SUMMARY
During infection, fungi frequently transition to a biofilm lifestyle, proliferating as communities of surface-adherent aggregates of cells. Phenotypically, cells in a biofilm are distinct from free-floating cells. Their high tolerance of antifungals and ability to withstand host defenses are two characteristics that foster resilience. Biofilm infections are particularly difficult to eradicate and most available antifungals have minimal activity. Therefore, the discovery of novel compounds and innovative strategies to treat fungal biofilms is of great interest. Although many fungi have been observed to form biofilms, the most well-studied is Candida albicans. Animal models have been developed to simulate common Candida device-associated infections, including those involving vascular catheters, dentures, urinary catheters, and subcutaneous implants. Models have also reproduced the most common mucosal biofilm infections, oropharyngeal and vaginal candidiasis. These models incorporate the anatomical site, immune components, and fluid dynamics of clinical niches and have been instrumental in the study of drug resistance and investigation of novel therapies. This chapter describes the significance of fungal biofilm infections, the animal models developed for biofilm study, and how these models have contributed to development of new strategies for eradication of fungal biofilm infections.
SIGNIFICANCE OF FUNGAL BIOFILMS IN INFECTION
Many fungal and bacterial pathogens build biofilms, establishing resilient communities on a variety of clinical surfaces (1, 2). Biofilm formation has become increasingly appreciated as one of the most common modes of growth. Medically, these adherent conglomerates of cells pose a serious obstacle for treatment of infection. Compared to non-biofilm, planktonic cells, they are extraordinarily tolerant to anti-infective therapies and resist killing by host defenses (3). Biofilm formation has been well-described for Candida albicans, the most common fungal pathogen in the hospital setting (4, 5). More recently, the majority of clinically encountered fungi have been shown to produce biofilms. This group includes filamentous fungi (Aspergillus, Fusarium, zygomycetes), Pneumocysitis, and yeasts (Blastoschizomyces, Saccharomyces, Malassezia, Trichosporon, Cryptococcus, and numerous Candida spp.) (Table 1) (6–16).
Table 1.
Medically important fungi forming biofilms
| Aspergillus |
| Blastoschizomyces |
| Candida |
| Cryptococcus |
| Fusarium |
| Malassezia |
| Pneumocystis |
| Trichosporon |
| Zygomycetes |
One of the distinguishing traits of biofilm communities is their ability to adhere to a surface. In the medical setting, indwelling devices, such as catheters, provide an ideal niche for biofilm formation (1, 17). As technology advances, the use of medical devices has continued to escalate. At least 35 million devices are implanted yearly in the United States alone and the majority of hospital-acquired infections are device-associated (18). Many types of devices are at risk for biofilm infection, including catheters, dentures, implants, pacemakers, artificial heart valves, and central nervous system shunts (1, 18). Biofilm infections may be catastrophic, resulting in device malfunction or life-threatening, systemic infection (1). Candidiasis in the hospital setting most frequently involves biofilm infection of a medical device. Candida spp. are the 4th most common nosocomial bloodstream pathogens and the 3rd most common cause of urinary tract pathogens (19–22). While biofilm infection was initially described for C. albicans, the majority of Candida spp., including C. dubliniensis, C. glabrata, C. krusei, C. tropicalis and C. parapsilosis, have now been shown to cause biofilm infections (23).
Mucosal candidiasis is widespread. Vaginal candidiasis effects approximately 30–50% of women and many suffer recurrent infections (24). The clinical relevance of biofilms on biotic surfaces has become increasingly evident and mucosal biofilms have been described for Candida. In an animal model of oropharyngeal candidiasis, Candida forms a biofilm of yeast, hyphae, and commensal bacterial within the epithelial surface (25). Candida biofilms growing on the vaginal epithelial lining similarly demonstrate a typical biofilm architecture with adherence cells embedded in an extracellular matrix (26). Mucosal biofilms appear to have many similarities to biofilms growing on abiotic surfaces, including sessile growth, protection from environmental factors, and variable access to nutrients (27). However, biofilms on a mucosal surface participate in a dynamic interaction with the adjacent epithelial lining. The host epithelial lining may deliver immune component, nutrients, and antifungal components. Therefore, the basal aspect of a mucosal biofilm would be expected to be exposed to a vastly different environment when compared to a biofilm on an abiotic device surface.
Like Candida, other fungal pathogens, including Cryptococcus and Aspergillus, have been found in biofilms adherent to abiotic device surfaces (15, 28). (For review, see Martinez, Casadevall and Latge, Beauvais chapters in this volume). However, for Aspergillus, the biofilm mode of growth is most commonly observed in the absence of a foreign body. Even in the absence of a device, Aspergillus has been shown to proliferate as a cellular aggregate encased in extracellular matrix with properties similar to device-associated biofilms (28). Clinical examples of these non-device associated biofilms include fungal sinusitis, pulmonary aspergillosis and aspergilloma (7). As the biofilm lifestyle of Aspergillus is a relatively new field of investigation, further investigations are needed to explore this growth mode.
FUNGAL BIOFILM TRAITS
Structure
Fungal biofilms are comprised of adherent cells covered by an extracellular polymeric matrix. The process of fungal biofilm formation in vitro was initially described for Candida albicans in three main stages (29). First, during early biofilm formation, Candida cells adhere to the biofilm substrate. For C. albicans, germ tube formation may be elicited. During intermediate biofilm formation stage, the extracellular matrix begins to appear and covers proliferating fungal colonies. Finally, mature biofilm formation is marked by extracellular matrix encasing the adherent yeast and developing hyphae. It is now recognized that dispersion is a key component of the dynamic nature of biofilms (30). Release of cells from biofilms is a regulated process by which organisms can disseminate throughout the host and establish new sites of infection. This developmental process appears to hold true for in vivo Candida biofilms (31, 32).
For Aspergillus, aerial, static conditions promote biofilm formation (33). Under these conditions, A. fumigatus grows as a cohesive mycelial structure. The cells become progressively encased in a hydrophobic extracellular material over time. Ultrastructure analysis reveals air channels embedded within the conglomerate (33). These multicellular communities, surrounded by acellular matrix material, also develop in vivo in a murine model of pulmonary aspergilloma and invasive aspergillosis (7).
Tolerance to antifungals
Biofilms are notoriously difficult to treat in the clinical setting and their physical removal is often required for eradication of infection (34). Available antifungal medications are seldom effective given the high tolerance of biofilms to these commonly used anti-infective therapies. Candida biofilms proliferate in the face of antifungal concentrations up to 1,000 fold higher than those needed to inhibit non-biofilm, planktonic cells (29, 35–37).
The biofilm lifestyle of C. albicans is associated with resistance to available drug classes compared to activity against planktonic cells (29, 35, 38–42). Resistance to the azoles (fluconazole, itraconazole, voriconazole, posaconazole) is particularly pronounced, while the echinocandins (caspofungin, micafungin, anidulafungin) and liposomal amphotericin are more effective. Various mechanisms have been shown to contribute to this resistant phenotype, including the production of an extracellular matrix, an increase in efflux pump activity, alteration of sterols, production of resistant “persister cells”, activation of stress responses, and an increase in cell density (40, 43–50).
