than gefitinib and erlotinib (Han et al., 2008, Butts et al., 2010, Mukherjea et al., 2011, Allen et al., 2012, Yang et al., 2013, Agustoni et al., 2014). Second and third generation ERBB inhibitors are irreversible, have higher binding affinities, inhibit multiple ERBB family members, and have better activity against T790 mutation. Therefore, these new inhibitors, such as canertinib, may provide additional clinical benefits by functioning as a pan-ERBB inhibitor (Fry, 2000, Slichenmyer et al., 2001, Allen et al., 2003, Janne et al., 2007). Although adverse effects such as diarrhea, asthenia, stomatitis, and persistent rash were observed in canertinib clinical trials (Janne et al., 2007), no clinical studies of ototoxicity for these new inhibitors are reported so far.
The NRG1-ERBB signaling pathway plays a significant role in the development and mature function of the inner ear. In the cochlea, NRG1 is expressed in spiral ganglion neurons (SGNs) (Morley et al., 1998, Bao et al., 2003, Bao et al., 2004); whereas hair cells, Schwann cells, and supporting cells express ERBB (Morley, 1998, Hansen et al., 2001, Zhang et al., 2002, Hume et al., 2003). Cochlear innervation is abnormal in NRG1 and ERBB2 null mice and these adult mice have progressive hearing loss (Lee et al., 1995, Meyer and Birchmeier, 1995, Fritzsch et al., 1997, Morley, 1998, Adlkofer and Lai, 2000, Chen et al., 2003, Fritzsch et al., 2004, Hellard et al., 2004, Stankovic et al., 2004, Morris et al., 2006). One chemical compound blocking ERBB signaling could lead to progressive hearing loss in mature female pigmented guinea pigs (Watanabe et al., 2010). Since canertinib can block ERBB receptors, we first assessed possible hair cell toxicity of canertinib in a zebrafish model, and then in two mouse models (C57BL/6J and CBA/CaJ) by auditory brainstem recording (ABR) thresholds, distortion product otoacoustic emissions (DPOAE), and hair cell counts. Canertinib resulted in significant ototoxicity in all three preclinical animal models.
2. Materials and Methods
2.1 Zebrafish Studies
Zebrafish (Danio rerio) embryos were produced by paired matings of adult fish maintained at the University of Washington zebrafish facility. We used AB wild-type zebrafish strains. Embryos were maintained in fish embryo media (EM; 1 mM MgSO4, 120 μM KH2PO4, 74 μM Na2HPO4, 1 mM CaCl2, 500 μM KCl, 15 μM NaCl, and 500 μM NaHCO3 in dH2O) at a density of 50 animals per 100 mm2 Petri dish and kept in an incubator at 28.5°C. At 4 days post-fertilization ( dpf), larvae were fed live paramecia. All zebrafish procedures described were approved by the University of Washington Animal Care and Use Committee.
Free-swimming 5 dpf zebrafish larvae were transferred into a 48 well plate at a density of 10–12 fish per well using a wide-bore glass pipette. Fish were then treated with variable doses of canertinib (0–500 μM) for one hour. After canertinib exposure, larvae were anesthetized with MS-222 (3-aminobenzoic acid ethyl ester, methanesulfonate salt; Sigma-Aldrich) and then fixed for 1 hr in 4% paraformaldehyde (PFA) at room temperature. After fixation in PFA, larvae were rinsed in PBS and then incubated in blocking solution [1% Triton-X, 5% normal goat serum (NGS) in PBS] for 1–2 hours at room temperature. Larvae were then incubated overnight at 4°C in anti-parvalbumin primary antibody (monoclonal, 1:400 in 1% Triton-X, 1% NGS in PBS; Millipore) to label hair cells. After primary antibody labeling, larvae were rinsed in 1% Triton-X in PBS and then incubated for 2–4 hours at room temperature in Alexa 488 goat anti-mouse fluorescent antibody solution (1:500 in 1% Triton-X, 1% NGS in PBS; Invitrogen) secondary antibody. The larvae were then rinsed and mounted between two coverslips in Fluoromount-G (Southern Biotech) for imaging. A Zeiss Axioplan II microscope using a FITC filter set at a final magnification of 200X was used to count hair cells from the SO1, SO2, O1, and OC1 neuromasts (Raible and Kruse, 2000). Results are presented as the mean hair cell survival as a percentage of the control group treated. Error bars in figures indicate ±1 standard error (SE).
