Abstract
Defective DNA damage response is a threat to genome stability and a proven cause of tumorigenesis. C21ORF2 (chromosome 21 open reading frame 2) is a novel gene on chromosome 21, and the C21ORF2 protein is found to interact with NEK1. Earlier studies showed that C21ORF2 might be associated with some human genetic diseases including Down syndrome. However, the cellular functions of C21ORF2 remain unknown. In the present study, we reported that C21ORF2 affected cell proliferation after DNA damage induced by ionizing radiation, and DNA repair was less efficient in C21ORF2-depleted cells compared with control cells. However, C21ORF2-knockdown cells did not show defects in the activation of the G2-phase DNA damage checkpoint. Furthermore, homologous recombination, but not non-homologous end joining repair, was found to be impaired after C21ORF2 attenuation, which could be rescued by the overexpression of NEK1, indicating that C21ORF2 functions in the same pathway as NEK1 in DNA damage repair.
Keywords: NEK1 interactor, C21ORF2, DNA damage repair, ionizing radiation, G2-phase checkpoint
Introduction
In a large-scale mass spectrometry screen to identify endogenous protein complexes [1], C21ORF2 (chromosome 21 open reading frame 2) was found to be in the same complex of NEK1. C21ORF2 is widely expressed in different types of tissues (GCID: GC21M045749). However, cellular functions of C21ORF2 are still elusive. Studies on the genetic locus suggested that C21ORF2 might be associated with a series of human genetic diseases, such as Down syndrome (DS), which is caused by trisomy of chromosome 21 [2]. A reduction of C21ORF2 expression was observed in DS patients, and these patients were reported to be more sensitive to DNA damage [2,3], suggesting a possible association between C21ORF2 expression and the DNA damage response (DDR). Given the interaction between C21ORF2 and NEK1 and the involvement of NEK1 in DDR [4,5], we sought to determine whether C21ORF2 is involved in DDR. Here we reported that C21ORF2 is required for efficient DNA damage repair, but not for DNA damage checkpoint activation. Furthermore, we provided evidence that C21ORF2 and NEK1 work together in the same pathway to regulate DNA damage repair.
Materials and Methods
Cell culture and treatment
HeLa cells, U2OS cells, and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Gaithersburg, USA), supplemented with 10% fetal bovine serum (Sigma, St Louis, USA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, USA). Cells were incubated under 5% CO2 at 37°C. pGIPZ-C21ORF2-shRNA plasmids were obtained from Open Biosystems (Waltham, USA) for the knockdown of the expression of C21ORF2. To generate the lentivirus for shRNA knockdown, shRNA vectors were co-transfected into 293T cells with virus-packaging plasmids pMD2.G and psPAX2 by Lipofectamine 2000 (Invitrogen). HeLa cells were infected with respective lentiviruses and selected with puromycin (5–10 μg/ml; Sigma). After selection, the cells were recovered for 1 day and subject to analyses.
For the siRNA knockdown assay, siRNA duplexes against C21ORF2 and NEK1 (Sigma; predesigned siRNA pools) were transfected into HeLa cells by Lipofectamine 2000, according to the manufacturer's instructions.
To synchronize HeLa cells at G2 phase, exponentially growing cells were first treated with 2.5 mM thymidine-containing medium for 16 h, washed with phosphate-buffered saline (PBS) twice, and released into fresh culture medium for 8 h. The cells were then subject to another round of thymidine treatment for 16 h and released into the fresh medium. After the release, the cells were monitored under a microscope and with fluorescence-activated cell sorting (FACS) analysis to ensure synchronization. The cells were in G2 phase at about 4.5 h after the release (Supplementary Fig. S1).
Construction of plasmids
To construct the C21ORF2-HA-expressing construct, C21ORF2 cDNA (Open Biosystems) was cloned into pENTR-EF1A vector (forward primer, 5′-CATGCCATGGGGCATATGAAGCTGACGCGGAAGAT-3′; reverse primer, 5′-GGTGGGCGCGCCCACCCTCGGCGTGCTCCTGCACCT-3′) and then transferred into pHAGE-EF-Puro-Dest vector by LR recombination using LR recombinase (Invitrogen). HA-tag was at the C-terminal end of the fusion protein.
