Abstract
Background
Despite initial in-hospital treatment for acute heart failure (HF), some patients experience worsening heart failure (WHF). There are limited data about the outcomes and characteristics of patients who experience in-hospital WHF.
Methods and Results
We assessed the characteristics and outcomes of patients with and without WHF in the ASCEND-HF trial. WHF was defined as at least 1 symptom or sign of new, persistent, or WHF requiring additional intravenous inotropic/vasodilator or mechanical therapy during index hospitalization. We assessed the relationship between WHF and 30-day mortality, 30-day mortality or HF hospitalization, and 180-day mortality. We also assessed whether there was a differential association between early (day 1–3) versus late (day ≥4) WHF and outcomes. Of 7141 patients with acute HF, 354 (5%) experienced WHF. Patients with WHF were more often male and had a history of atrial fibrillation or diabetes, lower blood pressure, and higher creatinine. After risk adjustment, WHF was associated with increased 30-day mortality (odds ratio [OR] 13.37; 95% confidence interval [CI] 9.85–18.14), 30-day mortality or HF rehospitalization (OR 6.78; 95% CI 5.25–8.76), and 180-day mortality (hazard ratio [HR] 3.90; 95% CI 3.14–4.86) (all p-values<0.0001). There was no evidence of a difference in outcomes between early versus late WHF (all p-values for comparison≥0.2).
Conclusions
WHF during index hospitalization was associated with worse 30- and 180-day outcomes. WHF may represent an important patient-centered outcome in acute HF and a focus of future treatments.
Clinical Trial Registration
ClinicalTrials.gov; unique identifier: NCT00475852.
Keywords: acute heart failure, worsening heart failure, hospitalization, outcomes
INTRODUCTION
Hospitalization for acute heart failure (HF) is common and associated with a high risk of post-discharge morbidity and mortality.1 Despite standard initial HF therapy, a subset of patients experience worsening HF (WHF) during hospitalization, defined as persistent or worsening signs of HF requiring an escalation of therapy.2 Multiple clinical trials evaluating acute HF therapies have largely focused on improvement in dyspnea or clinical outcomes other than WHF. For endpoints of rehospitalization, length of stay, or mortality, there have been few, if any, signals for improvement in outcomes.3–11 However, WHF during hospitalization for acute HF has gained increased attention as an important clinical event indicating failure of usual care.10 A recent secondary analysis of the RELAX-AHF (Relaxin in Acute Heart Failure) trial revealed a reduction in mortality or WHF in the serelaxin-treated group as compared with the placebo group (6.7% versus 12.2%).12
Despite the interest in WHF, there have been few studies characterizing this outcome. In registry data, WHF has been shown to occur in 11% of admissions among patients ≥65 years of age, with a substantially increased 30-day mortality risk.2 However, registry data are often limited due to the extent of clinical data collected, and long-term outcomes are often limited to older patients. Additional data on WHF come from secondary endpoints in clinical trials evaluating treatment strategies for acute HF, with prevalence of WHF ranging from 6.6% to 42%.3–11,13–15 Two studies have suggested an association between WHF and worse outcomes; however, these studies were limited by small sample sizes and earlier study periods.13,16
As interest in in-hospital WHF as an important clinical endpoint to approve new drug indications continues to grow, it will be critical to understand the context of WHF and its importance in the spectrum of patients with acute HF. To date, the potential indications ranging from dyspnea improvement to outcomes of cardiovascular morbidity and mortality have varied and are controversial. WHF may represent an easily measured and clinically important endpoint. We performed a retrospective analysis in ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure), the largest trial of acute HF with detailed characterization of patients with WHF, to define the prevalence and describe the clinical characteristics, qualifying features, and associated mortality of patients with in-hospital WHF.
METHODS
ASCEND-HF enrolled 7141 patients with acute HF within 24 hours of their first intravenous HF-related therapy. ASCEND-HF was a randomized, double-blind, placebo-controlled trial of nesiritide in addition to standard care. The study design and results have been previously reported.7,17 The 2 primary endpoints were a composite of all-cause mortality or HF readmission up to 30 days after randomization and the change in early dyspnea relief after study drug initiation. Each participating center’s ethics committee or institutional review board approved the study and all patients gave written informed consent. The present analysis focuses on patients enrolled in ASCEND-HF with WHF during their index hospitalization compared with patients who did not experience WHF. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting of the manuscript and its final contents.
