Abstract
Purpose
To determine the potential association between genitourinary (GU) toxicity and planning dose-volume parameters for GU pelvic structures after high-dose intensity-modulated radiotherapy (IMRT) in localized prostate cancer patients.
Methods and Materials
268 patients who underwent IMRT to a prescribed dose of 86.4 Gy in 48 fractions during 06/2004–12/2008 were evaluated with the International Prostate Symptom Score (IPSS) questionnaire. Dose volume histograms of the whole bladder, bladder wall, urethra, and bladder trigone were analyzed. The primary endpoint for GU toxicity was an IPSS sum increase ≥10 points over baseline. Univariate and multivariate analyses were done by Kaplan-Meier method and Cox proportional hazard models, respectively.
Results
Median follow-up was 5 years (range, 3–7.7 years). Thirty-nine patients experienced an IPSS sum increase ≥10 during follow-up; 84% remained event free at 5 years. After univariate analysis, lower baseline IPSS sum (P=0.006), the V90 of the trigone (P=0.006), and the maximal dose to the trigone (P=0.003) were significantly associated with an IPSS sum increase ≥10. After multivariate analysis, lower baseline IPSS sum (P=0.009) and increased maximal dose to the trigone (P=0.005) remained significantly associated. Seventy-two patients had both a lower baseline IPSS sum and a higher maximal dose to the trigone and were defined as high-risk and 68 patients had both a higher baseline IPSS sum and a lower maximal dose to the trigone and were defined as low-risk for development of an IPSS sum increase ≥10. Twenty-one of 72 high-risk (29%) and 5 of 68 low-risk (7%) patients experienced an IPSS sum increase ≥10 (P=0.001; odds ratio, 5.19).
Conclusions
The application of hot spots to the bladder trigone was significantly associated with relevant changes in IPSS during follow-up. Reduction of radiation dose to the lower bladder and specifically the bladder trigone appears to be associated with a reduction in late GU toxicity.
Keywords: dose volume histogram, urogenital abnormalities, radiotherapy, toxicity, prostatic neoplasms
Introduction
After high-dose intensity-modulated radiotherapy (IMRT), late genitourinary (GU) toxicity is more common than late gastrointestinal (GI) toxicity, and ≈20% of patients experience grade ≥2 late GU toxicity at 5 years [1]. GI toxicity has been reduced by better understanding the dose-response relationship for rectal toxicity and the development of appropriate treatment-planning rectal dose constraints. With IMRT achieving reduced exposure of the rectum to high doses of irradiation, decreased long-term GI toxicities have been well documented [2]. However, the relationship between radiotherapy (RT) dose to the bladder for prostate cancer and subsequent late GU toxicity is poorly understood [3]. Thus, despite improvements in highly conformal RT delivery, significant reductions of urinary-related toxicities after high-dose RT have not been observed.
We have suggested that image-guided RT (IGRT) can reduce GU toxicity, by decreasing the volume of the bladder neck region exposed to high radiation dose [4]. While some reports associated dose volume histogram (DVH) parameters with late GU toxicity [4], other studies did not [5]. Others have described bladder trigone dose as possibly associated with late urinary retention [6]. Thus, there is still a need to clarify the critical bladder component associated with GU-related toxicity and changes in quality of life (QoL) after high-dose IMRT. This can have profound implications for developing treatment-planning constraints that may achieve urinary toxicity reductions.
We carried out the present comprehensive DVH analysis related to GU structures including the bladder, the bladder trigone, and the urethra in localized prostate cancer patients followed using International Prostate Symptom Score (IPSS) after IMRT with 86.4 Gy.
Patients and Methods
Patient selection
During 08/1997–12/2008, 1002 consecutive localized prostate cancer patients were treated with definitive IMRT to a prescribed dose of 86.4 Gy in 48 fractions [1]. Of these, 269 were treated between June 2004 and December 2008, had available baseline IPSS data with ≥3 years of follow-up with ≥1 IPSS assessment during follow-up, and had available treatment-planning dosimetry previously described [7]. One patient who received salvage brachytherapy <3 years after IMRT was excluded and eight patients receiving salvage treatment ≥3 years post-IMRT were censored at salvage treatment. 268 patients were eligible. Research authorization was approved by our internal review board.