2.2 Mouse Studies
All experiments in mice were approved by the Animal Studies Committee at Washington University in St. Louis. The dosages used in the clinical studies varied based on which group the individuals were in: 1) 50 mg per day for 21 consecutive days; 2) 150 mg per day for 21 consecutive days; 3) 450 mg per day for 14 days followed by 7 days off. Dosages were subsequently titrated on an individual basis based on the National Cancer Institute Common Toxicity Criteria version 2 with the most common side effects being rash and diarrhea. However, no serum studies in humans were performed so a direct comparison between human effect and mouse effect was not possible. Furthermore, drug pharmacokinetics is vastly difference between humans and rodents, with a much faster rate in rodents for most of drugs. Therefore, our testing dosage in mice with ototoxicity could be much lower if compared to the clinical dosages in humans. This study started with a total of 40 C57BL/6J and 24 CBA/CaJ mice for preliminary dosage studies, which showed lethality with 120 mg/kg/day and higher dosages, but showed safe at 30 and 60 mg/kg/day with no loss of body weight after a 14-day treatment. We thus selected 30 mg/kg/day for this study. The data presented here were from 15 C57BL/6J and 13 CBA/CaJ mice, at two months old, purchased from the Jackson Laboratory (Bar Harbor, ME, USA).
2.3 Drug Application
Animals were subjected to one protocol with two arms, a control arm under which saline was administered and a treatment arm wherein canertinib was administered. The volume of saline was 0.1 ml/kg/day and the dose of canertinib (dissolved in saline) was 30 mg/kg/day. Canertinib was obtained from LC laboratories product number C-1201. All injections were administered intraperitoneally (i.p). ABR and DPOAEs were taken prior to and after each cycle of the drug administration. In clinical human studies, one common treatment cycle was 450 mg/day for 14 days followed by 7 days off. Accordingly, here, one cycle consisted of two weeks of daily i.p. injections followed by one week when ABRs and DPOAEs were assessed (Diagram 1).
Diagram 1.
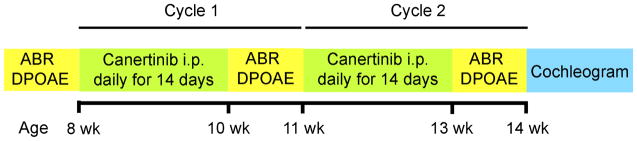
Two canertinib treatment cycles for C57BL/6J and CBA/CaJ mice. Timeline of functional and histological assays and canertinib treatments.
2.4 ABR Recording
ABR thresholds were obtained as described previously (Ohlemiller et al., 2000, Ohlemiller et al., 2000, Bao et al., 2005, Bao et al., 2013). Tucker-Davis Technologies (TDT) System II hardware and software (a Cambridge Electronic Design micro 1401, in conjunction with SIGNAL and custom signal averaging software, operating on a 120 MHz Pentium PC) were used for ABR recording. Animals were anesthetized with a combination of ketamine (80 mg/kg) and xylazine (15 mg/kg, i.p.) and positioned dorsally in a custom headholder. Subdermal platinum needle electrodes were placed in the mid-back (ground), behind the right pinna (active), and at the vertex (reference). Body temperature was monitored throughout testing using a rectal probe, and maintained at 37.5 ± 1.0°C using a DC current-based isothermal pad. In an anechoic chamber, the right ear of each mouse was stimulated in free-field using a TDT ES1 speaker placed 7 cm from the interaural axis. Stimuli were 5 ms tone bursts (1000 repetitions, 20/s, and 1.0 ms rise/fall time) at frequencies of 5k, 10k, 20k, 28.3k, and 40 kHz. To eliminate contributions by the unstimulated ear, the left external meatus was compressed using a spring-loaded clip. Responses were amplified X100, 000 and filtered at 100–10,000 Hz.
2.5 DPOAE Recording
For DPOAE recordings, animals were anesthetized with a combination of ketamine (80 mg/kg) and xylazine (15 mg/kg, i.p.) and positioned dorsally in a custom head holder. DPOAE thresholds were recorded at f2 frequencies (f2/f1=1.2) of 5k, 10k, 20k, 28.3k and 40k Hz with relative level fixed (L2–L1=10 dB) with ascending f2 level from 20 dB to 80 dB SPL in 5 dB steps. EMAV software and Tucker Davis Technologies (TDT) hardware were used to generate continuous tones presented to one ear using two TDT ES-2 transducers in a closed coupler. A modified Knowles low noise microphone and custom pre-amp were used to record the signal in the ear canal.
