Abstract
Introduction
Ulcerated melanomas may have a unique biology and microenvironment. We test whether markers of immune infiltration correlate with clinical outcome in ulcerated compared to non-ulcerated primary melanoma tumors.
Methods
Sixty-two stage II–III cutaneous melanomas, 32 ulcerated and 30 non-ulcerated, were analyzed for tumor-infiltrating lymphocytes (TILs). Immunohistochemistry (IHC) was performed for CD2, a marker previously shown to correlate with overall survival (OS) and recurrence-free survival (RFS) in this patient population. IHC using antibody, VE1, to BRAF V600E was also performed on a subset of 41 tumors to assess the relationship of BRAF mutation to immune markers.
Results
We found, using Cox regression models, that the presence of TILs was associated with improved OS (p = 0.034) and RFS (p = 0.002) in ulcerated melanoma tumors, but not in non-ulcerated melanoma (p = 0.632, 0.416). CD2 expression also was correlated with improved OS (p = 0.021) and RFS (p = 0.001) in ulcerated melanoma, but no relationship was seen in non-ulcerated melanoma (p = 0.427, 0.682). In this small population, BRAF status did not correlate with TILs or CD2+ count.
Conclusion
Our data show that immune markers including TILs and CD2 count correlate more closely with survival in ulcerated melanomas than that in non-ulcerated melanomas. We propose that immune biomarkers may be particularly relevant to ulcerated, as compared to non-ulcerated, melanomas and that this merits study in larger populations.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1726-0) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Ulceration, Biomarker, CD2, Tumor-infiltrating lymphocytes
Introduction
Primary cutaneous melanomas have heterogeneous morphology and clinical outcomes. Depth is the most critical prognostic feature with ulceration being the second most useful in clinical practice. Ulceration of the primary tumor was first defined as a poor prognostic feature in 1953 by Allen and Spitz [1]. More recently, the classification of ulcerated melanomas as a distinct biological category within melanoma has been advanced [2, 3]. Notably, ulcerated melanomas may respond more favorably to adjuvant interferon [3–5]. This observation raises the possibility that the immune microenvironment is distinct between ulcerated and non-ulcerated melanomas and that immune features may be more relevant to clinical outcomes in patients with ulcerated tumors.
The definition of “ulceration” in melanoma can be contentious, with some dermatopathologists proposing that ulcerated melanomas are merely excoriated. However, Spatz defined ulceration as a distinct entity characterized by a full-thickness epidermal defect (including the absence of stratum corneum and basement membrane), evidence of host response (i.e., fibrin deposition, neutrophils), and thinning, effacement, or reactive hyperplasia of the surrounding epidermis [6]. In 1980, Balch and colleagues showed that survival in stage I melanoma is reduced from 80 to 55 % if the primary tumor is ulcerated and from 53 to 12 % in stage II melanoma (p < 0.001) [7]. Subsequent studies have also demonstrated that ulceration correlates with poorer recurrence-free survival (RFS) and overall survival (OS) [8, 9]. Currently, ulceration is incorporated in American Joint Committee on Cancer (AJCC) staging and upstages a patient from stage IIa to IIb if the primary lesion is ulcerated [1, 10]. Many adjuvant studies enroll IIb but not IIa patients so that ulceration status does impact clinical decision-making. However, the biology of ulceration in melanoma is not fully understood. Ulceration has been proposed to relate to a higher propensity for invasion, as demonstrated by the tumors ability to invade the basement membrane. Further, melanomas with a higher vascular density have a higher rate of ulceration [11].
Thus, ulceration may reflect a distinct subtype of melanoma with a higher vascular density and greater local host response in the surrounding epidermis. Several groups have proposed that ulcerated melanomas have distinct gene profiles, increased vascularity, increased lymphovascular invasion, increased sentinel node positivity, and immunosuppression in primary lymph nodes even in the absence of tumor cells [2, 12]. In addition, it has been advanced that ulcerated melanomas show greater response rates to interferon therapy [3, 5, 13]. Work by Sarpa and colleagues demonstrated a significant relationship between the percentage of the tumor that is ulcerated and both sentinel lymph node status and OS; even minimal ulceration worsens the overall prognosis [14]. Moreover, ulceration, along with tumor thickness and disease stage, was shown to be one of the three risk factors most associated the development of brain metastasis [15].
There is growing evidence in the literature that certain subtypes of solid tumors may be more antigenic and, therefore, more immune stimulatory and potentially better targets for immunotherapy. Thus, tumor-infiltrating lymphocytes (TILs) are a positive prognostic factor in triple-negative breast cancer (TNBC), a more aggressive subtype of breast cancer, but not in other subtypes [16, 17]. With every 10 % increase in stromal TILs, a 14 % reduction was observed in recurrence and death, and for every 10 % increase in intratumoral TILs, a 28 % decrease in risk of recurrence was reported [16]. One hypothesis for this finding is that TNBC is more antigenic, thereby stimulating a stronger host immune response. Most recently, similar hypotheses have been advanced in lung cancer where immunotherapy is most effective in a subset of patients with a history of smoking [18]. In melanoma, it has been proposed that a higher tumor mutation burden correlates with response to immune therapy, and this could also correlate with the natural immune surveillance at the primary tumor site [19].
