Abstract
Objective
To examine the effect of neonatal morbidity on ATP breakdown in late preterm infants.
Study Design
Urinary hypoxanthine concentration, a marker of ATP breakdown, was measured from 82 late preterm infants on days of life (DOL) 3 to 6 using high-performance liquid chromatography. Infants were grouped according to the following diagnoses: poor nippling alone (n = 8), poor nippling plus hyperbilirubinemia (n = 21), poor nippling plus early respiratory disease (n = 26), and respiratory disease alone (n = 27).
Results
Neonates with respiratory disease alone had significantly higher urinary hypoxanthine over DOL 3 to 6 when compared with neonates with poor nippling (P = .020), poor nippling plus hyperbilirubinemia (P < .001), and poor nippling plus early respiratory disease (P = .017). Neonates with poor nippling who received respiratory support for 2 to 3 days had significantly higher hypoxanthine compared with infants who received respiratory support for 1 day (P = .017) or no days (P = .007).
Conclusions
These findings suggest that respiratory disorders significantly increase ATP degradation in late premature infants.
Keywords: ATP, hypoxanthine, late preterm, high-performance liquid chromatography, urine
Infants born before 37 weeks gestation are considered premature and have significantly higher morbidity and mortality rates compared with infants born between 37 and 41 weeks gestation. Much of the research involving premature infants is focused on very low birth weight (<1500 g) or infants less than 33 weeks gestation1; however, roughly 70% of all preterm infants are considered late preterm—infants born between 340/7 and 366/7 weeks gestation.2–4 Furthermore, this population is increasing in number. Much of the 30% increase in premature births observed between 1981 and 2003 can be accounted for by an increase in the rate of late preterm births.2,5 Although the rate of late preterm births declined from 9.1% in 2006 to 8.26% in 2011, it is still roughly 13% higher than it was in 1990.4 Despite the prevalence of this population, they are still relatively understudied compared with more premature or full-term infants.
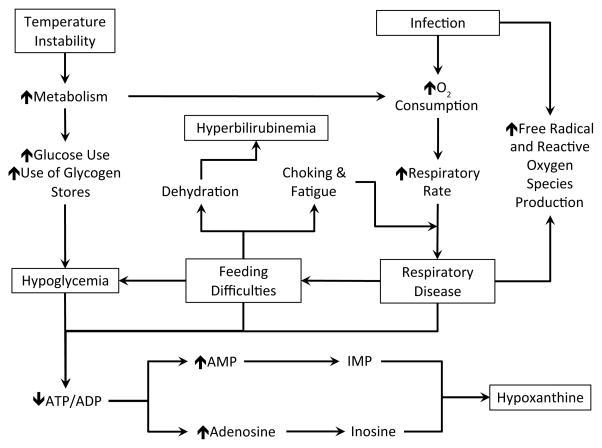
Late preterm infants tend to resemble full-term infants in size and shape; however, they are physiologically and metabolically immature compared with their term counterparts and are therefore at a higher risk for developing medical complications.6 Late preterm infants are 4 times more likely to be diagnosed with 1 medical condition and 3.5 times more likely to be diagnosed with 2 or more medical conditions compared with their term counterparts.7 Some of the most common morbidities experienced by late preterm infants include respiratory disease, temperature instability, hypoglycemia, hyperbilirubinemia, feeding difficulties, and infection.3,6,8–15 Many of the morbidities associated with late preterm neonates are linked to increased oxygen need or increased metabolic demand (Figure 1).16–21
Figure 1.
Pathway Depicting the Interrelationship Between Common Late Preterm Morbidities and Hypoxanthine, a Marker of ATP Breakdown.
Abbreviations: O2, oxygen; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; IMP, inosine monophosphate.
In addition to increased morbidity, late preterm infants have elevated resting energy expenditure compared with term infants.22 Few studies, if any, have measured total energy expenditure in late preterm infants; however, it is known that total energy expenditure is significantly higher in moderately premature infants, 30 to 33 weeks gestation, compared with term infants.23 Moreover, respiratory morbidity can further increase energy expenditure in premature infants.20,24 Despite the prevalence, increased morbidity rates, and higher energy expenditure observed in the late preterm population, a thorough understanding of the metabolic consequences of morbidity in this population is lacking.