Definitions
For the present analysis, WHF was defined as at least 1 sign, symptom, or radiologic evidence of new, persistent, or worsening acute HF requiring addition of a new intravenous therapy (inotrope or vasodilator) or mechanical support during a patient’s index hospitalization targeted specifically at HF symptoms. WHF status was documented by clinicians and coordinators on a standardized case report form. Based on average length of stay in ASCEND-HF, we defined early WHF as that which occurred in the first 3 days of index hospitalization; late WHF was defined as starting at 4 days and continuing through index hospital discharge or day 30. HF rehospitalization was defined as clinical manifestations of WHF and the addition or increased use of intravenous pharmacologic agents (inotrope or vasodilator), mechanical or surgical intervention or ultrafiltration, hemofiltration, or dialysis specifically aimed at managing persistent or WHF. Rehospitalization and fatal events within 30 days after randomization were reviewed and categorized by an independent, blinded clinical events committee. If patients remained hospitalized at 30 days because of ongoing HF, they were included in the rehospitalization for HF group.
Patient population
Patients enrolled in ASCEND-HF were required to have acute HF with dyspnea at rest or with minimal activity, ≥1 sign of HF, and ≥1 objective measure of HF. Signs of acute HF included tachypnea with respiratory rate ≥20 breaths/minute and pulmonary congestion/edema with rales or crackles/crepitations at least one-third above the lung base. Objective measures were defined as a chest X-ray with pulmonary congestion/edema, B-type natriuretic peptide ≥400 pg/mL or N-terminal pro-BNP (NT-proBNP) ≥1000 pg/mL at presentation, pulmonary capillary wedge pressure >20 mm Hg, or ejection fraction <40% measured by any modality within 12 months before randomization without intervening revascularization or cardiac surgery.
Outcomes of interest
The primary outcome for the present analysis was all-cause mortality or HF hospitalization through 30 days after randomization. Secondary outcomes were 30- and 180-day all-cause mortality. Although it would be interesting to look at dyspnea relief in patients who had WHF compared with those who did not develop WHF, dyspnea relief was not recorded in relationship to the initiation of WHF.
Statistical analysis and clinical endpoints
In this secondary analysis from ASCEND-HF, we evaluated the prespecified clinical endpoint of inpatient persistent or WHF. Demographics, physical and laboratory findings, medical history, and therapies were summarized as frequencies and percentages for categorical variables and by medians and 25th and 75th percentiles for continuous variables in patients with and without WHF. Baseline characteristics were compared using the Student t test or Wilcoxon rank sum test for continuous variables, and chi-square tests for categorical variables. We assessed the association between WHF and the clinical endpoints of 30-day mortality, 30-day mortality or rehospitalization for HF, and 180-day mortality. Logistic regression models were used in the analysis of the 30-day mortality and 30-day mortality or HF rehospitalization clinical endpoints; a Cox proportional hazards model was used for the 180-day mortality clinical endpoint. To account for dynamic patient characteristics that could influence the association between WHF and outcomes, we also performed a time-dependent analysis to adjust for daily changes in covariates, based on methods previously described.18 The following variables previously shown to be associated with the outcomes were used for the multivariable analyses: age, blood urea nitrogen (BUN), serum sodium, systolic blood pressure, creatinine, region, and HF hospitalization in the past year. The discrimination ability of each model was also assessed and a c-index was reported for the 30-day mortality and 30-day mortality or HF rehospitalization models. Multiple logistic regression models were also used to evaluate for the presence of a differential association between early (1–3 days) versus late (≥4 days) WHF. Statistical significance was assessed using 2-sided p-values, with values <0.05 considered statistically significant. Hazard ratios (HRs) or odds ratios (ORs) and their corresponding confidence intervals (CIs) associated with WHF were calculated relative to no WHF. A model was created to predict WHF in the ASCEND-HF trial, starting with a list of variables that have previously been used to predict WHF. Subsequently, the least significant variables were dropped out in a stepwise fashion until the remaining variables all contributed to the model. All statistical computations were generated using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting of the manuscript, and its final contents.
