Abstract
CSF abnormalities have been reported in CSF leakage syndrome. However, the mechanism for these CSF changes is actually unknown and they may indicate impaired CSF flow or blood-CSF barrier. Angiopoietin-2 (Ang-2), a protein which is expressed and released by endothelial cells, has been associated with increased vascular permeability. In the assumption that CSF changes are due to an impaired blood-CSF barrier, we hypothesized that subjects with persistent CSF leakage may have increased CSF Ang-2 levels. We enrolled 10 subjects with a clinically definite diagnosis of persisting CSF leakage syndrome and 10 control subjects. In CSF analyses, CSF to serum albumin ratio (Qalb) was the most frequently increased parameter indicating a disturbed blood-CSF barrier function. Comparison of the mean CSF Ang-2 levels, CSF to serum Ang-2 ratio (QAng-2), and QAng-2/Qalb between the control and CSF leakage patients did not show any significant difference. We suggest that the increase of Qalb results from a low CSF flow. Future studies with phase contrast-MRI in conjunction with CSF analyses before and after epidural blood patch treatment are required to address this question. It would be of particular interest whether Qalb can be used as a marker for successful nontargeted epidural blood patch treatment.
1. Introduction
Persistent cerebrospinal fluid (CSF) leakage syndrome is a rare cause of chronic headache. The common clinical presentation is orthostatic headache of dull aching nature, which, according to the International Headache Society Classification, occurs or worsens in less than 15 minutes after assuming the upright position and disappears or improves in less than 30 minutes after recumbency [1]. While secondary CSF loss can be an aftermath of a trauma, neurosurgical procedure, or even systemic dehydration, the etiology of spontaneous (primary) CSF loss remains unknown. Suspected factors are an underlying weakness of the meningeal sac/diverticula in certain regions and a trivial trauma [1]. The concept of such “loci minoris resistentiae” is supported by the fact that generalized connective tissue disorders predispose to the formation of dural defects allowing CSF leakage into the epidural or subdural space [1]. These leakages most often occur at the level of the thoracic spine or cervicothoracic junction but rarely at the skull base [2]. Elevated leukocyte counts and protein concentrations in the CSF have been reported in several cases and were attributed to hydrostatic CSF pressure changes [1, 3]. The exact mechanism of these CSF changes is not well understood and they may indicate alterations in the CSF flow or blood-CSF barrier. The blood-CSF barrier is constituted by several barriers and one of them is the choroid plexus (CP), a highly vascularized endothelial-epithelial convolute in the ventricular system and the main source of CSF production [4]. Epithelial cells of the CP are interconnected via dense tight junction strands which shield the CSF from the blood. In contrast, endothelial cells embedded in the extracellular matrix of the CP are constitutively fenestrated [4]. This fenestrated phenotype has been shown to be maintained by vascular endothelial growth factor (VEGF) [5, 6]. A gatekeeper of VEGF function is angiopoietin-2 (Ang-2) and has been shown to breach the endothelial barrier function by counteracting the inhibitory effect of Ang-1 [7]. Ang-2 has been reported to be expressed in endothelial cells of the mouse and buffalo CP suggesting that Ang-2 together with Ang-1 and VEGF is involved in the regulation of CSF production [4, 8, 9]. Ang-2 has also been shown to be expressed by ependymal cells which, in contrast to epithelial cells of the CP, form a less tight “cellular barrier” between CSF and brain parenchyma [4, 10]. Interestingly, increased levels of CSF Ang-2 have been reported in hypoxemic patients or those with traumatic brain injury/subarachnoid hemorrhage suggesting that these resulted by disruption/increased permeability of the blood-CSF or CSF-brain parenchyma barrier [11–13]. Less is known about Ang-2 in pathological conditions affecting the CSF compartment. We hypothesized that subjects with persistent CSF leakage may have altered CSF Ang-2 levels which may indicate an impaired or more permeable blood-CSF barrier, respectively.
