Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive disease characterised by dyspnea and loss of lung function.
Methods
Using pooled data from the replicate, randomized, 52-week, placebo-controlled INPULSIS® trials, we characterized the safety and tolerability of nintedanib 150 mg twice daily in patients with IPF and described how adverse events were managed during these trials.
Results
One thousand and sixty- one patients were treated (nintedanib 638; placebo 423). Higher proportions of patients in the nintedanib group than the placebo group had ≥1 dose reduction to 100 mg bid (27.9 % versus 3.8 %) or treatment interruption (23.7 % versus 9.9 %). Adverse events led to permanent treatment discontinuation in 19.3 % and 13.0 % of patients in the nintedanib and placebo groups, respectively. Diarrhea was the most frequent adverse event, reported in 62.4 % of patients in the nintedanib group versus 18.4 % in the placebo group; however, only 4.4 % of nintedanib-treated patients discontinued trial medication prematurely due to diarrhea. Monitoring of liver enzymes before and periodically during nintedanib treatment was recommended so that liver enzyme elevations could be managed through dose reduction or treatment interruption.
Conclusion
Nintedanib had a manageable safety and tolerability profile in patients with IPF. Recommendations for adverse event management minimized permanent treatment discontinuations in the INPULSIS® trials.
Trial registration
clinicaltrials.gov NCT01335464 and NCT01335477
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-015-0276-5) contains supplementary material, which is available to authorized users.
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrosing interstitial pneumonia, occurring primarily in the elderly population, which is characterized by increasing dyspnea and loss of lung function [1–3]. Patients with IPF have impaired quality of life and reduced exercise tolerance [4]. IPF is ultimately fatal, with a median survival time of only 2–3 years from diagnosis [3].
Our evolving understanding of the pathophysiology of IPF suggests complex interplay between multiple pathways. Protein tyrosine kinases are known to play a key role in intracellular signaling pathways involved in the pathogenesis of lung fibrosis [5–7]. Nintedanib is an intracellular inhibitor of tyrosine kinases, including the receptors for the fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) [8, 9]. In preclinical studies nintedanib interfered with processes associated with fibrosis including fibroblast proliferation, migration and differentiation and was associated with reduced secretion of extracellular matrix and reduced lung inflammation [9].
The efficacy and safety of nintedanib as a treatment for IPF have been studied in the Phase II, 52-week, TOMORROW trial [10] and in the two replicate randomized, placebo-controlled, 52-week Phase III INPULSIS® trials [11, 12]. Results from the TOMORROW trial, which involved 432 patients with IPF, suggested that compared with placebo, treatment with nintedanib 150 mg twice daily (bid) was associated with a reduced annual decline in forced vital capacity (FVC), fewer acute exacerbations, and preservation of health-related quality of life, assessed using the St George’s Respiratory Questionnaire (SGRQ) [10]. In both INPULSIS® trials, the primary endpoint of the annual rate of decline in FVC was significantly reduced by approximately 50 % in the nintedanib group compared with placebo, consistent with a slowing of disease progression. The adjusted annual rate of decline in FVC was −114.7 mL/year with nintedanib versus −239.9 mL/year with placebo (a difference of 125.3 mL/year [95 % confidence interval (CI): 77.7, 172.8]; p < 0.001) in INPULSIS®-1 and −113.6 mL/year with nintedanib versus −207.3 mL/year with placebo (a difference of 93.7 mL/year [95 % CI: 44.8, 142.7]; p < 0.001) in INPULSIS®-2 [11]. In INPULSIS®-2, there was a significant difference in favor of nintedanib for time to first acute exacerbation and change from baseline in SGRQ total score, both over 52 weeks. However, in INPULSIS®-1, no significant difference between groups was observed for either of these endpoints [11]. Gastrointestinal events, particularly diarrhea, were the most frequently reported adverse events in patients treated with nintedanib.
An analysis of pooled safety data from the two INPULSIS® trials was pre-specified. In this manuscript, we further characterize the safety and tolerability of nintedanib and describe how adverse events were reported and managed during these Phase III trials.
Methods
The INPULSIS® trials, performed at 205 sites in 24 countries in the Americas, Europe, Asia and Australia, recruited a broad range of patients with IPF [11]. Patients with IPF aged ≥40 years were eligible to participate if they had an FVC of ≥50 % of predicted value and a diffusing capacity of the lung for carbon monoxide (DLCO) of 30–79 % of predicted value. In the absence of a surgical lung biopsy, diagnosis of IPF required the presence of honeycombing and/or a combination of traction bronchiectasis and reticulation, without nodules or consolidation, on high-resolution computed tomography (HRCT), which was centrally reviewed. If available, surgical lung biopsies were centrally reviewed to confirm eligibility to enter the trials using a multidisciplinary approach. Patients with emphysema on HRCT were not excluded, but patients were required to have an FEV1/FVC ratio of ≥0.7. Concomitant therapy with prednisone ≤15 mg/day, or the equivalent, was permitted if the dose had been stable for ≥8 weeks before screening. Patients receiving other therapies for IPF, including pirfenidone, prednisone >15 mg/day, azathioprine, N-acetylcysteine and any investigational treatments for IPF, were excluded. Patients with elevated hepatic enzymes (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) or bilirubin >1.5 x upper limit of normal (ULN) were excluded.