RESULTS
WHF occurred in 354 (5%) patients enrolled in ASCEND-HF. The baseline characteristics of patients with and without WHF are presented in Table 1. Patients with WHF were more often male and white with a history of atrial fibrillation, diabetes, and HF admission compared with those without WHF. There was similar use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta-blockers in patients with and without WHF, but higher rates of aldosterone antagonism and baseline diuretic use were seen in patients with WHF. Ejection fraction and blood pressure were lower in patients with WHF, whereas natriuretic peptide levels and renal function markers were more likely to be elevated in the WHF group. The baseline characteristics and qualifying events of patients with WHF are presented in Table 2. Most patients had worsening dyspnea (72%) and worsening pulmonary congestion (53%), but fewer patients had worsening orthopnea (44%), paroxysmal nocturnal dyspnea (30%), or jugular venous distension (26%). Some patients required mechanical/non-pharmacologic intervention (26%) at randomization, whereas most patients required intravenous vasoactive therapy (65%) at randomization.
Table 1.
Baseline characteristics of patients with and without WHF
| Variable | WHF (N=354) | No WHF (N=6787) | P-Value |
|---|---|---|---|
| Age, median, (25th, 75th), yrs | 68 (57, 76) | 67 (56, 76) | 0.610 |
| Female sex, % | 29.4 | 34.4 | 0.082 |
| Race, % | 0.001 | ||
| Asian | 17.8 | 25.1 | |
| Black | 11.9 | 15.2 | |
| Other | 3.7 | 4.3 | |
| White | 66.6 | 55.3 | |
| Region, % | 0.001 | ||
| Asia-Pacific | 17.8 | 25.0 | |
| Central Europe | 17.2 | 13.3 | |
| Latin America | 8.2 | 9.4 | |
| Western Europe | 11.0 | 6.9 | |
| North America | 45.8 | 45.4 | |
| Medical history, % | |||
| Hypertension | 72.0 | 72.1 | 0.971 |
| Atrial fibrillation/Flutter | 45.8 | 37.0 | 0.001 |
| Diabetes | 50.3 | 42.3 | 0.003 |
| Cerebrovascular disease | 15.0 | 11.6 | 0.057 |
| Peripheral artery disease | 8.8 | 10.4 | 0.309 |
| Chronic respiratory disease | 19.8 | 16.3 | 0.089 |
| Coronary artery disease | 60.2 | 54.4 | 0.033 |
| Previous HF admission | 55.9 | 38.2 | <0.001 |
| Baseline vitals, median (25th, 75th) | |||
| BMI, kg/m2 | 28.6 (24.8, 32.7) | 27.5 (23.7, 32.6) | 0.020* |
| Heart rate, beats/min | 82.5 (72.0, 97.0) | 82.0 (72.0, 95.0) | 0.131* |
| Respiratory rate, breaths/min | 24.0 (22.0, 26.0) | 23.0 (21.0, 26.0) | <0.001* |
| Systolic BP, mm Hg | 120 (108, 130) | 124 (110, 140) | <0.001* |
| Diastolic BP, mm Hg | 72 (65, 80) | 74 (67, 84) | <0.001* |
| LVEF, % | 28 (20, 36) | 30 (20, 37) | |
| EF <40% | 20.0 | 20.8 | 0.753 |
| EF ≥40% | 80.0 | 79.2 | |
| Baseline laboratory studies, median (25th, 75th) | |||
| BNP, pg/mL | 1158 (676, 2233) | 986 (541, 1845) | 0.034* |
| NT-proBNP, pg/mL | 7409 (3062, 14000) | 4407 (2062, 8959) | <0.001* |
| Sodium, mmol/L | 138 (134, 141) | 139 (136, 141) | 0.002* |
| Creatinine, mg/dL | 1.49 (1.18, 1.90) | 1.21 (1.00, 1.55) | <0.001* |
| BUN, mg/dL | 37.1 (22.6, 53.1) | 25.1 (18.0, 38.1) | <0.001* |
| Hemoglobin, g/dL | 12.6 (11.1, 14.3) | 12.7 (11.3, 14.0) | 0.762* |
| Medical therapy on admission, % | |||
| ACE inhibitor/ARB | 59.0 | 60.9 | 0.488 |
| Aldosterone blocker | 35.9 | 27.5 | 0.001 |
| Beta-blocker | 59.6 | 58.2 | 0.594 |
| Admission diuretic use, % | |||
| No diuretic | 21.5 | 37.1 | <0.001 |