2.6 Hair Cell Quantification
At the end of experiment, the animals were decapitated under deep anesthesia for cochlear histological analysis. The procedures used for this analysis have been described previously (Ding et al., 2001). Briefly, the stapes was removed to open the oval window, the round window membrane was punctured, and the apex of the cochlea was also opened. The cochlea was then perfused with 10% formalin in phosphate-buffered saline (PBS) and immersed in the fixative for 24 hours. The cochlear temporal bone was decalcified with 3.6% hydrochloric acid overnight. After decalcification, the cochlear basilar membrane was micro-dissected out and stained with Harri’s hematoxylin solution for 5 minutes. The cochlear basilar membrane was mounted as a flat surface preparation in glycerin on a glass slide, coverslipped, and examined using light microscopy (Zeiss Standard, 400X magnifications). The number of missing inner (IHCs) and outer (OHCs) hair cells was counted along the entire length of the basilar membrane from the apical turn to the base. Individual cochleogram was constructed to show the percentage of hair cells missing as functions of frequency and the distance from the apex. The individual cochleograms in each group were averaged to obtain the mean cochleograms using the cochleogram software (Ding et al., 1999, Ding et al., 2001, Ding et al., 2011, Ding et al., 2013).
2.7 Data Analysis
All functional and histological analyses were collected without knowing animal identification. Data analysis was not blinded but the statistician was unaware of the purpose of this study. The statistical analyses of ABR and DPOAE thresholds for both C57BL/6J CBA/CaJ mice were performed by two-way ANOVA. Post-hoc tests were done using the Tukey-Kramer multiple comparison adjustment. Follow-up tests were performed to test for a group effect at each frequency after applying a Bonferroni multiple comparison adjustment. Data expressed as means +/− SEM, p<0.05 considered as statistical significant difference.
3. Results
3.1 Toxicity to Zebrafish Lateral Line Hair Cells
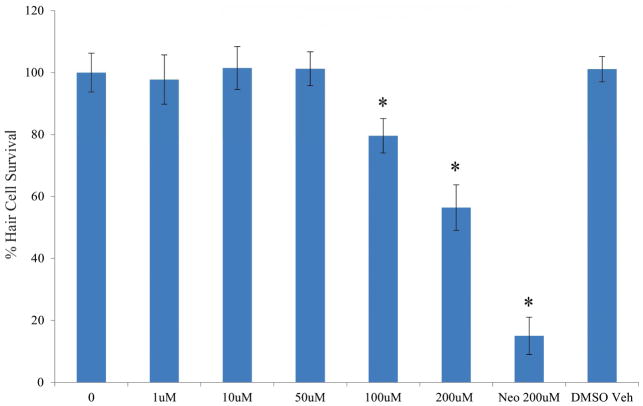
Since the zebrafish lateral line allows rapid assessment and quantification of hair cell toxicity (Hirose et al., 2011, Ou et al., 2012, Thomas et al., 2013), we first tested possible canertinib toxicity in zebrafish (Figure 1). At the lower concentrations from 0 to 50 μM, canertinib showed no obvious hair cell toxicity, while at the higher doses, it clearly demonstrated a dose-dependent loss of hair cell at 100 μM (p = 0.28 × 10−3), or 200 μM (p = 0.18 × 10−10). Doses above 200 μM were lethal to the fish. The quantitative data with both positive (neomycin at 200 μM) and negative controls (dimethyl sulfoxide) are also included in Figure 1.
Figure 1.
Canertinib toxicity in zebrafish. Lateral line hair cells were quantified and compared among different groups. Data plotted as means +/− standard error of the mean (SEM). Neomycin was used as a positive control (Neo 200 μM), while dimethyl sulfoxide was the negative control (DMSO Veh). Statistically significant difference between the control (0) and the group treated with canertinib at 100 μM (p = 0.28 × 10−3), 200 μM (p = 0.18 × 10−10) or neomycin at 200 μM (p = 0.11 × 10−16) were labelled.