While depth, ulceration, and mitotic rate are useful to predict clinical outcome, prognostic and predictive immune biomarkers are greatly needed in melanoma, the leading cause of skin cancer-related death worldwide, with an incidence that has increased 2000 % from 1930 to 2010 [20]. This is particularly true for patients with intermediate-risk stage II–III tumors, who face an uncertain prognosis with approximately 50 % recurrence risk. While the AJCC tumor staging has been used for many years, accurate risk assessment at the level of the individual patient remains a difficult clinical challenge. It is now evident that the immune system plays an important role in limiting melanoma progression and that tumors evolve multiple mechanisms to evade and suppress the immune system [21, 22]. Most evidence suggests that immune infiltration is a favorable prognostic indicator in melanoma. Tumor-infiltrating lymphocytes (TILs) have been associated with better outcomes of melanoma in all stages [23–25]. Immune-based biomarkers would be useful both to provide more accurate prognostic information and to select patients for adjuvant immune therapy trials. TILs, however, are not included in AJCC staging and only a small minority of patients have higher grade or absent TILs, whereas most have intermediate grade TILs, a category with uncertain prognostic value.
We recently demonstrated that the density of CD2+ cells is an independent predictor of RFS and OS in patients with primary cutaneous melanoma [26]. CD2 is a member of the immunoglobulin superfamily shown to be present on T cells and NK cells. CD2 has two functions. Firstly, it interacts with lymphocyte function-associated antigen-3 (LFA-3) on antigen-presenting cells leading to interleukin-2 production and antigen stimulation. Secondly, CD2 acts as a co-stimulatory molecule on T and NK cells [27]. CD2 has been implicated in immunosurveillance and anti-tumor immunity [28, 29]. CD2 is present on CD4+ and CD8+ T cells, as well as NK cells, indicating that CD2 count may be a more accurate measure of active immune infiltrate than TILs alone.
In this study, based on the hypothesis that the immune microenvironment may be distinct between ulcerated and non-ulcerated melanomas, we test whether inflammatory markers better correlate with RFS and OS in ulcerated melanoma compared to non-ulcerated melanoma. In a population of 62 stage II–III primary melanomas identified based on screen of dermatopathology databases at Geisinger Medical Center (GMC) and the Icahn School of Medicine at Mount Sinai (ISMMS), we found that TILs correlate with clinical outcomes in ulcerated melanomas more significantly than in non-ulcerated melanomas. Further, we found that CD2 levels also are closely associated with outcomes in ulcerated melanomas but not in non-ulcerated melanomas.
Materials and methods
Patient selection
A retrospective review of dermatopathology database records from 2001 to 2012 at the Icahn School of Medicine at Mount Sinai (ISMMS) and Geisinger Medical Center (GMC) was conducted. Patients with AJCC stage II or stage III primary melanoma were selected for possible inclusion. Nonrecurrence was defined as no further evidence of melanoma following excision of the primary lesion. A minimum follow-up of 2 years was required for all nonrecurrent patients. Patients were censored in March 2012. Median follow-up time was 50.5 months. All studies were conducted on melanoma tissues from previously untreated patients.
Patient demographics, tumor histopathologic features, and clinical follow-up were extracted from electronic medical records by authorized personnel at each institution following approval by the institutional review board (IRB). Due to the retrospective nature of the study, treatment and monitoring following the diagnosis of primary melanoma were dictated by each patient’s dermatologist or oncologist. Information was obtained from physician records of these visits. ISMMS patients with incomplete clinical records were contacted by mail and telephone under an IRB approved protocol by authorized personnel to obtain clinical follow-up.
Dermatopathology
All slides were reviewed by a dermatopathologist to confirm the presence of melanoma and integrity of samples. Slides stained with hematoxylin and eosin were reviewed for characterization of TILs. TILs were characterized according to existing criteria present or absent [30]. Present was defined by at least several lymphocytes per high-powered field (HPF). Ulceration status was determined by a dermatopathologist according to Spatz’s criteria (listed above) [6].
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 5-μm charged slides of formalin-fixed paraffin-embedded (FFPE) tissue obtained from eligible patients with known clinical follow-up according to standard procedures. For CD2 staining, sections were deparaffinized in xylene, rehydrated in ethanol, and stained with an anti-CD2 monoclonal antibody (pre-diluted, Ventana Medical Systems, Tucson, AZ) using the Ventana BenchMark XT immunostainer.
For BRAF staining, sections were obtained from FFPE tissue from a subset of 41 patients for whom tissue was available at ISMMS; FFPE charged slides were obtained from Mount Sinai Biorepository. The Leica BOND Rx automated system for immunohistochemistry was utilized. Primary antibodies used were VE1 (Spring Biosciences) diluted 1:100 in BOND Primary Antibody Diluent solution. Bond Epitope Retrieval solution 2 was used for 30 min. The slides were mounted with Permount and imaged. Slides were scored by intensity of staining 0, 1+, 2+, 3+, as documented previously in the literature [31], and confirmed by 2 pathologists in the department of dermatopathology. 0 and 1+ staining were graded as negative for the proteins, while 2+ and 3+ staining were considered positive.
All 62 FFPE samples were prepared as 5-μm charged slides and stained with anti-CD2 monoclonal antibody using the Ventana BenchMark XT. Once stained, each slide was evaluated twice, independently, by blinded investigators using an ocular micrometer with a 1 mm2 130 grid (Nikon Eclipse E400®) and the number of CD2+ cells in 8 HPFs per slide was counted. Scores for each slide were averaged to yield a single score for use in subsequent analyses.