Treatment
All patients were treated with a 5- to 7-field IMRT plan and 15-MV photon beams using dose constraints as previously described [1]. Briefly, the clinical target volume consisted of the prostate and seminal vesicles, with a 1-cm planning target volume (PTV) margin in all directions except posteriorly (0.6-cm). To delineate the bladder wall, the inner bladder wall was contoured and expanded 6 mm. A cylindrical structure ≈10 mm in diameter was placed near the center of the PTV as a surrogate for the urethra. Dmax was constrained to <60 Gy for the large intestine, <53 Gy for the small intestine, and <105% for the urethra. Volume receiving at least 47 Gy (V47) was constrained to <53%, V75.6 to <30% for the rectal wall and <53% for the bladder. Dmax to the PTV was limited to <110%, and typically >87% of the PTV received the prescribed dose or more. Where PTV and rectum overlap, the maximum point dose to the rectal wall was limited to 99% of the prescription dose (85.50 Gy). The rectum-prostate interface was the primary region of dose reduction to meet the rectal constraint.
Patient position was verified with weekly port films or daily using fiducial markers in most patients since 2007/2008 [7]. Patient positioning was prone prior to 2007 and supine subsequently. Patients were treated with an empty bladder protocol unless the small bowel did not separate well enough from the PTV at simulation, in which case patients were treated with a full bladder to further displace the small bowel from the PTV. Patients received a rectal catheter to remove rectal air during the planning CT.
Androgen-deprivation therapy (ADT) was used for either volume reduction pretherapy or for high-risk features (Gleason Score 8–10, prostate-specific antigen >20 ng/mL, or clinical cT3 disease). Generally a 6-month course of ADT (3 months neoadjuvantly and 3 months concurrently) was used for low- and intermediate-risk patients, and a 6-month to 2-year course for high-risk patients. All patients received 86.4 Gy in 48 fractions of 1.8 Gy, except two patients did not receive their last fraction.
Baseline symptoms and toxicity
The 7 IPSS questions and the QoL index (question 8, QoL due to urinary symptoms; Table 1) were asked at baseline and each subsequent visit. Patients were evaluated every 3 months for the first year, every 6 months for the next 5 years, and yearly thereafter. A clinically relevant IPSS sum increase was defined as an increase of ≥10 (IPSS+10) during follow-up, as this change has been rated as a “marked change” by patients [8]. We calculated that an IPSS+10 correlated with an average QoL index drop of 4 points (data not shown). A second endpoint was defined as an absolute IPSS sum of ≥20 points including an increase of ≥4 points over the baseline.
Table 1.
International prostate symptom score (IPSS)
| Name: | Date: | ||||||
|---|---|---|---|---|---|---|---|
| Not At All |
Less Than 1 Time In 5 |
Less Than Half The Time |
About Half The Time |
More Than Half The Time |
Almost Always |
Your Score |
|
| Incomplete emptying Over the past month, how often have you had a sensation of not emptying your bladder completely after you finish urinating? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Frequency Over the past month, how often have you had to urinate again less than two hours after you finished urinating? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Intermittency Over the past month, how often have you found you stopped and started again several times when you urinated? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Urgency Over the last month, how difficult have you found it to postpone urination? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Weak stream Over the past month, how often have you had a weak urinary stream? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Straining Over the past month, how often have you had to push or strain to begin urination? | 0 | 1 | 2 | 3 | 4 | 5 | |
| None | 1 Time | 2 Times | 3 Times | 4 Times | 5 Times Or More | Your Score | |
| Nocturia Over the past month, many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning? | 0 | 1 | 2 | 3 | 4 | 5 | |
| Total IPSS score | |||||||
| Quality of life due to urinary symptoms | Delighted | Pleased | Mostly Satisfied | Mixed — About Equally Satisfied And Dissatisfied | Mostly Dissatisfied | Unhappy | Terrible |
| If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
Total score: 0–7 Mildly symptomatic; 8–19 moderately symptomatic; 20–35 severely symptomatic.
At baseline, during treatment, and at each follow-up visit the GU morbidity/toxicity symptoms dysuria, incontinence, retention, frequency/urgency, and hematuria were also assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Acute toxicity was defined as ≤3 months post-IMRT. Late toxicities were defined as after 3 months; however, if there was acute toxicity, late toxicity was only assumed >6 months post-IMRT. To account for baseline symptoms, acute and late toxicities were defined as an increase over the baseline value for every symptom.