3.2 Ototoxicity in C57BL/6J Mice
After the first round of canertinib treatment, two-way ANOVA analysis of ABR threshold shifts across all five frequencies indicated no significant drug effect (F = 2.267, p = 0.137; Figure 2A). However, at 40 kHz, univariate tests showed a significant drug effect (F = 5.392, P = 0.024). For DPOAE threshold shifts, a significant difference was found between the control and drug-treated groups (F = 4.900, p = 0.032; Figure 2C). After the second round treatment, two-way ANOVA analysis of ABR threshold shifts across all five frequencies showed a significant drug effect (F = 2.267, p = 0.001; Figure 2B). At 40 kHz, the ABR threshold shift was increased about 25 dB in the drug treatment group (F = 22.155, p < 0.001). For DPOAE threshold shifts, a significant difference was again found between the control and drug-treated groups (F = 25.588, p < 0.001; Figure 2D). Thus, canertinib ototoxicity was clearly observed in C57BL/J6 mice, and the DPOAE data suggested that its main ototoxic effect could be the damage of OHCs.
Figure 2.
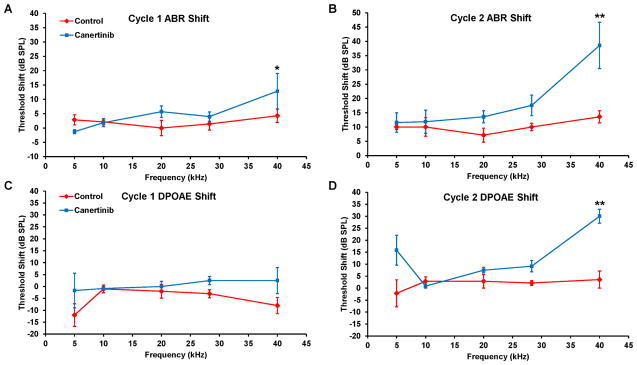
Hearing changes in C57BL/6J mice after canertinib treatment. ABR threshold shifts of C57BL/6J mice after the first (A) and second (B) cycle of the treatment. Control mice (red diamond; 3 males and 4 females) are compared to the drug-treated mice (blue square; 4 per gender). DPAOE threshold shifts were also quantified for both cycles (C and D). Data plotted as means +/− SEM. (* p < 0.05, ** p < 0.001).
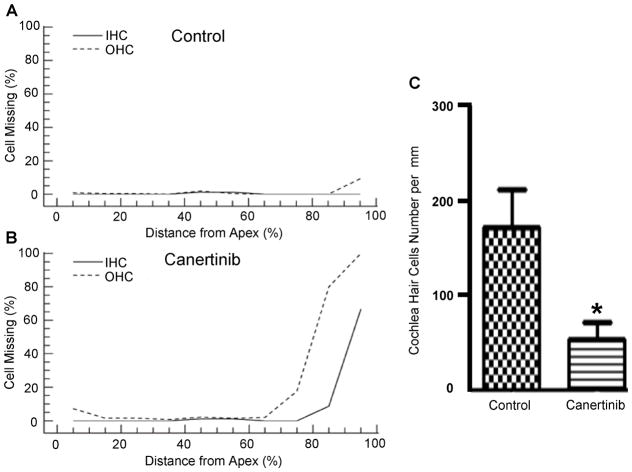
To directly identify possible cochlear targets for canertinib ototoxicity, cochleograms were performed to provide hair cell counts in both the control and canertinib-treated groups. While no loss of IHCs and only slight loss of OHCs at the basal high frequency region were observed in the cochleogram averaged from all control animals (Figure 3A), a clear loss of both OHCs and IHCs at the basal region of the drug-treated animals was observed (Figure 3B). A detailed quantification of OHCs from 70% to 100% of basal cochlear region showed a statistical significant difference between the control and drug-treated groups (Figure 3C). Thus, these quantitative histological data, consistent with ABR and DPOAE functional data, clearly indicated canertinib ototoxicity in C57BL/6J mice.
Figure 3.
Loss of hair cells after canertinib treatment. The same C57BL/6J mice were used for histological quantification of hair cells. Hair cell counts were graphically displayed for both the control (A) and canertinib-treated (B) mice from apex to base (the x-axis is % distance from the apex; n=7 for the control group, and n=8 for the drug-treated group). Quantitative data of OHC density was collected from the regions that were 70% distant from the cochlear apex, and compared between the control and the drug-treated group (C). Data plotted as mean +/− SEM. (* p < 0.05, ** p < 0.001).
