Abstract
The exposure to ultraviolet radiations (UVR) is the key source of skin sunburn; it may produce harmful entities, reactive oxygen species (ROS), leading to aging. The skin can be treated and protected from the injurious effects of ROS by using various pharmaceutical formulations, such as cream. Cream can be loaded with antioxidants to quench ROS leading to photo-protective effects. Moreover, modern medicines depend on ethnobotanicals for protection or treatment of human diseases. This review article summarizes various in vivo antioxidant studies on herbal creams loaded with phyto-extracts. These formulations may serve as cosmeceuticals to protect skin against injurious effects of UVR. The botanicals studied for dermatologic use in cream form include Acacia nilotica, Benincasa hispida, Calendula officinalis, Camellia sinensis, Camellia sinensis, Nelumbo nucifera, Capparis decidua, Castanea sativa, Coffea arabica, Crocus sativus, Emblica officinalis Gaertn, Foeniculum vulgare, Hippophae rhamnoides, Lithospermum erythrorhizon, Malus domestica, Matricaria chamomilla L., Moringa oleifera, Morus alba, Ocimum basilicum, Oryza sativa, Polygonum minus, Punica granatum, Silybum marianum, Tagetes erecta Linn., Terminalia chebula, Trigonella foenum-graecum, and Vitis vinifera. The observed anti-aging effects of cream formulations could be an outcome of a coordinating action of multiple constituents. Of numerous botanicals, the phenolic acids and flavonoids appear effective against UVR-induced damage; however the evidence-based studies for their anti-aging effects are still needed.
1. Etiologies and Types of Human Skin Aging
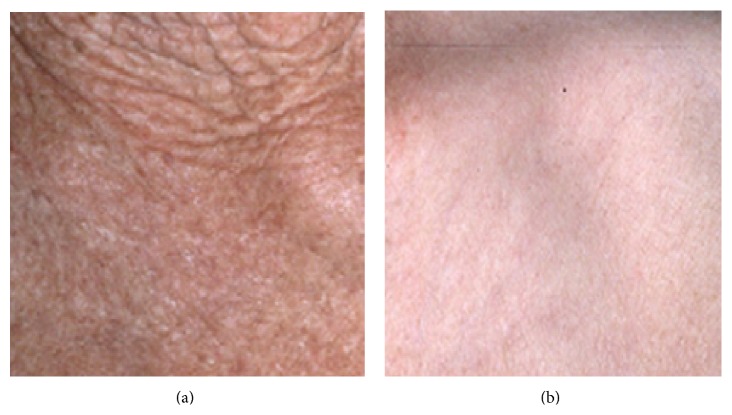
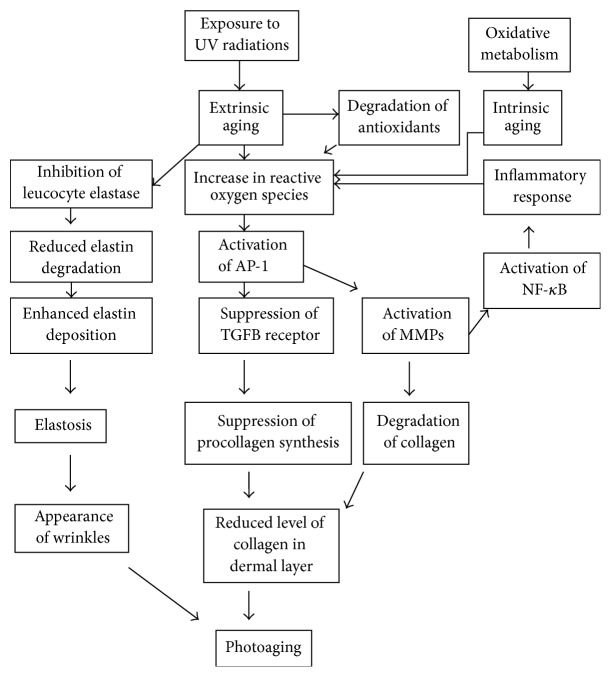
Skin aging is a dermatologic change that progresses as a person ages or is exposed to ultraviolet radiations (UVR) if no treatment is adopted. The extensive research activities are focused on this skin concern that involves the appearance of unpleasant, observable marks on skin surface due to proteolysis of cutaneous elastic fibers resulting in the reduced cell functions [1]. Skin aging can be divided into two types, that is, intrinsic aging or chronological aging (inevitable phenomenon) and extrinsic or premature or photoaging (evitable phenomenon) owing to the physiological and environmental factors, respectively [2–4]. Morphologically, photoaging is characterized by dry, rough, pigmented, and abraded skin especially of face and hands in individuals who live in sunny geographical regions and are chronically exposed to direct sunlight (Figure 1). Conversely, fine, smooth wrinkles on dry, pale skin impart the characteristics of intrinsic aging [1]. Diagnostically, intrinsic skin aging is identified by seborrheic keratosis which is not a biomarker of photoaging [5]. Pathologically, the photodamaged skin shows vascular damage that is absent in intrinsically aged skin. An increased skin vascularization and angiogenesis are observed in the photoaged skin [6]. Microscopically, thicker epidermis is another feature of the photoaged skin [4]. It is noteworthy that the strength and resiliency of skin depend on proper and uniform arrangement of collagen (types I and III) fibrils and elastin in the dermis [7]; thus, collagen deficiency may result in skin aging due to the production of collagenase and thymine dimer in skin on exposure to UVR. Histologically, the extracellular matrix of intrinsically aged skin possesses diminished levels of elastin [2], while the elastin amassing in the photoaged skin is observed just below the dermal-epidermal junction [8]. Elastin is a fibrous protein that is reduced in thickness from deeper to superficial dermis. It provides natural elasticity and strength to human body. It also plays a role in tissue repair [9]. The basic and the major molecular unit involved in the construction of human skin is collagen that is produced from procollagen. Collagen is a protein that is present in the connective tissues of human body. The dermal fibroblasts generate the procollagen under the effect of transforming growth factor-β (TGF-β) and activator protein-1 (AP-1), where TGF-β and AP-1 govern the production and breakdown of collagen, respectively. Under the effect of UVR received from sun, the upregulation of matrix metalloproteinases (MMPs) enzymes secreted by keratinocytes, fibroblasts, and other cells promotes breakdown of collagen by AP-1 as well as decrease in collagen synthesis (Figure 1) [10, 11]. It results in breakdown of the connective tissues during photoaging [12–14]. During adulthood, there is about 1% decrease in collagen content per year, but this rate is higher in the aged people since old age people have higher levels of MMP [7].
Figure 1.
Clinical appearance of extrinsic (a) and intrinsic (b) aging of skin.
2. Reactive Oxygen Species and Photoaging
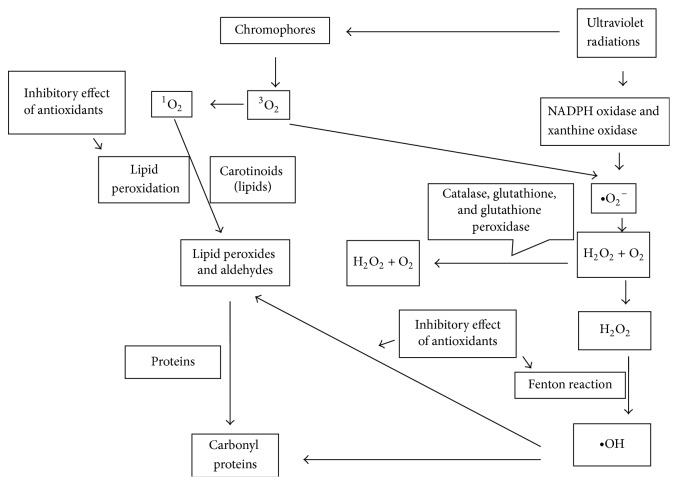
The exposure to UVR is the main cause of oxidative stress in the skin and thus is an important risk factor for development of skin problems, for example, wrinkle formation, lesions, and cancer. On exposure to sunlight, skin molecules absorb UVR resulting in the generation of reactive oxygen species (ROS). There are two types of ROS: type 1 consists of a single, excited oxygen molecule (1O2) (Figure 3), while oxygen molecules with unpaired electron constitute second type of ROS. The examples of second type are presented in Table 1 that also describes the enzymes which are involved in the generation of these ROS [16]. Reactive oxygen entities exert a damaging effect on cellular fractions including cell walls, lipid membranes, mitochondria, nucleus, and DNA producing “oxidative stress,” that is, a difference between ROS and antioxidants, ROS being in excess leading to tissue injury and development of disease including aging, cancer, ischemia, liver injury, arthritis, and Parkinson's syndrome (Figure 2).
Figure 3.
Production of ROS and its role in the initiation of oxidative chain reactions and target sites for antioxidant action.
Table 1.
Enzymes involved in the generation of ROS with an unpaired electron.
Figure 2.
Mechanism of aging.