Biofilms formed by Aspergillus and Cryptococcus also display this multi-drug resistance (8, 33, 51, 52). For Aspergillus biofilms, antifungal tolerance has been linked to the presence of an extracellular matrix, as well as an increase in efflux pump activity (52–54). Few investigations examined the mechanisms underlying the antifungal resistance of Cryptococcus biofilms, but this phenotype has been correlated with production of melanin (51).
Immune resistance
In addition to the well-described drug resistance, biofilm growth also appears to afford protection from the host immune response (55–58). Compared to planktonic cells, both neutrophils and mononuclear cells are less effective in killing Candida biofilm cells. Mononuclear cells become entrapped in biofilms, but do not efficiently activate or phagocytize fungal cells (57, 59) Neutrophils have impaired function against both C. albicans and C. parapsilosis biofilms (55, 56, 58). Although most studies involving the leukocyte response to fungal biofilms have been limited to in vitro studies, the phenotype appears clinically relevant. An intact immune response is not sufficient to clear Candida biofilms. When coupled with antifungal therapy, improved clinical outcomes are observed when Candida-infected medical devices are removed (34, 60). Studies examining the innate immune response to C. neoformans biofilms also demonstrated a resistant phenotype. In vitro, these biofilms are more tolerant of defensins and oxidative stress when compared to planktonic cells (61).
HOST FACTORS INFLUENCING FUNGAL BIOFILMS
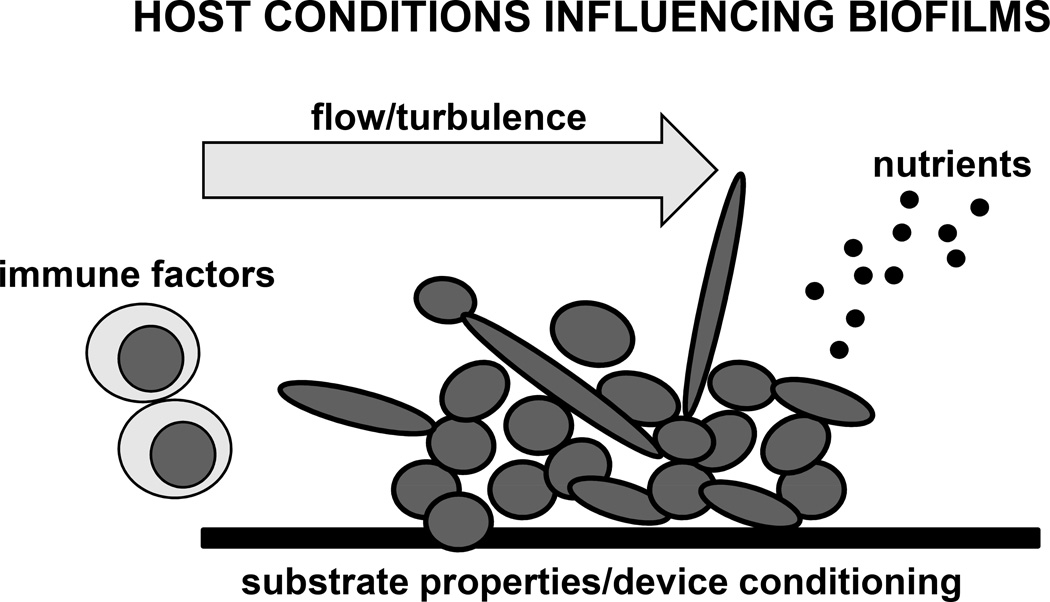
Fungal biofilms form in a variety of clinical niches. These sites of infection can vary quite significantly with regard to available nutrients, flow conditions, immune components, pH, and the substrate for cell adhesion and initiation of biofilm growth (Figure 1). Each of these factors is likely to influence the biofilm properties and structure, as discussed below. Models most closely mimicking the clinical niche are necessary to best reproduce the host environmental conditions and ultimately a clinical biofilm infection.
Figure 1. Host factors influence fungal biofilm formation.
This schematic depicts host conditions that may impact fungal biofilm formation and architecture. In vivo animal models can closely mimic biofilm infection niches, incorporating many of these host and environmental conditions.
Flow conditions
One of the greatest environmental differences among the sites of common fungal biofilms is the flow conditions. For example, Candida can form a biofilm in the face of a low rate of salivary flow (denture stomatitis), a rapid current of blood (endocarditis) or an intermittent flow (vascular and urinary catheter infection) (18). The Douglas group investigated the influence of flow conditions on C. albicans biofilm architecture using in vitro models (62, 63). Compared to biofilms grown in static conditions, those propagated in a continuous flow device were encased in a higher concentration of extracellular matrix. The continuous-flow biofilms also exhibited increased resistance to antifungals, including amphotericin B and fluconazole. Investigation of continuous flow by an independent laboratory confirmed the drug resistance phenotype, as well as the altered biofilm structure (64). Biofilms formed in the flow environment were more dense and compact. One might expect that this architectural change to greatly impact other aspects of biofilm physiology, such the availability of oxygen and nutrients.
As described above, Aspergillus has been shown to form biofilms during invasive pulmonary aspergillosis and pulmonary aspergilloma (7). In the lung, these biofilms proliferate in a static, aerial environment. Unlike Candida biofilms, the extracellular matrix production is mostly supported by these low flow conditions (33). The variability in key properties, including matrix production and drug resistance, of biofilms formed under a variety of flow conditions points to the importance of using models that best fit a particular physiologic niche.
Substrates and conditioning
One of the most influential factors for biofilm initiation is the substrate for adherence (4, 65). Although many materials have been shown to support biofilm formation, the topography and hydrophobicity of the substrate may greatly impact fungal adherence. Indwelling medical devices are often designed to resist microbial adherence. Compared to other plastics, these materials, such as silicone, may even require a preconditioning, or protein coating, for robust biofilm formation in vitro (66, 67). However, in vivo, medical devices are rapidly conditioned with host factors from the surrounding fluids, such as blood, saliva, urine, or other fluids (68–72). Several of the factors that may coat various devices, influencing adherence and biofilm formation, include fibrinogen, fibronectin, vitronectin, thrombospondin-1, albumin, and von Willebrand factor (VWF) (68–72). As the concentrations of these substances differ among clinical niche sites, in vivo models best account for the conditioning of medical devices prior to biofilm initiation. When considering mucosal biofilms, substrate representation becomes even more complex. Here, the epithelial layer of cells provides a surface for microbial adhesion which also involves receptor-ligand interactions (27).
Nutrient composition
Biofilm niche sites vary greatly with respect to availability of nutrients. For example, blood is a fairly nutrient-rich environment, while urine has a lower abundance of sugars and proteins. In addition, the conditions may be altered by both medical illness and diet. Untreated diabetes mellitus raises the glucose content throughout the host while a diet high in sugar primarily raises the glucose content in the oral cavity. The carbon source (galactose or glucose) and abundance can greatly influence biofilm integrity for both C. albicans and C. neoformans (65, 73). Another factor shown to influence Candida biofilm formation is the concentration of metal ions (Ni2+, Fe3+, Cr3+) (74). These variables can impact the rate of biofilm growth, production of extracellular matrix, and the strength of the biofilm. Animal models of biofilm infection which utilize an equivalent anatomical site provide the ideal composition of nutrients and minerals to mimic patient biofilm infections.