3.3 Ototoxicity in CBA/CaJ Mice
After the above finding that canertinib caused hearing loss in C57BL/6J mice, we repeated the experiment with CBA/CaJ mice, a well-studied mouse model without early age-related hearing loss (Frisina et al., 2011). After the first round of canertinib treatment, two-way ANOVA analysis of ABR threshold shifts across all five frequencies indicated a significant drug effect (F = 10.638, p = 0.002; Figure 4A). Univariate tests at each frequency showed a significant drug effect at 20 kHz (F = 13.298, P < 0.001) and at 40 kHz (F = 4.787, P = 0.033). For DPOAE threshold shifts, no significant difference was found between the control and drug-treated groups (F = 0.042, p = 0.839; Figure 4C). After the second round treatment, two-way ANOVA analysis of ABR shifts across all five frequencies showed a significant difference between the control and drug-treated groups (F = 8.848, p = 0.005; Figure 4B). Univariate tests at each frequency showed a significant drug effect at 20 kHz (F = 8.073, P = 0.007) and at 40 kHz (F = 13.549, P < 0.001). For DPOAE shifts, a significant difference was again found between the control and drug-treated groups (F = 11.314, p = 0.00158; Figure 4D). Thus, canertinib ototoxicity was again observed in CBA/CaJ mice, and the DPOAE data suggested that canertinib could damage OHCs. Lack of parallel changes in ABR and DPOAE threshold shifts, however, suggests that other structures could also be damaged.
Figure 4.
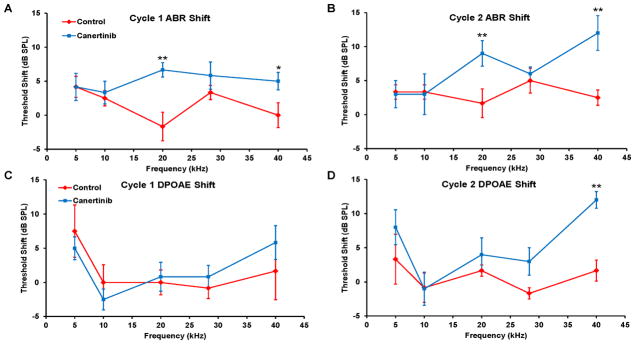
Hearing changes in CBA/CaJ mice after canertinib treatment. ABR threshold shifts of CBA/CaJ mice after the first (A) and second (B) cycle of the treatment. Control mice (red diamond; 3 per gender) were compared to the drug-treated mice (blue square; 4 males and 3 females). DPAOE threshold shifts were also quantified for both cycles (C and D). Data plotted as means +/− SEM. (* p < 0.05, ** p < 0.001)
4. Discussion
Based on a possible shared RTK molecular signaling mechanism, we examined possible canertinib ototoxicity in three preclinical animal models. The zebrafish studies clearly showed dose-dependent canertinib ototoxicity, which was further confirmed in two mouse models with different genetic backgrounds. Thus, our study strongly suggested an evolutionally preserved ERBB molecular mechanism underlying canertinib ototoxicity. Because canertinib is a promising NSCLC drug candidate for one of the deadliest cancers, our finding has an important clinical implication for future clinical monitoring of this class of cancer drugs.
Our observation of canertinib ototoxicity is consistent with one previous finding with 4557W, an ERBB2 and EGFR inhibitor (Watanabe et al., 2010), however, one major difference should be noted. In this study, after the treatment of canertinib, DPOAE data indicated a loss of OHC function with consistent histological evidence, while the previous study demonstrated preserved OHC function after the treatment of 4557W (Watanabe et al., 2010). Possible explanations of this difference are discussed below.
4.1 Possible Molecular and Cellular Targets in the Cochlea
In the mature inner ear, ERBB is important for auditory function (Hansen et al., 2001, Bao et al., 2003, Stankovic et al., 2004, Gomez-Casati et al., 2010, Watanabe et al., 2010). NRG1 and ERBB2 are expressed in SGNs and hair cells respectively, whereas ERBB2 and ERBB3 are expressed in the supporting cells of the organ of Corti (Morley et al., 1998, Stankovic et al., 2004). Chronic blocking of ERBB2 by 4557W, but not the specific EGFR blocker PD153035, causes progressive hearing impairment over the course of weeks as seen in ABR threshold shifts especially prominent at high frequencies (Watanabe et al., 2010). Based on the expression pattern of ERBB receptors in the cochlea, this functional outcome would lead to predict a damage of hair cells by 4557W, which would be consistent with our finding. In addition, after the treatment of 4557W, Neurotrophin-3 (NT-3) was decreased by ~65% compared to control, and NT-3 should have a more significant effect at the basal region of the cochlea (Fritzsch et al., 1997, Farinas et al., 2001, Fritzsch et al., 2004, Yang et al., 2011, Green et al., 2012). Surprisingly, no OHC damage was observed (Watanabe et al., 2010). This difference from our study could be explained by unknown protective molecular targets activated by 4557W in hair cells, unknown supporting factors from supporting cells activated by 4557W, or animal differences between the studies. Regardless, our data are consistent with current known NRG1-ERBB signaling pathways in the cochlea and the observed functional outcome is partially due to OHC loss. However, our data could not exclude the possibility that canertinib could also have direct effects on IHCs and supporting cells, and synaptic connections between hair cells and SGNs in part due to the difference between ABR and DPOAE threshold shifts in CBA/CaJ mice. Future studies will elucidate these possibilities.