3. Benefits and Types of Antioxidants
The oxidative stress-mediated development of diseases is manageable by prolonged usage of the safe antioxidants [17]. The literature study reveals that numerous compounds have been investigated with the intention of exploring evidence against ROS-induced damage and noted their antiaging effect on skin. These compounds are efficient for overcoming sunlight-induced skin problems and making it fresh, healthy, and young through collagen synthesis [18]. Generally, the antioxidants behave as antiaging compounds in action because they are capable of scavenging ROS leaving healthy effect on skin. Since living systems have capability to maintain homeostasis of ROS in cell, the human skin is protected from UVR through complex antioxidant defense system comprising of two types of antioxidants, that is, endogenous and exogenous (consumed) antioxidants. The former category constitutes a network of protective antioxidants in skin; it includes melanin and some enzymes. Manganese-superoxide dismutase is a mitochondrial enzyme that destroys the superoxide ions produced by respiratory chain activity [19]. In general, expression of antioxidant enzymes is found very high in the epidermal layer compared to that of stratum corneum and dermis. If there is imbalance between oxidants and endogenous antioxidants, exogenous antioxidants are helpful to restore the balance. The exogenous antioxidants comprise of compounds that cannot be synthesized by human body. Vitamins, ascorbate, carotenoids, and polyphenols constitute latter type of antioxidants which are also involved in the maintenance of oxidative homeostasis [20]. The endogenous antioxidants in dermal and epidermal layers of skin exposed to sunlight are depleted under the effect of elevated levels of UVR-generated ROS. Such depletion results in the diminished activity of these antioxidants leading to skin damage [21]. With age, endogenous antioxidants are steadily consumed increasing the risk of oxidative stress; then the use of exogenous antioxidants as prevention strategy is essential. It is evident from the above discussion that skin cells are damaged by oxidative stress which might be decreased by action of the antioxidants.
4. Exogenous Antioxidants
The exogenous antioxidants include synthetic and natural compounds. The synthetic exogenous antioxidants include monoethanolamine, diethanolamine, sodium laureth sulfate, and triethanolamine, but these compounds have undesired effects including allergic and irritant contact dermatitis and contact dermatitis [19]. On the other hand, natural exogenous antioxidants are nontoxic in nature and produce no unwanted effect on skin.
5. Phytoantioxidants
The phyto-kingdom includes vegetables, fruits, whole grains, and beverages, for example, tea, chocolate, and wine. These products are rich in natural antioxidants. An important class of natural exogenous antioxidants is phytoantioxidants, that is, antioxidants found in plants [21]. Phytoantioxidants include terpenes or polyphenols (Figure 4). After synthesis in plants, these compounds are found to have important role in the metabolism and defense system of plants. Terpenes are known to have potential for managing the oxidative stress through their free radical scavenging potential. Moreover, polyphenols occur in all parts (roots to leaves) of the plants and protect them from environmental stress through their free radical scavenging property. There are various types (>8000 phenolic structures) of polyphenols on the basis of molecular weight and polarity [64]. The structural formula of polyphenols contains phenol group(s), that is, benzene ring possessing hydroxyl group. The antioxidant activity of various polyphenols depends on number and position of phenol groups [65].
Figure 4.
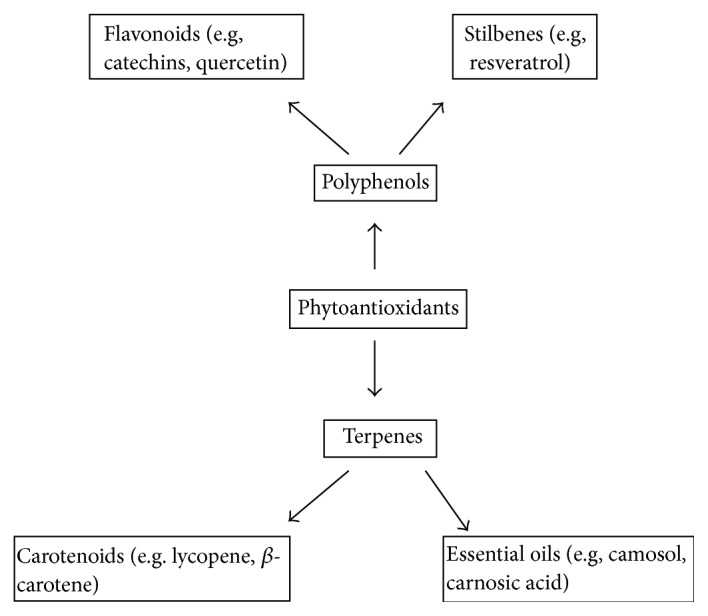
Classification of phytoantioxidants [15].
6. Stratum Corneum as Target Site for Antioxidants
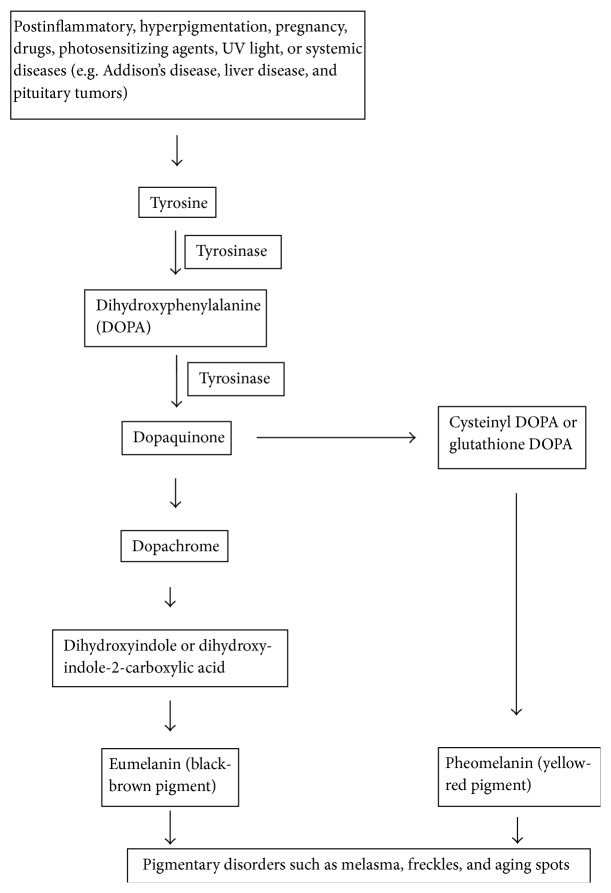
The normal human skin maintains homeostasis of water and other materials in body, principally due to the presence of stratum corneum [66]. The stratum corneum, a water barrier in function, consists primarily of lipids, that is, ceramides, cholesterol, free fatty acids, triglycerides, stearyl esters, and cholesterol sulfate. The cholesterol sulfate is responsible for intercellular adhesion, and its high concentration is known to inhibit desquamation. The synthesis of these lipids is affected by many factors, mainly related to enzymes, fatty acids, environment, cosmetics, and water contents. Other important constituents of stratum corneum are proteins (e.g., involucrin and loricrin), enzymes, and water (approximately 30%) [67]. Depending upon natural moisturizing factor of skin cells, some fraction of this water is tightly held in stratum corneum and is responsible for skin elasticity. The disturbance in level or nature of any of lipids, proteins, enzymes, and water might lead to skin problems including wrinkled and dry skin. Dry skin might be due to excessive transepithelial water loss that could be retained for maintaining the proper skin hydration by using skin moisturizer. It might exercise softening effect on skin. The skin moisturizer, however, should be inert, nonirritant, stable, and sterile [68]. On the other hand, skin wrinkles might be due to distorted elastic fibers, diminished collagen contents, and uneven types I and III collagen. There is decrease in type IV collagen protein at the wrinkle's base; it could be due to activation of MMPs, the collagen-degrading enzymes. Alternatively, the activation of MMPs may lead to upregulation of collagenase, gelatinase, and stromelysin [69]. Thus, the skin wrinkles could be treated by using topical formulations loaded with the bioactive compounds having potential of inhibiting MMPs, thus increasing the collagen level. Moreover, skin colour depends on the kind and allocation of melanin in the skin, in addition to number and amount of melanocytes [67, 70]. Figure 5 shows the melanin synthesis involving tyrosinase and a series of oxidative reactions that could be inhibited by usage of skin-whitening agents. Thus, the stratum corneum is a primary target site for topical phytoantioxidants for skin protection against UVR-mediated oxidative stress [71]. The phytoantioxidants might have capability of stimulating the regeneration of stratum corneum to protect itself and the underlying epidermis and dermis from the injurious effects of UVR and promote growth of the skin [72, 73].
Figure 5.
Melanin synthesis involving tyrosinase and a series of oxidative reactions.
7. Skin Care Products
The pharmaceutical formulations used for skin care, termed as the cosmetics, could be herbal in nature. The herbal cosmetics might contain the isolated bioactive compounds or the crude phytoextracts [74]. Currently, there are extensive research activities in progress involving development and characterization of extract loaded formulations to concurrently achieve various goals such as anti-inflammatory and antiaging effect [75]. There are three types of bioactive compounds present in various phytoextracts; the compounds include polyphenols, flavonoids, and carotenoids. These compounds exert both the antioxidant and the UV protection effect [76].
8. Pharmaceutical Creams
Skin care products could be solid, semisolid, or liquid. The semisolid formulations include creams, ointments, and pastes. Cream is an emulsion of oil and water, prepared for skin applications [77]. Emulsions represent a class of disperse systems which comprise of two insoluble, thermodynamically stable phases, that is, continuous and dispersed phase [78]. The emulsion is water-in-oil if the dispersed phase is oil and vice versa. This type of emulsion is termed as simple emulsion. If simple emulsion is further dispersed in the dispersed phase medium, such type of system is termed as the multiple emulsion. Based on globule size of dispersed phase, emulsions can be grouped into various classes including macro- and microemulsion [79]. Emulsions constitute an exclusive class of cosmetics that produce a pleasant feeling to skin on application, acceptable for long-term use, improved spreadability of the ingredients, and remain stable during long storage period [80]. Owing to these characteristics, emulsions are extensively used as a vehicle in drug delivery, particularly across the skin. In particular, for dry skin, water-in-oil (W/O) emulsions are more broadly used for the treatment of dermatological concerns [81]. The addition of antioxidants as active ingredients endows these emulsions with features of cosmetics. For improved cosmetic features, the botanical extracts can be added to the cosmetic creams since the extracts comprise of a number of antioxidants that might produce synergistic effect [82].