| Furosemide alone | 61.1 | 54.2 | |
| Torsemide alone | 9.6 | 5.4 | |
| Bumetanide alone | 3.7 | 2.3 | |
| Multiple diuretics | 1.1 | 0.9 | |
| Pre-randomization inotropes/vasodilators, % | |||
| Dobutamine | 3.95 | 3.2 | 0.434 |
| Dopamine | 1.41 | 1.22 | 0.625† |
| IV nitroglycerin | 17.2 | 13.94 | 0.03 |
| IV nitroprusside | 0.28 | 1.19 | 0.191† |
| Nitrates (oral or topical) | 28.0 | 23.3 | 0.044 |
| Hydralazine | 12.7 | 7.2 | <0.001 |
| Loop diuretic use from randomization through 24 hours, % | |||
| Furosemide | 87.9 | 84.2 | 0.061 |
| Torsemide | 6.78 | 4.76 | 0.085 |
| Bumetanide | 4.24 | 3.57 | 0.508 |
Non-parametric test.
Exact test.
ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; HF, heart failure; IV, intravenous; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; WHF, worsening heart failure.
Table 2.
Clinical characteristics of patients at time of qualification for WHF
| WHF (N=354) | |
|---|---|
| New or worsening dyspnea | 255 (72%) |
| New or worsening orthopnea | 156 (44.2%) |
| New or worsening paroxysmal nocturnal dyspnea | 107 (30.2%) |
| Worsening pulmonary congestion/edema with rales/crackles | 189 (53.4%) |
| New or worsening jugular venous distension | 90 (25.5%) |
| Radiological evidence of WHF | 84 (23.8%) |
| Renal hypoperfusion with no apparent cause other than WHF | 79 (26.4%) |
| Mechanical/non-pharmacologic intervention | 79 (26.3%) |
| IV nitroglycerin | 15 (19%) |
| IV nitroprusside | 1 (0.13%) |
| Dobutamine | 3 (3.8%) |
| Dopamine | 1 (0.13%) |
Values presented as number (%).
These characteristics are from time of definition of WHF.
IV indicates intravenous; WHF, worsening heart failure.
The unadjusted clinical event rates based on WHF status are displayed in Table 3. The observed 30-day mortality was higher in patients with WHF (29.7%) compared with those without WHF (2.5%). The observed 30-day mortality or HF rehospitalization event rate was 42.7% in patients with WHF versus 8.1% in patients without WHF. By 180 days, 146 patients (41.5%) with WHF had died compared with 754 patients (11.3%) without WHF. WHF was associated with a significant increase in 30-day mortality, 30-day mortality or HF rehospitalization, and 180-day mortality (all P<0.0001) (Table 3).
Table 3.
Association between WHF and outcomes (reference=no WHF)
| Endpoint | WHF n/N (%) | No WHF n/N (%) | Unadjusted OR/HR (95% CI) | Unadjusted P-Value | Adjusted OR/HR* (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| 30-day mortality | 105/354 (29.7) | 168/6764 (2.5) | 16.56 (12.58–21.79) | <0.0001 | 13.37 (9.85–18.14) | <0.0001 |
| 30-day mortality or HF rehospitalization | 150/351 (42.7) | 536/6587 (8.1) | 8.43 (6.70–10.60) | <0.0001 | 6.78 (5.25–8.76) | <0.0001 |
| 180-day mortality† | 146/352 (41.5) | 754/6653 (11.3) | 5.05 (4.23–6.03) | <0.0001 | 3.90 (3.14–4.86) | <0.0001 |
Adjustment variables for 30-day mortality or HF rehospitalization: age, BUN, serum sodium, systolic BP, creatinine, region, and HF hospitalization in the past year. Logistic regression modeling used. C-index for this model is 0.742; 30-day mortality: age, BUN, serum sodium and systolic BP. Logistic regression modeling used. C-index for this model is 0.799; 180-day mortality: age, serum sodium, BUN, systolic blood pressure, EF, multiple diuretics at baseline, creatinine, hospitalization for HF in the past year.