4.2 Future Ototoxicity Studies of Canertinib and other RTK Inhibitors
There are many second and third generation RTK inhibitors undergoing phase II and phase III clinical trials with promising results. Investigations into the human ototoxicity of these RTK inhibitors will be useful for developing ways to ameliorate this severe side effect. Previous detailed studies of cisplatin and aminoglycoside antibiotics have provided a blueprint for the future studies in this area (Schacht et al., 2012). First, genetic influences of these drug candidates are unknown and should be examined in certain subpopulations that may be more susceptible to pharmacologic induced damages (Oldenburg et al., 2007, Schacht et al., 2012). Second, more detailed pathology of the cochlear damage, particularly synaptic changes between hair cells and SGNs, should be examined since our previous study showed that NRG1 and ERBB signaling is particularly important at this site (Bao et al., 2004). Third, pharmacokinetics of these drug candidates in the cochlea should be studied because the metabolism and clearance of these second and third generation drugs may vary and have more or less presence in the inner ear. Finally, an understanding of downstream signaling pathways altered by these drug candidates in the cochlea, including the cell death pathway, would help to design hearing protection therapies in future without reducing their anti-cancer efficacy.
5. Conclusions
RTK inhibitors blocking ERBB signaling are a promising new treatment for advanced NSCLC. Given the importance of ERBB signaling in the cochlea, it is important to consider the potential ototoxicity of these new cancer drug candidates. Here, we discovered the ototoxic effects of canertinib, indicating the importance of assessing human ototoxicity in future therapies that inhibit ERBB family members.
Highlights.
Canertinib, a new cancer drug candidate blocking ERBB signaling, causes toxicity in hair cells of zebrafish lateral line.
Canertinib ototoxicity is confirmed in two mouse models.
One major target of its ototoxicity in mice is outer hair cells.
Ototoxicity should be monitored for cancer drug candidates with pan-ERBB inhibition properties.
Acknowledgments
The project was supported by grants to J.B. from the National Institute of Health (DC010489 and DC011793). We thank Debin Lei for his laboratory management and assistance with mouse husbandry.
Footnotes
Conflicts of Interests
All authors state that they have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Agustoni F, Platania M, Vitali M, Zilembo N, Haspinger E, Sinno V, Gallucci R, de Braud F, Garassino MC. Emerging toxicities in the treatment of non-small cell lung cancer: Ocular disorders. Cancer Treat Rev. 2014;40:197–203. doi: 10.1016/j.ctrv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Allen CT, Judd NP, Bui JD, Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012;122:144–157. doi: 10.1002/lary.21913. [DOI] [PubMed] [Google Scholar]
- Allen LF, Eiseman IA, Fry DW, Lenehan PF. CI-1033, an irreversible pan-erbB receptor inhibitor and its potential application for the treatment of breast cancer. Semin Oncol. 2003;30:65–78. doi: 10.1053/j.seminoncol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Almadori G, Cadoni G, Galli J, Ferrandina G, Scambia G, Exarchakos G, Paludetti G, Ottaviani F. Epidermal growth factor receptor expression in primary laryngeal cancer: an independent prognostic factor of neck node relapse. Int J Cancer. 1999;84:188–191. doi: 10.1002/(sici)1097-0215(19990420)84:2<188::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Awada A, Bozovic-Spasojevic I, Chow L. New therapies in HER2-positive breast cancer: a major step towards a cure of the disease? Cancer Treat Rev. 2012;38:494–504. doi: 10.1016/j.ctrv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bao J, Hungerford M, Luxmore R, Ding D, Qiu Z, Lei D, Yang A, Liang R, Ohlemiller KK. Prophylactic and therapeutic functions of drug combinations against noise-induced hearing loss. Hear Res. 2013;304:33–40. doi: 10.1016/j.heares.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacci EG, Pustilnik LR, Rossi AM, Emerson E, Miller PE, Boscoe BP, Cox ED, Iwata KK, Jani JP, Provoncha K, Kath JC, Liu Z, Moyer JD. The biological and biochemical effects of CP-654577, a selective erbB2 kinase inhibitor, on human breast cancer cells. Cancer Res. 2003;63:4450–4459. [PubMed] [Google Scholar]
- Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, Goss G, Ely G, Beier F, Soulieres D. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer. 2010;11:391–395. doi: 10.3816/CLC.2010.n.101. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Cowan-Jacob SW. Structural biology of protein tyrosine kinases. Cell Mol Life Sci. 2006;63:2608–2625. doi: 10.1007/s00018-006-6202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castria TB, da Silva EM, Gois AF, Riera R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2013;8:CD009256. doi: 10.1002/14651858.CD009256.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, Salvi RJ. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res. 2011;282:196–203. doi: 10.1016/j.heares.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, McFadden S, Salvi RJ. Cochlear hair cell densities and inner-ear staining techniques. In: Willott James F., editor. Handbook of Mouse Auditory Research. CRS Press; Florida: 2001. pp. 189–204. [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ, Katsuno K, Hsieh YH, Miyakawa T, Salvi R, Tanokura M, Someya S. Addition of exogenous NAD(+) prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS One. 2013;8:e79817. doi: 10.1371/journal.pone.0079817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, Mueller M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Singh A, Bak M, Bozorg S, Seth R, Zhu X. F1 (CBAxC57) mice show superior hearing in old age relative to their parental strains: hybrid vigor or a new animal model for “golden ears”? Neurobiol Aging. 2011;32:1716–1724. doi: 10.1016/j.neurobiolaging.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fry DW. Site-directed irreversible inhibitors of the erbB family of receptor tyrosine kinases as novel chemotherapeutic agents for cancer. Anticancer Drug Des. 2000;15:3–16. [PubMed] [Google Scholar]
- Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A. 2010;107:17005–17010. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SH, Bailey E, Wang Q, Davis RL. The Trk A, B, C’s of neurotrophins in the cochlea. Anat Rec (Hoboken) 2012;295:1877–1895. doi: 10.1002/ar.22587. [DOI] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Harrison RT, DeBacker JR, Bielefeld EC. A low-dose regimen of cisplatin before high-dose cisplatin potentiates ototoxicity. Laryngoscope. 2014;125:E78–83. doi: 10.1002/lary.24948. [DOI] [PubMed] [Google Scholar]
- Hellard D, Brosenitsch T, Fritzsch B, Katz DM. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null mice. Dev Biol. 2004;275:34–43. doi: 10.1016/j.ydbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Hirono Y, Tsugawa K, Fushida S, Ninomiya I, Yonemura Y, Miyazaki I, Endou Y, Tanaka M, Sasaki T. Amplification of epidermal growth factor receptor gene and its relationship to survival in human gastric cancer. Oncology. 1995;52:182–188. doi: 10.1159/000227455. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Simon JA, Ou HC. Hair cell toxicity in anti-cancer drugs: evaluating an anti-cancer drug library for independent and synergistic toxic effects on hair cells using the zebrafish lateral line. J Assoc Res Otolaryngol. 2011;12:719–728. doi: 10.1007/s10162-011-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Kirkegaard M, Oesterle EC. ErbB expression: the mouse inner ear and maturation of the mitogenic response to heregulin. J Assoc Res Otolaryngol. 2003;4:422–443. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Janne PA, von Pawel J, Cohen RB, Crino L, Butts CA, Olson SS, Eiseman IA, Chiappori AA, Yeap BY, Lenehan PF, Dasse K, Sheeran M, Bonomi PD. Multicenter, randomized, phase II trial of CI-1033, an irreversible pan-ERBB inhibitor, for previously treated advanced non small-cell lung cancer. J Clin Oncol. 2007;25:3936–3944. doi: 10.1200/JCO.2007.11.1336. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Klijn JG, Look MP, Portengen H, Alexieva-Figusch J, van Putten WL, Foekens JA. The prognostic value of epidermal growth factor receptor (EGF-R) in primary breast cancer: results of a 10 year follow-up study. Breast Cancer Res Treat. 1994;29:73–83. doi: 10.1007/BF00666183. [DOI] [PubMed] [Google Scholar]