2. Materials and Methods
In this study, 10 (8 female and 2 male) subjects with a clinically definite diagnosis of persisting CSF leakage syndrome were enrolled from February 2009 to April 2011 (http://www.ihs-classification.org/en/02_klassifikation/03_teil2/07.02.03_nonvascular.html). Beside the clinical symptoms, diagnosis was based on the findings in intrathecal gadolinium- (gd-) enhanced magnet resonance (MR) myelography and/or CSF scintigraphy (Table 1). For gd-enhanced MR myelography, gd (gadoterate meglumine, Dotarem, Guerbet, Villepinte, France) was injected intrathecally after obtaining informed consent. Subjects received a single dose of 0.2 mmol gd (≈0.4 mL 0.5 mmol/mL Gd-DOTA) and were maintained in a position of 35° head elevation. Conventional spin-echo sagittal and axial T1-weighted fat-suppressed sequences on a 1.5 T MR unit using a 35 mT/m gradient magnet (Siemens, Erlangen, Germany) enabled the detection of the site of the dural dehiscence. CSF scintigraphy was performed by administering 20 MBq (4.6 mCi) 111 indium-diethylene-triamine-pentaacetic acid via lumbar puncture. Images were obtained at 1 h and 4 h including the region of the urinary bladder and the area of lumbar puncture (posterior projection) after radioisotope injection. Early appearance of urinary bladder on the 4-hour image was noted (Table 1). At 24 and 48 h, images showed the activity distribution in the entire spinal subarachnoid space (dorsal projection) and the head (anterior, posterior, and both lateral projections). Images were obtained using large-field-of-view gamma cameras (DIACAM or ECAM, Siemens, Erlangen, Germany) equipped with medium-energy collimators. CSF and serum analyses were analyzed by standard methods before intrathecal administration of gd (Table 1) [14]. Ang-2 was measured in serum and CSF using the RayBio Human Ang-2 Elisa-Kit (Ray Biotech, distributed by Hoelzel Diagnostika GmbH, Köln, Germany) according to the manufacturer's instructions. All conditions were tested in duplicate for each independent measurement. The study was approved by the Institutional Review Board of the Hannover Medical School. All participants signed an informed consent form detailing the purpose of this study, the tests included in the exploratory protocol, and the permission to revoke the obtained data. Statistical analysis was performed using GraphPad Prism version 5.02. Normality of data was assessed by the Shapiro-Wilk test. Since the control cohort did not show a normal distribution of data, we performed the two-tailed Mann-Whitney U test to compare the mean Ang-2 values between the two cohorts. To analyze correlations to clinical measures two-tailed Pearson or Spearmen correlation coefficients were calculated. For each comparison, a p value < 0.05 was considered as statistically significant.
Table 1.
Overview of demographic, clinical, and radiological characteristics. CSF: cerebrospinal fluid; MRI: magnetic resonance imaging; BMI: body mass index; DD: disease duration (defined as the time period from first onset of symptoms); m: male; f: female; n.d.: not done; and n.a.: not applicable.
(a).
| Subjects | Demographic and clinical characteristics of subjects with CSF leakage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Gender | BMI | Relevant comorbidities | Symptoms | Orthostatic symptoms | DD (days) | Prior traumatic event | Other events before disease onset | |
| 1 | 50 | f | 24.7 | None | Headache left frontal | Yes | 30 | No | Physical exertion |
| 2 | 40 | f | 18.4 | Marfan's syndrome, Arnold-Chiari I malformation, and depressive disorder | Occipital and temporal headache | Yes | 14 | No | No |
| 3 | 43 | m | 26 | None | Occipital headache | Yes | 10 | No | No |
| 4 | 45 | f | 23.7 | Migraine with aura | Occipital headache, pressure feeling in left ear, diplopia, and paresthesia in left arm and leg | Yes | 173 | No | No |
| 5 | 72 | f | 14.7 | Subtotal colectomy, short bowel syndrome, cachexia, chronic gastritis, and numerous intestinal operations due to recurrent ileus | Occipital headache, vertigo | Yes | 360 | No | No |
| 6 | 30 | f | 21.5 | None | Holocephalic headache | Yes | 30 | No | No |
| 7 | 44 | m | 25.2 | Arterial hypertension | Holocephalic headache, vertigo, vomiting, diplopia, and paresthesia in left arm and leg | Yes | 196 | Fenestration of a spinal arachnoid cyst T2–4 | Decompression surgery due to disc herniation at the level of C5/6 |
| 8 | 23 | f | 22.8 | None | Occipital headache, vertigo, and photosensitivity | Yes | 4 | No | No |
| 9 | 44 | f | 20.2 | None | Occipital headache, vertigo, vomiting, and abducens nerve palsy | Yes | 2 | No | No |
| 10 | 52 | f | 23.4 | Cervical conisation due to cervix carcinoma and decompression surgery due to disc herniation at the level of L5/S1 | Holocephalic headache | Protracted | 202 | No | No |
(b).