DVH analysis
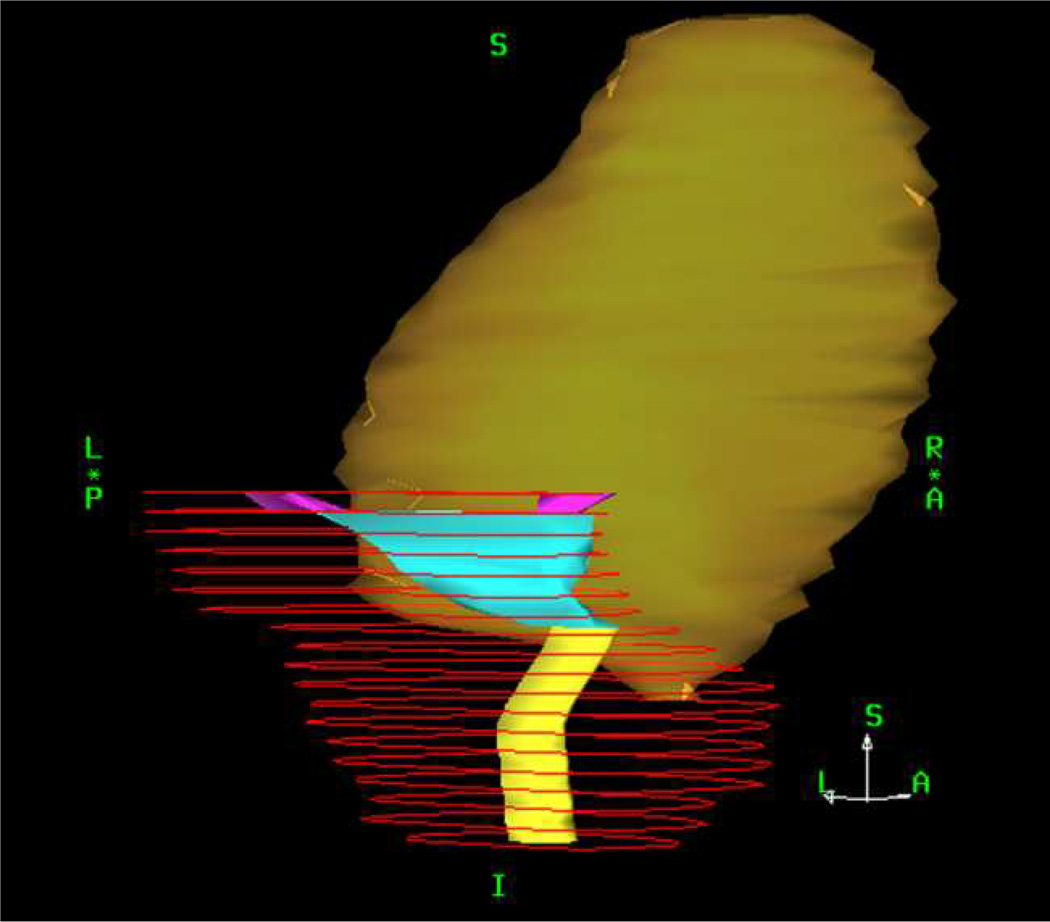
The full bladder, bladder wall, and urethral surrogate structure were prospectively delineated and available in all patients. The distal ends of both ureters were retrospectively contoured for this analysis and the bladder trigone was defined as the triangle-shaped structure between the transition of the urethra surrogate structure into the bladder wall caudally and the transition of the distal ureters in the bladder wall cranially (Figure 1). Full DVHs from the full bladder, bladder wall, trigone, and urethra were obtained. Thus, a total of 1072 DVHs (4 each for 268 patients) were analyzed. % volume at dose d (Vd) was examined for doses 5–100 Gy in 5 Gy steps. Dosimetric data were assumed to be exact, though we acknowledge uncertainties in the calculations and actual delivery.
Fig. 1.
A right-posterior (45°) view of an example delinea ted trigone (cyan), defined as the triangle-shaped structure between the transition of the urethra surrogate structure (light yellow) into the bladder wall (dark yellow) caudally and the transition of the distal ureter’s (magenta) in the bladder wall cranially. The planning target volume is depicted in red.