9. Preparation and Characterization of Phytoextract Loaded Creams
The creams are topically used to protect and treat the skin problems including hyperdepigmentation and wrinkles. Beside these advantages, the creams may produce skin problems such as infection, photosensitivity, erythema, contact dermatitis, cancer, and/or change in skin colour. During the development of such antiaging creams, the researchers should be more focused on the elucidated sources, structures, and interactive modes of the composite active constituents with the skin to achieve maximum formulation efficacy and skin safety [83].
The preparation of herbal creams may involve the modified methodology using isolated phytochemicals or the extracts along with appropriate composition of the mandatory constituents essentially employed for creams with desirable features [84]. Due to technical sophistication of cream development, it is accentuated that the phytochemicals maintain their bioactivity during extreme processing. To ensure effective and stable formulations, exhaustive analytical strategies are adopted. Various physicochemical characterization parameters include stability, pH, and viscosity testing [85]. Table 3 shows various types of equipment used for in vivo characterization of botanical creams.
Table 3.
Equipment used for in vivo characterization of botanical creams.
| Number | Equipment | Purpose of use |
|---|---|---|
| 1 | Mexameter | Erythema analysis |
| 2 | Tewameter | Transepidermal water loss (TEWL) evaluation |
| 3 | Corneometer | Detection of skin hydration |
| 4 | Evaporimeter | Barrier function test |
| 5 | Sebumeter | Assessment of skin surface sebum/lipid contents |
| 6 | Visiometer | Wrinkle test |
| 7 | Cutometer | Measurement of skin mechanical properties/elasticity |
| 8 | Chromameter | Skin colour test |
10. Phytoextract Loaded Creams
Due to the presence of numerous bioactive ingredients in phytoextracts, extract loaded creams are considered more efficacious with lesser side effects against aging in comparison to creams loaded with specific individual antioxidant. Owing to tremendous antioxidant potential, phytoextracts are extensively used in numerous cream formulations. Up to now, Acacia nilotica, Benincasa hispida, Calendula officinalis, Camellia sinensis, Nelumbo nucifera, Capparis decidua, Castanea sativa, Coffea arabica, Crocus sativus, Emblica officinalis Gaertn, Foeniculum vulgare, Hippophae rhamnoides, Lithospermum erythrorhizon, Malus domestica, Matricaria chamomilla L., Moringa oleifera, Morus alba, Ocimum basilicum, Oryza sativa, Polygonum minus, Punica granatum, Silybum marianum, Tagetes erecta Linn., Terminalia chebula, Trigonella foenum-graecum, and Vitis vinifera have successfully been used in developing the stable cream formulations with excellent antioxidant effect, possibly due to presence of multiple antioxidant phytochemicals. In this review article, the documented phytoextract loaded creams with their characterization on human skin have been discussed with special emphasis on their bioactive constituents (Table 2).
Table 2.
Phytoextracts loaded to creams with antioxidant features studied in human.
| Botanical name | Family | Part used | Nature of extracts | Antioxidants† | Nature of cream | Oil phase | Emulsifier | References |
|---|---|---|---|---|---|---|---|---|
|
Acacia
nilotica |
Mimosaceae | Bark | Ethanol | Phlobatannin, pyrocatechol, (+)-catechin, protocatechuic acid, (−)-epigallocatechin-7-gallate, and (−)-epigallocatechin-5, 7-digallate | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [27] |
|
| ||||||||
| Benincasa hispida | Cucurbitaceae | Fruit | Petroleum ether | Caffeic acid | Simple W/O cream†† | Cetyl alcohol | Polysorbate | [28] |
|
| ||||||||
|
Calendula
officinalis |
Compositae | Flowers | Ethanol | Isorhamnetin, quercetin, myricetin, and kaempferol | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [29, 30] |
|
| ||||||||
| Camellia sinensis | Theaceae | Leaves | Ethanol | Epigallocatechin gallate | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [31, 32] |
|
| ||||||||
| Camellia sinensis (green tea) and Nelumbo nucifera (lotus) | Camellia sinensis (Theaceae): Nelumbo nucifera (Nelumbonaceae) | Leaves of Camellia sinensis: whole plant material of Nelumbo nucifera | Ethanol for Camellia sinensis; methanol for Nelumbo nucifera | Epigallocatechin gallate in green tea; hyperin, isoquercetin, and astragalin in lotus | W/O/W nano-multiple-emulsions | Paraffin oil | ABIL EM 90, polyoxyethylene (20) cetyl ether, Cetomacrogol 1000 | [33–35] |
|
| ||||||||
| Capparis decidua | Capparidaceae | Full plant | Methanol | Isoginkgetin and ginkgetin | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [36] |
|
| ||||||||
| Castanea sativa | Fagaceae | Leaves | Ethanol | Catechin and myricetin derivatives | Simple W/O cream†† | Surfactant-free formulation | [37] | |
|
| ||||||||
|
Coffea
arabica |
Rubiaceae | Berry | Ethanol | Chlorogenic acid, condensed proanthocyanidins, quinic acid, and ferulic acid | Simple cream†† | Information not available | Information not available | [38] |
|
| ||||||||
| Crocus sativus | Iridaceae | Flowers | Ethanol | Zeaxanthin, lycopene, carotenes, crocetin, and picrocrocin | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [39] |
|
| ||||||||
| Emblica officinalis Gaertn | Euphorbiaceae | Fruit | Hydroalcoholic | Emblicanin A, emblicanin B, punigluconin, and pedunculagin | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [40] |
|
| ||||||||
| Foeniculum vulgare | Apiaceae | Seeds | Ethanol | Gallic acid, caffeic acid, ellagic acid, quercetin, and kaempferol | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [41, 42] |
|
| ||||||||
|
Hippophae
rhamnoides |
Elaeagnaceae | Fruit | Hydroalcoholic | Isorhamnetin, quercetin, myricetin, and kaempferol | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [43] |
|
| ||||||||
| Lithospermum erythrorhizon | Boraginaceae | Root | Ethanol | Naphthoquinone (shikonin, acetylshikonin, deoxyshikonin, b-acetoxyisovalerylshikonin, isobutylshikonin, b,b-dimethyl acrylshikonin, 2-methyl-n-butyrylshikonin, and isovalerylshikonin) | Simple O/W cream†† | Cyclomethicone, caprylic/capric triglyceride, phytosphingosine (0.005%), and cholesterol | Sodium lauroyl lactylate | [44] |
|
| ||||||||
| Malus domestica | Rosaceae | Fruit | Methanol : formic acid : double distilled water (70 : 2 : 28) |
Hesperetin | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [45, 46] |
|
| ||||||||
| Matricaria chamomilla L. | Asteraceae | While plant | Hydroalcoholic | α-Bisabolol and apigenin | Simple W/O cream†† | Cetyl alcohol | Sodium lauroyl lactylate | [47] |
|
| ||||||||
| Moringa oleifera | Moringaceae | Leaves | Hydroalcoholic | Epigallocatechin gallate, myricetin, quercetin, rutin, morin, taxifolin, chrysin, baicalein, fisetin, biochanin A, genistein, kaempferol, emodin anthraquinone, caffeic acid phenethyl ester, and octyl and dodecyl gallates | Simple O/W cream†† | Paraffin oil | ABIL EM 90 | [48–50] |
|
| ||||||||
| Morus alba | Moraceae | Fruit | Hydroalcoholic | Rutin, quercetin, isoquercitrin, and quercetin | Simple O/W cream†† | Paraffin oil | ABIL EM 90 | [51, 52] |
|
| ||||||||
| Ocimum basilicum | Lamiaceae | Seeds | Ethanol | Quercetin, isoquercetin, kaempferol, caffeic acid, rosmarinic acid, rutin, catechin, ferulic acid, rutinoside, and apigenin | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [53] |
|
| ||||||||
| Oryza sativa | Poaceae | Grains | Ethanol | Gallic acid, pyrogallol, apigenin, and rutin | Niosomes loaded cream | Information not available | Information not available | [54] |
|
| ||||||||
| Polygonum minus | Polygonaceae | Leaves | Aqueous | Caffeic acid and quercetin | Simple W/O cream†† | Isoparaffin | Laureth-7 | [55] |
|
| ||||||||
| Punica granatum | Punicaceae | Seeds | Ethanol | Ellagic acid | Nanotransfersomes loaded cream | Cetyl alcohol |
Span 60 and tween 80 | [56] |
|
| ||||||||
| Silybum marianum | Asteraceae | Seeds | Ethanol | Silymarin (silybin, silydianin, and silychristin) | W/O emulsion cream | Paraffin oil | ABIL EM 90 | [57, 58] |
|
| ||||||||
|
Tagetes erecta
Linn. |
Asteraceae | Flowers | Ethyl acetate | Lutein | Nanostructured lipid carrier loaded cream | Glyceryl monostearate, stearic acid, octyldodecanol, and mineral oil | Tween, span, or triethanolamine stearate | [59] |
|
| ||||||||
| Terminalia chebula | Combretaceae | Seeds | Methanol | Gallic acid, ellagic acid, tannic acid, ethyl gallate, chebulic acid, chebulagic acid, corilagin, and ascorbic acid | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [60] |
|
| ||||||||
| Trigonella foenum-graecum | Fabaceae | Seeds | Methanol | Kaempferol derivatives such as 3-O- -D-glucosyl(1 2) - -D-galactoside | Simple W/O cream†† | Paraffin oil | ABIL EM 90 | [61, 62] |
|
| ||||||||
| Vitis vinifera | Vitaceae | Shoot | Ethanol | Resveratrol, delphinidin, peonidin, petunidin, malvidin, and (+)-catechin | Simple W/O cream†† | Cetyl alcohol |
Span 60 | [63] |
†Including but not limited to.