The aim of this study was to evaluate, noninvasively, the effects of respiratory disease, poor nippling, and hyperbilirubinemia on ATP utilization in late preterm infants. Urinary hypoxanthine was used to evaluate the effects of morbidities on ATP utilization in late preterm infants for multiple reasons. First, hypoxanthine is a major breakdown product of ATP in most human tissues, with the exception of the intestine and liver, where it is readily oxidized to uric acid by the enzyme xanthine dehydrogenase/xanthine oxidase.25,26 Second, the normal circulatory concentration of hypoxanthine is relatively low, allowing for the detection of minor changes. Third, as described in the Methods section, urinary hypoxanthine is relatively stable under variable sampling conditions. Last, research demonstrates increased hypoxanthine under conditions of enhanced ATP breakdown like hypoxia or maximal exercise.27–30
Methods
Subject Enrollment and Sample Collection
Premature neonates, between 340/7 and 366/7 weeks gestation, who were admitted to Loma Linda University Children’s Hospital neonatal intensive care unit (NICU) were included in this study. The Loma Linda University Institutional Review Board approved study protocol and informed consent documents. Infants with congenital anomalies, congenital heart disease, metabolic acidosis, or more than 2 major morbidities were excluded from the study. These infants were excluded from the study because they (a) required surgery during the study period, (b) were known to have medical conditions associated with elevated hypoxanthine, or (c) have morbidities that may produce confounding results. Patient diagnoses were determined by a neonatologist (AH).
After parental consent was obtained, investigators collaborated with the clinical staff to obtain urine samples. Urine samples were collected by placing cotton balls over the urethral meatus. Urine-soaked cotton balls were removed from the diaper with every changing and stored at 4°C. Samples that were free of stool were combined into 24-hour aliquots over DOL 3 to 6. Validation studies performed in our laboratory show that hypoxanthine and creatinine remained stable under our sampling and processing conditions (Table 1). Urine was extracted from the cotton using pressure, centrifuged for 10 minutes at 20 000 × g and 4°C, filtered through a Millex syringe filter (Low Protein Binding Durapore PVD filter, 0.45 μm, 13 mm; Millipore Corp), and stored at −80°C until analysis.
Table 1.
Representative Time and Temperature Stability of Hypoxanthine and Creatinine.
| Hypoxanthine | Creatinine | Hypoxanthine/Creatinine | |
|---|---|---|---|
| Immediately after collection | |||
| Straight urine | 113.52 (0.00) | 3190.16 (0.00) | 0.0356 (0.00) |
| Extracted from cotton | 115.42 (1.67) | 3165.66 (−0.78) | 0.0365 (2.47) |
| Room temperature | |||
| 3 hours | 118.11 (4.04) | 3167.73 (−0.70) | 0.0373 (4.48) |
| 24 hours | 115.41 (1.66) | 3051.40 (−4.35) | 0.0374 (4.99) |
| 3 days | 113.90 (0.33) | 3270.32 (2.51) | 0.0328 (−2.13) |
| 1 week | 105.76 (−6.84) | 3173.32 (−0.53) | 0.0333 (−6.34) |
| 37°C | |||
| 3 hours | 114.29 (0.67) | 3189.22 (−0.03) | 0.0358 (0.70) |
| 6 hours | 115.75 (1.96) | 3136.34 (−1.69) | 0.0369 (3.71) |
| 0°C | |||
| 24 hours | 118.24 (3.98) | 3097.36 (−2.91) | 0.0381 (7.10) |
| 1 week | 102.24 (−9.94) | 3161.66 (−0.89) | 0.0323 (−9.12) |
| −80°C | |||
| 24 hours | 117.28 (3.31) | 3098.28 (−2.88) | 0.0379 (6.38) |
| 1 week | 102.08 (−10.08) | 3126.06 (−2.01) | 0.0327 (−8.24) |
Data are mean (% change from fresh urine). Data are representative of a single urine sample. The experiment was performed on 3 separate urine samples with similar results.
Subject Classification
Neonates in the poor nippling group were those with oral motor immaturity without signs and symptoms of feeding intolerance or necrotizing enterocolitis. Neonates classified as poor nippling plus hyperbilirubinemia were those with oral motor immaturity who received phototherapy at some point over the first 6 days of life. Neonates classified as poor nippling plus initial respiratory disease were those with oral motor immaturity and evidence of apnea or ventilatory support for 3 days or less. Neonates diagnosed with respiratory disease alone are those with transient tachypnea of the newborn (TTNB) or radiographic evidence of respiratory distress syndrome (RDS) or pneumonia requiring oxygen and/or ventilatory support over at least a successive 4-day period in the first 6 days of life.
Hypoxanthine and Creatinine Quantification
Urinary hypoxanthine and creatinine concentrations were determined using an adaptation of the high-performance liquid chromatography (HPLC) method described by George et al.31 Briefly, urine samples were thawed and sonicated before 200 μL was transferred to an Eppendorf tube containing 1 × 10−7 mol of 2-aminopurine (internal standard). The samples were then analyzed on an HPLC (Waters 996 PDA, Waters 600 controller, and 717plus autosampler; Millipore Corp) by injecting 35 μL onto a Supelcosil LC-18-S 15 cm × 4.6 mm, 5 μm column (SGE; Austin, TX), with the following isocratic conditions: 10 mM potassium dihydrogen phosphate buffer, pH 4.7, flow rate 1.0 mL/min. Creatinine, hypoxanthine, and 2-aminopurine were quantitated by obtaining peak areas at the appropriate retention times (~3.5, 8, and 13.5 minutes, respectively) and wavelengths (230, 248, and 305 nm, respectively). The area ratios of each compound to 2-aminopurine were determined and converted into concentration using standard curves. Samples were analyzed in triplicate and values with a coefficient of variation less than 10% were included in the final analysis. The limits of detection were 1.58 μM for hypoxanthine and 3.2 μM for creatinine.