Statistical considerations
Categorical variables were summarized using absolute and relative frequencies and continuous variables by descriptive statistics. The primary endpoint was IPSS+10. Only IPSS data obtained ≥3 months after the end of RT was considered, thus reflecting late toxicity. Follow-up duration was calculated from the baseline IPSS to the last available IPSS visit. The occurrence of IPSS+10 and grade ≥2 late GU toxicity was compared using the Chi-square test.
Time to GU toxicity was analyzed using the Kaplan-Meier method and univariate comparisons between dosimetric or clinical variables and GU toxicity endpoints were performed using the log-rank test. All continuous clinical variables were dichotomized according to median value and analyzed: age (>71 vs. ≤71 years), race (African-American vs. others), diabetes (yes vs. no), smoking (yes vs. no), prostate volume (>37 vs. ≤37 cm3), risk group (high vs. low and intermediate), ADT (yes vs. no), IGRT (yes vs. no), patient positioning (supine vs. prone), bladder volume (>148 vs. ≤148 cm3), cross-sectional rectal area (>7.3 vs. ≤7.3 cm2), cranial-caudal extent of seminal vesicles (>27 vs. ≤27 mm), and baseline IPSS sum (>7 vs. ≤7). Cross-sectional rectal area was calculated by dividing rectal volume by rectal length. Acute toxicity information was only available according to the CTCAE and thus compared with late CTCAE-based GU toxicity, not IPSS endpoints.
In addition to DVH variables, the median of both mean and maximal dose delivered to the four delineated GU structures were dichotomized and used in the univariate and multivariate analyses. Multivariate Cox proportional hazards models were applied to identify predictors of GU toxicity. Variables were analyzed for univariate log-rank P≤0.1. The database was closed for analysis in August 2012. Two-sided P<.05 was considered statistically significant. The data was analyzed in SPSS (SPSS Inc., Chicago, IL, version 19.0).
Results
Patients
Median follow-up was 5 years (range, 3–7.7 years). For the trigone, maximal dose received was 90.88 Gy (range, 78.04–94.38 Gy) and V90 (proportion of volume receiving 90 Gy) was 0.92% (range, 0.00–43.41%). Baseline demographics and clinical characteristics are summarized in Table 2. Median bladder volume at simulation was 148 cm3 (range, 31–1053 cm3), and 130 cm3 (range, 31–592 cm3) and 277 cm3 (range, 69–1053 cm3) for patients with empty and full bladder protocol. The median volumes of the bladder wall, the trigone, and the urethra were 56 cm3 (range, 18–240 cm3), 4 cm3 (range, 1–11 cm3), and 4 cm3 (range, 2–13 cm3). Median cross-sectional rectal area was 7 cm2 (range, 3–18 cm2) and median extent of the seminal vesicles was 27 mm (range, 15–45 mm). Larger bladder volume at simulation was associated with larger extent of the seminal vesicles (P<0.001).
Table 2.
Patient characteristics
| Total patients | n = 268 (%) |
|---|---|
| Age | |
| ≤71 years | 142 (53) |
| >71 years | 126 (47) |
| Race | |
| Caucasian | 227 (85) |
| African-American/Black | 24 (9) |
| Other | 17 (6) |
| Comorbidity | |
| Hypertension present | 119 (44) |
| Cardiovascular history present | 46 (17) |
| Diabetes present | 49 (18) |
| Smoking present* | 143 (53) |
| Prostate volume, cm3 (range)† | 36 (13–157) |
| Risk group* | |
| Low | 58 (22) |
| Intermediate | 144 (53) |
| High | 66 (25) |
| Androgen-deprivation therapy | |
| No | 134 (50) |
| Yes | 134 (50) |
| Median duration, months (range) | 7 (1–35) |
| IGRT using fiducial markers | |
| No | 213 (79) |
| Yes | 55 (21) |
| Bladder filling protol | |
| Empty | 196 (73) |
| Full | 72 (27) |
| Median FU, years (range) | 5 (3–7.7) |
Abbreviations: FU=follow-up; IGRT = image-guided radiation therapy.
Including former significant abuse.
Information lacking for 11 patients.
According to National Comprehensive Cancer Network.