††Simple cream is the cream that is not loaded with some novel carrier system such as nanotransfersomes.
10.1. Acacia nilotica
Acacia nilotica (Mimosaceae) contains tannins, gallic acid, phlobatannin, pyrocatechol, (+)-catechin, protocatechuic acid, (−)-epigallocatechin-7-gallate, and (−)-epigallocatechin-5, 7-digallate. All parts of this plant, from root to flowers, possess various medicinal activities such as antidiabetic, antiasthmatic, and anticancer [86]. Ali et al. prepared water-in-oil cream of bark extract of Acacia nilotica and applied it to photoaged skin [27]. They found improved mechanical features of skin, that is, reduced levels of roughness, scaliness, smoothness, and wrinkles of photoaged skin. The investigators attributed this antiaging activity to the phenolic compounds present in extract; the phenolics have capability to quench ROS.
10.2. Benincasa hispida
The major constituents of Benincasa hispida (Cucurbitaceae) are triterpenoids, flavonoids, glycosides, saccharides, carotenes, vitamins, β-sitosterin, and uronic acid [87]. The fruit of this plant is effective for different diseases including cardiac disease, diabetes, inflammation, and cancer [88]. The water-in-oil cream of fruit extract of Benincasa hispida showed antioxidant activity revealing its potential to retard symptoms of aging [28].
10.3. Calendula officinalis
Calendula officinalis belongs to family Compositae. This plant is rich in active compounds including terpenoids, carotenoids, flavonoids, and volatile oils [89]. The water-in-oil cream of flower extract of Calendula officinalis exhibited aptitude of stimulating skin tightness and improved skin elasticity leading to delayed aging process. Moreover, this preparation enhances the skin hydration level, as evident from reduced TEWL values, which is crucial for normal cutaneous metabolism; it prevents early aging [29]. In addition, the reduction in skin melanin contents and the decrease in skin sebum level were also observed after application of this formulation [30].
10.4. Camellia sinensis (Green Tea) and Nelumbo nucifera (Lotus)
Potent antioxidants have been isolated from both green tea and lotus. Green tea is rich in a polyphenol, catechins, especially EGCG which is a strong antioxidant [90]. The water-in-oil cream loaded with ethanolic leaf extract of Camellia sinensis significantly reduced the sebum production as compared to base formulation (formulation without extract) [31]. In another study, Mahmood et al. reported reduced TEWL values and thus improved skin hydration level using this formulation [32]. Furthermore, the W/O/W nano-multiple emulsion containing alcoholic extracts of Camellia sinensis (Theaceae) leaves and Nelumbo nucifera (Nelumbonaceae) plant, alone or in combination, was formulated and tested on human skin. Both extracts were found to have diminished sebum secretions for mono (green tea or lotus) and combined treatments (green tea plus lotus) after applying same formulations to volunteers' skin. Green tea plus lotus together produced statistically better antisebum effect [33]. It indicates that active ingredients in lotus add a synergistic effect to the activity of green tea that can be attributed to 5α-reductase inhibition activity of both extracts [91]. Zinc compounds, polyphenolics, flavonoids, tannic acid, and linoleic acid are excessively found in lotus [92]. Linoleic acid, a polyunsaturated fatty acid, and EGCG have an advantage of inhibiting sebum production due to their 5α-reductase inhibition activity [93]. In another study, the researchers noted the significant effectiveness of nano-multiple emulsion against skin wrinkles, roughness, and scaliness leading to skin revitalization effect. Green tea and lotus combined in multiple emulsions exerted a superior synergistic antiaging effect [34]. In addition, Mahmood and Akhtar also reported that this formulation had an advantage of reducing melanin and improving hydration contents of skin without causing erythema [35].
10.5. Capparis decidua
Capparis decidua belongs to family Capparidaceae [94]. This plant is rich in active compounds including isothiocyanate glucoside, glucocapparin, stachydrine, n-triacontane, β-carotene, and β-sitosterol [95]. The water-in-oil emulsion cream of plant extract of Capparis decidua exhibited reduction in skin sebum level after application on skin [36].
10.6. Castanea sativa
Castanea sativa (Fagaceae) contains catechin, myricetin 3-O-glucoside, quercetin 3-O-rutinoside, quercetin 3-O-glucoside, kaempferol 3-O-rutinoside, and kaempferol 3-O-glucoside. The surfactant-free cream of ethanolic extract of Castanea sativa leaves has been found to produce moisturizing effect on human skin by controlling transepithelial water loss [37].
10.7. Coffea arabica
Coffea arabica belongs to family Rubiaceae. This coffee plant is rich in active compounds including chlorogenic acid, condensed proanthocyanidins, quinic acid, and ferulic acid [96]. All of these polyphenolic compounds possess antioxidant property [97]. McDaniel prepared cream of berry extract of Coffea arabica and found reduced levels of MMP-1 and IL-1b [38]. Moreover, upregulated gene expression for four collagen structural proteins and downregulated gene expression for three MMPs were also observed concluding reparative effects of Coffea arabica extract upon photoaged skin.
10.8. Crocus sativus
Water-in-oil emulsion based cream loaded with ethanolic extract of Crocus sativus flowers has been found to produce moisturizing effect on human skin by controlling transepithelial water loss [39]. Crocus sativus (Iridaceae) contains zeaxanthin, lycopene, carotenes, crocetin, and picrocrocin.
10.9. Emblica officinalis Gaertn
Some potent antioxidants including gallotannins, for example, emblicanin A, emblicanin B, punigluconin, and pedunculagin, are present in Emblica officinalis Gaertn (Euphorbiaceae), generally known as Amla [98]. This plant exists in China, India, and Indonesia. All parts of this plant, from root to fruit, are medicinally effective in different health problems; for example, diarrhea and jaundice are effectively treated by using its fruit [99]. The hydroalcoholic fruit extract of Emblica officinalis has been formulated as water-in-oil cream that is found effective for reducing transepidermal water loss, as observed by using Tewameter [40]. Since transepidermal water loss plays crucial role in aging process this formulation can be employed as an antiaging product. This property of cream could be due to presence of strong antioxidants in Emblica officinalis.
10.10. Foeniculum vulgare
On application of water-in-oil emulsion based cream loaded with ethanolic extract of Foeniculum vulgare (Apiaceae) seeds for eight months, reduced transepithelial water loss was observed which resulted in improved moisture contents on human skin [41]. Moreover, there was an improvement in skin mechanical properties, that is, reduced levels of roughness, scaliness, smoothness, and wrinkles of photoaged skin. In another study, Rasul et al. applied same formulation on hyperpigmented human skin and observed the decreased melanin level, sebum production, and erythema of the treated skin [42]. The decrease in skin melanin level leads to skin-whitening effect. Beside flavonoids, they attributed this effect to linoleic acid present in the used extract. This unsaturated fatty acid, a main constituent of biological cell membranes, has an advantage of accelerating process of tyrosinase degradation resulting in reduced melanin synthesis due to low tyrosinase levels [100]. Furthermore, this cream may be useful for skin acne due to its diminishing effect on skin sebum level. Oleic acid, linolenic acid, and linoleic acid, present in Foeniculum vulgare extract, could be responsible for this effect [101]. These unsaturated fatty acids have inhibitory effect on sebum production owing to selective inhibition of 5-reductase which is involved in production of sebum [102]. Lastly, there was decrease in erythema on treated skin; this effect elaborates the anti-inflammatory action of this cream.
10.11. Hippophae rhamnoides
By using Cutometer, Khan et al. reported the improvement in facial skin mechanical parameters, for example, skin elasticity, indicating antiaging effect after using water-in-oil based hydroalcoholic cream loaded with fruit extract of Hippophae rhamnoides [43]. Another study reported the antisebum secretion effect of same formulation [103]. Moreover, the extracts of Hippophae rhamnoides and Cassia fistula were also found effective in the reduction of skin sebum content (antiacne effects) in human with grade I and grade II acne vulgaris [104]. Khan et al. reported that this formulation improves barrier function of human skin as tested by Tewameter and Corneometer [105]. The possible antiaging effect was pointed out as a feature of antioxidants such as carotene, particularly β-carotene, vitamin C, and vitamin E present in extract. Vitamin C occurs in a concentration of 28–2500 mg/100 g of Hippophae rhamnoides extract [106] and plays a role in the stimulation of dermal fibroblasts to synthesize collagen which is responsible for holding water contents in skin [107]. The Hippophae rhamnoides extract affects skin mechanical properties through increased expression of cell surface integrins that promote collagen contraction. Moreover, Khan et al. reported reduced TEWL and thus increased skin hydration level for same formulation [108]. Furthermore, same researchers also described reduction in skin melanin level and erythema by using this cream [109].
10.12. Lithospermum erythrorhizon
Oil-in-water emulsion based cream loaded with ethanolic extract of Lithospermum erythrorhizon root has been found to produce moisturizing effect on human skin by controlling transepithelial water loss. Lithospermum erythrorhizon (Boraginaceae) contains shikonin, acetylshikonin, deoxyshikonin, b-acetoxyisovalerylshikonin, isobutylshikonin, b,b-dimethyl acrylshikonin, 2-methyl-n-butyrylshikonin, and isovalerylshikonin [44].
