Abstract
Following the introduction of West Nile virus into California during the summer of 2003, public health and vector control programs expanded surveillance efforts and were in need of diagnostics capable of rapid, sensitive, and specific detection of arbovirus infections of mosquitoes to inform decision support for intervention. Development of a multiplex TaqMan or real-time semiquantitative reverse transcription polymerase chain reaction (RT-PCR) assay in which three virus specific primer–probe sets were used in the same reaction is described herein for the detection of western equine encephalomyelitis, St. Louis encephalitis and West Nile viral RNA. Laboratory validation and field data from 10 transmission seasons are reported. The comparative sensitivity and specificity of this multiplex assay to singleplex RT-PCR as well as an antigen detection (rapid analyte measurement platform) and standard plaque assays indicate this assay to be rapid and useful in providing mosquito infection data to estimate outbreak risk.
Keywords: multiplex, surveillance, West Nile virus, western equine encephalitis virus, St. Louis encephalitis virus
Arthropod-borne virus surveillance for decision support to direct intervention by public health and mosquito control agencies requires the rapid and accurate detection of viruses of human health importance. Although 14 mosquito-borne viruses were known to occur in California prior to 2003, only 2 caused significant human or equine disease and have been the focus of statewide surveillance and intervention efforts (Reeves et al. 1990). Western equine encephalomyelitis virus (WEEV) (Togaviridae Alphavirus) was isolated originally from an equine fatality during a large equine epizootic in the Central Valley of California in the 1930s (Meyer et al. 1931), was a frequent cause of summer encephalitis in children (Howitt 1939), and was responsible for a large epidemic of human encephalitis in 1952 (Reeves and Hammon 1962). St. Louis encephalitis virus (SLEV) (Flaviviridae: Flavivirus) was recognized as a cause of human encephalitis within the Central Valley during the 1930s (Howitt 1938, 1939) shortly after its discovery in Missouri (Muckenfuss et al. 1934) and was responsible for outbreaks as recently as 1984 in Los Angeles (Murray et al. 1985) and 1989 in Bakersfield (Reisen et al. 1992), CA. Both WEEV and SLEV persisted locally within enzootic foci, with periodic introductions or replacements by new genotypes (Kramer et al. 1997, Kramer and Fallah 1999, Reisen et al. 2002). West Nile virus (WNV) (Flaviviridae: Flavivirus) is genetically closely related to SLEV and was first isolated in California from a pool of Culex tarsalis Coquillett mosquitoes collected in Imperial Valley in July 2003 (Reisen et al. 2004). WNV rapidly expanded its geographic distribution to every county during the 2004 transmission season (Hom et al. 2005) and now is considered endemic throughout California, where it is an annual cause of human neuroinvasive disease.
The westward progression of WNV across North America led to large outbreaks of human and equine neuroinvasive disease and resulted in the increased submission of mosquito pools to track virus outbreak risk, necessitating increased laboratory diagnostic effort and efficiency (Nasci et al. 2003). In response to the anticipated incursion of WNV and following its discovery in 2003, the number of mosquito pools tested in California by the Center for Vectorborne Diseases (CVEC) at the University of California, Davis, and other state agencies increased from 3,901 during the 2000 surveillance season to 10,297 in 2003 (the year of WNV invasion) to a peak of 35,637 during 2008; almost an order of magnitude increase (Fig. 1).
Fig. 1.
Mosquito pools tested in California from 2000 to 2013. Shown are the annual number of pools tested and the number positive for WEEV, SLEV, and WNV viruses each year. Arrow shows the start of testing using only qRT-PCR.
Initially, mosquito pools submitted to CVEC by the Mosquito and Vector Control Districts (MVCD) of California were tested for WEEV and SLEV by an in situ–enzyme immunoassay (EIA; Graham et al. 1986, Chiles et al. 2004). This 96-well format assay detected viral antigen following amplification in Vero cell culture. Because SLEV requires up to 7 d to produce sufficient viral antigen for detection, an incubation of several days was required before tests could be read delaying turnaround time. Additionally, these tests required the use of virus-specific antibodies, and separation of WNV and SLEV was complicated by extensive serological cross-reactivity, necessitating additional confirmation methods (Baba et al. 1998). Initially, we adapted a singleplex reverse transcription polymerase chain reaction (RT-PCR) using gel visualization format and found that these results were superior to antigen-detection tests (Vectest, rapid analyte measurement platform [RAMP]), and in situ EIA (Chiles et al. 2004); however, this assay was not as suited for high-throughput as was real-time amplification assays (Shi et al. 2001, Lanciotti et al. 2000). In addition, it was possible for multiplex real-time assays to allow the simultaneous detection of RNA from more than one virus or genetic portions of the same virus (Lanciotti and Kerst 2001, Zink et al. 2013), including closely related viruses within the same serocomplex (Barros et al. 2013). Given the limitations of antigen detection and electrophoresis techniques, as well as the need for testing for multiple viruses concurrently, we developed a triplex real-time qRT-PCR for use by the California arbovirus surveillance program. The current paper describes the genetic composition and development of this multiplex assay to simultaneously detect WEEV, SLEV, and WNV RNA, its utility, and use in California, and comparisons to virus isolation and antigen tests.