Eligible patients were randomized using a 3:2 ratio to receive nintedanib 150 mg bid or placebo for 52 weeks, followed by a 4-week follow-up period. There was no requirement for dose titration on initiation of treatment. Treatment interruption and/or dose reduction from 150 mg bid to 100 mg bid were allowed for the management of adverse events. For patients who had a dose interruption, treatment could be reinstituted at a dose of 150 mg bid or 100 mg bid after resolution of the adverse event. After a dose reduction, the dose could be re-escalated to 150 mg bid. Specific recommendations were provided to the investigators for the management of diarrhea (Fig. 1) and hepatic enzyme elevations (Fig. 2).
Fig. 1.
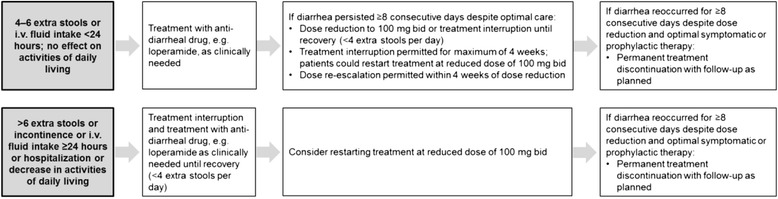
Algorithm for the management of diarrhea adverse events in the INPULSIS® trials. i.v., intravenous
Fig. 2.
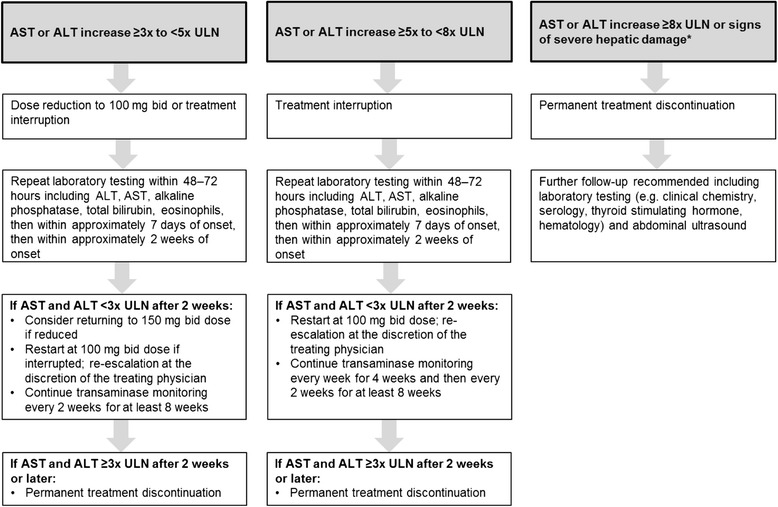
Algorithm for the management of hepatic enzyme elevations in the INPULSIS® trials. *Defined as increase in hepatic transaminases (AST or ALT) to ≥3 x ULN, and i) total bilirubin >1.5 ULN or ii) INR >1.5 or iii) appearance of fatigue, nausea, vomiting, right upper abdominal quadrant pain or tenderness, fever, rash and/or eosinophilia (>5%). In the INPULSIS® trials, routine clinical laboratory testing was undertaken at screening and weeks 2, 4, 6, 12, 18, 24, 30, 36, 44 and 52
For patients who had >1 treatment interruption, the duration of treatment interruption was calculated as total duration of all interruptions. Dose intensity was defined as the amount of drug administered over the study divided by the amount of drug that would have been received had the 150 mg bid dose been administered throughout the 52-week study or until discontinuation in patients who discontinued prematurely. A pre-specified subgroup analysis assessed the impact of dose intensity (≤90 % versus >90 %) on the annual rate of decline in FVC using pooled data from the two trials.
Compliance with study medication was defined as the number of capsules taken divided by the number of capsules that should have been taken over the trial period. Safety was assessed by means of clinical and laboratory evaluation, clinical assessment of vital signs and physical examination at study visits and the recording of adverse events, coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1. The analyses were descriptive and based on patients who received ≥1 dose of study medication. Patients with adverse events with onset after the first dose and up to 28 days after the last dose of trial medication were included in the analysis. The intensity of adverse events was rated by the investigators as mild (easily tolerated), moderate (enough discomfort to cause interference with usual activity) or severe (incapacitating or causing inability to work or to perform usual activities). For every adverse event, investigators reported whether they considered the event was related to study drug. For patients who experienced ≥1 diarrhea adverse event, the duration of diarrhea adverse events was calculated as the total duration of all the events. A specific diarrhea electronic case report form (eCRF) was introduced during the course of the trials to characterize diarrhea adverse events. Routine clinical laboratory testing was undertaken at screening and weeks 2, 4, 6, 12, 18, 24, 30, 36, 44 and 52.
Both trials were conducted in accordance with the principles of the Declaration of Helsinki and the Harmonised Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation and were approved by local authorities. The clinical protocol was approved by an independent ethics committee or institutional review board at each participating center. All patients provided written informed consent before study entry.
Results
Patients
A total of 1061 patients were treated in the two INPULSIS® trials, 638 in the nintedanib group and 423 in the placebo group. Demographics and baseline characteristics were comparable between the treatment groups (Table 1 and Additional file 1).
Table 1.