10.13. Malus domestica
The reduced transepithelial water loss has been observed leading to improved moisture contents in human skin after application of water-in-oil emulsion based cream loaded with hydroalcoholic extract of Malus domestica (Rosaceae) seeds for eight months [45]. Moreover, there was an improvement in skin mechanical properties, that is, reduced levels of roughness, scaliness, smoothness, and wrinkles of the photoaged skin. In another study, Khan et al. applied same formulation on the hyperpigmented human skin and observed decreased melanin level, sebum production, and erythema of the treated skin. These antiaging effects on skin could be attributed to flavonoid, quercetin, and hesperetin, present in Malus domestica extract [46].
10.14. Matricaria chamomilla L
After skin treatment with water-in-oil emulsion based cream loaded with hydroalcoholic extract of Matricaria chamomilla plant for eight weeks, reduced transepithelial water loss was observed. Since an increase in transepithelial water loss shows disruption of the stratum corneum and loss of intercellular lipids, accordingly this formulation possibly repairs the stratum corneum and improves the moisture contents in human skin [47]. Moreover, there was an improvement in skin mechanical properties, that is, diminished levels of roughness, scaliness, smoothness, and wrinkles of photoaged skin [110, 111]. The investigators attributed this antiaging activity to the phenolic compounds present in extract. Matricaria chamomilla belongs to Asteraceae family and contains some bioactive ingredients including terpenes, polysaccharides, and flavonoids such as α-bisabolol and apigenin.
10.15. Moringa oleifera
Moringa oleifera (Moringaceae) contains carotene, vitamin C, vitamin B, vitamin A, carotenoids, myricetin, quercetin, kaempferol, gallic acid, syringic acid, and rutin. All parts of this plant, from root to leaves, possess various medicinal activities such as antibacterial, anticancer, and antioxidant [112, 113]. Ali et al. prepared water-in-oil cream of hydroalcoholic extract of Moringa oleifera leaves and applied it to photoaged skin [48, 49]. The investigators found reduced undesirable skin sebum contents of skin and diminished skin transepidermal water loss leading to increased skin hydration, particularly for dry skin using Sebumeter and Corneometer, respectively. In addition, same formulation was also found effective against skin wrinkles, roughness, and scaliness leading to skin revitalization effect [50]. They tagged the antiaging characteristic of Moringa oleifera to the phenolic compounds present in extract, since the phenolics have capability to scavenge ROS.
10.16. Morus alba
Morus alba belongs to family Moraceae. This plant is rich in active compounds including anthocyanins, gallic acid, flavonoids and tannins, citric acid, vitamin C, and palmitic acid [114, 115]. Akhtar et al. prepared oil-in-water cream of hydroalcoholic extract of Morus alba fruit followed by application to the photoaged skin of the human volunteers [53]. After 8 weeks, the studied skin areas were tested using Mexameter and Corneometer. The results indicated the reduction in melanin contents of skin, without producing erythema, attributing this activity to the presence of anthocyanin and flavonoids. These phenolics have tyrosinase inhibition activity, one of the modes of antiaging activity [51, 52, 116]. In another study, Akhtar et al. reported decrease in erythema and melanin contents in the treated skin using same formulation [52].
10.17. Ocimum basilicum
Rasul and Akhtar reported the improvement in facial skin mechanical (viscoelasticity) and biochemical parameters (superoxide dismutase, catalase, total protein, and ascorbic acid level) when skin was treated with water-in-oil emulsion based cream loaded with ethanolic extract of Ocimum basilicum (Lamiaceae) seeds [53]. Moreover, the reduction in malondialdehyde level was also noted. The possible antiaging effect was pointed out as a feature of antioxidants such as quercetin, isoquercetin, kaempferol, caffeic acid, rosmarinic acid, rutin, catechin, ferulic acid, rutinoside, and apigenin present in extract [117].
10.18. Oryza sativa
Some strong antioxidants including ferulic acid, gamma-oryzanol, and phytic acid are present in Oryza sativa (Poaceae). The grains extract of Oryza sativa was loaded to niosomes followed by the preparation of water-in-oil cream using these niosomes. This cream was found effective for reducing transepidermal water loss, as observed by using Corneometer and Evaporimeter. Since transepidermal water loss plays crucial role in aging process by enhancing skin hydration, this formulation can be employed as an antiaging product. This feature of cream could be due to strong antioxidants present in Oryza sativa extract [54].
10.19. Polygonum minus
Haris et al. reported the improvement in facial skin elasticity as well as reduced wrinkles after treating with water-in-oil emulsion based cream loaded with aqueous extract of Polygonum minus (Polygonaceae) seeds [55]. The possible antiaging effect could be due to the antioxidants such as caffeic acid and quercetin [118].
10.20. Punica granatum
Kaur and Saraf reported the improvement in facial skin mechanical (viscoelasticity) and biochemical parameters (catalase and ascorbic acid concentration) [56]. Moreover, the reduction in malondialdehyde level was also noted. These results indicate the antiaging effect of nanotransfersomes loaded cream. The investigators used nanotransfersomes loaded with ethanolic extract of Punica granatum (Punicaceae) seeds to prepare a novel cream beside development of conventional cream. The antiaging effect of various formulations was in this decreasing order: nanotransfersomal cream > conventional cream > blank nanotransfersomal cream > base cream. The possible antiaging effect was pointed out as a feature of antioxidants such as anthocyanins, ellagic acid, and hydrolysable tannins present in extract.
10.21. Silybum marianum
On application of water-in-oil emulsion based cream loaded with ethanolic extract of Silybum marianum (Asteraceae) seeds for eight months, reduced transepithelial water loss was observed which resulted in improved moisture contents in human skin [57]. Moreover, there was an improvement in skin mechanical properties, that is, reduced levels of roughness, scaliness, smoothness, and wrinkles of photoaged skin. In another study, Rasul et al. applied same formulation on hyperpigmented human skin and observed the decreased melanin level, sebum production, and erythema of the treated skin [58].
10.22. Tagetes erecta Linn
Tagetes erecta Linn. (Asteraceae) contains quercetagetin, syringic acid, lutin, quercetin, and gallates. This plant possesses different medicinal activities including antiaging, anticancer, and anti-inflammatory [119–121]. Leelapornpisid et al. prepared ethyl acetate extract of Tagetes erecta flowers and loaded it to nanostructured lipid carriers [59]. These carriers were then formulated as cream and applied to photoaged skin. Using Visiometer, the researchers observed reduction in skin wrinkles of photoaged skin without producing skin irritation after eight weeks of cream usage. The investigators attributed this antiaging activity to the antioxidants present in extract.
10.23. Terminalia chebula
After skin treatment with water-in-oil emulsion based cream loaded with hydroalcoholic extract of Terminalia chebula plant for eight weeks, reduced transepithelial water loss was observed. Moreover, there was decrease in skin melanin contents also [60]. These features could be due to the phenolic compounds present in extract. Terminalia chebula belongs to Combretaceae family and contains some bioactive ingredients including gallic acid, ellagic acid, tannic acid, ethyl gallate, chebulic acid, chebulagic acid, corilagin, and ascorbic acid [122].
10.24. Trigonella foenum-graecum
Trigonella foenum-graecum belongs to family Fabaceae. This medicinal plant is rich in active compounds including polyphenols, galactomannans and flavonoid, protodioscin, trigoneoside, diosgenin, and yamogenin. Galactomannan has an advantage of improving skin hydration [123]. Waqas et al. and Akhtar et al. prepared water-in-oil cream of methanol extract of Trigonella foenum-graecum seeds [61, 62]. After applying this cream to human skin for predetermined time, the former authors observed an improvement in facial skin mechanical parameters without producing erythema, while the latter authors reported reduction in skin melanin contents and maintenance of skin hydration.
10.25. Vitis vinifera
Various bioactive compounds such as sarmentine are present in Vitis vinifera (Vitaceae). The hydroalcoholic shoot extract of Vitis vinifera was formulated as water-in-oil cream that was found effective for improving clinical signs of photoaged skin. This property of cream could be due to the presence of strong antioxidants in Vitis vinifera extract [63].