The HA-tagged NEK1-expressing (HA-NEK1) plasmid was a generous gift from Dr Lee Zou in Massachusetts General Hospital (MGH; Boston, USA) [5].
Growth curve analysis
For growth curve analysis, 3 × 105 cells were plated into 6 cm plates at day 0 and collected in subsequent days. The cells were counted using a cell counter (Beckman Coulter, Brea, USA), following the manufacturer's instructions.
Western blot and immunostaining analysis
For western blot analysis, cells were collected and lysed in RIPA buffer [50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.02% NaN3, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 1% NP-40], supplemented with protease-inhibitor mixture (Roche, Basel, Switzerland). Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, USA). Total protein (50 μg/sample) was separated in SDS–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane (Bio-Rad). The membrane was blocked in 5% milk/TBST at room temperature for 1 h and incubated overnight with the primary antibodies at 4°C. The primary antibodies used were anti-C21ORF2 antibody (homemade against full-length human protein), anti-NEK1 antibody (a generous gift from Dr Lee Zou at MGH), and anti-GAPDH, anti-MYC, and anti-HA antibodies (Santa Cruz Biotechnology, Santa Cruz, USA). After being washed with TBST, the membrane was incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (Bio-Rad) for 1 h at room temperature, and then the immunoreactive bands were visualized using the ECL detection kit (Thermo, Waltham, USA).
For immunostaining analysis, cells were fixed in 4% paraformaldehyde/PBS for 10 min, permeabilized in 0.4% Triton X-100/PBS for 15 min, and blocked in 5% bovine serum albumin (BSA)/PBS for 1 h at room temperature. Blocked cells were incubated with the primary antibody (diluted in 3% BSA/0.1% Triton X-100/PBS) at 4°C overnight and washed with PBS. Fluorochrome-conjugated secondary antibodies (Bio-Rad) were incubated for 1 h at room temperature in the dark. Cells were counterstained with DAPI (Sigma) for 5 min and washed with PBS. Fluorescent images were captured under the microscope (E800; Nikon, Tokyo, Japan) using a digital camera (SPOT-RT model 2.3.1; Diagnostic Instruments Inc., San Diego, USA). Antibodies used for immunostaining were as follows: anti-γH2AX (Cell Signaling, Beverly, USA), anti-MYC, and anti-HA (Santa Cruz Biotechnology).
Mitotic index analysis
For mitotic index analysis, cells were fixed with 70% ethanol at −20°C overnight, permeabilized in 0.4% Triton X-100/PBS for 15 min, and stained with propidium iodide (PI; Sigma). Percentages of mitotic cells (cells with DNA condensation) were counted under the microscope (E800).
Comet assay
Comet assay was performed according to the manufacturer's instructions of the Comet assay kit (Trevigen, Gaithersburg, USA). Briefly, cells were mixed with LMAgarose at 37°C and immobilized onto CometSlides. Then, the slides were immersed in the lysis solution for 1 h on ice and washed with the neutral electrophoresis buffer. After electrophoresis for 1 h at 4°C, the slides were immersed in the DNA precipitation solution for 30 min at room temperature, transferred to 70% ethanol for 30 min, dried in air, and stained with PI to visualize DNA. Fluorescent images were captured under the microscope (E800), and tail moments were analyzed by the software (TriTek Comet Score, free version).
Co-immunoprecipitation (co-IP) analysis
To perform co-IP analysis, empty vector and HA-NEK1 plasmids were transfected into HeLa cells by Lipofectamine 2000. Forty-eight hours after transfection, cells were collected and lysed in NETN buffer [20 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, and 0.5% NP-40], supplemented with protease-inhibitor mixture (Roche). The lysates were first incubated with anti-HA antibody (Millipore, Billerica, USA) at 4°C for 2 h and then with protein G agarose beads (GE Healthcare) at 4°C for 1 h. The immunoprecipitates were washed four times with NETN lysis buffer and eluted in 2× SDS loading buffer for subsequent western blot analysis. C21ORF2 expression was detected by western blot analysis as described earlier with anti-C21ORF2 antibody (homemade).