Baseline demographics and clinical characteristics in the INPULSIS® trials
| Nintedanib | Placebo | |
|---|---|---|
| (n = 638) | (n = 423) | |
| Male, n (%) | 507 (79.5) | 334 (79.0) |
| Age, years, mean (SD) | 66.6 (8.1) | 67.0 (7.9) |
| Weight, kg, mean (SD) | 79.2 (16.6) | 78.6 (16.5) |
| Race, n (%) | ||
| White | 360 (56.4) | 248 (58.6) |
| Asian | 194 (30.4) | 128 (30.3) |
| Black | 2 (0.3) | 0 (0.0) |
| Missinga | 82 (12.9) | 47 (11.1) |
| Smoking status, n (%) | ||
| Never smoked | 174 (27.3) | 122 (28.8) |
| Ex-smoker | 435 (68.2) | 283 (66.9) |
| Current smoker | 29 (4.5) | 18 (4.3) |
| Time since diagnosis of IPF, years, mean (SD)b | 1.7 (1.4) | 1.6 (1.3) |
| FVC, mL, mean (SD) | 2714 (757) | 2728 (810) |
| FVC, % predicted, mean (SD) | 79.7 (17.6) | 79.3 (18.2) |
| FEV1/FVC ratio, %, mean (SD) | 81.7 (5.8) | 81.7 (6.0) |
| DLCO, % predicted, mean (SD) | 47.4 (13.5) | 47.0 (13.4) |
| SpO2, %, mean (SD) | 95.9 (2.3) | 95.8 (2.0) |
| SGRQ total score, mean (SD)c | 39.5 (19.2) | 39.6 (18.5) |
Based on patients who received ≥1 dose of study medication. Data collected at screening unless otherwise stated
aIt was not permitted to collect data on race in France
bAt randomization
c n = 624 for nintedanib 150 mg bid and n = 419 for placebo
Exposure
The mean (standard deviation [SD]) duration of exposure was 10.3 (3.4) months in the nintedanib group and 10.8 (2.8) months in the placebo group. Compliance with study medication was 96.4 % in the nintedanib group and 96.7 % in the placebo group.
A summary of the proportion of patients requiring dose reductions and treatment interruptions is presented in Table 2. The proportion of patients who had ≥1 dose reduction from 150 mg bid to 100 mg bid was higher in the nintedanib group than in the placebo group (178 patients [27.9 %] versus 16 patients [3.8 %]); 15 patients (2.4 %) in the nintedanib group and none in the placebo group had ≥2 dose reductions from 150 mg bid to 100 mg bid. There was no distinct temporal pattern associated with dose reductions. Forty patients (6.3 %) in the nintedanib group and 7 patients (1.7 %) in the placebo group had ≥1 dose increase from 100 mg bid to 150 mg bid. In total, 76.3 % of patients in the nintedanib group and 97.9 % of patients in the placebo group received 150 mg bid as their last dose.
Table 2.
Dose reductions and treatment interruptions in the INPULSIS® trials
| N (%) | Nintedanib | Placebo |
|---|---|---|
| (n = 638) | (n = 423) | |
| Dose reductions | ||
| Patients with ≥1 dose reduction | 178 (27.9) | 16 (3.8) |
| Dose reductions per patient | ||
| 1 | 163 (25.5) | 16 (3.8) |
| 2 | 13 (2.0) | 0 (0.0) |
| > 2 | 2 (0.3) | 0 (0.0) |
| Time to first dose reduction | ||
| ≤ 1 month | 20 (3.1) | 1 (0.2) |
| > 1 month to ≤2 months | 28 (4.4) | 4 (0.9) |
| > 2 month to ≤3 months | 19 (3.0) | 0 (0.0) |
| > 3 month to ≤4 months | 18 (2.8) | 3 (0.7) |
| > 4 month to ≤5 months | 24 (3.8) | 1 (0.2) |
| > 5 month to ≤6 months | 22 (3.4) | 1 (0.2) |
| > 6 months | 47 (7.4) | 6 (1.4) |
| Patients with ≥1 dose increase to 150 mg bid | 40 (6.3) | 7 (1.7) |
| Patients who took 150 mg bid as last dose | 487 (76.3) | 414 (97.9) |
| Treatment interruptions | ||
| Patients with ≥1 treatment interruption | 151 (23.7) | 42 (9.9) |
| Treatment interruptions per patient | ||
| 1 | 113 (17.7) | 39 (9.2) |
| 2 | 30 (4.7) | 3 (0.7) |
| > 2 | 8 (1.3) | 0 (0.0) |
| Time to first treatment interruption | ||
| ≤ 1 month | 32 (5.0) | 3 (0.7) |
| > 1 month to ≤2 months | 18 (2.8) | 4 (0.9) |
| > 2 month to ≤3 months | 18 (2.8) | 6 (1.4) |
| > 3 month to ≤4 months | 21 (3.3) | 4 (0.9) |
| > 4 month to ≤5 months | 18 (2.8) | 2 (0.5) |
| > 5 month to ≤6 months | 11 (1.7) | 3 (0.7) |
| > 6 months | 33 (5.2) | 20 (4.7) |
| Patients re-introduced at the same dose of 100 mg bid after treatment interruptiona | 20 (13.2) | 1 (2.4) |
| Patients re-introduced at a reduced dose of 100 mg bid after treatment interruptiona | 81 (53.6) | 9 (21.4) |
| Patients re-introduced at the same dose of 150 mg bid after treatment interruptiona | 49 (32.5) | 32 (76.2) |
| Patients re-introduced at an increased dose of 150 mg bid after treatment interruptiona | 1 (0.7) | 0 (0.0) |
Based on patients who received ≥1 dose of study medication
aDose at last re-introduction
A greater proportion of patients treated with nintedanib had ≥1 treatment interruption than those treated with placebo (23.7 % versus 9.9 %); 38 patients (6.0 %) in the nintedanib group and 3 patients (0.7 %) in the placebo group had ≥2 treatment interruptions. There was no discernible accumulation of dose interruptions during a specific time period during the trials. The mean (SD) duration of interruption was 25.1 (18.8) and 25.6 (15.3) days in the nintedanib and placebo groups, respectively.