11. Conclusion
Due to constant exposure of human skin to the UV radiations present in sunlight, several pathobiological alterations in cells occur. The photoprotection is the main approach for managing the photoaging, but cosmeceuticals could also be used as an alternative therapy. The selection of therapeutic approaches depends on the nature of these injurious molecular changes. Large number of botanical extract loaded creams have been prepared and assessed (Table 1) for their antiaging potential. The observed antiaging effects of cream formulations could be an outcome of a coordinating action of multiple constituents. Of numerous botanicals, the phenolic acids and flavonoids appear effective against UV radiation-induced damage; however, the evidence-based studies for their antiaging effects are still needed. Since environment affects the skin-cream interaction, the cautious assessment of their clinical efficacy should be conducted in future.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Gilchrest B. A. Skin aging and photoaging: an overview. Journal of the American Academy of Dermatology. 1989;21(3):610–613. doi: 10.1016/s0190-9622(89)70227-9. [DOI] [PubMed] [Google Scholar]
- 2.Braverman I. M., Fonferko E. Studies in cutaneous aging. I. The elastic fiber network. Journal of Investigative Dermatology. 1982;78(5):434–443. doi: 10.1111/1523-1747.ep12507866. [DOI] [PubMed] [Google Scholar]
- 3.Montagna W., Kirchner S., Carlisle K. Histology of sun-damaged human skin. Journal of the American Academy of Dermatology. 1989;21(5):907–918. doi: 10.1016/s0190-9622(89)70276-0. [DOI] [PubMed] [Google Scholar]
- 4.Warren R., Gartstein V., Kligman A. M., Montagna W., Allendorf R. A., Ridder G. M. Age, sunlight, and facial skin: a histologic and quantitative study. Journal of the American Academy of Dermatology. 1991;25(5 I):751–760. doi: 10.1016/s0190-9622(08)80964-4. [DOI] [PubMed] [Google Scholar]
- 5.Bielenberg D. R., Bucana C. D., Sanchez R., Donawho C. K., Kripke M. L., Fidler I. J. Molecular regulation of UVB-induced cutaneous angiogenesis. Journal of Investigative Dermatology. 1998;111(5):864–872. doi: 10.1046/j.1523-1747.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 6.Yano K., Oura H., Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. Journal of Investigative Dermatology. 2002;118(5):800–805. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- 7.Seo J. Y., Chung J. H. Thermal aging: a new concept of skin aging. Journal of Dermatological Science Supplement. 2006;2:S13–S22. [Google Scholar]
- 8.Mera S. L., Lovell C. R., Russell Jones R., Davies J. D. Elastic fibres in normal and sun-damaged skin: an immunohistochemical study. British Journal of Dermatology. 1987;117(1):21–27. doi: 10.1111/j.1365-2133.1987.tb04086.x. [DOI] [PubMed] [Google Scholar]
- 9.Udompataikul M., Sripiroj P., Palungwachira P. An oral nutraceutical containing antioxidants, minerals and glycosaminoglycans improves skin roughness and fine wrinkles. International Journal of Cosmetic Science. 2009;31(6):427–435. doi: 10.1111/j.1468-2494.2009.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher G. J., Wang Z. Q., Datta S. C., Varani J., Kang S., Voorhees J. J. Pathophysiology of premature skin aging induced by ultraviolet light. The New England Journal of Medicine. 1997;337(20):1419–1428. doi: 10.1056/nejm199711133372003. [DOI] [PubMed] [Google Scholar]
- 11.Varani J., Warner R. L., Gharaee-Kermani M., et al. Vitamin A antagonizes decreased cell growth and elevated collagen- degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. Journal of Investigative Dermatology. 2000;114(3):480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 12.Fisher G. J., Datta S. C., Talwar H. S., et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 13.Chung J. H., Seo J. Y., Choi H. R., et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. Journal of Investigative Dermatology. 2001;117(5):1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin H. C., Jin Y. S., Mi K. L., et al. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo . Journal of Investigative Dermatology. 2002;119(2):507–512. doi: 10.1046/j.1523-1747.2002.01844.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheynier V. Polyphenols in foods are more complex than often thought. The American Journal of Clinical Nutrition. 2005;81(1):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 16.Masaki H. Role of antioxidants in the skin: anti-aging effects. Journal of Dermatological Science. 2010;58(2):85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Lavker R. M. Cutaneous aging: chronologic versus photoaging. In: Gilchrest B., editor. Photodamage. Vol. 1. Blackwell; 1995. pp. 123–135. [Google Scholar]
- 18.Kim Y. H., Kim K. S., Han C. S., et al. Inhibitory effects of natural plants of Jeju Island on elastase and MMP-1 expression. International Journal of Cosmetic Science. 2007;29:487–488. [PubMed] [Google Scholar]
- 19.Sander C. S., Chang H., Salzmann S., et al. Photoaging is associated with protein oxidation in human skin in vivo. Journal of Investigative Dermatology. 2002;118(4):618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- 20.Shindo Y., Witt E., Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. Journal of Investigative Dermatology. 1993;100(3):260–265. doi: 10.1111/1523-1747.ep12469048. [DOI] [PubMed] [Google Scholar]
- 21.McArdle F., Rhodes L. E., Parslew R., Jack C. I. A., Friedmann P. S., Jackson M. J. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radical Biology and Medicine. 2002;33(10):1355–1362. doi: 10.1016/s0891-5849(02)01042-0. [DOI] [PubMed] [Google Scholar]
- 22.Babior B. M., Lambeth J. D., Nauseef W. The neutrophil NADPH oxidase. Archives of Biochemistry and Biophysics. 2002;397(2):342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 23.Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. American Journal of Physiology. 1988;255(6, part 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 24.Fantel A. G., Person R. E., Tumbic R. W., Nguyen T.-D., Mackler B. Studies of mitochondria in oxidative embryotoxicity. Teratology. 1995;52(4):190–195. doi: 10.1002/tera.1420520404. [DOI] [PubMed] [Google Scholar]
- 25.Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radical Research. 1998;28(6):623–635. doi: 10.3109/10715769809065818. [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. F., Hibbs J. B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Current Opinion in Immunology. 1991;3(1):65–70. doi: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- 27.Ali A., Akhtar N., Khan H. M. S. Enhancement of human cheek skin texture by Acacia nilotica bark extract cream. Tropical Journal of Pharmaceutical Research. 2013;12(3):323–327. doi: 10.4314/tjpr.v12i3.8. [DOI] [Google Scholar]
- 28.Sabale V., Kunjwani H., Sabale P. Formulation and in vitro evaluation of the topical antiageing preparation of the fruit of Benincasa hispida . Journal of Ayurveda and Integrative Medicine. 2011;2(3):124–128. doi: 10.4103/0975-9476.85550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhtar N., Zaman S. U., Khan B. A., Amir M. N., Ebrahimzadeh M. A. Calendula extract: effects on mechanical parameters of human skin. Acta Poloniae Pharmaceutica—Drug Research. 2011;68(5):693–701. [PubMed] [Google Scholar]
- 30.Akhtar N., Zaman S. U., Khan B. A., et al. Evaluation of various functional skin parameters using a topical cream of Calendula officinalis extract. African Journal of Pharmacy and Pharmacology. 2011;5(2):199–206. [Google Scholar]
- 31.Mahmood T., Akhtar N., Khan B. A., Khan H. M. S., Saeed T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosnian Journal of Basic Medical Sciences. 2010;10(3):260–264. doi: 10.17305/bjbms.2010.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmood T., Akhtar N., Khan B. A., Ahmad M., Khan H. M. S., Zaman S. U. Application of stable green tea extract cream on human cheeks. International Journal of Academic Research. 2010;2(2):121–126. [Google Scholar]
- 33.Mahmood T., Akhtar N. Combined topical application of lotus and green tea improves facial skin surface parameters. Rejuvenation Research. 2013;16(2):91–97. doi: 10.1089/rej.2012.1380. [DOI] [PubMed] [Google Scholar]
- 34.Mahmood T., Akhtar N., Moldovan C. A comparison of the effects of topical green tea and lotus on facial sebum control in healthy humans. Hippokratia. 2013;17(1):64–67. [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmood T., Akhtar N. Short term study of human skin irritation by single application closed patch test: assessment of four multiple emulsion formulations loaded with botanical extracts. Cutaneous and Ocular Toxicology. 2013;32(1):35–40. doi: 10.3109/15569527.2012.700472. [DOI] [PubMed] [Google Scholar]
- 36.Zaman S. U., Akhtar N., Khan B. A., et al. Development of a sebum control cream from a local desert plant Capparis deciduas . Journal of Medicinal Plants Research. 2012;6(5):744–748. [Google Scholar]
- 37.Almeida I. F., Maleckova J., Saffi R., et al. Characterization of an antioxidant surfactant-free topical formulation containing Castanea sativa leaf extract. Drug Development and Industrial Pharmacy. 2013;41(1):148–155. doi: 10.3109/03639045.2013.850712. [DOI] [PubMed] [Google Scholar]
- 38.McDaniel D. H. Clinical safety and efficacy in photoaged skin with CoffeeBerry extract, a natural antioxidant. Cosmetic Dermatology. 2009;22(12):610–616. [Google Scholar]