Materials and Methods
Primer Selections and Design
Previously described TaqMan primer–probe sets for WNV (Lanciotti et al. 2000, Shi et al. 2001), SLEV (Lanciotti and Kerst 2001), and WEEV (Lambert et al. 2003) initially were selected for evaluation. Only the NY99 strain 3526221 of WNV was used for evaluation of the primer–probe sets because of the minimal genetic variation recognized in WNV in North America at that time (Beasley et al. 2003). SLEV and WEEV primer–probe sets were tested for sensitivity and specificity against representative WEEV and SLEV strains selected from different genetic clades described in California (Kramer et al. 1997, Kramer and Fallah 1999). Because one strain of WEEV did not react with the Lambert et al. (2003) assay, new primer–probe sets were developed for WEEV with Primer Express software (Applied Biosystems Inc., Foster City, CA) and were designed to recognize WEEV isolates from all the clades recognized from California (Kramer and Fallah 1999).
RNA Extraction
Viral RNA extraction methods followed protocols and chemistry provided by Applied Biosystems Inc. (ABI, Foster City, CA; now LifeTechnologies, Grand Island, NY) and changed over time. Initially, a series of lysis buffers were compared to streamline the sample handling and increase the product yield by the ABI Prism 6700 Automated Nucleic Acid extraction platform. The 6700 system later was replaced with the ABI 6100 Nucleic Acid Prep Station system in 2007 and then by the MagMAX Express-96 Deep Well Magnetic Particle Processor (Life Technologies) in 2010.
Multiplex Development
To reduce the assay cost and to improve conditions for the triplex reaction, optimal primer concentrations were determined using the aforementioned screening primer–probe sets and conditions with all possible combinations of forward and reverse primer concentrations; 50, 300 to 900 nm. Optimal FAM-TAMRA probe concentrations were determined similarly by altering its concentration from 0.05 to 0.25 µM in 50 nM increments. Originally, the linear dynamic range of detection for reactions containing one primer–probe set (singleplex) and multiple primer–probe sets for multiple targets (triplex) was determined using real-time RT-PCRs in triplicate with 10-fold serial dilutions of a single species of target RNA, optimal primer, and probe concentrations and thermocycler conditions of 30 min at 48°C for reverse transcription (RT), 10 min at 95°C for RT inactivation and Taq polymerase activation, followed by 45 cycles of 15 s at 95°C for denaturation and a 1 min, 60°C annealing and extension incubations. Since 2012, we have been using the SensiFAST Probe Lo-ROX One-Step Kit (Bioline USA Inc., Reno, NV) with our Life Technologies ViiA 7 platform that has reduced assay time to 10 min at 45°C for RT, 2 min at 95°C for polymerase activation, followed by 40 cycles of 5 s at 95°C for denaturation, and 20 s at 60°C for annealing and extension incubations. Ct values ≥40 were considered negative. Although the same primer–probe sequences were retained throughout, fluorophores were modified to enhance wavelength separation. Originally, we used FAM (excitation wavelength = 495 nm), VIC (538 nm), and TET (521 nm) fluorophores for WNV, SLEV, and WEEV, respectively, with our quencher conjugated to TAMRA (557 nm). Using this system, we occasionally would get false high Ct SLEV positives associated with samples with low Ct WNV positives. To correct this problem, our current assay uses FAM, Quasar670 (647 nm), and TAMRA for WNV, SLEV, and WEEV, respectively, with BHQ and BHQ2 nonfluorescent quenchers (Biosearch Technologies Inc., Petaluma, CA). Ct values were plotted as a function of virus concentration to ensure goodness of fit and linearity of slope and to determine the levels of detection. Single and multiplex assays were compared for sensitivity for each of the three viruses.
Operational Use
Mosquitoes trapped by participating vector control agencies were enumerated by sex and species into pools of up to 50 individual females and placed in a 5-ml mixer-mill tube containing two glass beads. Pools were shipped on dry ice and then, if necessary, stored at −80°C. Mosquito pools were thawed at room temperature, diluent containing 10% fetal bovine serum (FBS) and a full complement of the antibiotics (penicillin, streptomycin, and mycostatin) added, and the mixture homogenized for 3 min using a Spex Centriprep 8000D mixer-mill (SPEX SamplePrep, Metuchen, NJ). After homogenization, an aliquot of the mosquito slurry was removed, clarified by centrifugation, and RNA extraction and RT-PCR performed, as described above without replication. Results from RT-PCR initially were verified by virus isolation on Vero cell culture (obtained from the ATCC no. CCL-81) to ensure RNA detected in mosquitoes represented infectious virus. Results were also compared blindly to the RAMP (Response Biomedical Corp., Burnaby, BC, Canada) using field samples from the Turlock MVCD.