| Subjects | Radiological characteristics of subjects with CSF leakage | |||||
|---|---|---|---|---|---|---|
| Method of detection | Level of leakage | CSF scintigraphy | Early detection (in the bladder) | Meningeal enhancement (cranial MRI) | Subdural effusions (cranial MRI) | |
| 1 | Intrathecal gadolinium enhanced MR myelography | C1/2, T12 | Not detected | Yes | Yes | Yes |
| 2 | Intrathecal gadolinium enhanced MR myelography | L1/2, L2/3 | n.d. | n.a. | n.d. | Yes |
| 3 | Intrathecal gadolinium enhanced MR myelography | C7/T1, T1/2 | C7/T1 | Yes | Yes | Yes |
| 4 | Intrathecal gadolinium enhanced MR myelography | C6/7, T1/2, T11, 12, L1/2, L3/4 | L1/2, L3/4, T11/12 | Yes | No | No |
| 5 | Intrathecal gadolinium enhanced MR myelography | C5/6, C6/7 (nerve root cysts L4/5, S2/3, S3) | Not detected | Yes | Yes | Yes |
| 6 | Intrathecal gadolinium enhanced MR myelography | C1/2, C5/6, C6/7, T12/L1 | n.d. | n.a. | Yes | Yes |
| 7 | Intrathecal gadolinium enhanced MR myelography | (Weak) C4/5, T1/2 | Not detected | Yes | No | Yes |
| 8 | Intrathecal gadolinium enhanced MR myelography | C7/T1, T1/2, T2/3 | n.d. | n.a. | Yes | Yes |
| 9 | Intrathecal gadolinium enhanced MR myelography | C6/7, T1/2 | n.d. | n.a. | Yes | Yes |
| 10 | MRI of the spine | Not detected (multiple nerve root cysts, 10 mm T1/2 and 25 mm S1/2) | S1/2 | No | Yes | Yes |
(c).
| Controls | Demographic and clinical characteristics of control subjects | ||||
|---|---|---|---|---|---|
| Age (years) | Gender | BMI | Diagnosis | Relevant comorbidities | |
| 1 | 74 | f | 20.6 | Axonal polyneuropathy | Arterial hypertension, atrial fibrillation |
| 2 | 45 | m | 26.6 | Essential tremor | Alcohol dependence, recurrent depressive disorder |
| 3 | 48 | m | 27.2 | Multifocal motor neuropathy | Atopic dermatitis |
| 4 | 55 | f | 25.7 | Cervical vertigo | Disc herniation at the level of T6/7, asthma |
| 5 | 65 | m | 31.2 | Demyelinating neuropathy | Arterial hypertension, heart failure, psoriasis, gout |
| 6 | 45 | f | 42.0 | Axonal polyneuropathy | Arterial hypertension, diabetes mellitus, asthma, and anxiety disorder |
| 7 | 48 | f | 19.2 | Somatic symptom disorder | Depressive disorder, anxiety disorder |
| 8 | 31 | f | 28.7 | Axonal polyneuropathy | Borderline personality disorder |
| 9 | 24 | f | 20.5 | Somatic symptom disorder | None |
| 10 | 59 | m | 22.5 | Motor neuron disease | None |
3. Results
Demographic and clinical characteristics of the enrolled subjects with persistent CSF leakage and those of controls are summarized in Table 1. Control subjects were recruited from an inpatient population admitted to the hospital with different neurological diagnoses. They were on average five years older (49 ± standard deviation (SD) 15 years) than subjects with CSF leakage (44 ± SD 13 years) and the proportion of females outweighed that of males in both groups. In general, patients with CSF leakage had a normal or almost normal body weight (mean body mass index (BMI) 22.1 ± SD 3.5), while the mean BMI of control subjects was higher (26.4 ± 6.7). We included this parameter since it has been shown to correlate positively with the CSF to serum albumin ratio, also referred to as Qalb, and a relationship with the epidural fat storage has been suggested [15]. Considering all participating subjects, we did not find any significant correlation of BMI to Qalb (Spearman r = 0.19, p = 0.413). Exclusion of one outlier (CSF leakage subject number 5 with Qalb 48.5) did not result in a significant correlation either (Spearman r = 0.39, p = 0.096).