- 39.Akhtar N., Khan H. M., Ashraf S., Mohammad I. S., Saqib N., Bashir K. Moisturizing effect of stable cream containing Crocus sativus extracts. Pakistan Journal of Pharmaceutical Sciences. 2014;27(6):1881–1884. [PubMed] [Google Scholar]
- 40.Akhtar N., Zaman A., Ali A., et al. Effects of Emblica officinalis extract cream on human skin trans-epidermal water loss measured with non invasive probe. Journal of Pharmacy and Alternative Medicine. 2012;1(1):32–37. [Google Scholar]
- 41.Rasul A., Akhtar N., Khan B. A., Mahmood T., Uz Zaman S., Shoaib Khan H. M. Formulation development of a cream containing fennel extract: in vivo evaluation for anti-aging effects. Pharmazie. 2012;67(1):54–58. doi: 10.1691/ph.2012.1083. [DOI] [PubMed] [Google Scholar]
- 42.Rasul A., Akhtar N., Iqbal M., et al. Sebumetric and mexametric evaluation of a fennel based cream. ScienceAsia. 2012;38(3):262–267. doi: 10.2306/scienceasia1513-1874.2012.38.262. [DOI] [Google Scholar]
- 43.Ali Khan B., Akhtar N., Braga V. A. Anti-aging effects of Hippophae rhamnoides emulsion on human skin. Tropical Journal of Pharmaceutical Research. 2012;11(6):955–962. doi: 10.4314/tjpr.v11i6.12. [DOI] [Google Scholar]
- 44.Chang M.-J., Huang H.-C., Chang H.-C., Chang T.-M. Cosmetic formulations containing Lithospermum erythrorhizon root extract show moisturizing effects on human skin. Archives of Dermatological Research. 2008;300(6):317–323. doi: 10.1007/s00403-008-0867-9. [DOI] [PubMed] [Google Scholar]
- 45.Khan H. M. S., Akhtar N., Rasool F., Khan B. A., Mahmood T., Khan M. S. In vivo evaluation of stable cream containing flavonoids on hydration and TEWL of human skin. World Academy of Science, Engineering and Technology. 2010;47:896–899. [Google Scholar]
- 46.Shoaib Khan H. M., Rasool N. A. F., Madni A., Saeed T., Khan B. A., Mahmood T. Investigation of a new sebum control cream containing apple juice extract. Asian Journal of Chemistry. 2011;23(2):810–812. [Google Scholar]
- 47.Nóbrega A. T., Wagemaker T. A. L., Campos P. M. B. G. M. Antioxidant activity of Matricaria chamomilla L. extract and clinical efficacy of cosmetic formulations containing this extract and its isolated compounds. Biomedical and Biopharmaceutical Research. 2013;10(2):249–261. [Google Scholar]
- 48.Ali A., Akhtar N., Khan M. S., Khan M. T., Ullah A., Shah M. I. Effect of Moringa oleifera on undesireble skin sebum secretions of sebaceous glands observed during winter season in humans. Biomedical Research. 2013;24(1):127–130. [Google Scholar]
- 49.Ali A., Akhtar N., Khan M. S., et al. Moisturizing effect of cream containing Moringa oleifera (Sohajana) leaf extract by biophysical techniques: in vivo evaluation. Journal of Medicinal Plants Research. 2013;7(8):386–391. [Google Scholar]
- 50.Ali A., Akhtar N., Chowdhary F. Enhancement of human skin facial revitalization by moringa leaf extract cream. Postepy Dermatologii i Alergologii. 2014;31(2):71–76. doi: 10.5114/pdia.2014.40945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akhtar N., Hisham J., Khan H. M. S., Khan B. A., Mahmood T., Saeed T. Whitening and antierythemic effect of a cream containing Morus alba extract. Hygeia Journal for Drugs and Medicines. 2012;4(1):97–103. [Google Scholar]
- 52.Akhtar N., Hisham J., Khan B. A., et al. Cosmetic application of phenolic cream from mulberry bark extract. Asian Journal of Chemistry. 2012;24(4):1805–1808. [Google Scholar]
- 53.Rasul A., Akhtar N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non- invasive biophysical techniques. DARU Journal of Pharmaceutical Sciences. 2011;19(5):344–350. [PMC free article] [PubMed] [Google Scholar]
- 54.Manosroi A., Chutoprapat R., Sato Y., et al. Antioxidant activities and skin hydration effects of rice bran bioactive compounds entrapped in niosomes. Journal of Nanoscience and Nanotechnology. 2011;11(3):2269–2277. doi: 10.1166/jnn.2011.3532. [DOI] [PubMed] [Google Scholar]
- 55.Haris H. H. B., Ming Y. K., Perin F., Blanche C., Jinapong N. Split-face placebo controlled evaluation of the in vivo anti-ageing efficacy of lineminustm cream (Polygonum minus extract) in healthy asian skin type female subjects. Asian Journal of Pharmaceutical and Clinical Research. 2014;7(3):7–13. [Google Scholar]
- 56.Kaur C. D., Saraf S. Photoprotective herbal extract loaded nanovesicular creams inhibiting ultraviolet radiations induced photoaging. International Journal of Drug Delivery. 2011;3:699–711. [Google Scholar]
- 57.Rasul A., Akhtar N. Anti-aging potential of a cream containing milk thistle extract: formulation and in vivo evaluation. African Journal of Biotechnology. 2012;11(6):1509–1515. doi: 10.5897/ajb11.2678. [DOI] [Google Scholar]
- 58.Rasul A., Akhtar N., Khan B. A., et al. Assessment of anti erythmic and skin whitening effects of milk thistle extract. African Journal of Pharmacy and Pharmacology. 2011;5(20):2306–2309. [Google Scholar]
- 59.Leelapornpisid P., Chansakaow S., Na-Boonlong S., Jantrawut P. Development of cream containing nanostructured lipid carriers loaded marigold (Tagetes erecta Linn.) flowers extract for anti-wrinkles application. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(5):313–314. [Google Scholar]
- 60.Akhtar N., Khan A. B., Muhammad S., et al. Formulation and characterization of a cream containing terminalia chebula extract. Forschende Komplementarmedizin. 2012;19(1):20–25. doi: 10.1159/000335823. [DOI] [PubMed] [Google Scholar]
- 61.Cornacchione S., Sadick N. S., Neveu M., et al. In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract. Journal of Drugs in Dermatology. 2007;6(supplement 6):s8–s13. [PubMed] [Google Scholar]
- 62.Waqas M. K., Akhtar N., Ahmad M., et al. Natural drugs formulation and characterization of a cream containing extract of fenugreek seeds. Acta Poloniae Pharmaceutica—Drug Research. 2010;67(2):173–178. [PubMed] [Google Scholar]
- 63.Akhtar N., Waqas M. K., Ahmed M., et al. Effect of cream formulation of fenugreek seed extract on some mechanical parameters of human skin. Tropical Journal of Pharmaceutical Research. 2010;9(4):329–337. [Google Scholar]
- 64.Harborne J. B., Williams C. A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 65.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews. 1998;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 66.Lodén M., Andersson A.-C., Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm) British Journal of Dermatology. 1999;140(2):264–267. doi: 10.1046/j.1365-2133.1999.02660.x. [DOI] [PubMed] [Google Scholar]
- 67.Baumann L. Cosmetic Dermatology: Principles and Practice. Boston, Mass, USA: McGraw-Hill Companies Medical Publishing Division; 2002. [Google Scholar]
- 68.Rawlings A. V., Harding C. R. Moisturization and skin barrier function. Dermatologic Therapy. 2004;17(supplement 1):43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 69.Glogau R. G. Chemical peeling and aging skin. Journal of Geriatric Dermatology. 1994;12:31–36. [Google Scholar]
- 70.Lee E.-S., Kim J.-H., Im S., Lee K. B., Sohn S., Kang W. H. Application of computerized image analysis in pigmentary skin diseases. International Journal of Dermatology. 2001;40(1):45–49. doi: 10.1046/j.1365-4362.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- 71.Boelsma E., Hendriks H. F. J., Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. The American Journal of Clinical Nutrition. 2001;73(5):853–864. doi: 10.1093/ajcn/73.5.853. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto Y. Role of active oxygen species and antioxidants in photoaging. Journal of Dermatological Science. 2001;27:S1–S4. doi: 10.1016/s0923-1811(01)00120-7. [DOI] [PubMed] [Google Scholar]
- 73.Heinrich U., Tronnier H., Stahl W., Béjot M., Maurette J.-M. Antioxidant supplements improve parameters related to skin structure in humans. Skin Pharmacology and Physiology. 2006;19(4):224–231. doi: 10.1159/000093118. [DOI] [PubMed] [Google Scholar]
- 74.Jeon H. Y., Kim J. K., Kim W. G., Lee S. J. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and uv-induced skin damage. Skin Pharmacology and Physiology. 2009;22(3):137–141. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 75.Kapoor V. P. Herbal cosmetics for skin and hair care. Natural Product Radiance. 2005;4(4):306–314. [Google Scholar]
- 76.Kole P. I., Jadhav H. R., Thakurdesai P., Nagappa A. N. Cosmetics potential of herbal extracts. Natural Product Radiance. 2005;4(4):315–321. [Google Scholar]
- 77.Lieberman H. A., Rieger M. M., Banker G. S. Pharmaceutical Dosage Forms—Disperse Systems. Vol. 2. New York, NY, USA: Marcel Dekker; 1996. [Google Scholar]
- 78.Mahdavian A.-R., Sharifi-Sanjani N. Role of emulsifiers in preparation of core-shell particles via step-wise emulsion polymerization. Iranian Polymer Journal. 2001;10(4):243–249. [Google Scholar]
- 79.Kizling J., Kronberg B. On the formation of concentrated stable w/o emulsions. Advances in Colloid and Interface Science. 2001;89-90:395–399. doi: 10.1016/s0001-8686(00)00057-9. [DOI] [PubMed] [Google Scholar]
- 80.Al-Bawab A., Friberg S. E. Some pertinent factors in skin care emulsion. Advances in Colloid and Interface Science. 2006;123–126:313–322. doi: 10.1016/j.cis.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Waqas M. K., Akhtar N., Rasul A., et al. In vivo evaluation of a cosmetic emulsion containing soybean extract for anti-aging. Tropical Journal of Pharmaceutical Research. 2014;13(9):1401–1406. doi: 10.4314/tjpr.v13i9.4. [DOI] [Google Scholar]