Stability of Hypoxanthine and Creatinine
Because urine is stored in the bladder and urine soaked cotton can remain in the diaper for up to 3 hours, we determined the stability of hypoxanthine over time and at varying temperatures. Urine samples were collected from volunteers and hypoxanthine and creatinine were measured by HPLC in samples subjected to the following conditions: direct analysis, immediate cotton extraction and analysis, room temperature (3 hours, 24 hours, 3 days, and 1 week), 36°C (3 hours or 6 hours), 0°C (24 hours or 1 week), or −80°C (24 hours or 1 week). Hypoxanthine and creatinine as well as the ratio of hypoxanthine/creatinine were found to be stable under all conditions tested, with the mean concentration for each processing condition being within 15% of the mean for fresh urine (Table 1). When stability experiments were repeated, the direction of percent change was variable but never exceeded ±15%.
Statistics
To analyze the data, assumptions of normality and equal variance were assessed. Demographic data for categorical variables were analyzed using χ2 test. Repeated-measures ANOVA for 1 between-subject factor (diagnosis) and 1 within-subject factor (day of life) were assessed to evaluate the effect of the morbidity on urinary hypoxanthine concentrations over time. Repeated-measures ANOVA for 1 between-subject factor (respiratory support) and 1 within-subject factor (day of life) were assessed to evaluate the effect of the mode of respiratory support on urinary hypoxanthine concentrations over time in infants diagnosed as poor nippling plus initial respiratory support. All statistical analyses were performed using SPSS Statistics for Windows Version 21. Differences were considered significant at P < .05.
Results
Subject Enrollment
A total of 82 infants born between 340/7 and 366/7 weeks gestation were enrolled in this study and had adequate urine collected for assay. Of the infants enrolled in this study, 8 were classified as having poor nippling alone, 21 as having poor nippling plus hyperbilirubinemia, 26 as having poor nippling plus early respiratory disease, and 27 as having respiratory disease alone.
Subject Demographics
The subjects were heterogeneous for estimated gestational age (EGA), birth weight, length of NICU stay, 5-minute APGAR, race, mode of delivery, days on total parenteral nutrition (TPN), and days until oral/enteral feeds (Tables 2 and 3). Infants who were diagnosed with poor nippling plus hyperbilirubinemia had significantly lower estimated gestational age (P < .01) and birth weight (P < .01) compared with infants diagnosed with respiratory disease alone. Infants diagnosed with respiratory disease alone had significantly longer NICU stay compared with infants diagnosed with poor nippling plus hyperbilirubinemia (P < .01) and infants diagnosed with poor nippling plus early respiratory disease (P < .05). Infants diagnosed with respiratory disease, however, had significantly lower 5-minute APGAR scores compared with infants diagnosed with poor nippling plus hyperbilirubinemia (P < .01) and those diagnosed with poor nippling plus early respiratory disease (P < .01). Additionally, infants diagnosed with respiratory disease alone had significantly longer days on TPN and days until oral/enteral feeding compared with infants diagnosed with poor nippling (P < .01 for days on TPN, P = .043 for days until oral/enteral feeding), poor nippling plus hyperbilirubinemia (P < .01 for days on TPN, P = .002 for days until oral/enteral feeding), and infants diagnosed with poor nippling plus early respiratory disease (P < .01 for days on TPN, P = .003 for days until oral/enteral feeding).
Table 2.