Predictors for genitourinary toxicity
The median baseline IPSS sum was 7 (range, 0–35), and 137 (51%), 111 (41%), and 20 (8%) had mild (0–7), moderate (8–19) or severe (20–35) baseline symptoms, as previously described [3]. Thirty-nine patients experienced IPSS+10 after a median of 51.6 months (range, 3.4–86.3 months). The proportion remaining without IPSS+10 was 84% at 5 years. There were no IPSS questions over-represented in the rise of IPSS+10. In 19 of 39 patients with IPSS+10 this increase was observed only in one follow-up visit and durably improved in subsequent visits. In the remaining 20 patients, the endpoint was met in ≥1 subsequent visit, indicating persisting symptoms. The overall proportion of patients receiving an alpha-blocker at baseline, during acute follow-up, and during late follow-up was 23%, 49%, and 42%; for the 39 patients with IPSS+10 this was 23%, 44%, and 64%, respectively. A total of 39 patients had an IPSS ≥20 during follow-up. Of these, 6 patients had a similar or higher value at baseline. From the remaining 33 patients, 28 had an increase of ≥4 points over the respective IPSS baseline value.
Fifty-five and 2 patients experienced grade 2 and 3 late GU toxicity, with 5-year grade ≥2 late GU toxicity-free survival being 77% as previously described [3]. IPSS+10 during follow-up was associated with grade ≥2 late GU toxicity (P=0.000006).
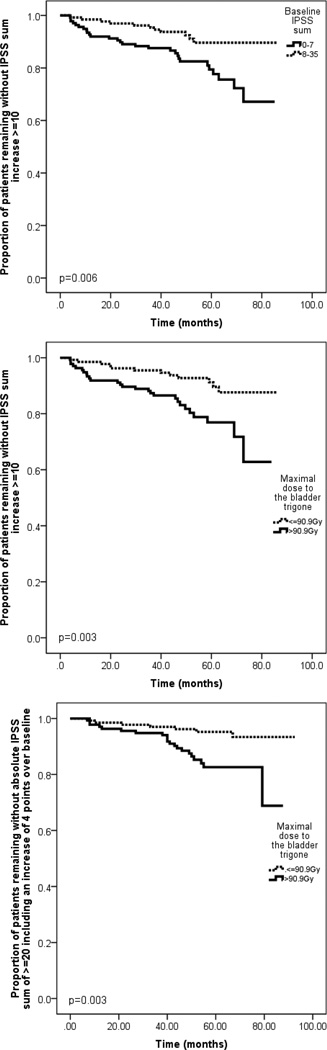
The univariate analysis for the IPSS sum +10 endpoint is summarized in Table 3; lower baseline IPSS sum (P=0.006), trigone V90 (P=0.006), and the maximal dose to the trigone (P=0.003) were significantly associated with IPSS+10. In multivariate analysis, lower baseline IPSS sum (P=0.009) and high maximal dose to the trigone (P=0.005) remained significantly associated with IPSS+10 (Table 4). Figure 2 demonstrates the impact of IPSS baseline sum and the maximal dose to the bladder trigone on late GU toxicity. Seventy-two patients had both a baseline IPSS sum ≤7 and a maximal dose to the trigone >90.9 Gy and were defined as high-risk, and 68 patients had both a baseline IPSS sum of >7 and a maximal dose to the trigone of ≤90.9 Gy and were defined as low-risk for development of an IPSS+10. Twenty-one of the 72 high-risk patients (29%) but only 5 of 68 (7%) low-risk patients experienced IPSS+10 (odds ratio, 5.19). When only the 20 patients with persisting IPSS elevation+10 (≥1 subsequent visit) were considered as events, the maximal dose to the trigone (P=0.025) but not the trigone V90 (P=0.147) remained significantly associated with IPSS+10 after univariate analysis. When the 28 patients with an IPSS of ≥20 points (including an increase of ≥4 points over baseline) were considered as events, the maximal dose to the trigone (P=0.003, Figure 2) but not the trigone V90 (P=0.073) remained associated with this endpoint after univariate analysis.
Table 3.