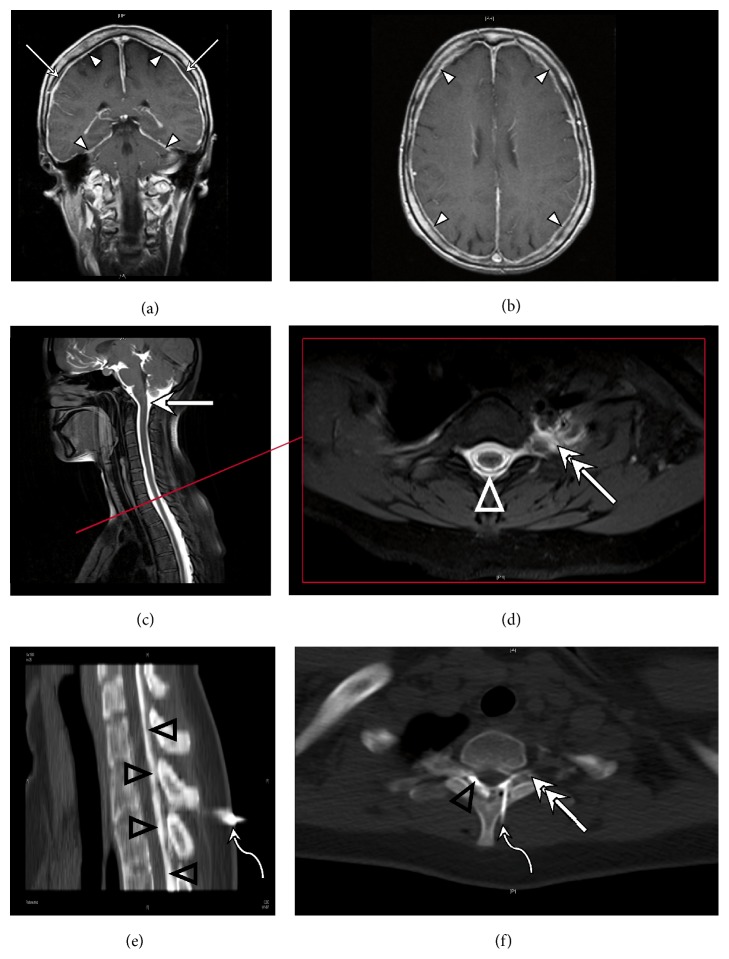
Figure 1 shows characteristic findings in cranial MRI as well as the diagnostic procedure with intrathecal gd MR myelography (Figure 1). CSF scintigraphy resulted in the localization of the level of CSF leakages in 3 of 6 subjects, while MR myelography with intrathecal gd was more efficient with 9 of 9 subjects (Table 1). The early appearance of the radioisotope in the bladder enabled the diagnosis of CSF leakage in 5 of 6 subjects. Moreover, intrathecal gd MR myelography revealed additional CSF leakages in 3 subjects who had CSF leakages already detected by scintigraphy. Cranial MRI demonstrated subdural effusions in 9 of 10 subjects, while after the application of gd contrast agent meningeal enhancement was found in 7 of 9 subjects suggesting that subdural effusions are at least as reliable sign as meningeal contrast enhancement (Table 1).
Figure 1.
Imaging in a patient with symptomatic CSF leakage. (a + b) MRI with intravenous contrast media displays enhancement of the meninges over the convexity and tentorium (arrow heads). A fluid collection separates the periosteal and the inner layer of the enhancing dura mater (arrows). (c + d) MRI with intrathecally applied gadolinium contrast agent. (c) Distribution of contrast agent in the subarachnoid space (solid arrow). (d) MRI depicts transforaminal contrast leakage as the diagnosis of meningeal tears in the left T2 nerve root (double arrow) with epidural distribution of contrast agent in the epidural space (triangle). (e + f) CT-guided blood patch. (e) Tuohy needle in the epidural space of T1/2 (curved arrows) used to inject a mixture of blood and iodine contrast agent into the epidural space (black triangles). (f) Epidural blood patch by means of a Tuohy needle (curved arrow) with epidural (triangle) and transforaminal (double arrow) distribution of the blood at the level of the T2 nerve root.
Orthostatic headache, which mostly occurred occipitally, was a constant and most reliable symptom in all CSF leakage subjects. Despite the typical orthostatic nature of headache, time from onset of complaints to diagnosis varied considerably (mean time 102 ± 123 days, expressed as disease duration in Table 1) and in only two cases a traumatic event or a trigger was identified. In one of these subjects, CSF leakage was clearly attributed to a decompression surgery due to disc herniation at the level of C5/6 and fenestration of a spinal arachnoid cyst at the level of T2 to T4. Concerning relevant comorbidities, one subject in the CSF leakage cohort had Marfan's disease as a predisposing factor for CSF leakage (Table 1).
CSF analyses revealed lymphocytic pleocytosis in 4 cases with a cell count up to 23 leukocytes per μL and CSF total protein was increased in 7 cases (normal range < 0.5 g/L). Qalb is a generally accepted measure of the blood-CSF barrier function and was increased in 7 subjects with CSF leakage [16]. In the control cohort, no subject revealed a CSF pleocytosis and the CSF total protein and Qalb were increased only in 4 and, respectively, in 3 cases. In each cohort, one subject exhibited type 2 oligoclonal bands, while in one subject of the CSF leakage cohort type 3 oligoclonal bands were detected. In none of the subjects, intrathecal immunoglobulin (Ig) G, IgA, or IgM synthesis was determined (Table 2).
Table 2.