Host immune components
Mounting evidence suggests a complex interaction between host immune cells and fungal biofilms. For example, leukocytes, important for controlling fungal infections, have also been shown to promote biofilm growth (59). In vitro, C. albicans biofilms were observed to proliferate in response to a soluble factor released by mononuclear peripheral blood cells. Ultimately, mononuclear cells became entangled within the basal level of a C. albicans biofilm and were not able to phagocytize the biofilm cells. When examining oral mucosal Candida biofilms, Dongari-Bagtzoglou et al. observed the migration of neutrophils throughout the biofilm (25). Host immune cells appear to incorporate into biofilm, even augment biofilm growth, but are most often unable to contain the infection. To understand how the immune system impacts the biofilm lifestyle, models encompassing immune components at the site of infection are optimal.
IN VIVO MODELS OF FUNGAL BIOFILMS AND DRUG DISCOVERY
Animal models best integrate the influence of host factors on the formation of biofilms and acquisition of their phenotypic traits. Utilization of animal models incorporates not only the influence of the immune system, but also niche-specific factors, such as the flow conditions, nutrients in the environment, and the substrate or surface of adherence. As Candida has served as a model organism for fungal biofilm infection in this arena, models involving this pathogen will be much of the focus of discussion in this chapter (Table 2 and Figure 2).
Table 2.
Animal models of Candida biofilm infection
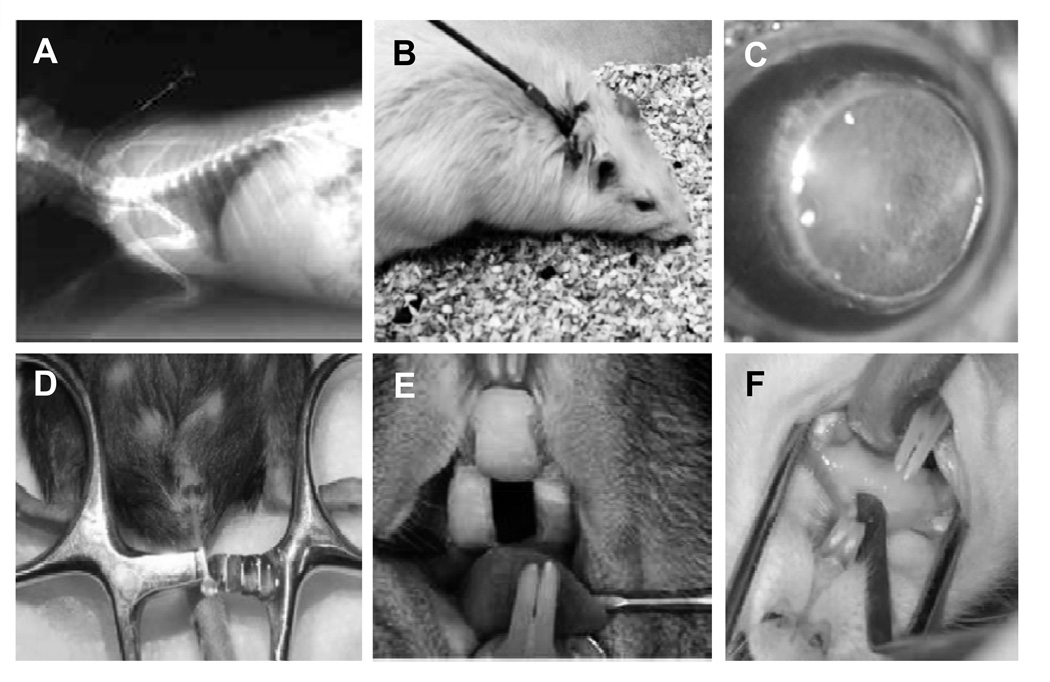
Figure 2. Animal models of fungal biofilm infection.
Rabbit venous catheter-associated Candida biofilm infection (A) (75). Rat venous catheter-associated Candida biofilm infection (B) (31). Mouse contact lens-associated Fusarium biofilm model (C) (102). Mouse urinary catheter-associated Candida biofilm infection (D) (98). Rat denture-associated Candida biofilm infection (removable intraoral device)(E) (87). Rat denture-associated Candida biofilm infection (32). Images adapted from prior publications (32, 75, 87, 98, 102, 114).
Vascular catheter model
Perhaps the most commonly used animal model for in vivo biofilm study is the venous catheter model. This model has been adapted for use in a rat, a rabbit, and a mouse and has been instrumental for examining the efficacy of antifungals against biofilms formed in vivo (31, 75, 76). As a close mimic one of the most common clinical biofilm infections, biofilms on the luminal catheter surface are exposed to host conditions, flow, serum proteins, and immune components. The model involves surgical vascular catheter insertion (often jugular vein) followed by subcutaneous tunneling and securing with a protective device. Performing the procedure prior to luminal inoculation allows for a period for host protein conditioning of the device surface. The influence of anti-infectives on biofilm growth can be assessed following systemic administration of drug or by instilling in the lumen as a lock therapy. Common techniques include microscopy for evaluation of biofilm extent and architecture or viable burden determination (Figure 3). Results of these studies have corroborated the multi-drug resistant phenotype of in vitro Candida biofilms and the need for discovery of new strategies and drugs to treat these infections (31).
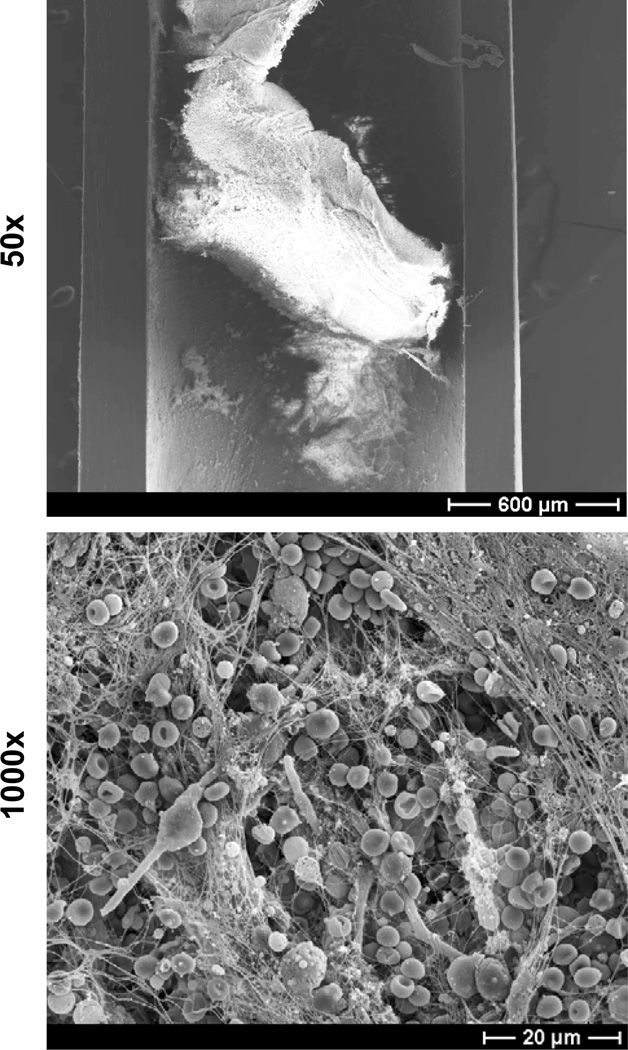
Figure 3. C. albicans biofilm infection of rat jugular venous catheter.