Subject Demographics.a
| Poor Nippling (n = 8) | Poor Nippling Plus Hyperbilirubinemia (n = 21) | Poor Nippling Plus Early Respiratory Disease (n = 26) | Respiratory Disease (n = 27) | P Value | |
|---|---|---|---|---|---|
| EGA (weeks) | 35.0 ± 0.6 | 34.4 ± 0.4 | 34.9 ± 0.7 | 35.1 ± 0.7 | .001b |
| Birth weight (g) | 2306.1 ± 854.2 | 2117.8 ± 460.5 | 2322.5 ± 428.5 | 2650.5 ± 489.0 | .005b |
| Length of stay (days) | 16.4 ± 5.8 | 15.1 ± 5.7 | 17.4 ± 7.8 | 28.7 ± 21.9 | .004b,c |
| APGAR, 1 minute | 7 ± 2 | 7 ± 2 | 7 ± 2 | 6 ± 3 | .363 |
| APGAR, 5 minute | 8 ± 1 | 9 ± 1 | 8 ± 1 | 7 ±2 | .000b,c |
| Gender, n (%) | .596d | ||||
| Male | 6 (75%) | 12 (57.1%0 | 15(57.7%) | 13 (48.1%) | |
| Female | 2 (625%) | 9 (42.9%) | 11 (42.3%) | 14 (51.9%) | |
| Race, n (%) | .024d | ||||
| White | 2 (25.0%) | 18 (85.7%) | 16 (61.5%) | 19 (70.4%) | |
| Hispanic | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.4%) | |
| African American | 0 (0.0%) | 2 (9.5%) | 3 (11.5%) | 2 (7.4%) | |
| Other | 6 (75.0%) | 1 (4.8%) | 7 (27.0%) | 4 (14.8%) | |
| Mode of delivery, n (%) | .049d | ||||
| Vaginal | 2 (25%) | 14 (66.7%) | 12 (46.2%) | 8 (29.6%) | |
| C/S | 6 (75%) | 7 (33.3%) | 14 (53.8%) | 19 (70.4%) | |
Abbreviations: EGA, estimated gestational age; C/S, Cesarean section.
Data are mean ± standard deviation.
Poor nippling plus Hyperbilirubinemia significantly different than Respiratory Disease.
Poor nippling plus Early Respiratory Disease significantly different than Respiratory Disease (one-way ANOVA).
Chi-square test.
Table 3.
Nutritional Intake and Dietary Additives.
| Poor Nippling (n = 8) | Poor Nippling Plus Hyperbilirubinemia (n = 21) | Poor Nippling Plus Early Respiratory Disease (n = 26) | Respiratory Disease (n = 27) | P Value | |
|---|---|---|---|---|---|
| Days on TPN | 2.4 ± 1.5 | 3.0 ± 2.4 | 3.5 ± 1.8 | 15.0 ± 13.5 | <.001a |
| Days until oral/enteral feeds | 0.1 ± 0.3 | 0.3 ± 0.5 | 1.0 ± 0.7 | 8.2 ± 12.5 | <.001a |
| Additives received during NICU stay | .280b | ||||
| None | 3 (37.5%) | 10 (47.6%) | 14 (53.8%) | 12 (44.4%) | |
| ECP | 3 (37.5%) | 2 (9.5%) | 5 (19.2%) | 6 (22.2%) | |
| HMF | 1 (12.5%) | 1 (4.8%) | 1 (3.8%) | 1 (3.7%) | |
| ECP & Prosobee lipil with Fe | 1 (12.5%) | ||||
| HMF & ECP | 2 (9.5%) | 3 (11.5%) | 4 (14.8%) | ||
| HMF, ECP, & LHMF | 3 (14.3%) | 2 (7.7%) | 1 (3.7%) | ||
| HMF & LHMF | 3 (14.3%) | ||||
| HMF & Bene protein | 1 (3.8%) | ||||
| HMF, ECP, MCT oil and pregestemil | 1 (3.7%) | ||||
| Pregestemil | 1 (3.7%) | ||||
| ECP & Enfamil AR | 1 (3.7%) | ||||
| Additives received during study period | .277b | ||||
| None | 6 (75.0%) | 16 (76.2%) | 24 (92.3%) | 27 (100.0%) | |
| ECP | 1 (12.5%) | 1 (4.8%) | 2 (7.7%) | 0 (0.0%) | |
| HMF | 1 (12.5%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | |
| LHMF | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | |
| HMF & ECP | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | |
| LHMF & HMF | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | |
| Day 3 nutritional intake, n (%) | <.001a | ||||
| TPN | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 21 (77.8%) | |
| Formula (F) | 1 (12.5%) | 3 (14.3%) | 2 (7.7%) | 0 (0.0%) | |
| Breast milk (BM) | 0 (0.0%) | 2 (9.5%) | 1 (3.8%) | 0 (0.0%) | |
| BM/F | 4 (50.0%) | 3 (14.3%) | 4 (15.4%) | 1 (3.7%) | |
| TPN/F | 3 (37.5%) | 2 (9.5%) | 11 (42.3%) | 3 (11.1%) | |