Overview of cerebrospinal fluid (CSF) and angiopoietin-2 (Ang-2) parameters. Q: quotient, that is, CSF to serum ratio; alb.: albumin; Ig: immunoglobulin; C: control subjects; LS: subjects with CSF leakage; LP: lymphocytic pleocytosis; N: normal; ABC: artificial blood contamination; n.a.: not applicable; and n.d.: not done.
| CSF characteristics | Ang-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell count per µL |
Cytology | CSF total protein (mg/L) | Qalb (CSF/serum × 103) |
Blood-CSF barrier function | IgG synthesis |
IgA synthesis |
IgM synthesis |
Oligoclonal bands | Lactate (mmol/L) |
QAng-2 (CSF/serum × 103) |
QAng-2/Qalb | |
| Subjects | LS | |||||||||||
|
| ||||||||||||
| 1 | 15.3 | LP | 1110 | 13.2 | Impaired | No | No | No | Type II | n.d. | 106.8 | 8.1 |
| 2 | 23.0 | LP | 896 | 8.8 | Impaired | No | No | No | Type I | 1.8 | 193.0 | 21.9 |
| 3 | 15.0 | LP | 1021 | 18.7 | Impaired | No | No | No | Type III | 1.7 | 403.6 | 21.6 |
| 4 | 1.3 | N | 476 | 5.9 | Intact | No | No | No | Type I | 1.6 | 203.6 | 34.5 |
| 5 | 0.3 | ABC | 2900 | 48.5 | n.a. | No | No | No | Type I | n.d. | 46.6 | 0.96 |
| 6 | 6.7 | LP | 365 | 4.8 | Intact | No | No | No | Type I | n.d. | 803.9 | 167.5 |
| 7 | 3.0 | N | 1033 | 15.4 | Impaired | No | No | No | Type I | 1.5 | 584.4 | 38.0 |
| 8 | 0.3 | ABC | 1044 | 17 | Impaired | n.a. | n.a. | n.a. | Type I | 1.8 | 418.6 | 24.6 |
| 9 | 2.0 | N | 729 | 9.9 | Impaired | No | No | No | Type I | 2.0 | 105.9 | 10.7 |
| 10 | 3.0 | N | 562 | 9.5 | Impaired | No | No | No | Type I | 1.7 | 542.4 | 57.3 |
|
| ||||||||||||
| Controls | C | |||||||||||
|
| ||||||||||||
| 1 | 0.7 | N | 404 | 5.3 | Intact | No | No | No | Type I | 1.7 | 218.3 | 41.5 |
| 2 | 1.0 | N | 413 | 6.3 | Intact | No | No | No | Type I | 1.9 | 235.8 | 37.7 |
| 3 | 2.3 | N | 796 | 14.8 | Impaired | No | No | No | Type I | 2.0 | 222.4 | 15.1 |
| 4 | 0.3 | N | 377 | 5.4 | Intact | No | No | No | Type I | 1.4 | 633.5 | 116.5 |
| 5 | 3.3 | N | 534 | 9.2 | Impaired | No | No | No | Type II | 1.7 | 254.4 | 27.5 |
| 6 | 0.3 | N | 654 | 10.1 | Impaired | No | No | No | Type I | 2.9 | 332.5 | 32.8 |
| 7 | 1.0 | ABC | 236 | 3.5 | Intact | No | No | No | Type I | 1.8 | 175.3 | 50.4 |
| 8 | 1.3 | N | 345 | 5.7 | Intact | No | No | No | Type I | 1.5 | 243.5 | 43.1 |
| 9 | 3.3 | N | 338 | 4.4 | Intact | No | No | No | Type I | 1.6 | 443.1 | 101.4 |
| 10 | 0.3 | N | 506 | 7.2 | Intact | No | n.d. | No | Type I | 1.3 | 371.0 | 51.5 |
The mean CSF Ang-2 concentration of all subjects was 0.31 ± SD 0.15 pg/mL without any difference between control and CSF leakage subjects (control 0.27 ± SD 0.09 pg/mL versus CSF leakage subjects 0.35 ± SD 0.20 pg/mL, p = 0.272, U = 35.0; Tables 2 and 3). The mean serum Ang-2 concentration of all subjects (1.23 ± SD 0.84 pg/mL) was approximately four times higher than the mean CSF Ang-2 concentration and again we did not detect any difference between the control and CSF leakage subjects (control 1.01 ± SD 0.49 pg/mL versus CSF leakage subjects 1.46 ± SD 1.10 pg/mL, p = 0.405, U = 38.5; Tables 2 and 3). The comparison of the CSF to serum ratio multiplied by 103 of Ang-2 (expressed as QAng-2 in Tables 2 and 3) did not result in any statistical difference (control 313.0 ± SD 138.9 versus CSF leakage subjects 340.9 ± SD 249.8, p = 0.791, U = 46.0). Normalizing of QAng-2 by dividing by Qalb (QAng-2/Qalb) did not yield a statistical difference either (control 51.74 ± SD 32.19 versus CSF leakage subjects 38.52 ± SD 48.15, p = 0.105, U = 28.0; Tables 2 and 3). Moreover, there were no significant correlations of QAng-2 (Spearman r = −0.14, p = 0.565) and CSF Ang-2 concentrations (Spearman r = −0.05, p = 0.848) with Qalb. CSF IgG, IgA, and IgM concentrations and CSF albumin did not correlate with CSF Ang-2 concentrations or QAng-2 either (data not shown).