Univariate predictors for IPSS sum increase after therapy
| Factor | Log-rank p-value IPSS sum increase ≥10 All patients n=268 |
|---|---|
| Univariate analysis | |
| Age: >71 years | 0.748 |
| Race: African American | 0.710 |
| Diabetes: yes | 0.621 |
| Smoking†: yes | 0.427 |
| Prostate volume‡: >37 cm3 | 0.497 |
| Risk group§: high | 0.083 |
| ADT: yes | 0.857 |
| IGRT: yes | 0.573 |
| Patient positioning: supine | 0.480 |
| Bladder volume¶: >148 cm3 | 0.188 |
| Cross-sectional rectal area¶: >7.3 cm2 | 0.385 |
| Extent of seminal vesicles: >27 mm | 0.189 |
| IPSS sum baseline: >7 | 0.006 |
| Full bladder max dose: >91.7 Gy | 0.211 |
| Full bladder V90: >0.98 % | 0.247 |
| Bladder wall max dose: >91.8 Gy | 0.217 |
| Bladder wall V90: >1.6 % | 0.177 |
| Trigone max dose: >90.9 Gy | 0.003 |
| Trigone V85: >61.6 % | 0.064 |
| Trigone V90: >4.5 % | 0.006 |
| Urethra mean dose: >88.4 Gy | 0.109 |
| Urethra max dose: >90.5 Gy | 0.793 |
Abbreviations: ADT = androgen deprivation therapy; IGRT = image-guided radiotherapy; IPSS = international prostate symptom score; Vx= proportion of volume receiving × Gy.
Current or significant history.
Only available for 257 patients.
According to the National Comprehensive Cancer Network.
At simulation.
Table 4.
Predictors for IPSS sum increase ≥10 after therapy after multivariate Cox regression
| Factor | Hazard ratio (95% confidence interval, P value) |
|---|---|
| IPSS sum baseline: >7 | 0.39 (0.20, 0.79) (0.009) |
| Trigone max dose: >90.9 Gy | 2.70 (1.35, 5.32) (0.005) |
Abbreviations: IPSS = international prostate symptom score; The same variables were predictive for increase in IPSS score ≥ 10 when the analysis was repeated without the 55 patients who had IGRT (data not shown).
Fig. 2.
Kaplan-Meier plot showing (A) the proportion of patients remaining without an International Prostate Symptom Score (IPSS) sum increase of ≥10 points stratified into baseline IPSS sum, (B) stratified according to the maximal dose to the bladder trigone and showing (C) the proportion of patients remaining without an absolute IPSS sum of ≥ 20 points including a ≥ 4 points increase over baseline stratified according to the maximal dose to the bladder trigone.
When clinical and dosimetric variables were compared in a univariate analysis for grade ≥2 late GU toxicity-free survival, grade ≥2 acute CTCAE-based GU toxicity (P=0.01) and increased trigone V90 (P=0.047) were associated with increased grade ≥2 late GU toxicity. There was only a trend for increased toxicity in the presence of larger extent of the seminal vesicles (P=0.08) and increased maximal dose to the trigone (P=0.1). In the multivariate model, late grade ≥2 GU toxicity was associated with grade ≥2 acute GU toxicity (P=0.014; hazard ratio [HR], 1.96; 95% confidence interval [CI], 1.15, 3.35) and high V90 of the trigone (P=0.055; HR, 1.68; CI, 0.99, 2.87).
DISCUSSION
On multivariate analysis we found a significant association between IPSS+10 during follow-up and increased maximal dose to the bladder trigone and lower baseline IPSS sum. Moreover, CTCAE-based grade ≥2 late GU toxicity-free survival was univariately associated with grade ≥2 acute GU toxicity and increased bladder trigone V90. Our study is noteworthy, consisting of homogenously treated patients using patient-reported toxicity, analyzing the bladder and also more distinct pelvic GU structures, such as the bladder trigone and prostatic urethra, and analyzing the full spectrum of captured DVH, not only point doses. Furthermore, minimal followup was 3 years, and baseline GU symptoms were accounted for by toxicity event definitions. IPSS score is sensitive to the GU symptoms caused by benign prostate hyperplasia and prostate cancer. Treatment can relieve these problems in some patients [7]. Our multivariate model accounting for baseline IPSS controls for these effects, which are obviously large for prostate cancer patients. Our study (consistent with our previous paper on the time development of IPSS scores [7]) underlines the importance of controlling for baseline bladder function when modeling bladder complications.