Cox proportional hazards model used.
BP indicates blood pressure; BUN, blood urea nitrogen; EF, ejection fraction; HF, heart failure; HR, hazard ratio; OR, odds ratio; WHF, worsening heart failure.
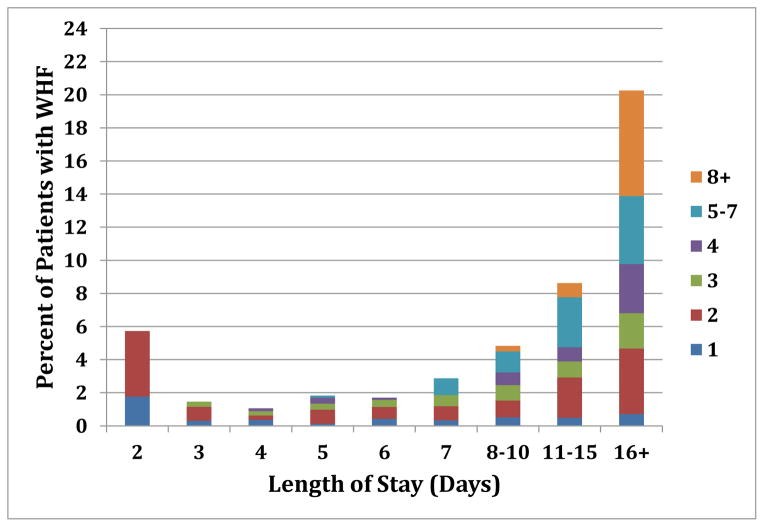
On risk-adjusted analysis, WHF was associated with increased 30-day mortality (OR 13.37, 95% CI 9.85–18.14; P<0.0001), 30-day mortality or HF hospitalization (OR 6.78, 95% CI 5.28–8.76; P<0.0001), and 180-day mortality (HR 3.9, 95% CI 3.14–4.86; P<0.0001) compared with no WHF. Using time-dependent models, the adjusted analyses of 30-day mortality and 30-day mortality or HF hospitalization yielded similar findings compared with the models based on admission characteristics alone (OR 6.91, 95% CI 5.11–9.33; P<0.0001 and OR 3.38, 95% CI 2.76–4.14; P<0.0001, respectively). For the model of 30-day mortality, the c-index was 0.799; the c-index for the model of 30-day mortality or hospitalization was 0.742. Early versus late WHF had no differential association on the clinical endpoints (Table 4). The time to WHF is graphically displayed to demonstrate the wide-ranging date of qualification for WHF and length of stay (Figure 1). BUN, respiratory rate, hospitalization for HF in the past year, and systolic blood pressure most accurately predicted which patients developed WHF (Table 5 with c-index of 0.726 for this model). When available, the addition of the laboratory value NT-proBNP increased the c-index to 0.735. Post-hoc analyses revealed that patients randomized to treatment with nesiritide compared with placebo had an OR of 0.77 for WHF (95% CI 0.61–0.97, p= 0.025).
Table 4.
Association between timing of WHF and outcomes (reference=no WHF)
| Endpoint | Adjusted OR/HR
|
P-Value | |
|---|---|---|---|
| Day 1–3 | Day ≥4 | ||
| 30-day mortality | 14.56 | 12.26 | 0.40 |
| 30-day mortality or HF rehospitalization | 6.13 | 7.54 | 0.22 |
| 180-day mortality | 4.29 | 3.58 | 0.20 |
Adjustment variables for 30-day mortality: age, BUN, serum sodium, and systolic BP. Logistic regression modeling used. C-index for this model is 0.799. Adjustment variables for 30-day mortality or HF rehospitalization: age, BUN, serum sodium, systolic BP, creatinine, region, and HF hospitalization in the past year. Logistic regression modeling used. C-index for this model is 0.742. Adjustment variables for 180-day mortality: age, serum sodium, BUN, systolic BP, EF, multiple diuretics at baseline, creatinine, hospitalization for HF in the past year. Cox proportional hazards model used.