Table 3.
Summary of the statistics. CSF: cerebrospinal fluid; C: control subjects; LS: subjects with CSF leakage; Ang-2: angiopoietin-2; Q: quotient, that is, CSF to serum ratio; alb.: albumin; Ig: immunoglobulin.
| LS | C | p | |||||
|---|---|---|---|---|---|---|---|
| Median | 25% percentile | 75% percentile | Median | 25% percentile | 75% percentile | ||
| Cell count | 3.00 | 1.05 | 15.08 | 1.00 | 0.30 | 2.55 | 0.092 |
| CSF total protein (g/L) | 0.74 | 0.45 | 1.02 | 0.41 | 0.34 | 0.56 | 0.063 |
| CSF lactate (mmol/L) | 1.74 | 1.57 | 1.83 | 1.73 | 1.44 | 1.88 | 0.625 |
|
| |||||||
| Qalb | 11.55 | 8.08 | 17.43 | 5.96 | 5.04 | 9.47 | 0.028 |
| Mean CSF Ang-2 (pg/mL) | 0.33 | 0.21 | 0.43 | 0.25 | 0.22 | 0.31 | 0.272 |
| Mean serum Ang-2 (pg/mL) | 1.04 | 0.76 | 2.00 | 0.93 | 0.58 | 1.36 | 0.405 |
| QAng-2 | 0.30 | 0.11 | 0.55 | 0.25 | 0.22 | 0.39 | 0.791 |
| QAng-2/Qalb | 23.3 | 10.1 | 42.8 | 42.3 | 31.5 | 63.9 | 0.105 |
|
| |||||||
| IgG ratio | 7.90 | 4.43 | 10.64 | 2.99 | 2.44 | 6.56 | 0.036 |
| IgA ratio | 4.40 | 2.66 | 7.07 | 1.77 | 1.32 | 2.98 | 0.013 |
| IgM ratio | 1.60 | 1.00 | 2.72 | 0.48 | 0.35 | 0.72 | 0.002 |
We did not detect any difference in mean CSF lactate concentrations between the two cohorts (control 1.8 ± SD 0.5 mmol/L versus CSF leakage subjects 1.7 ± SD 0.2 mmol/L, p = 0.625, U = 29.5; Tables 2 and 3). However, we found significant negative correlations for QAng-2 (Spearman r = −0.66, p = 0.003) and QAng-2/Qalb (Spearman r = −0.49, p = 0.045) with CSF lactate concentrations, while there was no significant correlation for CSF Ang-2 with CSF lactate concentration (Spearman r = 0.17, p = 0.520). There were no significant relationships when cell count was correlated with CSF Ang-2 concentrations, QAng-2, or QAng-2/Qalb (Spearman r ranged from −0.13 to 0.33; p ranged from 0.331 to 0.876). Furthermore, we could not find any association of Ang-2 with the obtained clinical or radiologic parameters.
4. Discussion
Our rationale for initiating this study was the observation of increased Qalb in several subjects with CSF leakage that attended our clinic. Qalb is generally used to evaluate blood-CSF barrier function and was indeed more frequently increased in our CSF leakage cohort. Albumin (66–69 kDa) is the most abundant protein in CSF comprising up to 67% of total CSF proteins and exclusively originates from blood [16, 17]. To reach CSF, albumin must cross the blood-brain and blood-CSF barrier [16, 17]. However, it is speculated that albumin is not able to pass the blood-brain barrier because of the presence of tight junctions and needs to be extracted from the plasma via fenestrated endothelial cells and to enter the CSF by crossing the epithelial cells of the CP via transcellular transport [18]. There is also speculation that the crossing of the epithelial cells is a passive process and may depend on the size of the CP [17]. However, there is also the view that all molecules are able to pass the blood-brain barrier and that the extent of protein transfer depends on the molecular size-dependent diffusion [16]. Moreover, caution must be taken in interpreting the increase of Qalb regarding the integrity of the brain barriers since elevation of Qalb can also be construed as a decline in the CSF secretion rate/turnover [16].