In total, 24.5 % of patients in the nintedanib group and 18.9 % in the placebo group prematurely discontinued trial medication; 19.3 % and 13.0 % of patients treated with nintedanib and placebo, respectively, prematurely discontinued trial medication due to an adverse event.
Mean dose intensity was high in both treatment groups. In the subgroup of patients with dose intensity >90 % (n = 484), mean (SD) dose intensity was 99.3 (2.2) % in patients treated with nintedanib and 99.8 (1.6) % in patients treated with placebo. In the subgroup of patients with dose intensity ≤90 % (n = 154), mean (SD) dose intensity was 76.1 (10.1) % in patients treated with nintedanib and 80.5 (7.5) % in patients treated with placebo. The adjusted annual rate of decline in FVC was of similar magnitude in patients treated with nintedanib with dose intensity >90 % as in patients treated with nintedanib with dose intensity ≤90 % (mean [standard error] 112.7 [12.8] and 72.7 [24.3] ml/year, respectively).
Adverse events
A summary of adverse events is presented in Table 3. In total, 95.5 % of patients treated with nintedanib and 89.6 % of patients treated with placebo experienced ≥1 adverse event. The proportion of patients who had ≥1 serious adverse event was similar between the nintedanib (30.4 %) and placebo (30.0 %) groups.
Table 3.
Patients with adverse events in the INPULSIS® trials
| N (%) | Nintedanib | Placebo |
|---|---|---|
| (n = 638) | (n = 423) | |
| Any adverse event(s) | 609 (95.5) | 379 (89.6) |
| Most frequent adverse eventsa | ||
| Diarrhea | 398 (62.4) | 78 (18.4) |
| Nausea | 156 (24.5) | 28 (6.6) |
| Nasopharyngitis | 87 (13.6) | 68 (16.1) |
| Cough | 85 (13.3) | 57 (13.5) |
| Progression of idiopathic pulmonary fibrosisb | 64 (10.0) | 61 (14.4) |
| Bronchitis | 67 (10.5) | 45 (10.6) |
| Dyspnea | 49 (7.7) | 48 (11.3) |
| Decreased appetite | 68 (10.7) | 24 (5.7) |
| Vomiting | 74 (11.6) | 11 (2.6) |
| Adverse event(s) leading to permanent dose reduction | 101 (15.8) | 2 (0.5) |
| Adverse event(s) leading to permanent treatment discontinuation | 123 (19.3) | 55 (13.0) |
| Adverse events that most frequently led to permanent treatment discontinuationc | ||
| Progression of idiopathic pulmonary fibrosisb | 13 (2.0) | 21 (5.0) |
| Diarrhea | 28 (4.4) | 1 (0.2) |
| Nausea | 13 (2.0) | 0 (0.0) |
| Decreased appetite | 9 (1.4) | 1 (0.2) |
| Pneumonia | 6 (0.9) | 1 (0.2) |
| Weight decreased | 6 (0.9) | 1 (0.2) |
| Abdominal pain | 5 (0.8) | 1 (0.2) |
| Vomiting | 5 (0.8) | 0 (0.0) |
| Asthenia | 4 (0.6) | 0 (0.0) |
| Increased alanine aminotransferase | 4 (0.6) | 0 (0.0) |
| Drug-related adverse events (as reported by the investigators) | 455 (71.3) | 120 (28.4) |
| Severe adverse eventsd | 174 (27.3) | 99 (23.4) |
| Serious adverse eventse | 194 (30.4) | 127 (30.0) |
| Fatal adverse event(s) | 37 (5.8) | 31 (7.3) |
Based on patients who received ≥1 dose of study medication
aAdverse events reported in >10 % of patients in either treatment group
bCorresponds to the MedDRA term ‘IPF’, which included disease worsening and IPF exacerbations
cAdverse events leading to permanent treatment discontinuation in >0.5 % of patients in either treatment group, by preferred term
dA severe adverse event was related to intensity and was defined as an event that was incapacitating or that caused an inability to work or to perform usual activities
eA serious adverse event was defined as any adverse event that resulted in death, was immediately life-threatening, resulted in persistent or clinically significant disability or incapacity, required or prolonged hospitalization, was related to a congenital anomaly or birth defect or was deemed serious for any other reason
Diarrhea was the most frequently reported adverse event in the nintedanib group, reported in 62.4 % of patients compared with 18.4 % of patients in the placebo group. A summary of diarrhea adverse events and their clinical consequences is presented in Table 4. Almost all patients who reported diarrhea adverse events (nintedanib: 94.5 %, placebo: 97.4 %) reported events of mild or moderate intensity. Of patients who had ≥1 completed eCRF specific for diarrhea (n = 185 in the nintedanib group; n = 25 in the placebo group), most had <4 extra stools per day (nintedanib: 111 patients [60.0 %], placebo: 19 patients [76.0 %]). Bowel movements were most commonly characterized as watery with or without formed stool. Among patients who experienced a diarrhea adverse event, anti-diarrheal therapies (most commonly loperamide) were used by 55.3 % of patients in the nintedanib group and 25.6 % of patients in the placebo group during the on-treatment period. For most patients who reported diarrhea adverse events (nintedanib: 78.6 %, placebo: 98.7 %), the events resolved without the need for dose reduction or treatment interruption. A small proportion of patients had a permanent dose reduction (nintedanib: 68 patients [10.7 %], placebo: no patients) and/or discontinued trial medication prematurely due to diarrhea (nintedanib: 28 patients [4.4 %], placebo: 1 [0.2 %]). Among patients who experienced any diarrhea adverse events, most reported a single event (nintedanib: 60.3 %; placebo: 76.9 %) or 2 events (nintedanib: 26.4 %; placebo: 15.4 %). The median (minimum, maximum) duration of diarrhea events was 138.5 (1, 473) days in the nintedanib group and 7.0 (1, 453) days in the placebo group. For most patients with a diarrhea adverse event, the first onset occurred within the first 3 months of treatment (Fig. 3).