BP indicates blood pressure; BUN, blood urea nitrogen; EF, ejection fraction; HF, heart failure; HR, hazard ratio; OR, odds ratio.
Figure 1.
Time to WHF. Colors on the right side of the table correspond to the number of days that patients qualified for WHF.
Table 5.
Characteristics associated with WHF
| Parameter | OR (95% CI) | P-Value |
|---|---|---|
| BUN (log–OR per doubling) | 1.53 (1.27–1.84) | <.0001 |
| Respiratory rate | 1.07 (1.04–1.10) | <.0001 |
| Systolic BP (per 10 mm Hg) | 0.87 (0.82–0.93) | <.0001 |
| Diabetes | 1.30 (1.02–1.64) | 0.031 |
| Hospitalized for HF in past year | 1.62 (1.28–2.05) | <.0001 |
| Creatinine (log–OR per doubling) | 1.41 (1.07–1.86) | 0.0159 |
| Sodium (<140) | 1.05 (1.03–1.08) | <.0001 |
Adjusted results by region with c-index = 0.726.
BP indicates blood pressure; BUN, blood urea nitrogen; CI, confidence interval; HF, heart failure; OR, odds ratio.
Discussion
In the largest clinical trial of acute HF, we characterized the endpoint of WHF, providing insight into a key population that has been poorly studied. Patients who experienced WHF had markedly worse outcomes at 30 days and 180 days. Specifically, patients with WHF had more than a 13-fold increase in 30-day mortality and nearly a 7-fold increase in 30-day mortality or rehospitalization for HF. By 180 days, 41.5% of patients with WHF had died compared with 11.3% of patients without WHF. We found no differential association between the timing of WHF during hospitalization and the increased risk for subsequent adverse events. Thus, WHF during hospitalization for acute HF is an event of great concern to patients and clinicians and is associated with markedly worse outcomes, regardless of when it occurs during hospitalization. Patients who experience WHF may represent an important subgroup that could benefit from targeted therapies in order to improve the very high adverse event rate.
In particular, regulatory agencies such as the European Medicines Agency and the U.S. Food and Drug Administration are interested in understanding the clinical endpoint of WHF. RELAX-AHF was the first study of acute HF to demonstrate improvement of an intermediate patient-centered endpoint, dyspnea relief. The trial also revealed a significant reduction in overall mortality at 180 days; however, the study was not powered to address overall mortality.10 With incomplete results, the regulatory agencies are left to grapple with approving a new drug class targeting acute HF via an intermediate clinical endpoint that improves symptoms associated with WHF, while awaiting a therapy that demonstrates improvement in hard clinical endpoints such as mortality, cardiovascular mortality, hospital length of stay, or rehospitalization for HF. Of patients with WHF, those treated with nesiritide had improved clinical outcomes compared to patients treated with placebo. This finding adds support to the use of WHF as an important clinical endpoint and suggests that short-term use of an in-hospital pharmacologic agent might improve clinical outcomes. The results of our analysis of ASCEND-HF should add significant weight to the value of targeting WHF with a mechanical or pharmaceutical intervention, given the poor adverse events associated with WHF. However, regulatory agencies appear to be waiting for clinical trial results that reflect improvement in the entire hospital clinical course through discharge and subsequent survival at home.