Ang-2 forms multimeric structures composed of monomers of 55 kDa and the native form is mainly present as disulfide-linked dimers, but variable oligomeric forms may exist as well [19]. So far, it remains unknown whether the CSF Ang-2 is an extraction from plasma, secreted by ependymal cells, or both. There is increasing evidence that Ang-2 plays a role in various conditions of plasma leakage by loosening interendothelial junctions [7, 20–22]. This activity at the “vessel wall” prompted us to hypothesize that the increase of Qalb is due to higher permeability of the blood-brain barrier and may explain increased Qalb values in subjects with CSF leakage syndrome. Consequently, we examined Ang-2 concentrations in serum and CSF of subjects with clinically definite and persistent CSF leakage syndrome. We found neither any difference in the mean CSF Ang-2, QAng-2, and QAng-2/Qalb as compared to our control cohort nor any correlation/association with the obtained CSF, clinical, or radiological parameters. In our study, the Ang-2 concentrations assessed in the CSF and serum concentrations are quite low suggesting quiescent ependymal or endothelial cells. The CSF Ang-2 levels of patients who suffered from subarachnoid hemorrhage have been reported to be 2.7-fold higher than control subjects, while only slightly elevated CSF Ang-2 concentrations have been observed in hypoxemic patients [12, 13]. In hypoxemic subjects, lactate that is abundantly released upon hypoxemia and known to activate receptor tyrosine kinases Axl, Tie2, and VEGF receptor 2 may have induced the release of Ang-2 as a compensatory response to hypoxia [23].
Among other factors, biomechanical stimulation has been shown to be a reliable trigger for the release of Ang-2 [24]. This is of particular interest since VEGF, whose function is mediated in corporation with Ang-2, has been implicated in the pathogenesis of hydrocephalus [25]. Accordingly, shear stress on arterial wall due to altered CSF flow or hemodynamics is considered as a possible trigger [26]. Since CSF leakage or, as a sort of biomechanical stimulation, pressure changes due to CSF leakage apparently do not lead to an increased release of Ang-2 into the CSF, the question of how and why Qalb increases in CSF leakage syndrome arises. In this regard, it should be mentioned that subjects with CSF leakage syndrome have been shown to exhibit a lower CSF flow as demonstrated by phase contrast-MRI [27]. We suggest that this is most probably the cause for the increase in Qalb which is supported by the fact that the concentrations of IgG, IgA, and IgM show that the molecular size-related selectivity of the barrier function is maintained in spite of blood-CSF barrier dysfunction (data not shown). Thus, the entry of plasma proteins via the leakage in CSF leakage syndrome seems rather unlikely. It should be mentioned that all CSF leakage subjects received an epidural blood patch treatment and intrathecal gd MR myelography provided a successful targeted approach in 9 of these subjects. The technique of intrathecal gd MR myelography is only available in specialized centers, while the epidural blood patch treatment is more common and mostly done in a nontargeted manner. It remains elusive whether Qalb can be used as a marker for successful nontargeted blood patching. Certainly, a constraining factor in this context is that CSF analyses are increasingly omitted since lumbar punctures are considered as a potential new source for a leakage. Further studies which will include CSF analyses and phase contrast-MRI before and after epidural blood patch treatment may shed light on the origin of the observed CSF changes in CSF leakage syndrome and whether Qalb can be used as a treatment marker.
Ang-2 and related molecules are of high interest for the CSF compartment and may help to elucidate our understanding of the mechanism of the brain barriers and CSF hemodynamics. In this pilot study, we did not find any involvement of Ang-2 in CSF leakage syndrome. There are several limitations inherent in this study. One limitation concerns the exploratory analysis of correlations which are post hoc exploratory analyses that need validation from independent studies. The second limitation is that the control group consisted of subjects who were not healthy. Therefore, a difference in CSF Ang-2 levels between healthy controls and subjects with CSF leakage syndrome cannot be excluded. The control group was deliberately chosen in order to find out whether other conditions, which may lead to an increase of Qalb, will be also accompanied by changes in CSF Ang-2 levels. Unexpectedly, we did not find any difference in CSF Ang-2 levels despite the significantly higher Qalb values and ratios of immunoglobulins in subjects with CSF leakage syndrome suggesting that secretion and elimination of Ang-2 are different from these proteins. Further limitations of the study are the small number of enrolled patients and that we assessed only a single marker of endothelial activation.
Conflict of Interests
All authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Corinna Trebst and Frank Donnerstag contributed equally to this paper.