Results
Primer Selections and Design
WEEV primer–probe set 10,248–10,314c (Lambert et al. 2003) did not detect California isolate BFN 3804 from the Sacramento Valley, so new primer–probe sets WEEV1 and WEEV2 were developed (Table 1), based on sequence analyses of California WEEV isolates representative of the different clades differentiated by Kramer and Fallah (1999) (Table 2). WEEV1 was consistently the most sensitive (i.e., had the lowest Ct score) of the three primer–probe sets, detected all virus strains, including BFN3804, which was not detected by the Lambert 10,248–10,314c set, and was selected for the multiplex assay.
Table 1.
Genetic sequences of the WEEV primer–probe sets used in the evaluations described in Table 2
| Primer–probe set | Genomic orientation | Primer sequences |
|---|---|---|
| WEEV-1 | forward | 5′-GTCTTCAACTCGCCGGATCTTA-3′ |
| Forward (probe) | 5′-FAM-CACACAGACCACTCAGTGCAAGGTAAACTGC-TAMRA-3′ | |
| reverse | 5′- GGTGTCAAGCGGAATGGAA-3′ | |
| WEEV-2 | forward | 5′- AGGTAAACTGCACATTCCATTCC-3′ |
| Forward (probe) | 5′-FAM-CCGACAGTCTGCCCGGTTCCG-TAMRA-3′ | |
| reverse | 5′- TTCGTGACTGTAGGCGTGTGA-3′ |
Table 2.
Ct values for 15 California strains of WEEV tested using 3 primer–probe sets
| Strain | Titer | LAMB | WEEV1 | WEEV2 |
|---|---|---|---|---|
| CNTR | 3.2 | 18.31 | 17.23 | 18.33 |
| BFN3060 | 3.6 | 19.58 | 18.75 | 19.91 |
| Lake 43 | 4.6 | 12.39 | 10.77 | 11.76 |
| S81-22 | 3.3 | 24.95 | 23.60 | 24.63 |
| Sac 74 | 3.0 | 18.29 | 16.51 | 17.73 |
| A7712 | 4.3 | 17.08 | 15.89 | 17.26 |
| CHLV 33 | 2.8 | 23.63 | 17.26 | 18.77 |
| E14416 | 5.4 | 16.00 | 15.17 | 16.28 |
| CHLV 592 | 4.6 | 15.90 | 15.44 | 16.75 |
| Bc28cl5 | 2.8 | 17.35 | 16.12 | 18.76 |
| CHLV 129 | 3.0 | 18.15 | 17.18 | 18.60 |
| Fleming | 5.3 | 14.71 | 13.96 | 14.86 |
| COAV 746 | 3.3 | 18.61 | 17.34 | 18.94 |
| BFS 1703 | 6.8 | 16.25 | 15.10 | 16.41 |
| BFN 3804 | 4.6 | >40.00 | 13.29 | 15.23 |
| Mean | 17.94 | 16.24 | 17.61 | |
| SE | 3.14 | 2.73 | 2.73 |
Shown are virus titers in log10 PFU/0.1 ml. Extraction performed by ABI 6700, RT-PCR on ABI 7900.
LAMB sequences = 10,248–10,314c (Lambert et al. 2003).
WEEV1 and WEEV2 sequences in Table 1.
Both published SLEV primers detected all representative California isolates and there were no significant differences (P > 0.05) between Ct scores for the same viruses when tested by paired t-test (Table 3). The SLE-1 primer–probe set was selected for the multiplex assay.
Table 3.
Ct values for two published primer–probe sets against six strains of SLEV from California
| Strain | Titer | SLE1 | SLE2 |
|---|---|---|---|
| COAV 353 | 6.3 | 18.54 | 18.14 |
| COAV 750 | 5.3 | 27.95 | 30.09 |
| COAV 608 | 4.4 | 13.51 | 18.78 |
| COAV 477 | 5.9 | 17.63 | 16.34 |
| KERN 217 | 4.6 | 18.75 | 26.61 |
| KERN 1750 | 4.0 | 26.72 | 34.00 |
| Mean | 20.50 | 24.00 | |
| SE | 2.30 | 2.97 |
SLE1 = 2420/2487c/2444.
SLE2 = 834/905c/857.