Immunofluorescence
Charged slides from a subset of primary melanomas were deparaffinized in xylene, rehydrated in ethanol, and heated in EDTA pH 9.0 for antigen retrieval. Slides were then co-stained with anti-CD2 (pre-diluted), anti-CD3 (pre-diluted), and anti-CD56 (pre-diluted) monoclonal antibodies (Ventana Medical Systems, Oro Valley, AZ) and anti-FoxP3 monoclonal antibodies (Abcam, Cambridge, MA). Staining was visualized using fluorochrome-conjugated secondary antibodies (Invitrogen, Grand Island, NY). Slides were sealed using fluorescence mounting medium (Dako, Glostrup, Denmark). Slides were visualized using a Nikon A1 confocal microscope and NIS-Elements software at the Confocal and Specialized Microscopy Facility of Herbert Irving Comprehensive Cancer Center at Columbia University.
Statistical analysis
CD2 count was analyzed as a continuous variable, while TILs, ulceration status, and BRAF status were analyzed as discrete variables. The Kaplan–Meier method was used in Graph Pad Prism version 6.0 (GraphPad Software, Inc) to calculate OS and RFS curves. XLStat (Addinsoft) was used for calculation of Cox regression. p values of t test for continuous data and Chi-square or Fisher’s exact tests for categorical data were calculated from http://www.graphpad.com.
Results
Patients
In order to test the hypothesis that the immune microenvironment in ulcerated stage II–III melanomas is distinct from non-ulcerated melanomas, we first screened the databases at ISMMS and GMC for cases where tissue and corollary clinical information were available. These included 26 samples from ISMMS and 36 samples from GMC. Patient demographics are shown in Table 1. The median age at biopsy was 69 years with a range from 27 to 87 years. The median depth was 2.88 mm (range 1.20–13.00 mm). In all, 37 patients presented with stage II disease and 25 presented with stage III disease. Fifty-two percentage of patients recurred (n = 32). Among patients developing recurrent disease, the median time to recurrence was 14.5 months. Nonrecurrent patients have a median follow-up time of 50.5 months. Forty-eight percentage of the primary melanomas were not ulcerated (n = 30), while 52 % were ulcerated (n = 32). There are no significant differences between ulcerated and non-ulcerated melanoma in most clinicopathologic variables (Supplementary Table S1), except depth (Supplementary Table S1, p = 0.015). The median thickness of ulcerated melanomas was 3.24 mm (range 1.30–13.00 mm), and the median thickness of non-ulcerated melanoma was 2.65 mm (range 1.20–6.20 mm). Among this population, tumor thickness was predictive of OS in a univariate Cox regression (Table 1, p = 0.014), and AJCC stage and ulceration trended toward significance (Table 1, p = 0.050, 0.057, respectively). Notably, this was a population of stage II–III patients where patients with tumors between 1.00 and 2.00 mm were included only if ulcerated based on current AJCC staging. Thus, in this population of 62 patients, with the exception of depth, ulcerated melanomas were not significantly different in terms of standard risk features and measures of immune infiltration (TILs) although, as expected, ulcerated tumors trended to have an inferior clinical outcome (Table 1).
Table 1.
Clinicopathologic characteristics of 62 patients with primary melanoma in correlation with OS
| Characteristic | Value | p value (univariate Cox regression) |
|---|---|---|
| Age (years), median (range) | 69 (27–87) | 0.062 |
| Sex, no. (%) | 0.581 | |
| Male | 41 (66) | |
| Female | 21 (34) | |
| Site of primary lesion, no. (%) | 0.094 | |
| Axial | 40 (65) | |
| Extremity | 22 (35) | |
| Thickness (mm) | 0.014 | |
| Mean | 3.60 | |
| Median (range) | 2.88 (1.20–13.00) | |
| Ulceration, no. (%) | 0.057 | |
| Yes | 32 (52) | |
| No | 30 (48) | |
| Stage, no. (%) | 0.050 | |
| Stage II | 37 (60) | |
| Stage III | 25 (40) | |
| Tumor-infiltrating lymphocytes, no. (%) | 0.018 | |
| Absent | 9 (14.5) | |
| Present | 53 (85.5) | |
| CD2+ cells | 0.029 | |
| Mean | 65.79 | |
| Median (range) | 54.25 (3.875–391.44) | |
| Status, no. (%) | ||
| Alive | 36 (58) | |
| Dead | 26 (42) | |
| Time to death (months), median (range) | 29 (6–139) | |
| Follow-up for nonrecurrence (months), median (range) | 50.5 (4–132) |
Bold values are statistically significant with p ≤ 0.05
Presence of TILs is associated with RFS and OS in ulcerated but not non-ulcerated melanomas
It has previously been reported that patients with ulcerated melanomas derive greater benefit from adjuvant interferon. Based on this, we hypothesized that the immune system may play a more protective role in ulcerated melanomas. TILs are a standard marker of inflammation and have been previously correlated with patient outcomes. In a cohort of 1865 patients with melanomas at least 0.75 mm in depth, patients with absent or almost absent TILs had a shorter recurrence-free interval (p < 0.001) [32]. Based on these data, we tested whether TILs correlate with OS and RFS among all of the patients and among ulcerated and non-ulcerated melanomas. In the population as a whole, patients with positive TILs have improved OS compared to absent TILs patients (Fig. 1a, p = 0.0120). In ulcerated melanoma, patients with TILs present had longer OS and RFS (Fig. 1c, d, absent TILs n = 6, present TILs n = 26, p = 0.0355, 0.0005, respectively). In contrast, non-ulcerated melanoma patients with absent TILs showed no difference in OS or RFS compared to patients with present TILs (Fig. 1e, f, absent TILs n = 3, present TILs n = 27). These data show that the presence or absence of TILs correlates more closely with outcomes in the ulcerated tumors in this population. Note that while TILs were more predictive of outcome in patients with ulcerated melanomas, there was no significant difference in quantity of TILs between ulcerated and non-ulcerated tumors (p = 0.475, Supplementary Table S1).