C. albicans was instilled in the lumen of a subcutaneously tunneled jugular venous catheter and allowed to dwell for 6 hours. After a growth period of 24 hours, the catheter was harvested, fixed, and dehydrated. Catheter segments were imaged by scanning electron microscopy on a JEOL JSM-6100 at 10 kV (50× and 1000×). The biofilm is composed of both yeast and hyphae encased in an extracellular matrix. Host components, including red blood cells, appear to associate with the biofilm.
One approach to circumvent the drug tolerance of biofilms is to directly administer antifungal in the form of lock therapy (75, 77–79) (Table 3). By avoiding the majority of systemic toxicities, significantly higher drugs doses may be safely delivered. Vascular catheter animal models have been valuable for analyzing the efficacy of various antifungal lock therapies against Candida biofilms in vivo. When instilled in the lumen of C. albicans infected catheters, several lock therapies were found to successfully treat the biofilm infections. Of the clinically available antifungals, the more efficacious solutions have included liposomal amphotericin B (10 mg/ml), amphotericin B lipid complex (5 mg/ml), and caspofungin (6.67 mg/ml) (75, 77–79). As might be predicted by in vitro biofilm susceptibility studies, the azole drugs and lower doses of echinocandin drugs have significantly less activity against vascular catheter biofilms and are not ideal for catheter lock therapy (75, 76). One concern regarding use of available antifungals for lock therapy is the potential for fostering a resistance-promoting environment. As a method to avoid this possibility, studies have also explored the use of alternative agents, such as biocides. Encouraging results have been observed in vitro for ethanol, ethylenediaminetetraacetic acid (EDTA), ethanol, and high dose minocycline (3 mg/ml) lock solutions (80–82).
Table 3.
Strategies for treatment of Candida biofilms with demonstrated efficacy in animal models
A second tactic to overcome the profound antifungal tolerance of biofilms is delivery of combination drug therapy. The rat vascular catheter biofilm infection model has successfully been used to evaluate the in vivo efficacy of several combination therapy lock solutions (48, 49). These studies have explored the impact of adding agents targeting cellular stress responses on azole drug resistance. Uppuluri et al. demonstrated the efficacy of combining calcineurin inhibitors and fluconazole for lock therapy treatment of C. albicans biofilms (48). An agent inhibiting the calcineurin pathway (tacrolimus) was found to augment the activity of fluconazole against C. albicans catheter biofilms. Subsequent investigations suggest calcineurin inhibitors similarly potentiate the activity of agents in other drug classes, including the echinocandins and amphotericinB (83). Robbins et. al also used a rat venous catheter model to test the efficacy of combination lock therapy (49). Combining an inhibitor of the Hsp90 pathway 17-AAG) with fluconazole improved the activity against C. albicans biofilms. The mechanism of this action is thought to involve a decrease in biofilm extracellular matrix, limiting the capacity of the matrix to sequester antifungal.
Denture model
Denture stomatitis involves biofilm formation on a denture surface and inflammation of the adjacent oral mucosal surface (84, 85). These infections are common, occurring in up to 70% of denture-wearers, and are often quite painful, even impairing the ability to eat. Biofilms are frequently polymicrobial with Candida spp. playing a key role. Several models have been developed to explore the pathogenesis and treatment of denture stomatitis (32, 86, 87). Early models included primarily Macacairus monkeys with custom-fitted acrylic plates and Wistar rats fitted with prefabricated acrylic devices (88–90). The focus of these investigations was examination of the mucosal inflammatory process associated with the infected device. Candida-infected animals with oral devices were observed to develop mucosal lesions similar to those seen in patients with denture stomatitis (88–90). Although both models were useful for describing the host response to denture biofilms, the rat model was more suited for drug efficacy studies, primarily related to cost. In these investigations, the incorporation of either chlorhexidine or miconazole to the denture acrylic material prevented the development of mucosal lesions of palatal candidiasis (88, 89). However, the chlorhexidine product was poorly tolerated with rat undergoing weight loss from poor dietary intake.
With the discovery of the role of biofilms in device-associated infections, there has been renewed interest in animal models to mimic denture stomatitis (32, 87, 91). Two models have been developed to replicate this clinical scenario in rats. In the first model, a Sprague-Dawley rat undergoes placement of an acrylic dental device over the hard palate, which is secured in place by orthodontic wire (32). As the device is fitted to the individual rat, there is close approximation of the device with the oral mucosa and this space can be inoculated with Candida to produce a biofilm device infection and associated mucosal inflammation over the course of 24–72 hours (Figure 4). This model represents an acute infection in the setting of immunosuppression, as rats are treated with a single dose of cortisone prior to infection. In a second rat model, Wistar rats are custom fitted to palatal acrylic devices retained by orthodontic wires (87, 91). However, a portion of the device is secured by embedded magnets and is easily removable throughout the experimental course. Following inoculation of Candida, biofilm develops on the device surface over weeks. In addition, mucosal biofilm infection and inflammation ensue, mimicking clinical infection occurs. As the devices can remain in place for an extended time (8 weeks), this model offers the opportunity to longitudinally follow the course of an individual animal with a chronic infection.
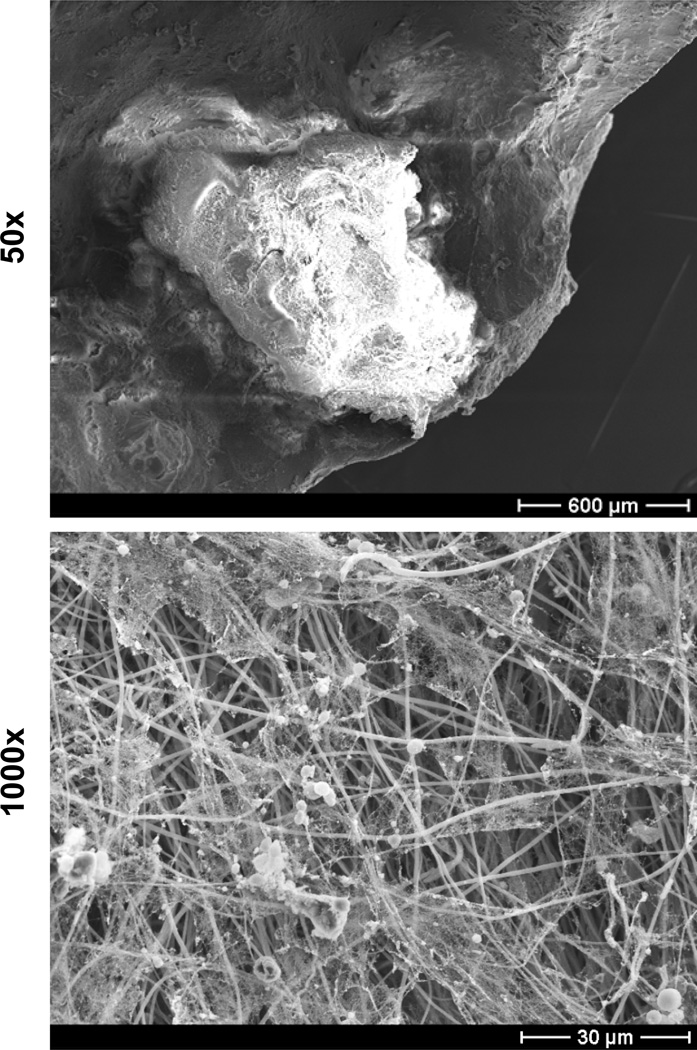
Figure 4. C. albicans biofilm infection in a rat denture model.