- 82.Waqas M. K., Akhtar N., Khan H. M. S., Mustafa R., Murtaza G. Stability study of a cosmetic emulsion loaded with Tamarindus indica seeds extract. Latin American Journal of Pharmacy. 2014;33(5):731–738. [Google Scholar]
- 83.Gao X.-H., Zhang L., Wei H., Chen H.-D. Efficacy and safety of innovative cosmeceuticals. Clinics in Dermatology. 2008;26(4):367–374. doi: 10.1016/j.clindermatol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 84.Sahu A. N., Jha S., Dubey S. D. Formulation and evaluation of curcuminoid based herbal face cream. Indo Global Journal of Pharmaceutical Sciences. 2011;1(1):77–84. [Google Scholar]
- 85.Paithankar V. V. Formulation and evaluation of herbal cosmetic preparation using safed musli. International Journal of PharmTech Research. 2010;2(4):2261–2264. [Google Scholar]
- 86.Ali A., Akhtar N., Khan B. A., et al. Acacia nilotica: a plant of multipurpose medicinal uses. Journal of Medicinal Plants Research. 2012;6:1492–1496. [Google Scholar]
- 87.Aslokar L. V., Kakkar K. K., Chakre O. J. Indian Medicinal Plants with Active Principles. Part I. 1st. New Delhi, India: CSIR; 1992. [Google Scholar]
- 88.Yashizumi S., Murakam T., Kadoya M., Matsuda H., Yamahara J., Yoshikava M. Histamine release inhibitors from wax gourd the fruits of Benincasa hispida (thunb) Yakugaku Zassi. 1998;118:188–192. doi: 10.1248/yakushi1947.118.5_188. [DOI] [PubMed] [Google Scholar]
- 89.Joanne B., Linda A. A., David P. J. Herbal Medicines. 2nd 2002. [Google Scholar]
- 90.Mahmood T., Akhtar N., Khan B. A., Khan H. M. S., Saeed T. Changes in skin mechanical properties after long-term application of cream containing green tea extract. Aging Clinical and Experimental Research. 2011;23(5-6):333–336. doi: 10.1007/bf03325232. [DOI] [PubMed] [Google Scholar]
- 91.Choe J.-H., Jang A., Choi J.-H., et al. Antioxidant activities of lotus leaves (Nelumbo nucifera) and barley leaves (Hordeum vulgare) extracts. Food Science and Biotechnology. 2010;19(3):831–836. doi: 10.1007/s10068-010-0117-8. [DOI] [Google Scholar]
- 92.Ottaviani M., Alestas T., Flori E., Mastrofrancesco A., Zouboulis C. C., Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. Journal of Investigative Dermatology. 2006;126(11):2430–2437. doi: 10.1038/sj.jid.5700434. [DOI] [PubMed] [Google Scholar]
- 93.Liao S. The medicinal action of androgens and green tea epigallocatechin gallate. Hong Kong Medical Journal. 2001;7(4):369–374. [PubMed] [Google Scholar]
- 94.Vyas G. K., Sharma R., Kumar V., Sharma T. B., Khandelwal V. Diversity analysis of Capparis decidua (Forssk.) Edgew. using biochemical and molecular parameters. Genetic Resources and Crop Evolution. 2009;56(7):905–911. doi: 10.1007/s10722-009-9488-1. [DOI] [Google Scholar]
- 95.Singh P., Mishra G., Srivastava S., Jha K. K., Khosa R. L. Traditional uses, phytochemistry and pharmacological properties of Capparis decidua: an overview. Der Pharmacia Lettre. 2011;3(2):71–82. [Google Scholar]
- 96.Lupo M. P., Draelos Z. D., Farris P. K. CoffeeBerry: a new, natural antioxidant in professional antiaging skin care. Cosmetic Dermatology. 2007;20(supplement 4):2–9. [Google Scholar]
- 97.Draelos Z. A double-blind, randomized clinical trial evaluating the dermatologic benefits of coffee berry extract. Journal of the American Academy of Dermatology. 2008;58(2, supplement 2):p. AB64. doi: 10.1016/j.jaad.2007.10.287. [DOI] [Google Scholar]
- 98.Bhattacharya S. K., Bhattacharya D., Sairam K., Ghosal S. Effect of bioactive tannoid principles of Emblica officinalis on ischemia-reperfusion-induced oxidative stress in rat heart. Phytomedicine. 2002;9(2):171–174. doi: 10.1078/0944-7113-00090. [DOI] [PubMed] [Google Scholar]
- 99.Adil M. D., Kaiser P., Satti N. K., Zargar A. M., Vishwakarma R. A., Tasduq S. A. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. Journal of Ethnopharmacology. 2010;132(1):109–114. doi: 10.1016/j.jep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 100.Ando H., Mary S., Ichihashi M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. International Journal of Molecular Sciences. 2010;11:2566–2575. doi: 10.3390/ijms11062566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He W., Huang B. A review of chemistry and bioactivities of a medicinal spice: foeniculum vulgare. Journal of Medicinal Plants Research. 2011;5(16):3595–3600. [Google Scholar]
- 102.Shigeta Y., Imanaka H., Ando H., et al. Skin whitening effect of linoleic acid is enhanced by liposomal formulations. Biological and Pharmaceutical Bulletin. 2004;27(4):591–594. doi: 10.1248/bpb.27.591. [DOI] [PubMed] [Google Scholar]
- 103.Akhtar N., Khan B. A., Mahmood T., et al. Formulation and evaluation of antisebum secretion effects of sea buckthorn w/o emulsion. Journal of Pharmacy and Bioallied Science. 2010;2(1):13–17. doi: 10.4103/0975-7406.62698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan B. A., Akhtar N. Clinical and sebumetric evaluation of topical emulsions in the treatment of acne vulgaris. Postępy Dermatologii i Alergologii. 2014;31(4):229–234. doi: 10.5114/pdia.2014.40934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan B. A., Akhtar N. Hippophae rhamnoides oil-in-water (O/W) emulsion improves barrier function in healthy human subjects. Pakistan Journal of Pharmaceutical Sciences. 2014;27(6):1919–1922. [PubMed] [Google Scholar]
- 106.Zeb A. Chemical and nutritional constituents of Sea Buckthorn juice. Pakistan Journal of Nutrition. 2006;3:99–106. [Google Scholar]
- 107.Draaijers L. J., Botman Y. A. M., Tempelman F. R. H., Kreis R. W., Middelkoop E., van Zuijlen P. P. M. Skin elasticity meter or subjective evaluation in scars: a reliability assessment. Burns. 2004;30(2):109–114. doi: 10.1016/j.burns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 108.Khan B. A., Akhtar N., Mahmood T., et al. In-vivo study of stratum corneum water content and transepideramal water loss using a newly formulated topical cream of hippophae rhamnoides fruit extract. African Journal of Pharmacy and Pharmacology. 2011;5(8):1092–1095. [Google Scholar]
- 109.Khan B. A., Akhtar N., Mahmood T., Shoaib H. M., Qayum M., Saeed T. Effects of antioxidants and flavonoids of sea buckthorn on skin whitening and skin erythema. Asian Journal of Chemistry. 2011;23(2):903–906. [Google Scholar]
- 110.Nile S. H., Khobragade C. N., Park S. W. Optimized and comparative antioxidant assays and its applications in herbal and synthetic drug analysis as an antioxidants. Mini-Reviews in Medicinal Chemistry. 2012;12(10):1007–1014. doi: 10.2174/138955712802762310. [DOI] [PubMed] [Google Scholar]
- 111.Ohno H., Nishimura N., Yamada K., et al. Effects of water nanodroplets on skin moisture and viscoelasticity during air-conditioning. Skin Research and Technology. 2013;19(4):375–383. doi: 10.1111/srt.12056. [DOI] [PubMed] [Google Scholar]
- 112.Iqbal S., Bhanger M. I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. Journal of Food Composition and Analysis. 2006;19(6-7):544–551. doi: 10.1016/j.jfca.2005.05.001. [DOI] [Google Scholar]
- 113.Anwar F., Latif S., Ashraf M., Gilani A. H. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy Research. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 114.Chen P. N., Chu S. C., Chiou H. L., Kuo W. H., Chiang C. L., Hsieh Y. S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Letters. 2006;235(2):248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 115.Bae S.-H., Suh H.-J. Antioxidant activities of five different mulberry cultivars in Korea. LWT—Food Science and Technology. 2007;40(6):955–962. doi: 10.1016/j.lwt.2006.06.007. [DOI] [Google Scholar]
- 116.Yu Z.-R., Hung C.-C., Weng Y.-M., Su C.-L., Wang B.-J. Physiochemical, antioxidant and whitening properties of extract from root cortices of mulberry as affected by membrane process. LWT—Food Science and Technology. 2007;40(5):900–907. doi: 10.1016/j.lwt.2006.05.008. [DOI] [Google Scholar]
- 117.Marwat S. K., Khan M. S., Ghulam S., Anwar N., Mustafa G., Usman K. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae) Asian Journal of Chemistry. 2011;23(9):3773–3782. [Google Scholar]
- 118.Maizura M., Aminah A., Wan Aida W. M. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. International Food Research Journal. 2011;18:529–534. [Google Scholar]
- 119.Pérez Gutiérrez R. M., Luna H. H., Garrido S. H. Antioxidant activity of Tagetes erecta essential oil. Journal of the Chilean Chemical Society. 2006;51(2):883–886. [Google Scholar]
- 120.Li W., Gao Y., Zhao J., Qi W. Phenolic, flavonoid, and lutein ester content and antioxidant activity of 11 cultivars of Chinese marigold. Journal of Agricultural and Food Chemistry. 2007;55(21):8478–8484. doi: 10.1021/jf071696j. [DOI] [PubMed] [Google Scholar]
- 121.Phrutivorapongkul A., Kiattisin K., Jantrawut P., Chansakaow S., Vejabhikul S., Leelapornpisid P. Appraisal of biological activities and identification of phenolic compound of African marigold (Tagetes erecta) flower extract. Pakistan Journal of Pharmaceutical Sciences. 2013;26(6):1071–1076. [PubMed] [Google Scholar]
- 122.Mishra A. K., Chattopadhyay P. Herbal cosmeceuticals for photoprotection from ultraviolet B radiation: a review. Tropical Journal of Pharmaceutical Research. 2011;10(3):351–360. doi: 10.4314/tjpr.v10i3.7. [DOI] [Google Scholar]
- 123.Aburjai T., Natsheh F. M. Plants used in cosmetics. Phytotherapy Research. 2003;17(9):987–1000. doi: 10.1002/ptr.1363. [DOI] [PubMed] [Google Scholar]