Table 4.
Gastrointestinal adverse events and clinical consequences in the INPULSIS® trials
| Diarrhea | Nausea | Vomiting | ||||
|---|---|---|---|---|---|---|
| Nintedanib | Placebo | Nintedanib | Placebo | Nintedanib | Placebo | |
| (n = 398) | (n = 78) | (n = 156) | (n = 28) | (n = 74) | (n = 11) | |
| Intensity of adverse eventa | ||||||
| Mild | 226 (56.8) | 60 (76.9) | 116 (74.4) | 26 (92.9) | 49 (66.2) | 9 (81.8) |
| Moderate | 150 (37.7) | 16 (20.5) | 38 (24.4) | 2 (7.1) | 21 (28.4) | 2 (18.2) |
| Severe | 21 (5.3) | 2 (2.6) | 2 (1.3) | 0 (0.0) | 4 (5.4) | 0 (0.0) |
| Outcome of adverse eventa | ||||||
| Recovered | 350 (87.9) | 72 (92.3) | 143 (91.7) | 22 (78.6) | 69 (93.2) | 11 (100.0) |
| Not yet recoveredb | 43 (10.8) | 6 (7.7) | 12 (7.7) | 6 (21.4) | 5 (6.8) | 0 (0.0) |
| Fatal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 5 (1.3) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Consequence for dosingc | ||||||
| No permanent dose reduction or discontinuationd | 313 (78.6) | 77 (98.7) | 135 (86.5) | 28 (100.0) | 64 (86.5) | 11 (100.0) |
| Permanent dose reduction of trial drug | 57 (14.3) | 0 (0.0) | 8 (5.1) | 0 (0.0) | 5 (6.8) | 0 (0.0) |
| Permanent discontinuation of trial drug | 28 (7.0) | 1 (1.3) | 13 (8.3) | 0 (0.0) | 5 (6.8) | 0 (0.0) |
| Number of adverse events | ||||||
| 1 | 240 (60.3) | 60 (76.9) | 116 (74.4) | 27 (96.4) | 54 (73.0) | 11 (100.0) |
| 2 | 105 (26.4) | 12 (15.4) | 31 (19.9) | 1 (3.6) | 14 (18.9) | 0 (0.0) |
| 3 | 29 (7.3) | 3 (3.8) | 8 (5.1) | 0 (0.0) | 4 (5.4) | 0 (0.0) |
| ≥ 4 | 24 (6.0) | 3 (3.8) | 1 (0.6) | 0 (0.0) | 2 (2.7) | 0 (0.0) |
| Duration of events, days, median (minimum, maximum)e | 138.5 (1, 473) | 7.0 (1, 453) | 44.0 (1, 400) | 51.0 (1, 404) | 6.0 (1, 390) | 1.0 (1, 4) |
Data are N (%) of patients with ≥1 diarrhea or nausea or vomiting adverse events unless otherwise stated
aFor patients with more with one event, the intensity/outcome of the worst event is displayed
bThe patient has not yet returned to his/her previous health status, continues to be followed for the adverse event, but is expected to recover without sequelae
cFor patients with more than one event, the last consequence for dosing is displayed
dIncludes patients with temporary dose reductions or treatment interruptions
eFor patients who experienced ≥1 adverse event, the duration of adverse events was calculated as the total duration of all the events; the definition of a single event was not specified to the investigators in the trial protocol
Fig. 3.
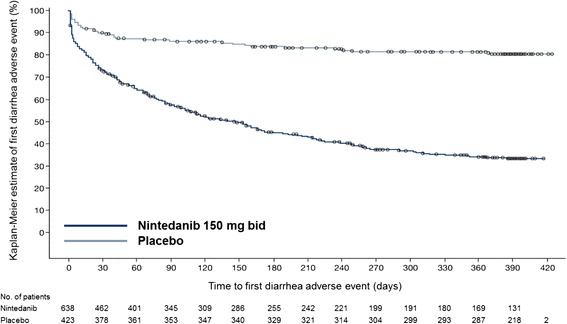
Time to first diarrhea adverse event in the INPULSIS® trials
In a post-hoc analysis, the absolute mean (SD) change from baseline in FVC at week 52 was −67.7 (271.4) ml for nintedanib-treated patients who experienced ≥1 diarrhea adverse event (n = 398) and −129.4 (246.0) ml for nintedanib-treated patients without any diarrhea events (n = 240). For patients treated with placebo, the absolute mean (SD) change from baseline in FVC at week 52 was −227.7 (283.6) ml for patients who experienced ≥1 diarrhea adverse event (n = 78) and −197.3 (294.7) ml for patients without any diarrhea events (n = 345).