| TPN/BM | 0 (0.0%) | 7 (33.3%) | 4 (42.3%) | 0 (0.0%) | |
| TPN/BM/F | 0 (0.0%) | 4 (10.9%) | 3 (11.5%) | 2 (7.4%) | |
| Day 4 nutritional intake, n (%) | <.001b | ||||
| TPN | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 14 (51.9%) | |
| Formula (F) | 2 (25.0%) | 3 (14.3%) | 5 (19.2%) | 0 (0.0%) | |
| Breast milk (BM) | 0 (0.0%) | 5 (23.8%) | 2 (7.7%) | 1 (3.7%) | |
| BM/F | 3 (37.5%) | 5 (23.8%) | 6 (23.1%) | 0 (0.0%) | |
| TPN/F | 1 (12.5%) | 1 (4.8%) | 3 (11.5%) | 3 (11.1%) | |
| TPN/BM | 0 (0.0%) | 2 (9.5%) | 5 (19.2%) | 3 (11.1%) | |
| TPN/BM/F | 2 (25.0%) | 5 (23.8%) | 5 (19.2%) | 6 (22.2%) | |
| Day 5 Nutritional Intake, n (%) | <.001b | ||||
| TPN | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 13 (48.1%) | |
| Formula (F) | 1 (12.5%) | 3 (14.3%) | 5 (19.2%) | 0 (0.0%) | |
| Breast milk (BM) | 0 (0.0%) | 6 (28.6%) | 4 (15.4%) | 1 (3.7%) | |
| BM/F | 7 (87.5%) | 6 (28.6%) | 11 (42.3%) | 1 (3.7%) | |
| TPN/F | 0 (0.0%) | 1 (4.8%) | 2 (7.7%) | 3 (11.1%) | |
| TPN/BM | 0 (0.0%) | 4 (19.0%) | 2 (7.7%) | 3 (11.1%) | |
| TPN/BM/F | 0 (0.0%) | 1 (4.8%) | 2 (7.7%) | 6 (22.2%) | |
| Day 6 nutritional intake, n (%) | <.001b | ||||
| TPN | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 11 (40.7%) | |
| Formula (F) | 1 (12.5%) | 2 (9.5%) | 7 (26.9%) | 1 (3.7%) | |
| Breast milk (BM) | 0 (0.0%) | 8 (38.1%) | 6 (23.1%) | 1 (3.7%) | |
| BM/F | 7 (87.5%) | 8 (38.1%) | 9 (34.6%) | 1 (3.7%) | |
| TPN/F | 0 (0.0%) | 1 (4.8%) | 1 (3.8%) | 2 (7.4%) | |
| TPN/BM | 0 (0.0%) | 1 (4.8%) | 0 (0.0%) | 6 (22.2%) | |
| TPN/BM/F | 0 (0.0%) | 1 (4.8%) | 3 (11.5%) | 5 (18.5%) | |
Abbreviations: ECP, Enfacare powder; HMF, human milk fortifier; LHMF, liquid human milk fortifier; MCT oil, medium-chain triglyceride oil; EMAR, Enfamil AR; TPN, total parenteral nutrition; F, formula; BM, breast milk.
Significantly different from Respiratory Disease; one-way ANOVA with Bonferroni correction.
Chi-square test.
No significant differences were observed among groups for gender, 1-minute APGAR scores, dietary additives over the course of the NICU stay, or dietary additives over the study period. We noted, however, that with the exception of poor nippling plus hyperbilirubinemia, there was a trend for more of the infants in each group to have been born via cesarean section (P = .049). There was a significantly higher number of white infants enrolled in the study compared with Hispanic, African American, or other (P = .024). Last, the nutritional intake varied significantly over every day of the study period (χ2, P < .001). No infants in the study suffered from asphyxia, intraventricular hemorrhage, or patent ductus arteriosus.
Urinary Hypoxanthine and Subject Diagnoses
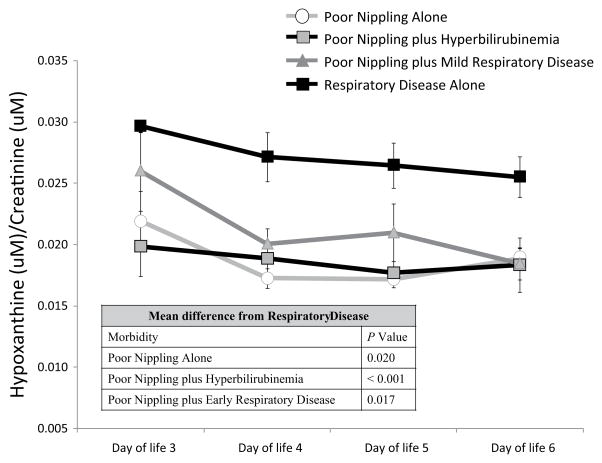
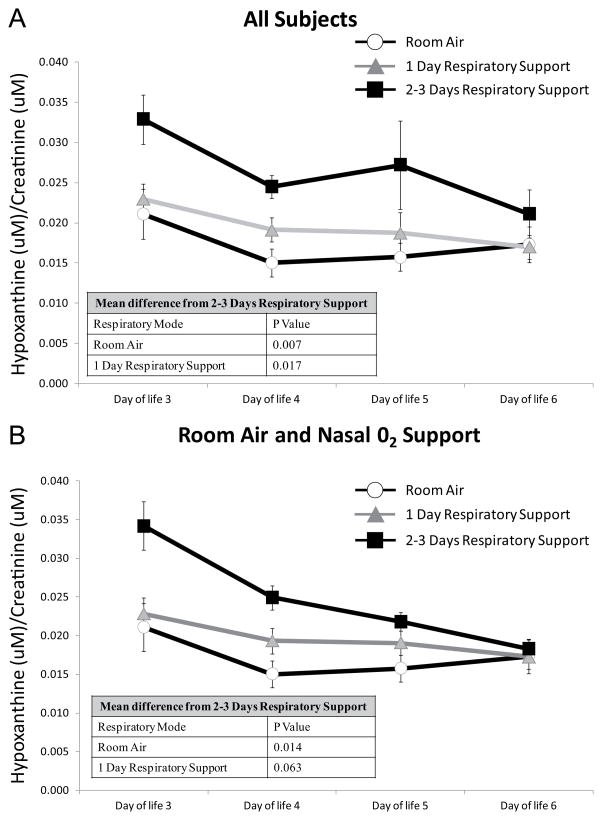
Repeated-measures analysis of urinary hypoxanthine over DOL 3 to 6 revealed significantly higher hypoxanthine over time for infants diagnosed as having respiratory disease alone compared with infants diagnosed with poor nippling (P = .020), poor nippling plus hyperbilirubinemia (P < .001), and poor nippling plus early respiratory disease (P = .017; Figure 2). There was a trend, although not significant, for infants with poor nippling plus early respiratory disease to have higher urinary hypoxanthine on DOL 3 to 5 when compared with