HR and NHEJ assay
Homologous recombination (HR) assay was performed as described previously [6]. Briefly, U2OS cells expressing the DR-GFP reporter were transfected with siRNA against C21ORF2 or NEK1 (predesigned siRNA pools). Forty-eight hours later, cells were co-transfected with pCBA-SceI and pCAGGS-mCherry (pCBA-SceI to induce double-strand breaks and pCAGGS-mCherry as a control for transfection efficiency). The cells were collected 48 h after transfection for flow cytometry analysis to detect GFP-positive and mCherry-positive cells with LSRFortessa and analyzed by BD FACSDiva software (Franklin Lakes, USA) at Cytometry and Cell Sorting Center of Baylor College of Medicine.
Non-homologous end joining (NHEJ) assay was performed as described previously [7]. In brief, U2OS cells were transfected with siRNA against C21ORF2 or NEK1. Seventy-two hours later, cells were co-electroporated with linearized pCSCMV:tdTomato (digested with BamHI between the promoter and the tdTomato coding sequence) and pEGFP-C1 plasmid (as a control for electroporation efficiency). Five hours after electroporation, cells were collected for FACS analysis to detect tdTomato-positive and GFP-positive cells.
Cells for HR and NHEJ assays were obtained from Dr Shiaw-Yih Lin's laboratory [6]. Plasmids for HR and NHEJ assays were obtained from Addgene (plasmid nos 26477, 41583, and 30530; Cambridge, USA).
Statistical analysis
Statistical analysis of data was performed by Student's t-test. A value of P < 0.05 was considered statistically significant.
Results
C21ORF2 depletion leads to the decreased growth potential and delayed mitotic entry after IR treatment
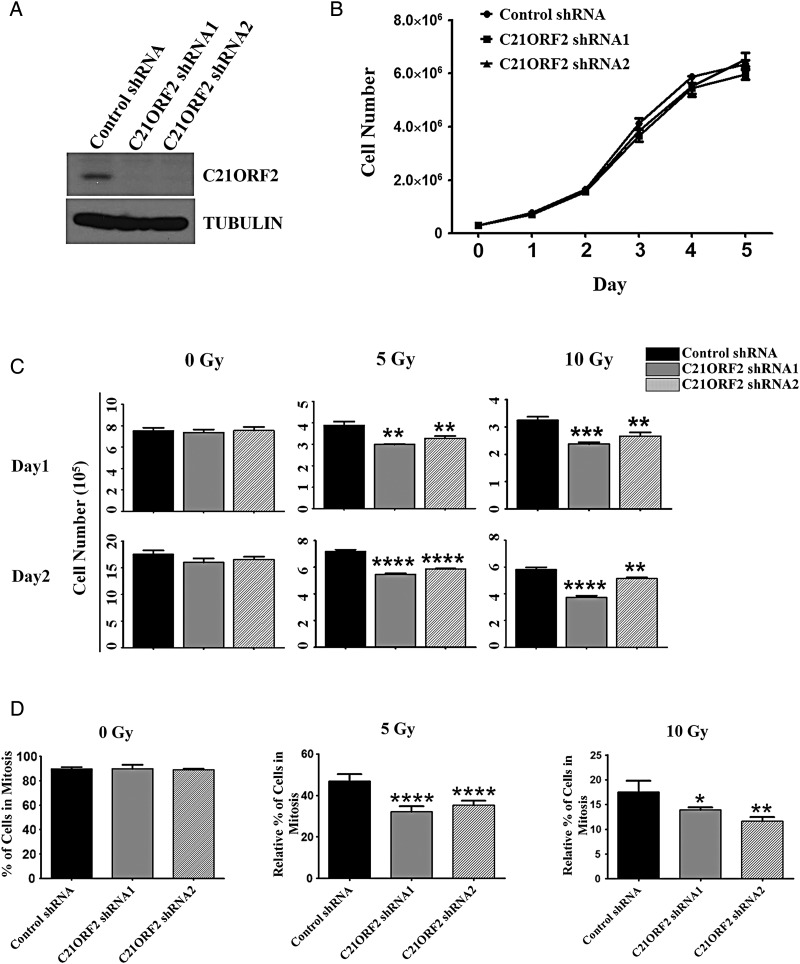
The interaction between C21ORF2 and NEK1 (Supplementary Fig. S2) was confirmed by co-IP. To determine whether C21ORF2 is involved in the DDR, we examined whether C21ORF2-knockdown cells were more sensitive to DNA damage than control cells. First, whether C21ORF2 was required for cell proliferation under normal conditions was tested. Two independent C21ORF2-stable-knockdown cell lines were generated by infecting HeLa cells with lentivirus expressing shRNAs against C21ORF2 (Fig. 1A). From the growth curve analysis, no significant difference in growth potential among control and C21ORF2-knockdown cells was observed (Fig. 1B), indicating that the C21ORF2 status did not affect the proliferation of cells. Next, whether cell proliferation was affected after ionizing radiation (IR) in C21ORF2-depeleted cells was examined. Control and C21ORF2-knockdown cells were synchronized at G1/S phase by double thymidine treatment and released into G2 phase. The synchronized cells were irradiated at different dosages, and the growth curve analysis was performed. A significant reduction in cell proliferation was found in C21ORF2-knockdown groups compared with control groups after treating cells with IR at both 5 and 10 Gy (Fig. 1C), indicating an increased sensitivity to IR and defects in the DDR in C21ORF2-knockdown cells.