A summary of nausea and vomiting adverse events is presented in Table 4. Almost all nausea and vomiting adverse events were mild or moderate in intensity. Most patients who experienced nausea reported only 1 event (nintedanib: 74.4 %; placebo: 96.4 %). Similarly, most patients who experienced vomiting reported only 1 event (nintedanib: 54 patients [73.0 %], placebo: 11 patients [100 %]). The majority of nausea adverse events had an early onset; vomiting adverse events occurred at any time relative to the start of treatment.
A summary of the proportions of patients with elevations in hepatic enzymes is shown in Table 5. For ALT, AST and bilirubin, the proportion of patients who had normal values at baseline but maximum values above the ULN during treatment was higher in the nintedanib group than in the placebo group. In the majority of patients, values had returned to values within the normal range by the end of treatment. Nevertheless, a greater proportion of patients in the nintedanib group than in the placebo group had a last value on treatment above the ULN for ALT (4.4 % versus 3.4 %) and AST (5.0 % versus 1.7 %). While it is difficult to identify a pattern due to the low number of patients with ALT and/or AST elevations ≥3x ULN, the elevations appeared not to occur at a particular time relative to the start of treatment. Thirty-two patients (5.0 %) in the nintedanib group and 3 patients (0.7 %) in the placebo group had ALT and/or AST elevations ≥3x ULN. Hy’s law criteria were met in 1 patient (in the placebo group).
Table 5.
Hepatic enzyme elevation adverse events in the INPULSIS® trials
| N (%) | Nintedanib | Placebo |
|---|---|---|
| (n = 638) | (n = 423) | |
| Elevations in ALT and/or AST | ||
| ≥ 3x ULN | 32 (5.0) | 3 (0.7) |
| ≥ 5x ULN | 14 (2.2) | 1 (0.2) |
| ≥ 8x ULN | 5 (0.8) | 1 (0.2) |
| Elevations in maximum total bilirubin | ||
| ≥ 1.5x ULN | 15 (2.4) | 3 (0.7) |
| ≥ 2x ULN | 3 (0.5) | 2 (0.5) |
| Elevations in maximum alkaline phosphatase | ||
| ≥ 1.5x ULN | 37 (5.8) | 4 (0.9) |
| ≥ 2x ULN | 17 (2.7) | 1 (0.2) |
| Normal ALT values at baseline but maximum value on treatment > ULN on treatmenta | 169 (27.3) | 30 (7.2) |
| Normal AST values at baseline but maximum value on treatment > ULNb | 134 (21.4) | 22 (5.3) |
| Normal bilirubin values at baseline but maximum value on treatment > ULNc | 48 (7.7) | 22 (5.3) |
| Normal alkaline phosphatase values at baseline but maximum value on treatment > ULNd | 94 (15.3) | 28 (6.8) |
Based on patients who received ≥1 dose of study medication. Categories are cumulative
anintedanib n = 620, placebo n = 416; bnintedanib n = 625, placebo n = 418; cnintedanib n = 621, placebo n = 413; dnintedanib n = 615, placebo n = 412
Hy’s law criteria were met in no patients in the nintedanib group and in 1 patient in the placebo group. Hy’s law criteria: AST or ALT ≥3x ULN and total bilirubin ≥2x ULN measured in the same blood sample, and no other reason found to explain the combination of increased hepatic transaminases and bilirubin, such as viral hepatitis A, B or C; pre-existing or acute hepatic disease; or the use of another drug capable of causing the observed injury
With regards to other adverse events of interest, bleeding events were reported in 10.3 % of patients in the nintedanib group and 7.8 % of patients in the placebo group. Epistaxis, which occurred in 26 patients (4.1 %) in the nintedanib group and 13 (3.1 %) in the placebo group and contusion, which occurred in 10 patients (1.6 %) in the nintedanib group and 4 (0.9 %) in the placebo group, were the most frequently reported bleeding events. Serious bleeding events occurred with similar incidences in both treatment groups (8 patients [1.3 %] in the nintedanib group and 6 [1.4 %] in the placebo group. Gastrointestinal perforation was reported in 2 patients (0.3 %) in the nintedanib group and no patients in the placebo group. Hypertension was reported in 33 patients (5.2 %) in the nintedanib group and 17 (4.0 %) in the placebo group.
Cardiac disorder adverse events were reported in 10.0 % of patients treated with nintedanib and 10.6 % of patients treated with placebo (Additional file 2). Serious cardiac disorder adverse events were reported in 5.0 % of patients in the nintedanib group and 5.4 % of patients in the placebo group; fatal cardiac disorders occurred in 3 patients (0.5 %) and 6 patients (1.4 %) in the nintedanib and placebo groups, respectively. The MedDRA category of ‘ischemic heart disease’ was reported in a similar proportion of patients in the nintedanib (4.2 %) and placebo (4.0 %) groups (Additional file 2). Within the MedDRA category of ‘ischemic heart disease’, a higher proportion of patients reported an event in the subcategory ‘myocardial infarction’ in the nintedanib group (17 patients [2.7 %]) compared with the placebo group (5 patients [1.2 %]), but a lower proportion of patients reported an event in the other subcategory, ‘other ischemic heart disease’, in the nintedanib group (11 patients [1.7 %]) than in the placebo group (13 patients ([3.1 %]) (Additional file 2). The MedDRA preferred terms of myocardial infarction or acute myocardial infarction were reported in 10 patients (1.6 %) treated with nintedanib and 2 patients (0.5 %) treated with placebo.