Titer is log10 PFU/0.1 ml estimated by Vero cell plaque assay. Extraction by ABI 6700, RT-PCR on ABI 7900 platform.
WNV primer–probe sets WNV-ENV and WNV3111v-3239c were both capable of detecting the WNV isolate 3526221; however, the primer–probe set targeting the 3′ noncoding region, WNV-3′NC, was not, supporting previous findings (Shi et al. 2001). As such, the WNV-ENV set (Lanciotti et al. 2000) was selected for use in the multiplex assay.
To determine the loss of sensitivity of the primer–probe sets when combined for our multiplex assay, we compared Ct scores in single and multiplex assays against WNV99, SLEV Kern217, and WEEV Kern1703 viruses grown to 6 log10 plaque-forming units (PFU)/0.1 ml in Vero cell culture, and then serially diluted 10-fold. Viral RNA was extracted using the MagMax system and RNA tested by RT-PCR using an ABI7900 platform (Fig. 2). Ct scores for all viruses were lower when the assay was run in singleplex than multiplex, and these differences varied among viruses, ranging from ∼1–2 Ct for WNV (<1 log10 PFU) to 4–7 Ct for SLEV (1–1.5 log10 PFU). Because most infected Culex mosquitoes develop virus titers >3 log10 PFU (Reisen et al. 2005), we felt that our multiplex was sufficiently sensitive for operational use.
Fig. 2.

Sensitivity of the primer–probe sets run in duplicate as single or multiplex. Plotted are mean qRT-PCR Ct scores as a function of virus titer assayed by Vero cell plaque titration as log10 PFU per 0.1 ml. RNA extraction by MagMax, RT-PCR by ABI7900.
Comparison of Sensitivity and Specificity to Plaque Assay, in situ EIA and RAMP
The switch from the ABI6100 manifold system to the MagMax Express system significantly improved WNV RNA extraction from mosquito pools, as indicated by Ct scores for pools tested on the same ABI7900 PCR platform. When 22 pools were triturated in virus diluent with 10% fetal bovine serum and antibiotics, the mean Ct for subsamples extracted using the ABI6100 (Ct = 30.3) was significantly greater (paired t = 6.85; P < 0.01) than subsamples with RNA extracted using the MagMax express (Ct = 25.8), indicating that the MagMax Express improved sensitivity by more than an order of magnitude.
To verify that the RT-PCR assay was detecting infectious virus and not simply RNA, we attempted to isolate WNV by Vero cell culture from 60 mosquito pools collected by three collaborating mosquito control agencies that tested positive for WNV RNA by the multiplex assay. WNV was isolated from 54 of the 60 pools (90%). When compared by t-test, the average Ct score for the positive pools from which virus was isolated (mean Ct = 27.2; SE = 0.45; range 19–34, n = 54) was significantly lower (P < 0.001) than the Ct score for the positive pools from which virus was not isolated (mean Ct = 34.4; SE = 2.36; range 33–39; n = 6). However, we could not explain our inability to isolate WNV from some pools with the same Ct score as those where virus was isolated, but this may indicate the approximate limits of sensitivity of our Vero cell plaque assay or perhaps how the mosquito samples were handled during processing. As this was an operational evaluation, we did not attempt virus recovery by duplicate or sequential cell culture. In addition, during 2007, 154 pools testing negative by qRT-PCR were retested by Vero cell plaque assay with negative results. This evaluation was expanded during 2008, as part of an effort to detect additional viral taxa, and 2,041 pools submitted by nine agencies that initially tested negative by our multiplex assay were retested by Vero cell plaque assay on six well plates, of which 21 (1%) yielded plaques; typically <5 per well, indicating low viral titer. Of these plaque-positive cultures, 20 tested positive for WNV when retested by singleplex RT-PCR; one isolate from Cx. tarsalis collected in the Coachella Valley was not detected by our WNV RT-PCR. The following year, 1,147 multiplex negative pools from two of the same agencies again were tested by Vero cell plaque assay with negative results. Overall, our WNV RT-PCR processing produced 0.6% false negatives during this 3-yr period (i.e., pools reported as negative by RT-PCR, but positive by plaque assay); WEEV or SLEV was not detected by either method. One possible contributing factor to these false negatives was the detection of a mutation within the envelope gene in the probe-binding region of WNV that lowered sensitivity of our assay by an order of magnitude (Brault et al. 2012).
During 2003, 356 mosquito pools from two agencies were tested by both the in situ EIA and qRT-PCR assays (RNA extracted by ABI6700, RT-PCR by ABI7900). Overall, 32 pools tested positive and 317 tested negative by both assays (98% agreement); 5 pools were positive by qRT-PCR but were negative by EIA, whereas the 2 pools positive by EIA were negative by qRT-PCR. Based on the previous isolation attempts, we feel that these 5 negatives by the EIA were related to lower Vero cell plaque assay sensitivity, whereas the 2 positive by EIA may have again contained the mutation discussed above.