Most existing studies are not directly comparable with ours, due to differences in endpoints and follow-up time. Heemsbergen et al. [6] previously described 40/557 patients with urinary obstruction based on the Dutch dose-escalation trial, after median follow-up of 71 months. Each patient was mapped to a selected reference patient, three-dimensional dose maps were constructed, and a dose-difference map was established. The dose-difference map indicated highly significant dose differences in the trigonal region in patients with vs. without late obstruction. A dose >47 Gy to a chosen point within the trigone predicted obstruction, especially for events >2 years post-therapy [6]. Differences between our study and theirs include that our cohort was treated with a higher dose, using IMRT, and only 2/268 patients had urinary obstruction events as defined for the other cohort. Late GU toxicity events in our series (both for IPSS endpoints and CTCAE) were defined as an increase over baseline, but in theirs baseline function was not accounted for in acute or late GU toxicity definitions. Moreover, the Dutch investigators used a crude approach, dividing events occurring <2 from >2 years post-treatment. They also used the dose to a defined ‘trigone point’ as predictive of complication while we used maximal dose to the trigone. In our study no patient underwent previous transurethral prostate resection and the V80 of the bladder wall, the craniocaudal extent of the seminal vesicles, and the bladder volume at simulation were not associated with IPSS sum increase, as was the case for the urinary obstruction endpoint in their cohort. However, both studies suggest that increased dose to the bladder trigone impacts late obstructive voiding side effects.
Little is known about the role of the bladder trigone in micturition. It was suggested that the trigone contracts during bladder filling, helping to keep the ureteral orifices open and the bladder neck shut [9]. Micturition may be initiated by trigone relaxation and consecutive funneling of urine into the urethra [9]. Bladder irradiation might lead to increased early or late GU toxicity by damaging different tissues including the urothelium, smooth muscle, and vasculature, and GU toxicity after RT might also involve nerve activation changes [10]. Alteration of the physiological role of the trigone may lead to obstructive voiding side effects; thus, sparing the trigone during RT planning might improve late GU toxicity after high-dose IMRT, and patient QoL. We previously suggested IGRT can reduce GU toxicity, potentially by keeping less of the bladder neck/bladder trigone region exposed to high radiation dose [6].
Data on dose-GU/dysfunction have been conflicting; a few studies showed an association between dose to the bladder and GU toxicity, generally unconfirmed by other studies. Limitations in these analyses include volume and position changes during treatment, distinction between urethral and bladder symptoms, duration of follow-up, and physician-reported toxicity scales [4]. Rosewall et al. identified further pitfalls, including the influence of genetic variations on radiation response, total prescribed dose as a surrogate for bladder dose, single DVH points rather than full DVH information, GU baseline function, symptom aggregation and dichotomization, and temporal variation of toxicity [5]. Also, determining whether GU symptoms were of urethral or bladder origin is usually difficult. In our cohort, urethral dose was not associated with significant changes in IPSS or CTCAE-based late GU toxicity; however, we cannot exclude that it may contribute to GU toxicity.
One of the few positive correlative studies regarding dose response in the bladder, by Harsolia et al., studied 331 patients who underwent three-dimensional conformal radiotherapy (median prescribed dose, 75.6 Gy; median follow-up, 1.6 years). They found volume of the bladder wall receiving ≥30 (V30) and ≥82 (V82) Gy predicted CTCAEv2 grade ≥2 late retention and grade 3 late retention, and recommended limiting the bladder wall V30 to <30 cm3 and the V82 to <7 cm3 [11]. These correlations could not be reproduced in our cohort.
Other studies usually found baseline GU function, acute GU toxicity, higher age, presence of diabetes, and transurethral resection of the prostate [12] to be associated with increased late GU toxicity. As previously described we believe the association between baseline GU function and late GU toxicity is an artifact of not considering baseline function when defining late toxicity events (e.g., score a toxicity event only when increase over baseline is present). In fact, our patients with excellent baseline function were more likely to experience late GU toxicity, probably because their superior baseline function increases the potential for loss of that function. We also found acute toxicity predicted for late CTCAAE-based GU toxicity, but not age, diabetes, and all other clinical variables [7].