C. albicans was inoculated between the hard palate and an acrylic device secured with orthodontic wire. After a growth period of 48 hours, the denture was harvested, fixed, and dehydrated. Oral devices were imaged by scanning electron microscopy on a JEOL JSM-6100 at 10 kV (50× and 1000×). The biofilm is composed of both yeast and hyphae encased in an extracellular matrix. Larger, host cells were observed as well. Microbiologic evaluation identified a polymicrobial infection consisting of C. albicans and various bacteria.
Studies have only just begun to explore the antifungal treatment in the rodent denture models. As might be predicted from clinical scenarios and other infection models, the C. albicans biofilms on the denture surface were found to exhibit high tolerance of both fluconazole and micafungin upon either topical or systemic administration (32). The model has also been helpful for exploring the role of gene products on denture biofilm infection in vivo. Chen et al. described the importance of the calcineurin pathway for C. dubliniensis in the processes of both filamentation and biofilm formation in a rat denture biofilm (92). This suggests that calcineurin inhibitors may be a viable option for treatment of C. dubliniensis. As drugs in this class exert synergistic activity with azole and echinocandin drugs, combination therapy is an attractive possibility for treatment of C. dubliniensis biofilm infections (92).
Subcutaneous implant model
To study the activity of a novel antifungal formulation against biofilms, Zumbuehl et al. developed a murine model of subcutaneous Candida biofilm infection (93). Disks containing amphogel, a dextran-based hydrogel loaded with amphotericin B, were inoculated with C. albicans and surgically implanted in the subcuntaneous flank tissue of BALB/c mice. After 3 days, Candida had been cleared from the surface of the disks containing amphogel. In contrast, control disks with hydrogel only were coated with C. albicans biofilm and host cells. The amphogel was well-tolerated, eliciting only a minimal or mild inflammatory response. As this antifungal hydrogel maintains efficacy for over 50 days, it is ideally suited for prevention of device-associated infection.
As an alternative, simpler model for study of Candida biofilm infections, Riciova et al. fabricated a subcutaneous catheter implant model. In this model, a polyurethane catheter segment is inoculated with Candida and implanted under the skin of a rat (94). Over the course of 1–6 days, a multi-layer biofilm consisting of both yeast and hyphae forms on the catheter surface. Compared to the vascular catheter model, this procedure is less invasive and requires a shorter period of anesthesia. In terms of mimicking patient infection, the model has similarities to both vascular catheter infections and wound infections. The implanted catheter material is a close mimic of the vascular catheter material used in patients. The model is avascular, so biofilm cells are not subjected to blood flow conditions and not exposed to the same concentrations of serum protein and blood cells. However, the devices can be treated with serum prior to implantation to partially mimic this exposure. The anatomical location of the implantation is most similar to a biofilm wound infection. The model allows for the interaction between host immune components and Candida biofilms in the subcutaneous tissue. One clear advantage is that the procedure allows for placement of multiple segments of catheter for potential comparison of several Candida strains or conditions within the same animal.
The subcutaneous implant model has also proven to be efficacious for evaluation of anti-biofilm treatments. Bink et al. used this mode to test the efficacy of combining a non-steroidal anti-inflammatory drug (NSAID) and an echinocandin for treatment of C. albicans biofilms in vivo (95). NSAIDs impair prostaglandin synthesis by targeting mammalian cycloogenases. Agents in this class are available for the treatment of pain and inflammation. However, the activity of this drug class is not limited to mammalian systems, as they have also been shown to disrupt filamentation and biofilm formation in C. albicans, likely though inhibition of prostaglandin E2 synthesis (96, 97). To examine the impact of disrupting this pathway in vivo, rats received diclofenac treatment in the setting of subcutaneous catheter implant infection (95). In rats that had been treated with diclofenac prior to development of C. albicans biofilm infection, the anti-biofilm activity of caspofungin was enhanced. It is of great interest to identify an available drug class able to potentiate the activity of echinocandins. However, the animals in the study received diclofenac prior to infection and whether or not it would be a helpful adjuvant later in the course of infection is not known.
Urinary catheter model
To investigate catheter-associated urinary tract infections and candiduria, Wang et al. developed a murine model representing this clinical scenario (98). In this model, a guide wire is inserted through the urethra of a female mouse and a catheter segment is threaded over a guide wire and into the bladder. The segment is secured by suture through the bladder wall. After 5–7 days, the animal is infected with C. albicans intravesicularly. Candiduria is detectable quickly after infection and persists for 28 days. A dense biofilm of adherent yeast and hyphae forms on both the luminal and extraluminal surfaces. To increase the susceptibility to Candida infection, mice lacking lysozyme M, an important effector for mucosal innate immunity, can be utilized. The model closely mimics patient Candida biofilm formation with regard to the use of biofilm substrate (catheter) and anatomic location (bladder). As only a segment of catheter is in place, the flow conditions are likely less than would be observed for a patient catheter functioning to drain the bladder. The model incorporates the mammalian immune system with the option of using wildtype or immunocompromised animals. To our knowledge, this model has not yet been used for investigating the activity of anti-biofilm therapies.
Mucosal candidiasis models
Biofilms have frequently been described in association with medical devices and abiotic surfaces. However, there is mounting evidence that Candida spp. exhibit similar characteristics when growing on mucosal surfaces (25, 26). Murine models of both oropharyngeal and vaginal candidiasis demonstrate that Candida produces of conglomerates of yeast, hyphae, and extracellular material associated with mucosal surfaces. In an oropharyngeal candidiasis model, the biofilms appear to be complex, involving commensal bacteria, neutrophils, and keratin (25). A murine model of vaginal candidiasis shows C. albicans regulators of biofilm formation on abiotic surfaces are similar to those required for development of vaginal biofilms. Although mucosal biofilms share many characteristics with device-associated biofilms, it is not clear they exhibit the same degree of drug resistance. Clinically, mucosal biofilms are most often responsive to antifungal therapies, including azoles (99, 100).