During 2006, 1,042 pools collected by the Turlock MVCD were ground in mosquito diluent and aliquots tested by RAMP test at the Turlock MVCD and then sent to CVEC for confirmation by qRT-PCR (RNA extraction by ABI6100, and RT-PCR by ABI7900). Similar to the in situ-EIA which also tested for antigen, there were 26 positive and 996 pools that were negative by both tests (98% agreement), as well as 16 pools negative by RAMP but positive by RT-PCR, and 4 pools positive by RAMP but negative by RT-PCR. Again, we felt the 16 pools determined to be negative by RAMP but positive by qRT-PCR reflected the lower RAMP sensitivity; cutoff at ∼3–4 log10 PFU/ml based on repeated tests on WNV dilution series by multiple agencies. Most of these RAMP negative pools were detected early in the season when temperatures are cooler and virus replication rates within Culex were slower (Reisen et al. 2006). We could not explain the 4 RAMP positives negative by RT-PCR, but these samples had RAMP scores between 52 and 168 and false positives with similar intermittent scores were reported previously (Kesavaraju et al. 2012).
Mosquito Surveillance
In 2003, following the detection of WNV in California, an unprecedented number of agencies submitted mosquito pools to CVEC. Overall, 39 different agencies submitted 10,297 pools from 25 different mosquito species. In total, 37 mosquito pools were positive by the in situ-EIA for at least one arbovirus: 32 WNV, 4 SLEV, and 1 WEEV. One mosquito pool was identified by the in situ-EIA to have contained both WNV and SLEV.
From 2004 through 2013, 271,889 mosquito pools were tested by qRT-PCR, of which 15,629 were positive for WNV, 55 were positive for WEEV, and none were positive for SLEV (Fig. 1). In 2005, our multiplex assay identified two pools of Cx. tarsalis positive for both WEEV and WNV RNA. From 817 to 2,866 pools tested positive for WNV each year. Of these total pools, 186,576 (69%) were tested by multiplex at CVEC. In 2004, all pools were tested by CVEC; however, over time, some MVCDs constructed their own laboratories and began testing for WNV by singleplex or multiplex RT-PCR, and by 2013, 47% of the total 30,144 state pools were tested by other agencies. Currently, all mosquito control agencies use the same primer–probe sets, extract RNA by MagMax express, detect virus using the ABI7500 platform, and annually pass a blinded proficiency panel administered by CVEC. Agencies that initially failed the proficiency panel were provided training and then were retested until their assay results were considered sufficiently sensitive and produced a linear dose–response curve similar to results in Fig. 3, the 2013 RT-PCR proficiency panel results for nine agencies and CVEC.
Fig. 3.
Results of blind proficiency panel testing for WNV RNA by nine local agencies and CVEC (fitted line) in 2013. Shown are the RT-PCR Ct score plotted as a function of WNV titer estimated by plaque assay on Vero cell culture. Note variation in sensitivity. At CVEC, RNA was extracted by MagMax Express and RT-PCR run using a one-step fast kit on an ABI Vii7a platform.
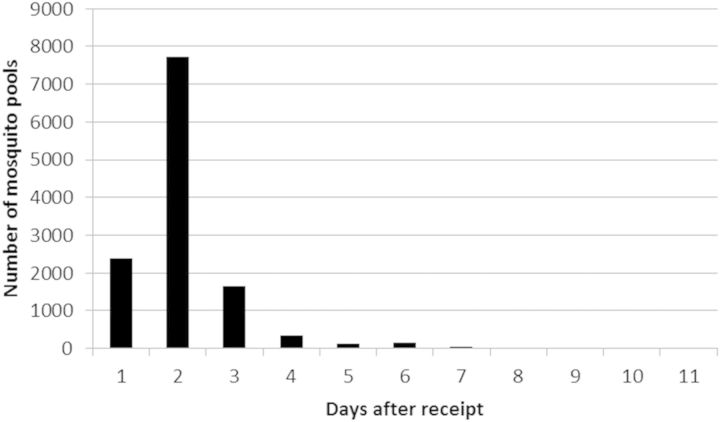
During 2006, we estimated the time required to test each of 12,706 pools after they were received at our laboratory (Fig. 4). Overall, 8,958 pools (65%) were processed and reported back to the submitting agency within 48 h. In our testing paradigm at that time, negative pools (Ct score >40) were reported immediately, whereas pools positive for WNV by the ENV gene primer–probe set were confirmed by a second assay using primer–probe from the NS1 region. If the first and second tests did not agree, then the sample was held until the following week, RNA was reextracted and retested with the ENV primer–probe set. Samples positive by the second assay run were reported as positive. Those negative by the second assay after reextraction and retesting were considered to be negative. Since this time, we have expedited the turnaround time by attempting confirmation only for pools with Ct values >30, those with values <30 are now immediately classified as positive based ∼100% confirmation and our ability to isolate infectious virus from these pools. Although variable among years, during 2012, for example, there were 3,002 WNV RT-PCR positive pools, of which 788 (26%) had Ct scores >30 and were confirmed by the paradigm described above; most confirmed by retesting with the NS1 primer–probe.