A higher proportion of patients experienced weight loss as an adverse event in the nintedanib group (9.7 %) than in the placebo group (3.5 %). The mean change from baseline in body weight at week 52 was −3.1 kg in the nintedanib group and −1.4 kg in the placebo group. The proportion of patients with infection adverse events was similar in both treatment groups (nintedanib: 56.3 %; placebo: 53.9 %) as was the proportion of patients with adverse events related to respiratory, thoracic and mediastinal disorders (nintedanib: 39.8 %; placebo: 41.8 %).
Discussion
We report collective safety data for nintedanib from the two Phase III INPULSIS® trials. These data demonstrate that nintedanib has a manageable safety and tolerability profile in patients with IPF.
In the INPULSIS® trials, the investigators were provided with recommendations for the management of adverse events, including symptomatic measures as well as the option of a flexible dosing regimen. These recommendations provide guidance for the management of adverse events associated with the use of nintedanib in clinical practice. Although a greater proportion of patients in the nintedanib group had a dose reduction or treatment interruption compared with patients in the placebo group, mean dose intensity in the nintedanib group was high. A pre-specified subgroup analysis demonstrated that nintedanib reduced FVC decline both in patients with a dose intensity of ≤90 % and in patients with a dose intensity >90 %, suggesting that dose reductions or treatment interruptions made to manage adverse events had no major impact on the efficacy of nintedanib.
As expected based on data from previous studies in patients with IPF [10] and patients with solid tumors [13, 14], the most frequently reported adverse events in patients treated with nintedanib in the INPULSIS® trials were gastrointestinal in nature, particularly diarrhea, nausea, vomiting and decreased appetite. Almost all such adverse events were mild or moderate in intensity and most resolved without the need for dose reduction or treatment interruption. A limitation of our studies was that the definition of a single diarrhea event was not specified to the investigators in the trial protocol. Thus, for patients with intermittent diarrhea over a prolonged period, some investigators may have reported this as several events and others as a single event, reducing the precision of our estimate of the number and the duration of diarrhea events. Only 4.4 % of patients in the nintedanib group discontinued trial medication prematurely due to diarrhea, suggesting that recommendations for the management of diarrhea, including symptomatic treatment with anti-diarrheal therapies, were successful in minimizing permanent treatment discontinuations. However, only 55.3 % of patients treated with nintedanib who experienced a diarrhea adverse event received symptomatic anti-diarrheal treatment during the on-treatment period, indicating that there is a need to provide adequate information to physicians in clinical practice and patients about the early management of diarrhea to enable patients to continue taking the drug and so receive maximum benefit from nintedanib treatment. This also applies to anti-emetic treatments. It is recommended that patients treated with nintedanib who experience diarrhea should maintain hydration and take antidiarrheal therapy (e.g., loperamide) as soon as symptoms occur. Dose reduction from 150 mg bid to 100 mg bid or temporary treatment interruption can also be considered. If diarrhea persists, nintedanib should be discontinued. The exact mechanism by which tyrosine kinase inhibition causes nausea or diarrhea is unknown; however, VEGF and VEGF receptors (VEGFR) are known to be highly expressed in the endocrine glands, stomach and intestines, suggesting that VEGF inhibition may cause morphometric changes in the bowel mucosa [15, 16]. A post-hoc analysis of data from the INPULSIS® trials showed that nintedanib was effective both in patients who had ≥1 diarrhea adverse event and patients who had no diarrhea adverse events, with a lower mean decline in FVC in patients who had ≥1 diarrhea adverse event. Although the limitations of this descriptive analysis do not allow firm conclusions to be drawn regarding the lower mean decline in FVC in patients who had diarrhea, trials investigating the use of tyrosine kinase inhibitors in oncology have reported an association between drug-related adverse events such as rash and diarrhea and efficacy [17, 18].
In addition to diarrhea, adverse events associated with inhibition of VEGF or VEGFR include arterial hypertension, gastrointestinal perforations, thromboembolism and bleeding [19, 20] and therefore these are potential risks of nintedanib treatment. Patients treated with full-dose anticoagulation or at known risk for bleeding were excluded from the INPULSIS® studies. This has led to recommendations stating that patients at known risk for bleeding should be treated with nintedanib only if the anticipated benefit outweighs the potential risk.
The mechanism behind the elevations in hepatic enzymes or bilirubin following treatment with nintedanib is not well understood, but requires laboratory testing before and periodically during treatment (e.g., during regular patient visits). In general, elevations in hepatic enzymes and total bilirubin were uncommon and returned to normal following dose reduction or treatment interruption.
Although cardiac disorders adverse events were balanced between the nintedanib and placebo groups, a higher proportion of patients in the nintedanib groups had myocardial infarctions. Conversely, a lower proportion of patients in the nintedanib groups had other ischemic heart disease. The clinical significance of this finding is unknown, and further observation is needed. However, it should be noted that the prevalence of myocardial infarctions in the INPULSIS® trials was low and below that observed in patients with IPF based on data from two US health claims databases [21].