Fusarium keratitis
Outbreaks of Fusarium keratitis have prompted interest in models to investigate this contact lens-associated biofilm infection (101). Two murine models have been developed to investigate the pathogenesis of this process (102, 103). Sun et al. demonstrated the ability of F. oxysporum to form a biofilm on the surface of a contact lens and for this to induce keratitis on an injured mouse cornea (102). The model has successfully been implemented for study of the host aspects of Fusarium keratitis. Zhang et al. developed a similar model of murine fungal keratitis employing F. solani (103). Although this model also involves inoculation of an injured cornea, the organisms are directly seeded and no contact lens is involved. The model utilizes fluorescently-labeled fungi for visualization of the infective process.
FUTURE DIRECTIONS
Recognition of the importance of animal models for the discovery of anti-biofilm drugs has only recently emerged. Most investigations have focused on C. albicans as a model pathogen and the vascular catheter models of biofilm infection have been the most popular. It will be interesting to see how biofilms formed under the conditions of other clinically relevant niches respond to antifungal therapies. The murine urinary catheter model, the rat subcutaneous model, and rat denture models will be of great value for these investigations for Candida (32, 87, 94, 98). The ocular lens model should be similarly useful for identification of preventative and therapeutic compounds for fungal keratitis. The models allow for testing of anti-biofilm compounds under physiologic conditions very similar to those encountered clinically. In addition to these animal models of device associated infections, models of mucosal Candida biofilms will surely be helpful for study of these common infections (25, 26).
As it is becoming increasingly clear that infections caused by diverse fungal pathogens involve biofilm communities, animal models of these infections will be beneficial for pathogenesis and drug discovery studies. Models are underway for several of the fungal pathogens. One example, discussed earlier, is Fusarium keratitis in the setting of contact lens biofilm infection. Murine models have been developed to study both host and pathogen aspects of this process and could be utilized to evaluate novel anti-biofilm therapies in this unique clinical niche (102, 103). Also, Aspergillus spp., including A. fumigatus, have been shown to produce fungal aggregates during pulmonary infection (7). Models mimicking aspergilloma or invasive aspergillosis will be helpful for exploring the impact of antifungal treatment on this mode of growth.
Although C. albicans has been the model pathogen for many in vivo biofilm investigations, the in vivo biofilm models can likely be adapted to biofilm infections caused by a variety of non-albicans Candida spp. and other yeasts, such as Cryptococcus. Of note, the rat vascular catheter model has been successfully used for study of C. parapsilosis and C. glabrata, while a rat denture model has been employed for investigation of C. dubliniensis (92, 104).
There are many approaches to the discovery of new anti-infectives. One strategy is to screen large libraries of compounds. Using in vitro models, the mining of pharmaceutical and natural product libraries has identified novel compounds with antibiofilm activity (105–107). An alternative approach is to determine a mechanism leading to drug resistance and identify or develop an anti-infective that disrupts the process. For C. albicans, the biofilm property most closely linked to resistance is the extracellular matrix. Enzymatic degradation of key matrix components, such as extracellular DNA and β-1,3 glucan, has been shown to enhance antifungal activity, suggesting these as potential drug targets (67, 108). In fact, a therapy directed at extracellular DNA degradation has shown to be a beneficial for patients with cystic fibrosis. It is thought dornase alfa (Pulmozyme), a clinically available inhaled enzymatic treatment, works by degrading extracellular DNA of bacterial biofilms (109). Regardless of the path of drug discovery, animal models will be beneficial for testing the efficacy of compounds against clinical biofilms and establishing safety.
One of the unique aspects of exploring anti-biofilm activity of drugs in animal models is the opportunity to vary the mode of antifungal delivery. For example, compounds may be systemically administered, topically administered, coated on a device, or embedded in a device. Another interesting delivery method is direct administration of a gel with prolonged elution of high antifungal concentrations, such as was developed for amphotericin B (110). Systemic administration is feasible to test in all models, while direct, topical administration of a compound is easily achievable in either the denture models via topical therapy or the venous catheter models via lock therapy. The subcutaneous tissue model may be ideal for exploring the utility of embedding or coating a device with an anti-biofilm compound, as numerous devices can be tested in a single animal. Another potential application is the investigation of vaccine efficacy, such as the NDV-3 vaccine in clinical trials, vaccines found to be efficacious in non-biofilm models of infections, or future vaccines designed specifically to inhibit the biofilm mode of growth (111–113).
ACKNOWLEDGEMENTS
J.N. is supported by the Burroughs Wellcome Fund and NIH K08 AI108727. D.A. is supported by NIH R01 AI073289. The authors acknowledge use of instrumentation supported by the UW MRSEC (DMR-1121288) and the UW NSEC (DMR-0832760).
REFERENCES
- 1.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyle BD, Jass J, Costerton JW. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–5. doi: 10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Hawser SP, Baillie GS, Douglas LJ. Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol. 1998;47:253–256. doi: 10.1099/00222615-47-3-253. [DOI] [PubMed] [Google Scholar]
- 4.Douglas LJ. Medical importance of biofilms in Candida infections. Rev Iberoam Micol. 2002;19:139–143. [PubMed] [Google Scholar]
- 5.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 6.Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int J Microbiol. 2012;2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, Kauffmann-Lacroix C, Latge JP, Beauvais A. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010;12:405–410. doi: 10.1111/j.1462-5822.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 8.Seidler MJ, Salvenmoser S, Muller FM. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–4136. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis LE, Cook G, Costerton JW. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis. 2002;8:376–379. doi: 10.3201/eid0804.010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Shivaprakash MR, Chakrabarti A. Biofilm formation by zygomycetes: quantification, structure and matrix composition. Microbiology. 2011;157:2611–2618. doi: 10.1099/mic.0.048504-0. [DOI] [PubMed] [Google Scholar]
- 11.D'Antonio D, Parruti G, Pontieri E, Di Bonaventura G, Manzoli L, Sferra R, Vetuschi A, Piccolomini R, Romano F, Staniscia T. Slime production by clinical isolates of Blastoschizomyces capitatus from patients with hematological malignancies and catheter-related fungemia. Eur J Clin Microbiol Infect Dis. 2004;23:787–789. doi: 10.1007/s10096-004-1207-4. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds TB, Fink GR. Bakers' yeast, a model for fungal biofilm formation. Science. 2001;291:878–881. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- 13.Cannizzo FT, Eraso E, Ezkurra PA, Villar-Vidal M, Bollo E, Castella G, Cabanes FJ, Vidotto V, Quindos G. Biofilm development by clinical isolates of Malassezia pachydermatis. Med Mycol. 2007;45:357–361. doi: 10.1080/13693780701225767. [DOI] [PubMed] [Google Scholar]