Fig. 4.
Average processing time in days for mosquito pools after receipt at CVEC during 2006.
Discussion
In response to expanded mosquito surveillance to estimate human infection risk and direct intervention, we developed a multiplex TaqMan qRT-PCR-based assay that detected RNA from WEEV, SLEV, and WNV strains known to circulate in California. In combination with efficient data management through the Surveillance Gateway net-based reporting system (Park et al. 2008, Lozano-Fuentes et al. 2011), we were able to provide efficient, near real-time risk estimates. If mosquito traps were set on Monday, specimens identified and shipped on Tuesday, and received by our laboratory by Wednesday, then the results were available to submitting agencies sometimes by Wednesday afternoon, but usually by Thursday or Friday at the latest, and within time to coordinate intervention efforts based on the California Mosquito-Borne Virus Surveillance and Response Plan (Kramer 2014). In general, our WNV RT-PCR results for mosquito pools have preceded or accompanied increases in the risk of human infection estimated by the Response Plan and the occurrence of human cases (Reisen et al. 2008, 2009; Kwan et al. 2010).
West Nile virus has demonstrated very low genetic variability since its introduction into North America in 1999 (Lanciotti et al. 2002, Beasley et al. 2003). The primer–probe set designed against the envelope gene of a WNV isolate from 1999 (Lanciotti et al. 2000) demonstrated a sensitivity level of 0.1 PFU per mosquito pool and was designated as the screening primer for WNV. A primer–probe combination from the NS1 gene region was demonstrated to have a sensitivity of 1.0 PFU and was used for confirmation of positives by the envelope set (Lanciotti et al. 2000). Unlike WNV, multiple genotypes of SLEV have been identified to circulate in California since the 1950s (Kramer et al. 1997, Reisen et al. 2002). The SLEV TaqMan assay had a detection level of less than a single PFU for all of the recently circulating SLEV genotypes; however, reduced sensitivity was identified for viral genotypes that differed from the prototype strain from which the primers were designed. New primer and probes were designed for WEEV because the previously published reagents were unable to identify all strains known to have circulated in California. Alignments of 55 partial sequences from the E2 envelope glycoprotein of Californian WEEV isolates were performed and two primer–probe sets were identified that detected WEEV at a sensitivity level of 0.01 PFU.
The sensitivity of our multiplex assay was greater than standard virus isolation by Vero cell culture and antigen detection assays. Improvements in RNA extraction and use of the One-Step Fastkit using the Vii7a RT-PCR platform has further enhanced original sensitivity and also shortened the processing time. Multiplexing of the reagents for the concurrent testing for three viral RNA species in the same reaction was associated with some reduced sensitivity, but did not affect specificity of the assay with new fluorophores having distinct wavelength activation.
The use of molecular approaches such as the assays described herein for the detection of viral RNA has the potential disadvantage of not detecting subtle genetic change due to the high level of specificity afforded by specific primer–probe sets. This fact was exemplified by the finding of reduced sensitivity of our SLEV probe for the different strains of SLEV that have circulated in California over the past 50 yr. Similarly, a spontaneous WNV mutation within the probe sequence reduced the assay sensitivity by an order of magnitude (Brault et al. 2012). Changes to the sequence of the primer and probe binding regions from these viruses indicated that only one nucleotide difference within the probe region can affect the sensitivity of the assay and that only a small number of mutations can be tolerated in the primers for the maintenance of sensitivity. The minimal effects on relative sensitivity owing to multiplexing could easily allow future assays to target multiple gene regions for individual viruses to reduce the negative impact of similar subtle genetic variation on assay sensitivity.
Although excellent for decision support to direct intervention against a specific suite of viruses that utilize the same vectors, reliance of highly specific and high-throughput assays precludes the ability to detect the emergence of local or introduction of novel viruses, for which the assays were not intended. Our future research and development efforts will target the use of new sequencing tools to provide a balanced approach to track a variety of viruses; however, these methods currently are cost-prohibitive to provide the necessary spatial and temporal resolution to direct intervention strategies. Future surveillance paradigms may use an eclectic approach of modified multiplexable platforms that can serve this traditional role for arboviral surveillance in concert with deep sequencing analyses for the detection of novel genetic variants with potentially altered vector infection and/or virulence phenotypes.