References
- 1.Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache. 2013;53(7):1034–1053. doi: 10.1111/head.12149. [DOI] [PubMed] [Google Scholar]
- 2.Schievink W. I., Schwartz M. S., Maya M. M., Moser F. G., Rozen T. D. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. Journal of Neurosurgery. 2012;116(4):749–754. doi: 10.3171/2011.12.jns111474. [DOI] [PubMed] [Google Scholar]
- 3.Wiesemann E., Berding G., Goetz F., Windhagen A. Spontaneous intracranial hypotension: correlation of imaging findings with clinical features. European Neurology. 2006;56(4):204–210. doi: 10.1159/000096487. [DOI] [PubMed] [Google Scholar]
- 4.Wolburg H., Paulus W. Choroid plexus: biology and pathology. Acta Neuropathologica. 2010;119(1):75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 5.Esser S., Wolburg K., Wolburg H., Breier G., Kurzchalia T., Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. The Journal of Cell Biology. 1998;140(4):947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts W. G., Palade G. E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. Journal of Cell Science. 1995;108(part 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 7.Parikh S. M., Mammoto T., Schultz A., et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Medicine. 2006;3(3):356–370. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nico B., Mangieri D., Corsi P., et al. Vascular endothelial growth factor-A, vascular endothelial growth factor receptor-2 and angiopoietin-2 expression in the mouse choroid plexuses. Brain Research. 2004;1013(2):256–259. doi: 10.1016/j.brainres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Scala G., Maruccio L. Angiogenesis of buffalo choroid plexuses: structural and immunocytochemical study. Microscopy Research and Technique. 2012;75(8):1104–1112. doi: 10.1002/jemt.22037. [DOI] [PubMed] [Google Scholar]
- 10.Nourhaghighi N., Teichert-Kuliszewska K., Davis J., Stewart D. J., Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Laboratory Investigation. 2003;83(8):1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt S., Patkar S., Ogunshola O. O. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. British Journal of Pharmacology. 2014;171(5):1210–1230. doi: 10.1111/bph.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreau C., Gosset P., Brunaud-Danel V., et al. CSF profiles of angiogenic and inflammatory factors depend on the respiratory status of ALS patients. Amyotrophic Lateral Sclerosis. 2009;10(3):175–181. doi: 10.1080/17482960802651725. [DOI] [PubMed] [Google Scholar]
- 13.Chittiboina P., Ganta V., Monceaux C. P., Scott L. K., Nanda A., Alexander J. S. Angiopoietins as promising biomarkers and potential therapeutic targets in brain injury. Pathophysiology. 2013;20(1):15–21. doi: 10.1016/j.pathophys.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Skripuletz T., Schwenkenbecher P., Pars K., et al. Importance of follow-up cerebrospinal fluid analysis in cryptococcal meningoencephalitis. Disease Markers. 2014;2014:10. doi: 10.1155/2014/162576.162576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyfert S., Kunzmann V., Schwertfeger N., Koch H. C., Faulstich A. Determinants of lumbar CSF protein concentration. Journal of Neurology. 2002;249(8):1021–1026. doi: 10.1007/s00415-002-0777-2. [DOI] [PubMed] [Google Scholar]
- 16.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restorative Neurology and Neuroscience. 2003;21(3-4):79–96. [PubMed] [Google Scholar]
- 17.Chen R.-L., Chen C. P.-C., Preston J. E. Elevation of CSF albumin in old sheep: relations to CSF turnover and albumin extraction at blood-CSF barrier. Journal of Neurochemistry. 2010;113(5):1230–1239. doi: 10.1111/j.1471-4159.2010.06689.x. [DOI] [PubMed] [Google Scholar]
- 18.Balslev Y., Dziegielewska K. M., Møllgård K., Saunders N. R. Intercellular barriers to and transcellular transfer of albumin in the fetal sheep brain. Anatomy and Embryology. 1997;195(3):229–236. doi: 10.1007/s004290050042. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.-T., Choi H.-H., Steinmetz M. O., et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. The Journal of Biological Chemistry. 2005;280(20):20126–20131. doi: 10.1074/jbc.m500292200. [DOI] [PubMed] [Google Scholar]
- 20.Benest A. V., Kruse K., Savant S., et al. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070459.e70459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Weg C. A. M., Pannuti C. S., van den Ham H.-J., et al. Serum angiopoietin-2 and soluble VEGF receptor 2 are surrogate markers for plasma leakage in patients with acute dengue virus infection. Journal of Clinical Virology. 2014;60(4):328–335. doi: 10.1016/j.jcv.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Roviezzo F., Tsigkos S., Kotanidou A., et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. The Journal of Pharmacology and Experimental Therapeutics. 2005;314(2):738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 23.Ruan G.-X., Kazlauskas A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/AKT and promote angiogenesis. The Journal of Biological Chemistry. 2013;288(29):21161–21172. doi: 10.1074/jbc.m113.474619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goettsch W., Gryczka C., Korff T., et al. Flow-dependent regulation of angiopoietin-2. Journal of Cellular Physiology. 2008;214(2):491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 25.Shim J. W., Sandlund J., Han C. H., et al. VEGF, which is elevated in the CSF of patients with hydrocephalus, causes ventriculomegaly and ependymal changes in rats. Experimental Neurology. 2013;247:703–709. doi: 10.1016/j.expneurol.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Shay-Salit A., Shushy M., Wolfovitz E., et al. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(14):9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tung H., Liao Y.-C., Wu C.-C., et al. Usefulness of phase-contrast magnetic resonance imaging for diagnosis and treatment evaluation in patients with SIH. Cephalalgia. 2014;34(8):584–593. doi: 10.1177/0333102413519513. [DOI] [PubMed] [Google Scholar]