Fig. 1.
TILs correlate with improved survival. Kaplan–Meier survival curve for all patients demonstrates improved a OS (p = 0.0120) and b RFS (p = 0.0015), by log rank for patients with TILs present (blue), compared to TILs absent (red). Brisk and non-brisk infiltrate was defined as “present,” and absent TILs were defined as “absent” (see “Materials and methods”). In ulcerated melanoma, TILs correlate with improved c OS (p = 0.0355) and d RFS (p = 0.0005). In non-ulcerated melanoma, TILs showed no correlation with OS or RFS, e and f (p = 0.626, and p = 0.406, respectively). Representative slides are shown demonstrating brisk TILs g and absent TILs h in melanoma, stained with hematoxylin and eosin (shown at 40×, 100× inset)
High CD2+ count is a prognostic indicator in ulcerated but not non-ulcerated melanoma
Recently, we defined CD2 as a predictor for RFS and OS in stage II and III melanoma [26]. We reasoned that CD2 would give us a better indication than TILs alone whether immune biomarkers are more accurate in ulcerated relative to non-ulcerated primary melanoma tumors (Table 2).
Table 2.
Univariate Cox regression analysis: predictors of overall survival (OS) and recurrence-free survival (RFS) in ulcerated and non-ulcerated melanomas
| Total (n = 62) | Ulcerated (n = 32) | Non-ulcerated (n = 30) | ||||
|---|---|---|---|---|---|---|
| OS | RFS | OS | RFS | OS | RFS | |
| CD2 | 0.029 | 0.020 | 0.021 | 0.001 | 0.427 | 0.682 |
| TILs | 0.018 | 0.003 | 0.034 | 0.002 | 0.632 | 0.416 |
CD2 and TILs are associated with improved OS and RFS in ulcerated melanoma tumors but not in non-ulcerated melanomas
Bolded figures are statistically significant, p < 0.05
Quantitative variable: CD2. Categorical variable: TILs
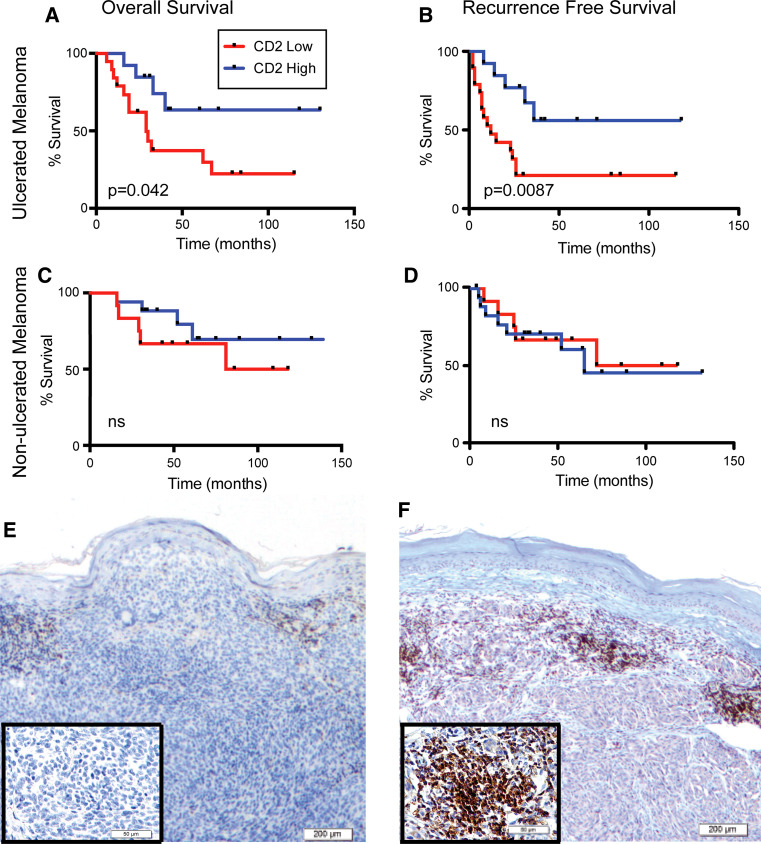
We found that CD2 correlated with OS in the overall population (p = 0.029) of patients from ISMMS and GMC, including both ulcerated and non-ulcerated melanomas. Next, we compared the predictive value of CD2 count in ulcerated and in non-ulcerated melanomas. Patients were then divided into CD2 high and CD2 low based on the median CD2 count. Representative slides showing “high” and “low” CD2 staining are shown in Fig. 2e, f. Patients with high CD2+ demonstrate longer RFS and OS (Fig. 2a, b, p = 0.042, 0.009). Among patients with non-ulcerated melanoma, the CD2+ count was not predictive of OS or RFS (Fig. 2c, d, p = 0.283, 0.817). These data show that CD2 count correlates more closely with recurrence and survival in ulcerated than in non-ulcerated melanomas, consistent with findings for TILs.