Acknowledgments
This work was supported by grants from the California Mosquito Research Program, as well as the Pacific Southwest NIH Regional Centers for Excellence (PSWRCE), National Institute of Allergy and Infectious Diseases, National Institute of Health grant RO1AI-055607, and Epidemiology and Laboratory Capacity funds from the Centers for Disease Control and Prevention to the California Department of Public Health. We thank the Mosquito and Vector Control Districts within the state of California for providing speciated mosquito pools that were used for standardization and testing of these assay systems. Robert Chiles (former CVEC Laboratory Manager), Sandra Garcia, Emily N. Green, Marzi Shafii, Sharon Clark, Siranoosh Ashtari, Nadira Chouicha, Helen Lu, Maureen Dannen, Amy Roth, Keira Simmons, and Andrew Chow provided technical assistance.
Reference Cited
- Baba S. S., Fagbami A. H., Olaleye O. D. 1998. Antigenic relatedness of selected flaviviruses: study with homologous and heterologous immune mouse ascitic fluids. Rev. Inst. Med. Trop. Sao Paulo 40: 343–349. [DOI] [PubMed] [Google Scholar]
- Barros S. C., Ramos F., Ze-Ze L., Alves M. J., Fagulha T., Duarte M., Henriques M., Luis T., Fevereiro M. 2013. Simultaneous detection of West Nile and Japanese encephalitis virus RNA by duplex TaqMan RT-PCR. J. Virol. Methods 193: 554–557. [DOI] [PubMed] [Google Scholar]
- Beasley D. W., Davis C. T., Guzman H., Vanlandingham D. L., Travassos da Rosa A. P., Parsons R. E., Higgs S., Tesh R. B., Barrett A. D. 2003. Limited evolution of West Nile virus has occurred during its southwesterly spread in the United States. Virology 309: 190–195. [DOI] [PubMed] [Google Scholar]
- Brault A. C., Fang Y., Dannen M., Anishchenko M., Reisen W. K. 2012. A naturally occurring mutation within the probe-binding region compromises a molecular-based West Nile virus surveillance assay for mosquito pools (Diptera: Culicidae). J. Med. Entomol. 49: 939–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiles R. E., Green E. N., Fang Y., Goddard L., Roth A., Reisen W. K., Scott T. W. 2004. Comparison of in situ enzyme immunoassay, RT-PCR and the VecTest wicking assay to detect West Nile and St. Louis encephalitis viruses in a blinded laboratory evaluation. J. Med. Entomol. 41: 539–544. [DOI] [PubMed] [Google Scholar]
- Graham R. R., Hardy J. L., Presser S. B. 1986. Use of the in situ enzyme immunoassay for the rapid detection of arbovirus infections in mosquitoes in California. Proc. Calif. Mosq. Vector Control Assoc. 54: 10. [Google Scholar]
- Hom A., Marcus L., Kramer V. L., Cahoon B., Glaser C., Cossen C., Baylis E., Jean C., Tu E., Eldridge B. F., et al. 2005. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2004. Proc. Mosq. Vector Control Assoc. Calif. 73: 66–77. [Google Scholar]
- Howitt B. F. 1938. Recovery of the virus of equine encephalomyelitis from the brain of a child. Science 88: 455. [DOI] [PubMed] [Google Scholar]
- Howitt B. F. 1939. Viruses of equine and St. Louis encephalitis in relationship to human infections in California 1937–1938. Am. J. Publ. Health 29: 1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B., Farajollahi A., Lampman R. L., Hutchinson M., Krasavin N. M., Graves S. E., Dickson S. L. 2012. Evaluation of a rapid analyte measurement platform for West Nile virus detection based on United States mosquito control programs. Am. J. Trop. Med. Hyg. 87: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer V. L. 2014. California Mosquito-borne Virus Surveillance and Response Plan ( http://westnile.ca.gov/resources.php). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L. D., Fallah H. M. 1999. Genetic variation among isolates of western equine encephalomyelitis virus from California. Am. J. Trop. Med. Hyg. 60: 708–713. [DOI] [PubMed] [Google Scholar]
- Kramer L. D., Presser S. B., Hardy J. L., Jackson A. O. 1997. Genotypic and phenotypic variation of selected Saint Louis encephalitis viral strains in California. Am. J. Trop. Med. Hyg. 57: 222–229. [DOI] [PubMed] [Google Scholar]