- 14.Di Bonaventura G, Pompilio A, Picciani C, Iezzi M, D'Antonio D, Piccolomini R. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother. 2006;50:3269–3276. doi: 10.1128/AAC.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TJ, Schlegel R, Moody MM, Costerton JW, Salcman M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery. 1986;18:373–375. doi: 10.1227/00006123-198603000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Dyavaiah M, Ramani R, Chu DS, Ritterband DC, Shah MK, Samsonoff WA, Chaturvedi S, Chaturvedi V. Molecular characterization, biofilm analysis and experimental biofouling study of Fusarium isolates from recent cases of fungal keratitis in New York State. BMC Ophthalmol. 2007;7:1. doi: 10.1186/1471-2415-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passerini L, Lam K, Costerton JW, King EG. Biofilms on indwelling vascular catheters. Crit Care Med. 1992;20:665–673. doi: 10.1097/00003246-199205000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, Armstrong D. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med. 1993;119:1168–1174. doi: 10.7326/0003-4819-119-12-199312150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40:1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Low Genit Tract Dis. 2013;17:340–345. doi: 10.1097/LGT.0b013e318273e8cf. [DOI] [PubMed] [Google Scholar]
- 25.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:e7967. doi: 10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dongari-Bagtzoglou A. Mucosal biofilms: challenges and future directions. Expert Rev Anti Infect Ther. 2008;6:141–144. doi: 10.1586/14787210.6.2.141. [DOI] [PubMed] [Google Scholar]
- 28.Ramage G, Rajendran R, Gutierrez-Correa M, Jones B, Williams C. Aspergillus biofilms: clinical and industrial significance. FEMS Microbiol Lett. 2011;324:89–97. doi: 10.1111/j.1574-6968.2011.02381.x. [DOI] [PubMed] [Google Scholar]
- 29.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun. 2010;78:3650–3659. doi: 10.1128/IAI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, Paris S, Mallet A, Prevost MC, Latge JP. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 34.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawser SP, Douglas LJ. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun. 1994;62:915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O'Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 37.O'Toole GA. To build a biofilm. J Bacteriol. 2003;185:2687–2689. doi: 10.1128/JB.185.9.2687-2689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis RE, Kontoyiannis DP, Darouiche RO, Raad II, Prince RA. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob Agents Chemother. 2002;46:3499–3505. doi: 10.1128/AAC.46.11.3499-3505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46:3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 44.Kumamoto CA. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci U S A. 2005;102:5576–5581. doi: 10.1073/pnas.0407097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khot PD, Suci PA, Miller RL, Nelson RD, Tyler BJ. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and beta-1,6-glucan pathway genes. Antimicrob Agents Chemother. 2006;50:3708–3716. doi: 10.1128/AAC.00997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–3846. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother. 2007;51:2454–2463. doi: 10.1128/AAC.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:1127–1132. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins N, Uppuluri P, Nett J, Rajendran R, Ramage G, Lopez-Ribot JL, Andes D, Cowen LE. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011;7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee PK, Zhou G, Munyon R, Ghannoum MA. Candida biofilm: a well-designed protected environment. Med Mycol. 2005;43:191–208. doi: 10.1080/13693780500107554. [DOI] [PubMed] [Google Scholar]
- 51.Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006;50:1021–1033. doi: 10.1128/AAC.50.3.1021-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mowat E, Lang S, Williams C, McCulloch E, Jones B, Ramage G. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J Antimicrob Chemother. 2008;62:1281–1284. doi: 10.1093/jac/dkn402. [DOI] [PubMed] [Google Scholar]
- 53.Rajendran R, Williams C, Lappin DF, Millington O, Martins M, Ramage G. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot Cell. 2013;12:420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bugli F, Posteraro B, Papi M, Torelli R, Maiorana A, Paroni Sterbini F, Posteraro P, Sanguinetti M, De Spirito M. In vitro interaction between alginate lyase and amphotericin B against Aspergillus fumigatus biofilm determined by different methods. Antimicrob Agents Chemother. 2013;57:1275–1282. doi: 10.1128/AAC.01875-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katragkou A, Chatzimoschou A, Simitsopoulou M, Georgiadou E, Roilides E. Additive antifungal activity of anidulafungin and human neutrophils against Candida parapsilosis biofilms. J Antimicrob Chemother. 2011;66:588–591. doi: 10.1093/jac/dkq466. [DOI] [PubMed] [Google Scholar]
- 56.Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine. 2011;55:330–334. doi: 10.1016/j.cyto.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Katragkou A, Kruhlak MJ, Simitsopoulou M, Chatzimoschou A, Taparkou A, Cotten CJ, Paliogianni F, Diza-Mataftsi E, Tsantali C, Walsh TJ, Roilides E. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J Infect Dis. 2010;201:1941–1949. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, Dongari-Bagtzoglou A. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J Infect Dis. 2012;206:1936–1945. doi: 10.1093/infdis/jis607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun. 2007;75:2612–2620. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 61.Martinez LR, Casadevall A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun. 2006;74:6118–6123. doi: 10.1128/IAI.00995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Fattani MA, Douglas LJ. Penetration of Candida biofilms by antifungal agents. Antimicrob Agents Chemother. 2004;48:3291–3297. doi: 10.1128/AAC.48.9.3291-3297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 64.Uppuluri P, Chaturvedi AK, Lopez-Ribot JL. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia. 2009;168:101–109. doi: 10.1007/s11046-009-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73:4592–4601. doi: 10.1128/AEM.02506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 67.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francois P, Schrenzel J, Stoerman-Chopard C, Favre H, Herrmann M, Foster TJ, Lew DP, Vaudaux P. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J Lab Clin Med. 2000;135:32–42. doi: 10.1016/s0022-2143(00)70018-7. [DOI] [PubMed] [Google Scholar]
- 69.Proctor RA. Toward an understanding of biomaterial infections: a complex interplay between the host and bacteria. J Lab Clin Med. 2000;135:14–15. doi: 10.1016/s0022-2143(00)70015-1. [DOI] [PubMed] [Google Scholar]
- 70.Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res. 2000;49:435–447. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 71.Brash JL, Ten Hove P. Protein adsorption studies on 'standard' polymeric materials. J Biomater Sci Polym Ed. 1993;4:591–599. doi: 10.1163/156856293x00230. [DOI] [PubMed] [Google Scholar]
- 72.Yanagisawa N, Li DQ, Ljungh A. Protein adsorption on ex vivo catheters and polymers exposed to peritoneal dialysis effluent. Perit Dial Int. 2004;24:264–273. [PubMed] [Google Scholar]