- Koutras AK, Mastronikolis NS, Evans TR, Papadeas ES, Makatsoris T, Kalofonos HP. Irreversible ototoxicity associated with the use of erlotinib in a patient with pancreatic cancer. Acta Oncol. 2008;47:1171–1173. doi: 10.1080/02841860802213328. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Leung HY, Weston J, Gullick WJ, Williams G. A potential autocrine loop between heregulin-alpha and erbB-3 receptor in human prostatic adenocarcinoma. Br J Urol. 1997;79:212–216. doi: 10.1046/j.1464-410x.1997.30412.x. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Hartmann JT. Platinum compounds: metabolism, toxicity and supportive strategies. Praxis (Bern 1994) 2005;94:187–198. doi: 10.1024/0369-8394.94.6.187. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–120. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majem M, Pallares C. An update on molecularly targeted therapies in second- and third-line treatment in non-small cell lung cancer: focus on EGFR inhibitors and anti-angiogenic agents. Clin Transl Oncol. 2013;15:343–357. doi: 10.1007/s12094-012-0964-2. [DOI] [PubMed] [Google Scholar]
- Meden H, Kuhn W. Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor. Eur J Obstet Gynecol Reprod Biol. 1997;71:173–179. doi: 10.1016/s0301-2115(96)02630-9. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Morley BJ. ARIA is heavily expressed in rat peripheral auditory and vestibular ganglia. Brain Res Mol Brain Res. 1998;54:170–174. doi: 10.1016/s0169-328x(97)00355-0. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Li HS, Hiel H, Drescher DG, Elgoyhen AB. Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Brain Res Mol Brain Res. 1998;53:78–87. doi: 10.1016/s0169-328x(97)00272-6. [DOI] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Rybak LP, Sheehan KE, Kaur T, Ramkumar V, Jajoo S, Sheth S. The design and screening of drugs to prevent acquired sensorineural hearing loss. Expert Opin Drug Discov. 2011;6:491–505. doi: 10.1517/17460441.2011.562887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA, C57BL, and BALB inbred strains. Hear Res. 2000;149:239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- Ou H, Simon JA, Rubel EW, Raible DW. Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear Res. 2012;288:58–66. doi: 10.1016/j.heares.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou SH. Second-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol. 2012;83:407–421. doi: 10.1016/j.critrevonc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I. Tyrosine kinase blockers: new hope for successful cancer therapy. Anticancer Agents Med Chem. 2009;9:66–76. doi: 10.2174/187152009787047752. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- Sainsbury JR, Malcolm AJ, Appleton DR, Farndon JR, Harris AL. Presence of epidermal growth factor receptor as an indicator of poor prognosis in patients with breast cancer. J Clin Pathol. 1985;38:1225–1228. doi: 10.1136/jcp.38.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 2012;295:1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Jr, Caceres C, Morote J, De Torres I, Rodriguez-Vallejo JM, Gonzalez J, Reventos J. Gains of the relative genomic content of erbB-1 and erbB-2 in prostate carcinoma and their association with metastasis. Int J Oncol. 1999;14:367–371. doi: 10.3892/ijo.14.2.367. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L National Cancer Institute of Canada Clinical Trials G. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin Oncol. 2001;28:80–85. doi: 10.1016/s0093-7754(01)90285-4. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci. 2013;33:4405–4414. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- Watanabe F, Kirkegaard M, Matsumoto S, Gont C, Mannstrom P, Ulfendahl M, Fridberger A. Signaling through erbB receptors is a critical functional regulator in the mature cochlea. Eur J Neurosci. 2010;32:717–724. doi: 10.1111/j.1460-9568.2010.07347.x. [DOI] [PubMed] [Google Scholar]
- Yang JC, Hirsh V, Schuler M, Yamamoto N, O’Byrne KJ, Mok TS, Zazulina V, Shahidi M, Lungershausen J, Massey D, Palmer M, Sequist LV. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–3350. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res. 2011;278:21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JG, Fayad JN, Kalinec F. Understanding drug ototoxicity: molecular insights for prevention and clinical management. Expert Opin Drug Saf. 2006;5:383–399. doi: 10.1517/14740338.5.3.383. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ding D, Salvi R. Expression of heregulin and ErbB/Her receptors in adult chinchilla cochlear and vestibular sensory epithelium. Hear Res. 2002;169:56–68. doi: 10.1016/s0378-5955(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Zinner RG, Nemunaitis J, Eiseman I, Shin HJ, Olson SC, Christensen J, Huang X, Lenehan PF, Donato NJ, Shin DM. Phase I clinical and pharmacodynamic evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2007;13:3006–3014. doi: 10.1158/1078-0432.CCR-06-1958. [DOI] [PubMed] [Google Scholar]