This analysis has limitations, including IGRT using fiducial markers in only 21% of patients. Therefore, it might be that the estimated dose to structures at simulation does not represent the real treatment dose distribution. Because a previous analysis of the 86 Gy patients showed that IGRT lowered grade ≥2 urinary toxicity rates, we repeated the analysis without the 55 IGRT patients, and found the same variables predicted increase in IPSS+10 (data not shown). Centers using conventionally fractionated IMRT with prescribed doses of 78–80 Gy may regard the hot-spots around 90 Gy as irrelevant for their practices. However, the biologically effective doses (BED) of the two treatment regimens may be comparable: conservatively assuming an α/β ratio of 3 for late bladder effects, the BED is 138 Gy for 48 × 1.8 Gy (= 86.4 Gy) and 133 Gy for 40 × 2 Gy (= 80 Gy). The effective doses may be significantly higher with slight or radical hypofractionation regimens. The median value of the max trigone dose in our cohort (90.9 Gy in 48 fractions) has a linear-quadratic equivalent dose of 86.3 Gy in 40 fractions. Also, the limited follow-up should be mentioned as a limitation but despite these limitations, our findings suggest that it might be possible to decrease late GU toxicity by limiting the inferior bladder dose and specifically the bladder trigone. Our results suggest a constraint of maximal dose to the bladder trigone <90.9 Gy or < 86.3 Gy when using 40×2 Gy, based on the significant difference in IPSS+10 (Figure 2) and the calculations above. For 3 patients with the highest maximal doses to the trigone, new plans were generated that obeyed the constraint that maximal trigone dose should be <91 Gy without compromising other requirements, showing that this constraint can be implemented clinically.
CONCLUSIONS
The application of high doses to small volumes of the bladder trigone was significantly associated with relevant changes in IPSS sum during follow-up. Our findings suggest that late GU toxicity might be decreased by limiting the dose to the bladder trigone.
SUMMARY.
This study was performed to determine the association between genitourinary toxicity and dose delivered to genitourinary pelvic structures after intensity-modulated radiotherapy (IMRT) for prostate cancer. Patients were evaluated for an IPSS sum increase of ≥10 points over baseline. An increased maximal dose to the trigone (P=0.005) was significantly associated with an IPSS sum increase of ≥10 suggesting that the reduction of dose to the trigone may reduce urinary toxicity in patients receiving high dose IMRT.
ACKNOWLEDGEMENT
PG was supported financially by the Swiss Foundation for Medical-Biological Scholarships (SSMBS) as well as the Eugen & Elisabeth Schellenberg Foundation. AJ was supported by NIH R01CA129182.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
The authors declare that there are no financial disclosures or conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan AN, Yorke ED, Marks LB, et al. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–S122. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Rosewall T, Catton C, Currie G, et al. The relationship between external beam radiotherapy dose and chronic urinary dysfunctionda methodological critique. Radiother Oncol. 2010;97:40–47. doi: 10.1016/j.radonc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Heemsbergen WD, Al-Mamgani A, Witte MG, et al. Urinary obstruction in prostate cancer patients from the Dutch trial (68 Gy vs. 78 Gy): Relationships with local dose, acute effects, and baseline characteristics. Int J Radiat Oncol Biol Phys. 2010;78:19–25. doi: 10.1016/j.ijrobp.2009.07.1680. [DOI] [PubMed] [Google Scholar]
- 7.Ghadjar P, Jackson A, Oh J, et al. Predictors and patterns of amelioration of genitourinary toxicity after high-dose intensity-modulated radiation therapy for localized prostate cancer: Implications for defining post-radiotherapy urinary toxicity. Eur Urol. 2013;64:931–938. doi: 10.1016/j.eururo.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, Lepor H. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 9.Roosen A, Wu C, Sui G, Chowdhury RA, Patel PM, Fry CH. Characteristics of spontaneous activity in the bladder trigone. Eur Urol. 2009;56:346–353. doi: 10.1016/j.eururo.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Marks LB, Carroll PR, Dugan TC, Anscher MS. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257–1280. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]
- 11.Harsolia A, Vargas C, Yan D, Brabbins D, Lockman D, Liang J, Gustafson G, Vicini F, Martinez A, Kestin LL. Predictors for chronic urinary toxicity after the treatment of prostate cancer with adaptive three-dimensional conformal radiotherapy: dose-volume analysis of a phase II dose-escalation study. Int J Radiat Oncol Biol Phys. 2007;69:1100–1109. doi: 10.1016/j.ijrobp.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 12.Budäus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, Wiegel T. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–1127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]