infants diagnosed with poor nippling alone and those diagnosed with poor nippling plus hyperbilirubinemia. However, we found that hypoxanthine concentration on DOL 3 to 6 in neonates with poor nippling plus early respiratory disease was significantly higher for infants who required respiratory support for the first 2 to 3 days of life when compared with infants who received no respiratory support (P = .007) or were on respiratory support for only 1 day (P = .017; Figure 3A). When we restricted our analysis to only nasal respiratory support, infants who received respiratory support for more than 2 days had significantly higher hypoxanthine on DOL 3 to 6 compared with infants who were on room air (P = .014; Figure 3B).
Figure 2.
Difference Between Days of Life 3 to 6 Urinary Hypoxanthine for Late Preterm Infants Diagnosed With Poor Nippling Alone (n = 8), Poor Nippling Plus Hyperbilirubinemia (n = 21), Poor Nippling Plus Early Respiratory Disease (n = 26), or Respiratory Disease Alone (n = 27).
P values represent significant differences between the indicated group and infants with respiratory disease over time.
Figure 3.
Effect of Number of Days of Respiratory Support on Urinary Hypoxanthine in Infants Diagnosed as Poor Nippling Plus Early Respiratory Disease.
(A) Infants on room air, receiving nasal oxygen support (high flow nasal cannula, nasal continuous positive airway pressure, or nasal intermittent positive pressure ventilation) or synchronized intermittent mandatory ventilation. (B) Infants on room air or receiving nasal oxygen support (high flow nasal cannula, nasal continuous positive airway pressure, or nasal intermittent positive pressure ventilation) only. P values represent significant differences between the indicated group and infants on 2 to 3 days respiratory support over time.
Discussion
Our preliminary work shows that hypoxanthine and creatinine are stable in urine and can be used to evaluate the effect of morbidity on ATP metabolism in late preterm infants. More important, we found that late preterm infants diagnosed with respiratory disease alone had significantly higher urinary hypoxanthine concentrations over time when compared with infants diagnosed with poor nippling alone, poor nippling plus hyperbilirubinemia, or poor nippling plus early respiratory disease. This indicates that, within this late preterm population, specific disorders can change ATP metabolism and induce enhanced purine breakdown.
We further observed a trend for infants diagnosed with poor nippling plus early respiratory disease to have higher urinary hypoxanthine compared with infants diagnosed with poor nippling alone or poor nippling plus hyperbilirubinemia over day 3 through 5 of life. Although the difference was not significant, we hypothesized that the higher urinary hypoxanthine was most likely a reflection of early respiratory support in this population. On investigating the effects of respiratory support within the poor nippling plus early respiratory disease population, we found that infants who received 2 to 3 days of respiratory support had significantly higher urinary hypoxanthine compared with infants who received only 1 day of respiratory support or were on room air. This further indicates enhanced ATP breakdown as a result of respiratory issues, even within this subpopulation.