- Kwan J. L., Kluh S., Madon M. B., Reisen W. K. 2010. West Nile virus emergence and persistence in Los Angeles, California, 2003–2008. Am. J. Trop. Med. Hyg. 83: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A. J., Martin D. A., Lanciotti R. S. 2003. Detection of North American eastern and western equine encephalitis viruses by nucleic Acid amplification assays. J. Clin. Microbiol. 41: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kerst A. J. 2001. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis Encephalitis viruses. J. Clin. Microbiol. 39: 4506–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kerst A. J., Nasci R. S., Godsey M. S., Mitchell C. J., Savage H. M., Komar N., Panella N. A., Allen B. C., Volpe K. E., et al. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Ebel G. D., Deubel V., Kerst A. J., Murri S., Meyer R., Bowen M., McKinney N., Morrill W. E., Crabtree M. B., et al. 2002. Complete genome sequences and phylogenetic analysis of West Wile virus strains isolated from the United States, Europe, and the middle East. Virology 298: 96–105. [DOI] [PubMed] [Google Scholar]
- Lozano-Fuentes S., Barker C. M., Coleman M., Coleman M., Park B. K., Reisen W. K., Eisen L. 2011. Emerging information technologies to provide improved decision support for surveillance, prevention, and control of vector-borne diseases, pp. 89–114. In Jao C. S. (ed.), Efficient decision support systems: Practice and challenges-from current to future. InTech, Open Access. [Google Scholar]
- Meyer K. F., Haring C. M., Howitt B. 1931. The etiology of epizootic encephalomyelitis of horses in the San Joaquin Valley, 1930. Science 74: 227–228. [DOI] [PubMed] [Google Scholar]
- Muckenfuss R. S., Armstrong C., Webster L. T. 1934. Etiology of the 1933 epidemic of encephalitis. JAMA 103: 731–733. [Google Scholar]
- Murray R. A., Habel L. A., Mackey K. J., Wallace H. G., Peck B. A., Mora S. J., Ginsberg M. M., Emmons R. W. 1985. Epidemiological aspects of the 1984 St Louis encephalitis epidemic in southern California. Proc. Calif. Mosq. Vector Control Assoc. 53: 5–9. [Google Scholar]
- Nasci R. S., Gottfried K. L., Burkhalter K. L., Ryan J. R., Emmerch E., Dave K. 2003. Sensitivity of the VecTest antigen assay for eastern equine encephalitis and western equine encephalitis viruses. J. Am. Mosq. Control Assoc. 19: 440–444. [PubMed] [Google Scholar]
- Park B., Eldridge B. F., Barker C. M., Reisen W. K. 2008. Building upon California's Surveillance Gateway. Proc. Mosq. Vector Control Assoc. Calif. 76: 27–28. [Google Scholar]
- Reeves W. C., Hammon W. M. 1962. Epidemiology of the arthropod-borne viral encephalitides in Kern County, California, 1943–1952. Univ. Calif. Berkeley Publ. Publ. Hlth. 4: 1–257. [PubMed] [Google Scholar]
- Reeves W. C., Asman S. M., Hardy J. L., Milby M. M., Reisen W. K. 1990. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. California MosquitoVector Control Association, Sacramento, CA. [Google Scholar]
- Reisen W. K., Meyer R. P., Milby M. M., Presser S. B., Emmons R. W., Hardy J. L., Reeves W. C. 1992. Ecological observations on the 1989 outbreak of St. Louis encephalitis virus in the southern San Joaquin Valley of California. J. Med. Entomol. 29: 472–482. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Lothrop H. D., Chiles R. E., Cusack R., Green E.-G. N., Fang Y., Kensington M. 2002. Persistence and amplification of St. Louis encephalitis virus in the Coachella Valley of California, 2000–2001. J. Med. Entomol. 39: 793–805. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Lothrop H. D., Chiles R. E., Madon M. B., Cossen C., Woods L., Husted S., Kramer V. L., Edman J. D. 2004. West Nile Virus in California. Emerg. Infect. Dis. 10: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W. K., Fang Y., Martinez V. M. 2005. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 42: 367–375. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Fang Y., Martinez V. M. 2006. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43: 309–317. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Lothrop H. D., Wheeler S. S., Kennsington M., Gutierrez A., Fang Y., Garcia S., Lothrop B. 2008. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J. Med. Entomol. 45: 494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W. K., Carroll B. D., Takahashi R., Fang Y., Garcia S., Martinez V. M., Quiring R. 2009. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J. Med. Entomol. 46: 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P. Y., Kauffman E. B., Ren P., Felton A., Tai J. H., DuPuis A. P., Jones S. A., Ngo K. A., Nicholas D. C., Maffei J., et al. 2001. High-throughput detection of West Nile virus RNA. J. Clin. Microbiol. 39: 1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink S. D., Jones S. A., Maffei J. G., Kramer L. D. 2013. Quadraplex qRT-PCR assay for the simultaneous detection of Eastern equine encephalitis virus and West Nile virus. Diagn. Microbiol. Infect. Dis. 77: 129–132. [DOI] [PubMed] [Google Scholar]