Fig. 2.
CD2+ count predicts OS and RFS. a Kaplan–Meier survival curves for patients with ulcerated melanoma, stratified using median CD2+. Each slide was evaluated twice, independently, by blinded investigators using an ocular micrometer with a 1 mm2 130 grid (Nikon Eclipse E400®) and the number of CD2+ cells in eight high-powered fields (HPF) was averaged to yield a single score for use in subsequent analyses. High CD2+ count correlates with longer OS (p = 0.042) and b RFS (p = 0.0087). In non-ulcerated melanoma, CD2+ count does not correlate with c OS (p = 0.283) or d RFS (p = 0.846). e Representative images of e CD2+ low and f CD2+ high stained with anti-CD2 antibody (shown at 40×, 100×, inset) magnifications
BRAF mutation status may not correlate with TILs or CD2+ count
The significance of BRAF mutational status on RFS and OS has been debated [33, 34]. To assess whether BRAF status correlates with TILs and CD2+ count, BRAF staining was carried out with VE1 antibody to BRAF V600E mutation in a subset of 41 patients with available tissue. Of these 41 melanoma tumors, 14 samples were scored as BRAF negative, and 27 were scored as BRAF positive. BRAF status may inversely correlate with younger age (p = 0.031), but showed no correlation with CD2 count, TILs, or ulceration (Supplementary Figure S1C–E). In conclusion, in this small number of patient samples, BRAF status did not appear to impact ulceration status, or immune response as measured by TILs or CD2+ count.
CD2+ count and TILs are the most predictive clinicopathologic variable in ulcerated melanoma for OS and RFS, whereas mitotic rate is more significant in non-ulcerated melanomas
In order to test the value of CD2 as a prognostic marker in the setting of other clinicopathologic features in ulcerated and non-ulcerated melanomas, we conducted a multivariate Cox regression analysis. After inclusion of other clinicopathologic predictors, CD2 count was found to be an independent predictor of OS and RFS in ulcerated melanoma using a multivariate Cox regression model (Table 3, p = 0.009, 0.001). In contrast, in non-ulcerated melanomas, CD2 is not predictive of OS or RFS (p = 0.323, 0.807). Instead, in non-ulcerated melanomas, mitotic index is the most predictive variable of OS (p = 0.003) and RFS (p = 0.004).
Table 3.
Multivariate Cox regression analysis including CD2: predictors of overall survival (OS) and recurrence-free survival (RFS) in ulcerated and non-ulcerated melanomas
| Non-ulcerated (n = 30) | Ulcerated (n = 32) | |||
|---|---|---|---|---|
| OS | RFS | OS | RFS | |
| Age | 0.295 | 0.306 | 0.038 | 0.055 |
| Depth | 0.240 | 0.234 | 0.024 | 0.048 |
| Mitotic index | 0.003 | 0.004 | 0.037 | 0.044 |
| CD2 | 0.323 | 0.807 | 0.009 | 0.001 |
| Gender | 0.259 | 0.291 | 0.440 | 0.369 |
| Stage | 0.057 | 0.065 | 0.051 | 0.035 |
CD2 associated with improved OS and RFS in ulcerated melanoma tumors but not in non-ulcerated melanomas
Bolded figures are statistically significant, p < 0.05
Quantitative variables: age, depth, mitotic index, CD2. Categorical variables: gender (M or F), stage (II or III)
The presence of TILs was also found to be an independent predictor of OS and RFS in ulcerated melanomas using multivariate Cox regression analysis (Supplementary Table S2, p = 0.004, 0.001, respectively), but not in non-ulcerated melanomas (p = 0.237, 0.209, respectively). Again, in non-ulcerated melanomas, mitotic index is the most predictive variable of OS (Supplementary Table S2, p = 0.003) and RFS (p = 0.003). Similarly, in a multivariate Cox model, CD2 predicted OS (p = 0.009) and RFS (p = 0.001) but not in non-ulcerated tumors (p = 0.323, 0.807), respectively. It is noted that when both CD2 and TILs are included in the multivariate Cox regression model, TILs explain away the correlation between CD2 and survival time [35] (Supplementary Table S3), because TILs correlates with CD2 (p = 7.295e-05). These data suggest that immune biomarkers may be most useful in ulcerated melanomas in the context of known clinicopathologic predictors.