As WHF during a hospitalization for acute HF is gaining attention, it is important to understand potential differences in definitions and related incidences. Previous estimates of the incidence of WHF range from 6.6% to 42%.4–6,8–11,13,19 The wide range in reported incidences and prevalence is due in large part to the broad range of definitions across registries and clinical trials. In ASCEND-HF, the prevalence is lower because the definition of WHF during the index hospitalization was more rigorous than other studies, with requirements for escalation of therapy beyond diuretic use for treatment of worsening symptoms. In other studies, definitions varied significantly with simply symptoms and escalation of diuretics.5,10,11,13,19 For example, Massie et al. reported a 10% incidence of WHF by day 3–7 in 2033 patients with persistent dyspnea at rest or with minimal activity, impaired renal function, elevated natriuretic peptide levels, and ongoing intravenous loop-diuretic therapies.5 On the contrary, in 2 smaller studies, which are difficult to generalize but provide insight into emerging clinical problems, the incidence is much larger (29–42%), but their definitions were much more inclusive with WHF defined as recurrent acute HF symptoms or failure to improve requiring rescue intravenous therapy or mechanical support.13,16 Devore et al. used a more exclusive definition of WHF requiring initiation of inotropic or intravenous vasodilators, intensive care unit transfer, or mechanical support, which reduced the prevalence of WHF to 11%.2 There is also a significant regional variation in diuretic use during hospitalization for acute HF,20 and up-titration of diuretics alone may not be an adequate criterion for the diagnosis of WHF. The strength of the definition used in our analysis of ASCEND-HF is the requirement of a sign or symptom of persistent or WHF requiring a specific intensified therapy beyond increased intravenous diuretic use.
The primary finding of this analysis was the striking association between WHF and increased post-discharge morbidity and mortality in acute HF patients. In the ASCEND-HF dataset, WHF was a predictor of poor outcomes, independent of known prognostic variables. The present study adds further support to previous smaller studies demonstrating an association between WHF and worse outcomes.14,21 Thus, the occurrence of WHF in patients with acute HF should be recognized as a critical juncture for patients. These patients may benefit from early identification of the etiology of WHF and targeted interventions to correct the underlying pathophysiology. Interestingly, we found that there were relatively few baseline differences between those who did and did not go on to experience WHF. However, there were some key variables that best predict who goes on to experience WHF: patients who have been hospitalized for HF in the last year; those with elevated serum levels of BUN, creatinine, and NT-proBNP; and those with lower serum sodium and elevated respiratory rate. Although our logistic regression modeling adjusted for covariates known to be associated with the outcomes of mortality and rehospitalization for HF in the ASCEND-HF dataset, the comorbidity burden in patients with WHF is such that these patients may have received additional therapies that altered their disease course.
While WHF may be challenging to predict, once it does occur, the prognostic utility of the event should be recognized. Although there is disagreement over how best to define WHF clinically and in the case report form, there is a clear signal associated with worse outcomes, and regulatory agencies are optimistically awaiting clinical trial results that demonstrate an improvement from the inpatient setting through survival at home before a new therapy is approved. WHF portends an unfavorable prognosis, there is no definite predictive or diagnostic pathway, and there is no clear therapy that provides benefit in patients who succumb to WHF at any time during their hospitalization for acute HF. Future studies should evaluate the causes of WHF, attempt to predict who will develop WHF, and identify new therapies or specific algorithms to optimally treat WHF (e.g., early mechanical support).
The findings of our analysis should be considered in light of a few key limitations. This was a retrospective analysis from a clinical trial. We used adjustment covariates that have been used in prior ASCEND-HF analyses. Despite covariate adjustment, other measured and unmeasured variables may have influenced these results. ASCEND-HF is also a clinical trial with a set of inclusion and exclusion criteria that may differ from other populations. Despite these potential limitations, the strong association of WHF and outcomes warrants additional study, particularly related to early patient identification and potential therapies targeting WHF.
Conclusions
The results from this retrospective analysis of a large, international, clinical trial of patients with acute HF patients extends the findings of previous smaller acute HF studies. WHF is strongly associated with significantly increased risk of rehospitalization and mortality at 30 days and 180 days. WHF portends a poor prognosis regardless of whether it occurs early or late during hospitalization. WHF in hospitalized patients with acute HF represents a patient-centered outcome that should be recognized for its prognostic utility and may represent a focus of future treatment.
Acknowledgments
Funding source
The ASCEND-HF study was funded by Scios. This analysis was funded by the Duke Clinical Research Institute.