In conclusion, pooled safety data from the INPULSIS® trials demonstrate that the dosing regimen utilized and the recommendations given for the management of adverse events, including symptomatic measures and hepatic enzyme monitoring, were successful in minimizing permanent treatment discontinuations. Nintedanib had a manageable safety and tolerability profile in patients with IPF. Physicians, nurses and patients will benefit from clear instructions on how to monitor patients and manage adverse events that may be associated with treatment with nintedanib, consistent with those implemented in the INPULSIS® trials.
Acknowledgments
Editorial assistance, supported financially by Boehringer Ingelheim, was provided by Julie Fleming and Wendy Morris of Fleishman-Hillard Group, Ltd, London, UK, during the preparation of this article. The authors were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development and have approved the final version.
Additional files
Baseline conditions and therapies in the INPULSIS ® trials. (DOCX 15 kb)
Cardiac disorders and ischaemic heart disease in the INPULSIS ® trials. (DOCX 14 kb)
Footnotes
Competing interests
The INPULSIS® trials were funded by Boehringer Ingelheim. CC, KP and MQ are employees of Boehringer Ingelheim. TC has received fees for consulting and serving on advisory boards for Boehringer Ingelheim and AstraZeneca; for contributing to educational materials for Boehringer Ingelheim and Actelion; travel support from Actelion, GlaxoSmithKline and Boehringer Ingelheim, and has received unrestricted educational grants from Actelion, Boehringer Ingelheim and InterMune. FB reports receipt of travel grants and speaker honoraria from Boehringer Ingelheim, as well as serving on advisory boards. BC reports receipt of grants, personal fees and non-financial support from InterMune and Boehringer Ingelheim; personal fees and non-financial support from Sanofi; grants from Cardif and MedImmune, and personal fees from AstraZeneca. MGD reports receipt of personal fees from Boehringer Ingelheim for being a member of the Data Monitoring Committee for the INPULSIS® trials. LR reports receipt of grants and personal fees from Boehringer Ingelheim for being a member of the INPULSIS® steering committee and co-principal investigator of the trials; grants and personal fees from InterMune for being a member of an advisory board; personal fees from MedImmune (advisory board member), Biogen-Idec (consulting), Sanofi-Aventis (consulting), Roche (advisory board member), Takeda (advisory board member), ImmuneWorks (consulting), Shionogi (speaker honoraria) and GlaxoSmithKline (advisory board member). JAL reports compensation from Boehringer Ingelheim for participation in a Data Monitoring Committee and advisory committee, as well as educational speaker programs.
Authors’ contributions
TC, BC and LR were investigators in the INPULSIS® trials. LR contributed to the design of the INPULSIS® trials through participation in the Steering Committee. MGD and JAL were co-chairs of the Data Monitoring Committee for the INPULSIS® trials. All authors contributed to the interpretation of data and the development of this manuscript and have approved the final version.
Contributor Information
Tamera Corte, Phone: +61295156120, Email: tameracorte@mac.com.
Francesco Bonella, Email: francesco.bonella@ruhrlandklinik.uk-essen.de.
Bruno Crestani, Email: bruno.crestani@bch.aphp.fr.
Maurits G. Demedts, Email: maurits.demedts@uz.kuleuven.ac.be
Luca Richeldi, Email: l.richeldi@soton.ac.uk.
Carl Coeck, Email: carl.coeck@boehringer-ingelheim.com.
Katy Pelling, Email: katy.pelling@boehringer-ingelheim.com.
Manuel Quaresma, Email: manuel.quaresma@boehringer-ingelheim.com.
Joseph A. Lasky, Email: jlasky@tulane.edu
References
- 1.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M, King TE, Pardo A, American Thoracic Society. European Respiratory Society. American College of Chest Physicians Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 6.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9:956–970. doi: 10.1038/nrd3297. [DOI] [PubMed] [Google Scholar]
- 7.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis: aiming to rein in runaway wound healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 9.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 10.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 11.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 12.Richeldi L, Cottin V, Flaherty KR, Kolb M, Inoue Y, Raghu G, et al. Design of the INPULSIS™ trials: Two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir Med. 2014;108:1023–1030. doi: 10.1016/j.rmed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Reck M, Kaiser R, Eschbach C, Stefanic M, Love J, Gatzemeier U, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol. 2011;22:1374–1381. doi: 10.1093/annonc/mdq618. [DOI] [PubMed] [Google Scholar]
- 14.Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res. 2010;16:311–319. doi: 10.1158/1078-0432.CCR-09-0694. [DOI] [PubMed] [Google Scholar]
- 15.Bowen JM. Mechanisms of TKI-induced diarrhea in cancer patients. Curr Opin Support Palliat Care. 2013;7:162–167. doi: 10.1097/SPC.0b013e32835ec861. [DOI] [PubMed] [Google Scholar]
- 16.Fan L, Iseki S. Immunohistochemical localization of vascular endothelial growth factor in the endocrine glands of the rat. Arch Histol Cytol. 1998;61:17–28. doi: 10.1679/aohc.61.17. [DOI] [PubMed] [Google Scholar]
- 17.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 18.Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45:e155–e160. doi: 10.1016/j.oraloncology.2009.05.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 20.Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl. 2013;11:172–191. doi: 10.1016/j.ejcsup.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15:829–835. doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]