In the tumor microenvironment, CD2+ cells are most likely to be T lymphocytes
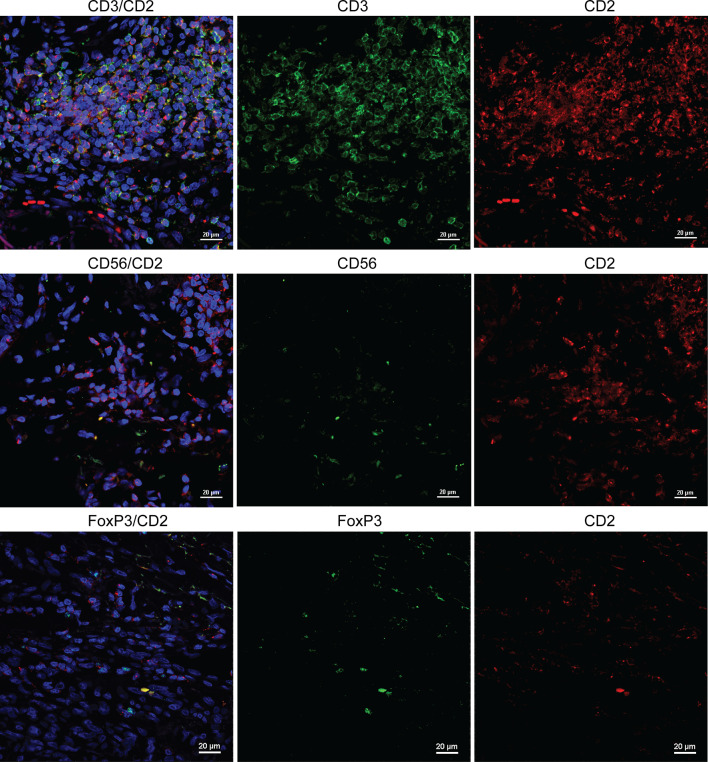
A salient question is what immune subtypes expressing CD2 were observed in our study. Because CD2 have been observed on both T cells and NK cells, we performed immunofluorescence to determine whether co-localization of CD2 is predominantly with CD3 or CD56. A significant amount of CD2/CD3 co-localized cells were observed in tumor specimens. In comparison, CD2/CD56 co-localization was relatively scarce (Fig. 3). This is consistent in specimens regardless of their levels of CD2 counts (Supplementary Figure S2). Previous studies have also shown strong co-localization of CD2 with CD4 and CD8 in T lymphocytes [26]. In addition, co-staining of CD2 and FoxP3 revealed that most CD2+ T lymphocytes do express FoxP3 (Fig. 3). The percentage of FoxP3+ T cells in our primary melanoma specimens was validated by CD3 and FoxP3 co-localization and shown consistent with published studies where approximately 10 % of infiltrating CD3+ cells were reported to be Foxp3+ [36] (Supplementary Figure S3). While it appeared that there was limited co-expression of CD2 and FoxP3, larger studies are needed to quantitatively compare percentages of Foxp3+ CD3+ cells and Foxp3+ CD2+ cells.
Fig. 3.
Co-localization of CD2/CD3 is more prevalent than CD2/CD56 and CD2/FoxP3 in melanoma specimens. In the top row, significant overlap of CD3+ (FITC) and CD2+ (Texas Red) cells was shown by immunofluorescence. In the middle row, expression patterns of CD56 (FITC) and CD2 (Texas Red) were dissimilar. In the bottom row, approximately 10 % of CD3+ (FITC, surface) cells express FoxP3 (Texas Red, intracellular). The results are representative of staining in three slides with high CD2 counts and three with low CD2 counts
Discussion
In this work, we studied immune infiltration in ulcerated and non-ulcerated melanoma and found that, in a multivariable model, CD2 was most predictive of OS and RFS only in ulcerated melanoma, while mitotic index was most predictive of outcome in non-ulcerated melanoma. Notably, while TILs quantification provides prognostic evidence, the majority of patients fall into the intermediate-risk category, with few patients having “brisk” or “absent” TILs. Thus, CD2 staining becomes more useful for patients with intermediate risk. While the significance of BRAF on progression has been debated, we found no correlation between BRAF and these inflammatory markers. These data would support the hypothesis that the immune microenvironment differs between ulcerated and non-ulcerated melanomas. Ulcerated melanoma may interact differently with the immune system than non-ulcerated melanoma, making it well suited for the application of immune biomarkers. Given the urgent need for biomarkers for stage II and III patients who face an approximate 50 % survival rate, as compared to >90 % for stage I disease and <10 % for stage IV disease, selectively testing immune biomarkers in ulcerated tumors may be a useful strategy [37].
Intriguingly, the definition of ulceration proposed by Spatz includes evidence of host response in the surrounding epidermis. Ulceration is associated with angiogenesis and inflammation on a molecular level, which again indicates its connection with immune surveillance. For example, angiopoietin 2 (ang-2), a mediator of angiogenesis, has similarly been correlated with ulceration status [38]. Inflammatory cytokines can promote tumorigenesis and tumor growth. A recent study by the Newton-Bishop group defined a gene panel that correlating with ulceration in melanoma, and some of them, including IL-6 pro-inflammatory cytokine and certain chemokines (data not shown), plays distinctive roles in angiogenesis and tumor growth [12]. However, these genes may serve paradoxical functions among different tumor types and may act as adjuvants in the context of vaccination or immunotherapy, triggering anti-tumor responses [39]. It has been shown that ulcerated melanomas respond more strongly to interferon therapy in the 18991 and 18952 European Organization for Research and Treatment of Cancer (EORTC) registry which included 2644 patients with stage IIB or III melanoma of whom 849 had ulcerated and 1336 had non-ulcerated tumors (RFS, p < 0.001 and OS, p < 0.001) [40]. Another study by Baurain has shown the association of ulceration with clinical benefit of adjuvant vaccination with tumor-specific antigenic peptides in primary melanoma [41]. Ulceration has been known to be a histopathologic variable that portends increased metastatic risk [15, 42]. In this setting, ulceration may be more than a phenotype, but may in fact reflect, a more aggressive and immunogenic subtype of melanoma. Thus, a vascular, inflammatory microenvironment may predispose toward increased activity of tumor-infiltrating lymphocytes and to response to immune therapy, which rationalizes the possibility of greater prognostic application of TILs in ulcerated melanoma.