- 73.Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Ronsani MM, Mores Rymovicz AU, Meira TM, Trindade Gregio AM, Guariza Filho O, Tanaka OM, Ribeiro Rosa EA. Virulence modulation of Candida albicans biofilms by metal ions commonly released from orthodontic devices. Microb Pathog. 2011;51:421–425. doi: 10.1016/j.micpath.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, Ghannoum MA. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004;48:1727–1732. doi: 10.1128/AAC.48.5.1727-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother. 2009;64:567–570. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]
- 77.Mukherjee PK, Long L, Kim HG, Ghannoum MA. Amphotericin B lipid complex is efficacious in the treatment of Candida albicans biofilms using a model of catheter-associated Candida biofilms. Int J Antimicrob Agents. 2009;33:149–153. doi: 10.1016/j.ijantimicag.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 78.Shuford JA, Rouse MS, Piper KE, Steckelberg JM, Patel R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis. 2006;194:710–713. doi: 10.1086/506452. [DOI] [PubMed] [Google Scholar]
- 79.Walraven CJ, Lee SA. Antifungal lock therapy. Antimicrob Agents Chemother. 2013;57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother. 2007;51:78–83. doi: 10.1128/AAC.00154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sherertz RJ, Boger MS, Collins CA, Mason L, Raad II. Comparative in vitro efficacies of various catheter lock solutions. Antimicrob Agents Chemother. 2006;50:1865–1868. doi: 10.1128/AAC.50.5.1865-1868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raad I, Chatzinikolaou I, Chaiban G, Hanna H, Hachem R, Dvorak T, Cook G, Costerton W. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob Agents Chemother. 2003;47:3580–3585. doi: 10.1128/AAC.47.11.3580-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinde RB, Chauhan NM, Raut JS, Karuppayil SM. Sensitization of Candida albicans biofilms to various antifungal drugs by cyclosporine A. Ann Clin Microbiol Antimicrob. 2012;11:27. doi: 10.1186/1476-0711-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 85.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J. 1998;43:160–166. doi: 10.1111/j.1834-7819.1998.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 86.Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson CC, Yu A, Lee H, Fidel PL, Jr, Noverr MC. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect Immun. 2012;80:1736–1743. doi: 10.1128/IAI.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norris MM, Lamb DJ, Craig GT, Martin MV. The effect of miconazole on palatal candidosis induced in the Wistar rat. J Dent. 1985;13:288–294. doi: 10.1016/0300-5712(85)90023-5. [DOI] [PubMed] [Google Scholar]
- 89.Lamb DJ, Martin MV. An in vitro and in vivo study of the effect of incorporation of chlorhexidine into autopolymerizing acrylic resin plates upon the growth of Candida albicans. Biomaterials. 1983;4:205–209. doi: 10.1016/0142-9612(83)90012-1. [DOI] [PubMed] [Google Scholar]
- 90.Budtz-Jorgensen E. Denture stomatitis. IV. An experimental model in monkeys. Acta Odontol Scand. 1971;29:513–526. doi: 10.3109/00016357109026330. [DOI] [PubMed] [Google Scholar]
- 91.Lee H, Yu A, Johnson CC, Lilly EA, Noverr MC, Fidel PL., Jr Fabrication of a multi-applicable removable intraoral denture system for rodent research. J Oral Rehabil. 2011;38:686–690. doi: 10.1111/j.1365-2842.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF, Jr, Nett JE, Andes DR, Solis NV, Filler SG, Averette A, Heitman J. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot Cell. 2011;10:803–819. doi: 10.1128/EC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zumbuehl A, Ferreira L, Kuhn D, Astashkina A, Long L, Yeo Y, Iaconis T, Ghannoum M, Fink GR, Langer R, Kohane DS. Antifungal hydrogels. Proc Natl Acad Sci U S A. 2007;104:12994–12998. doi: 10.1073/pnas.0705250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ricicova M, Kucharikova S, Tournu H, Hendrix J, Bujdakova H, Van Eldere J, Lagrou K, Van Dijck P. Candida albicans biofilm formation in a new in vivo rat model. Microbiology. 2010;156:909–919. doi: 10.1099/mic.0.033530-0. [DOI] [PubMed] [Google Scholar]
- 95.Bink A, Kucharikova S, Neirinck B, Vleugels J, Van Dijck P, Cammue BP, Thevissen K. The nonsteroidal antiinflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J Infect Dis. 2012;206:1790–1797. doi: 10.1093/infdis/jis594. [DOI] [PubMed] [Google Scholar]
- 96.Alem MA, Douglas LJ. Prostaglandin production during growth of Candida albicans biofilms. J Med Microbiol. 2005;54:1001–1005. doi: 10.1099/jmm.0.46172-0. [DOI] [PubMed] [Google Scholar]
- 97.Ghalehnoo ZR, Rashki A, Najimi M, Dominguez A. The role of diclofenac sodium in the dimorphic transition in Candida albicans. Microb Pathog. 2010;48:110–115. doi: 10.1016/j.micpath.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Fries BC. A murine model for catheter-associated candiduria. J Med Microbiol. 2011;60:1523–1529. doi: 10.1099/jmm.0.026294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, Reed BD, Summers PR. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 100.Graybill JR, Vazquez J, Darouiche RO, Morhart R, Greenspan D, Tuazon C, Wheat LJ, Carey J, Leviton I, Hewitt RG, MacGregor RR, Valenti W, Restrepo M, Moskovitz BL. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am J Med. 1998;104:33–39. doi: 10.1016/s0002-9343(97)00307-0. [DOI] [PubMed] [Google Scholar]
- 101.Chang DC, Grant GB, O'Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FM, Schaffzin JK, Kainer MA, Genese CA, Alfonso EC, Jones DB, Srinivasan A, Fridkin SK, Park BJ Fusarium Keratitis Investigation T. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 102.Sun Y, Chandra J, Mukherjee P, Szczotka-Flynn L, Ghannoum MA, Pearlman E. A murine model of contact lens-associated Fusarium keratitis. Invest Ophthalmol Vis Sci. 2010;51:1511–1516. doi: 10.1167/iovs.09-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Wang L, Li Z, Liu S, Xie Y, He S, Deng X, Yang B, Liu H, Chen G, Zhao H, Zhang J. A novel murine model of Fusarium solani keratitis utilizing fluorescent labeled fungi. Exp Eye Res. 2013;110:107–112. doi: 10.1016/j.exer.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Nett J, Lincoln L, Marchillo K, Andes D. Beta-1,3 glucan as a test for central venous catheter biofilm infection. J Infect Dis. 2007;195:1705–1712. doi: 10.1086/517522. [DOI] [PubMed] [Google Scholar]
- 105.Lafleur MD, Sun L, Lister I, Keating J, Nantel A, Long L, Ghannoum M, North J, Lee RE, Coleman K, Dahl T, Lewis K. Potentiation of azole antifungals by 2-adamantanamine. Antimicrob Agents Chemother. 2013;57:3585–3592. doi: 10.1128/AAC.00294-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sherry L, Jose A, Murray C, Williams C, Jones B, Millington O, Bagg J, Ramage G. Carbohydrate Derived Fulvic Acid: An in vitro Investigation of a Novel Membrane Active Antiseptic Agent Against Candida albicans Biofilms. Front Microbiol. 2012;3:116. doi: 10.3389/fmicb.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol. 2010;5:321–332. doi: 10.1021/cb900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012;55:80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006;95:1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- 110.Hudson SP, Langer R, Fink GR, Kohane DS. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials. 2010;31:1444–1452. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE, Jr, Hennessey JP., Jr NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–7600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201:1718–1728. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cassone A. Development of vaccines for Candida albicans: fighting a skilled transformer. Nat Rev Microbiol. 2013;11:884–891. doi: 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]
- 114.Nett JE, Marchillo K, Andes DR. Modeling of fungal biofilms using a rat central vein catheter. Methods Mol Biol. 2012;845:547–556. doi: 10.1007/978-1-61779-539-8_40. [DOI] [PMC free article] [PubMed] [Google Scholar]