It is well documented that respiratory issues are common in the late preterm infants.3,32,33 This population is known to have a higher incidence of transient tachypnea of the newborn, respiratory distress syndrome, persistent pulmonary hypertension of the newborn, apnea, and respiratory failure.13,34–37 Khashu et al report that late preterm infants have 4.4 times the relative risk of respiratory morbidity than term infants.38 In addition, due to their prematurity, late preterm infants have lower energy stores and higher energy needs at birth compared with term infants.14,22,23,39 The available energy stores can be further decreased when the newborn is challenged by additional stressors, such as respiratory disease.18,20,24,40 Due to the increased energy needs in this population, particularly when combined with respiratory disease, it may be prudent to adjust the diet of late preterm infants so these energy demands are more adequately met.
Studies involving very low birth weight infants indicate that early and aggressive introduction of total parenteral nutrition and enteral feeding can result in better growth, reduced nutritional deficits, enhanced achievement of full enteral feedings, decreased morbidity, and improved neurodevelopment.41–43 Furthermore, it was reported that simultaneous low-dose amino acid infusion and breast milk feeding reduces the time of mechanical ventilation in premature infants with respiratory distress syndrome, possibly by ensuring improved energy support to aid respiratory muscle strength.44,45 These studies highlight the advantages of optimizing nutritional support in premature neonates through the implementation of dietary additives. More important, these data suggest that adjusting the diet of late preterm infants to resemble the higher calorie, lipid, and protein diet administered to more premature infants may provide better nutritional support for this population to combat respiratory challenges. Additionally, the utilization of dietary additives such as human milk fortifier, medium-chain triglyceride oil, or whey protein may help supplement the energy needs of this population. Further research is needed to determine if optimizing daily energy and protein intake can reduce ATP breakdown in late preterm infants.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 2.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of Vital Statistics: 2010–2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2006. Natl Vital Stat Rep. 2009;57(7):1–102. [PubMed] [Google Scholar]
- 6.Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120:1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 7.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandrappa A, Jain L. Health issues of the late preterm infant. Pediatr Clin North Am. 2009;56:565–577. doi: 10.1016/j.pcl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Darcy AE. Complications of the late preterm infant. J Perinat Neonatal Nurs. 2009;23:78–86. doi: 10.1097/JPN.0b013e31819685b6. [DOI] [PubMed] [Google Scholar]
- 10.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30:28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. A multicenter study on incidence and fatality rates of neonatal acute respiratory disorders according to gestational age, maternal age, pregnancy complications and type of delivery. Italian Group of Neonatal Pneumology. Biol Neonate. 1998;74(1):7–15. doi: 10.1159/000014005. [DOI] [PubMed] [Google Scholar]
- 12.Henderson-Smart DJ. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 1981;17:273–276. doi: 10.1111/j.1440-1754.1981.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 13.Dudell GG, Jain L. Hypoxic respiratory failure in the late preterm infant. Clin Perinatol. 2006;33:803–830. doi: 10.1016/j.clp.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Garg M, Devaskar SU. Glucose metabolism in the late preterm infant. Clin Perinatol. 2006;33:853–870. doi: 10.1016/j.clp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl 2):S12–S17. doi: 10.1038/jp.2009.41. [DOI] [PubMed] [Google Scholar]
- 16.Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J Pineal Res. 2009;46:128–139. doi: 10.1111/j.1600-079X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapman AG, Westerberg E, Siesjö BK. The metabolism of purine and pyrimidine nucleotides in rat cortex during insulin-induced hypoglycemia and recovery. J Neurochem. 1981;36:179–189. doi: 10.1111/j.1471-4159.1981.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 18.McGowan JE. Neonatal hypoglycemia. Pediatr Rev. 1999;20(7):e6–e15. [Google Scholar]
- 19.Laiakis EC, Morris GA, Fornace AJ, Howie SR. Metabolomic analysis in severe childhood pneumonia in the Gambia, West Africa: findings from a pilot study. PloS One. 2010;5(9) doi: 10.1371/journal.pone.0012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer J, Maier K, Muehlbauer B, Poeschl J, Linderkamp O. Energy expenditure and plasma catecholamines in preterm infants with mild chronic lung disease. Early Hum Dev. 2003;72:147–157. doi: 10.1016/s0378-3782(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 21.Brown L. Heart rate variability in premature infants during feeding. Biol Res Nurs. 2007;8:283–293. doi: 10.1177/1099800406298542. [DOI] [PubMed] [Google Scholar]