It is becoming clear that biomarkers do not apply equally to all subsets of cancers and tailoring immune biomarkers to select populations of melanoma patients may improve their predictive value. Recent work has shown that among patients with complete melanoma clearance in response to ipilimumab, there is a higher prevalence mutations resulting in antigenic neoepitopes. One hypothesis for this response is that these neoepitopes are more antigenic and therefore more conducive to generate a robust immune response capable of halting disease progression, or even leading to complete cure, than the mutations in the non-responding patients [19]. Similar results have been found in lung cancer where smokers have both higher mutations rates and higher rates of response to immunotherapy [18]. It would be expected based on these findings that immunogenic tumors would also attract a denser immune infiltrate.
In line with the above findings, TILs are well validated as prognostic markers, at least in univariate models [30, 32]. However, characterization of TILs can be difficult due to the heterogeneity of infiltrate throughout the tumor, as well as the nature of the infiltrate, including CD4+ “helper cells,” CD8+ cytotoxic T cells, B cells, NK cells, or immunosuppressive T regulatory cells. Therefore, there are many ongoing attempts to quantify and qualify immune infiltrate of solid tumors more precisely. We previously found staining for CD2 to be a predictor of OS and RFS in melanoma [26]. CD2 expression is found primarily on CD3 positive cells, both CD4+ and CD8+, and rarely on CD56 positive cells. Co-staining with CD2 and FoxP3 was also very rare. Thus, we hypothesize that CD2 is expressed primarily on activated T cells. While localization of the infiltrate has been reported to have prognostic implications, we were not able to reproduce this specific finding in our patient populations. Despite the recognition that immune infiltrate is a significant prognostic indicator in solid tumors [25, 43], TILs classification is currently not included in the AJCC staging of melanoma and there is no standard clinically applicable test of immune infiltration into the tumor. Applying immune biomarkers such as TILs and CD2 staining to select populations of melanoma patients such as those with ulcerated tumors may improve the accuracy of prediction of immune biomarkers.
Our findings would suggest that the evaluation of immune biomarkers may be particularly applicable to ulcerated melanomas and should be studied in larger populations within this subtype. It may also serve useful in the selection of patients for trials of adjuvant therapy, by highlighting a subgroup most at-risk for recurrence and death. The limitations of our study include the small population size and the retrospective nature of our study. Further exploration of the differences between ulcerated and non-ulcerated melanoma is needed. An understanding of the genetic differences between ulcerated and non-ulcerated melanoma will provide even greater insight into the biology driving the poorer clinical outcomes and different response patterns to treatment. Given the poor prognosis of ulcerated melanoma, there is a great need to identify predictive markers of recurrence and survival. In light of the growing evidence to support differences in immunogenicity among subtypes of cancer, the role of immune biomarkers in the clinical management of ulcerated melanoma warrants further exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 200,267 kb)
Acknowledgments
The study was funded by Dermatology Foundation (Career Development Award), American Association for Cancer Research (Landon Cancer Immunology Innovator Award), Herbert Irving Comprehensive Cancer Center at Columbia University, and Tisch Cancer Center at the Icahn School of Medicine at Mount Sinai.
Conflict of interest
The authors have no conflict of interest to declare.
Abbreviations
- AJCC
American Joint Committee on Cancer
- Ang-2
Angiopoietin 2
- EORTC
European Organisation for Research and Treatment of Cancer
- FFPE
Formalin fixed paraffin embedded
- GMC
Geisinger Medical Center
- HPF
High-powered field
- IHC
Immunohistochemistry
- IRB
Institutional review board
- ISMMS
Icahn School of Medicine at Mount Sinai
- LFA-3
Lymphocyte function-associated antigen-3
- OS
Overall survival
- RFS
Recurrence-free survival
- TILs
Tumor-infiltrating lymphocytes
- TNBC
Triple-negative breast cancer
Footnotes
Ellen H. de Moll and Yichun Fu have contributed equally to this article and should be considered co-first authors.