Ms. Elizabeth Cook and Ms. Morgan Deblecourt provided editorial assistance.
Footnotes
Disclosures:
All authors have approved the final article.
Kelly: Receives funding from Duke’s T32 Training grant (NIH Ruth L. Kirschstein NRSA Institutional Research Training Grant: 5 T32 HL 7101-39).
Mentz: Honoraria from Thoratec; Research support from Amgen, AstraZeneca, BMS, GSK, Gilead, Novartis, Otsuka, and ResMed.
Hasselblad: Nothing to report.
Ezekowitz: Consulting fees from AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Abbott Labs, Medtronic, Pfizer, and Servier; Research grants from Alere, Amgen, Merck, Ortho-Biotech/Johnson & Johnson, and Trevena.
Armstrong: Consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, F. Hoffmann-LaRoche Ltd., Axio/Orexigen, Eli Lilly, and Bayer; Research grants from Boehringer Ingelheim, Merck Sharp & Dohme, GlaxoSmithKline, Amylin Pharmaceuticals, Merck, Sanofi-Aventis, and Regado Biosciences.
Zannad: Consulting fees/Honoraria from Novartis, Servier, ResMed, Pfizer, biomérieux, Mitsubishi, Gambro, Biotronik, Janssen, St Jude Medical, Takeda, and Bayer; Speaker’s bureau for AstraZeneca and Daiichi Sankyo; Ownership/Partnership/Principal in CardioRenal Diagnostics; Research grants from Boston Scientific.
Felker: Consulting from Novartis, Amgen, Celladon, Trevena, Singulex, Roche Diagnostics, Medtronic; Grant support from NIH, Novartis, Amgen, Ostuka.
Califf: Full disclosures available at: https://www.dcri.org/about-us/conflict-of-interest/Califf-COI_Jan-March_2014.docx.
O’Connor: Consulting fees/Honoraria from Actelion Pharmaceuticals Ltd., and Amgen; Research/Research grants from BG Medicine, Critical Diagnostics, Gilead, Otsuka, ResMed, and Roche Diagnostics; Ownership interest/Partnership in Biscardia, LLC.
Hernandez: Research funding: Amgen, BMS, Janssen, Novartis; Honoraria: Amgen, Janssen, Novartis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 2.DeVore AD, Hammill BG, Sharma PP, et al. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001088. pii: e001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 4.Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16:922–30. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 5.Massie BM, O’Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 8.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–11. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Teerlink JR, Metra M, Voors AA, et al. Prevention of worsening heart failure by serelaxin in patients admitted for acute heart failure: Results from RELAX-AHF. Presented at: European Society of Cardiology Congress; September 2014; Barcelona, Spain. [Google Scholar]
- 13.Torre-Amione G, Milo-Cotter O, Kaluski E, et al. Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. J Card Fail. 2009;15:639–44. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor CM, Mentz RJ, Cotter G, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–12. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 15.Cotter G, Metra M, Weatherley BD, et al. Physician-determined worsening heart failure: a novel definition for early worsening heart failure in patients hospitalized for acute heart failure--association with signs and symptoms, hospitalization duration, and 60-day outcomes. Cardiology. 2010;115:29–36. doi: 10.1159/000249280. [DOI] [PubMed] [Google Scholar]
- 16.Weatherley BD, Milo-Cotter O, Felker GM, et al. Early worsening heart failure in patients admitted with acute heart failure--a new outcome measure associated with long-term prognosis? Fundam Clin Pharmacol. 2009;23:633–9. doi: 10.1111/j.1472-8206.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez AF, O’Connor CM, Starling RC, et al. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) Am Heart J. 2009;157:271–7. doi: 10.1016/j.ahj.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute Inc; 1995. [Google Scholar]
- 19.McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298:2009–19. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 20.Mentz RJ, Cotter G, Cleland JG, et al. International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short-term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial. Eur J Heart Fail. 2014;16:614–24. doi: 10.1002/ejhf.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotter G, Dittrich HC, Weatherley BD, et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. 2008;14:631–40. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]