- 22.Dechert R, Wesley J, Schafer L, et al. Comparison of oxygen consumption, carbon dioxide production, and resting energy expenditure in premature and full-term infants. J Pediatr Surg. 1985;20:792–798. doi: 10.1016/s0022-3468(85)80045-2. [DOI] [PubMed] [Google Scholar]
- 23.Olhager E, Forsum E. Total energy expenditure, body composition and weight gain in moderately preterm and full-term infants at term postconceptional age. Acta Paediatr. 2003;92:1327–1334. doi: 10.1080/08035250310005396. [DOI] [PubMed] [Google Scholar]
- 24.de Meer K, Westerterp KR, Houwen RHJ, Brouwers HAA, Berger R, Okken A. Total energy expenditure in infants with bronchopulmonary dysplasia is associated with respiratory status. Eur J Pediatr. 1997;156:299–304. doi: 10.1007/s004310050605. [DOI] [PubMed] [Google Scholar]
- 25.Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest. 1996;74:48–56. [PubMed] [Google Scholar]
- 26.Saugstad OD. Oxidative stress in the newborn—a 30-year perspective. Biol Neonate. 2005;88:228–236. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- 27.Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res. 1988;23:143–150. doi: 10.1203/00006450-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Plank MS, Boskovic DS, Sowers LC, Angeles DM. Biochemical markers of neonatal hypoxia. Pediatr Health. 2008;2:485–501. [Google Scholar]
- 29.Ketai LH, Simon RH, Kreit JW, Grum CM. Plasma hypoxanthine and exercise. Am Rev Respir Dis. 1987;136:98–101. doi: 10.1164/ajrccm/136.1.98. [DOI] [PubMed] [Google Scholar]
- 30.Hellsten-Westing Y, Sollevi A, Sjödin B. Plasma accumulation of hypoxanthine, uric acid and creatine kinase following exhausting runs of differing durations in man. Eur J Appl Physiol. 1991;62:380–384. doi: 10.1007/BF00634977. [DOI] [PubMed] [Google Scholar]
- 31.George SK, Dipu MT, Mehra UR, Singh P, Verma AK, Ramgaokar JS. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:134–137. doi: 10.1016/j.jchromb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 32.Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, Defranco EA. Late preterm birth. Rev Obstet Gynecol. 2010;3(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- 33.Engle WA. A recommendation for the definition of “late preterm” (near-term) and the birth weight-gestational age classification system. Semin Perinatol. 2006;30:2–7. doi: 10.1053/j.semperi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Heritage CK, Cunningham MD. Association of elective repeat cesarean delivery and persistent pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1985;152(6 pt 1):627–629. doi: 10.1016/s0002-9378(85)80034-x. [DOI] [PubMed] [Google Scholar]
- 35.Roth-Kleiner M, Wagner BP, Bachmann D, Pfenninger J. Respiratory distress syndrome in near-term babies after caesarean section. Swiss Med Wkly. 2003;133:283–288. doi: 10.4414/smw.2003.10121. [DOI] [PubMed] [Google Scholar]
- 36.Ventolini G, Neiger R, Mathews L, Adragna N, Belcastro M. Incidence of respiratory disorders in neonates born between 34 and 36 weeks of gestation following exposure to antenatal corticosteroids between 24 and 34 weeks of gestation. Am J Perinatol. 2008;25:79–83. doi: 10.1055/s-2007-1022470. [DOI] [PubMed] [Google Scholar]
- 37.Jain L. Respiratory morbidity in late-preterm infants: prevention is better than cure! Am J Perinatol. 2008;25:75–78. doi: 10.1055/s-2007-1022471. [DOI] [PubMed] [Google Scholar]
- 38.Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 weeks’ gestation: a population-based cohort study. Pediatrics. 2009;123:109–113. doi: 10.1542/peds.2007-3743. [DOI] [PubMed] [Google Scholar]
- 39.Bier DM, Leake RD, Haymond MW, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26:1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 40.Cornblath M, Ichord R. Hypoglycemia in the neonate. Semin Perinatol. 2000;24:136–149. doi: 10.1053/sp.2000.6364. [DOI] [PubMed] [Google Scholar]
- 41.Dinerstein A, Nieto RM, Solana CL, Perez GP, Otheguy LE, Larguia AM. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006;26:436–442. doi: 10.1038/sj.jp.7211539. [DOI] [PubMed] [Google Scholar]
- 42.Merritt TA, Pillers D, Prows SL. Early NICU discharge of very low birth weight infants: a critical review and analysis. Semin Neonatol. 2003;8:95–115. doi: 10.1016/S1084-2756(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenkranz RA, Das A, Wrage LA, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–529. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho MY, Yen Yu, Hsieh MC, Chen HY, Chien SC, Hus-Lee SM. Early versus late nutrition support in premature neonates with respiratory distress syndrome. Nutrition. 2003;19:257–260. doi: 10.1016/s0899-9007(02)01110-3. [DOI] [PubMed] [Google Scholar]
- 45.Ho MY, Yen YH, Chen HY, Chien SC, Hsieh MC, Yang YS. Effect of aggressive early high-dose intravenous amino acid infusion and early trophic enteral nutrition on very low birth weight infants. Food Nutr Sci. 2012;3:1604–1608. [Google Scholar]