References
- 1.Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6:1–45. doi: 10.1002/1097-0142(195301)6:1<1::AID-CNCR2820060102>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AM, Spatz A, Lazar V, Robert C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol. 2012;24:137–140. doi: 10.1097/CCO.0b013e32834fcb0d. [DOI] [PubMed] [Google Scholar]
- 3.McMasters KM, Edwards MJ, Ross MI et al (2010) Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Ann Surg. 252:460–465; discussion 465–466. doi: 10.1097/SLA.0b013e3181f20bb1 [DOI] [PubMed]
- 4.Suciu S, Ives N, Eggermont AM, Kirkwood JM, Lorigan P, Markovic S, Garbe C, Wheatley K (2014) Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): AN individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). J Clin Oncol 32 (suppl; abstr 9067)
- 5.Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 6.Spatz A, Batist G, Eggermont AM. The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol. 2010;22:163–168. doi: 10.1097/CCO.0b013e328337fe8f. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA. The prognostic significance of ulceration of cutaneous melanoma. Cancer. 1980;45:3012–3017. doi: 10.1002/1097-0142(19800615)45:12<3012::AID-CNCR2820451223>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Aviles-Izquierdo JA, Lazaro-Ochaita P. Histological ulceration as a prognostic factor in cutaneous melanoma: a study of 423 cases in Spain. Clin Transl Oncol. 2012;14:237–240. doi: 10.1007/s12094-012-0790-6. [DOI] [PubMed] [Google Scholar]
- 9.Eigentler TK, Buettner PG, Leiter U, Garbe C, Central Malignant Melanoma Registry of the German Dermatological S Impact of ulceration in stages I to III cutaneous melanoma as staged by the American Joint Committee on Cancer Staging System: an analysis of the German Central Malignant Melanoma Registry. J Clin Oncol. 2004;22:4376–4383. doi: 10.1200/JCO.2004.03.075. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 11.Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR., 3rd Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–1831. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- 12.Jewell R, Elliott F, Laye J, et al. The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res. 2015;28:94–104. doi: 10.1111/pcmr.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suciu S, Ives N, Eggermont AM, Kirkwood JM, Lorigan P, Markovic S, Garbe C, Wheatley K (2014) Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): an individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). J Clin Oncol 32 (suppl; abstr9067)
- 14.Grande Sarpa H, Reinke K, Shaikh L, Leong SP, Miller JR, 3rd, Sagebiel RW, Kashani-Sabet M. Prognostic significance of extent of ulceration in primary cutaneous melanoma. Am J Surg Pathol. 2006;30:1396–1400. doi: 10.1097/01.pas.0000213262.61855.7d. [DOI] [PubMed] [Google Scholar]
- 15.Qian M, Ma MW, Fleming NH, Lackaye DJ, Hernando E, Osman I, Shao Y. Clinicopathological characteristics at primary melanoma diagnosis as risk factors for brain metastasis. Melanoma Res. 2013;23:461–467. doi: 10.1097/CMR.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldsmith LA, Fitzpatrick TB (2012) Fitzpatrick’s dermatology in general medicine. McGraw-Hill Medical, New York. Access Medicine. Web. September 28, 2014
- 21.Gallois A, Bhardwaj N. Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol. 2013;4:436. doi: 10.3389/fimmu.2013.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashani-Sabet M. Tumor progression by immune evasion in melanoma. Cancer. 2010;116:1623–1625. doi: 10.1002/cncr.24909. [DOI] [PubMed] [Google Scholar]
- 23.Mandala M, Imberti GL, Piazzalunga D, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer. 2009;45:2537–2545. doi: 10.1016/j.ejca.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Burton AL, Roach BA, Mays MP, et al. Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg. 2011;77:188–192. [PubMed] [Google Scholar]
- 25.Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci USA. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harcharik S, Bernardo S, Moskalenko M, et al. Defining the role of CD2 in disease progression and overall survival among patients with completely resected stage-II to -III cutaneous melanoma. J Am Acad Dermatol. 2014;70:1036–1044. doi: 10.1016/j.jaad.2014.01.914. [DOI] [PubMed] [Google Scholar]
- 27.Green JM, Karpitskiy V, Kimzey SL, Shaw AS. Coordinate regulation of T cell activation by CD2 and CD28. J Immunol. 2000;164:3591–3595. doi: 10.4049/jimmunol.164.7.3591. [DOI] [PubMed] [Google Scholar]
- 28.Tibaldi EV, Salgia R, Reinherz EL. CD2 molecules redistribute to the uropod during T cell scanning: implications for cellular activation and immune surveillance. Proc Natl Acad Sci USA. 2002;99:7582–7587. doi: 10.1073/pnas.112212699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford K, Stark A, Kitchens B, et al. CD2 engagement induces dendritic cell activation: implications for immune surveillance and T-cell activation. Blood. 2003;102:1745–1752. doi: 10.1182/blood-2002-07-2206. [DOI] [PubMed] [Google Scholar]
- 30.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 31.Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11–19. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 32.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 33.Safaee Ardekani G, Jafarnejad SM, Khosravi S, Martinka M, Ho V, Li G. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320–328. doi: 10.1111/bjd.12351. [DOI] [PubMed] [Google Scholar]
- 34.Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, Aaronson SA. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. [PubMed] [Google Scholar]
- 35.van Rijn P, Rijmen F. On the explaining-away phenomenon in multivariate latent variable models. Br J Math Stat Psychol. 2015;68:1–22. doi: 10.1111/bmsp.12046. [DOI] [PubMed] [Google Scholar]
- 36.De Panfilis G, Campanini N, Santini M, et al. Phase- and stage-related proportions of T cells bearing the transcription factor FOXP3 infiltrate primary melanoma. J Invest Dermatol. 2008;128:676–684. doi: 10.1038/sj.jid.5701046. [DOI] [PubMed] [Google Scholar]
- 37.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–122. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 39.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 40.Eggermont AM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–225. doi: 10.1016/j.ejca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Baurain J, Stas M, Hammouch F, et al. Association of primary melanoma ulceration and clinical benefit of adjuvant vaccination with tumor-specific antigenic peptides. ASCO Meet Abstr. 2009;27:3022. [Google Scholar]
- 42.Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31:4387–4393. doi: 10.1200/JCO.2013.50.1114. [DOI] [PubMed] [Google Scholar]
- 43.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 200,267 kb)