Figure 1.
C21ORF2-depleted cells show the decreased proliferation and delayed mitotic entry after IR (A) Western blot analysis of C21ORF2 expression in HeLa cells. (B) Growth curve analysis of control and C21ORF2-knockdown cells. Control and C21ORF2-shRNA-knockdown cells (3 × 105) were plated into 6 cm plates. Cells were collected at indicated time points after plating, and the cell number in each group was counted. (C) Growth curve analysis of control and C21ORF2-knockdown cells after IR treatment. Control and C21ORF2-knockdown cells (3 × 105) were arrested at G1/S phase by double-thymidine (2.5 mM) treatment and released into G2 phase. The cells were then treated with IR at indicated dosages and plated in six-well plates. The cells were collected at indicated time points and counted. (D) Mitotic index analysis. Control and C21ORF2-shRNA-knockdown cells were synchronized at G2 phase as described previously, treated with IR at indicated dosages, and then released into the medium containing 100 ng/ml nocodazole. Cells were collected at 18 h after IR and prepared for mitotic index analysis. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Checkpoint activation and DNA repair are the two most important aspects of the DDR. Upon DNA damage, the DNA damage checkpoint is activated and cell cycle is arrested until the damage is repaired. The increased sensitivity to DNA damage in C21ORF2-depleted cells could be a result of defects either in checkpoint activation or in damage repair. To address this issue, control and C21ORF2-shRNA-knockdown cells were synchronized at G1/S phase, released into G2 phase, and irradiated. Immediately after the irradiation treatment, the culture media were switched to nocodazole-containing medium to arrest cells at mitosis. The mitotic cells were counted 18 h after IR under a microscope, and the mitotic indices were determined. Without IR treatment, there was no difference in the percentage of mitotic cells between control and C21ORF2-knockdown cells, indicating that knockdown of C21ORF2 did not affect G2/M transition in cells (Fig. 1D). As expected, IR blocked mitotic entry. However, both control and C21ORF2-depleted cells showed nearly identical mitotic blockage at two different IR dosages of 5 and 10 Gy (Fig. 1D). Similar results were obtained with siRNA-mediated knockdown of C21ORF2 (Supplementary Fig. S3). These results indicated that the G2-phase DNA damage checkpoint does not need C21ORF2 to function and suggested that the IR-induced reduction in proliferation of C21ORF2-depleted cells is likely caused by defects in DNA damage repair.
C21ORF2 is required for proficient DNA damage repair
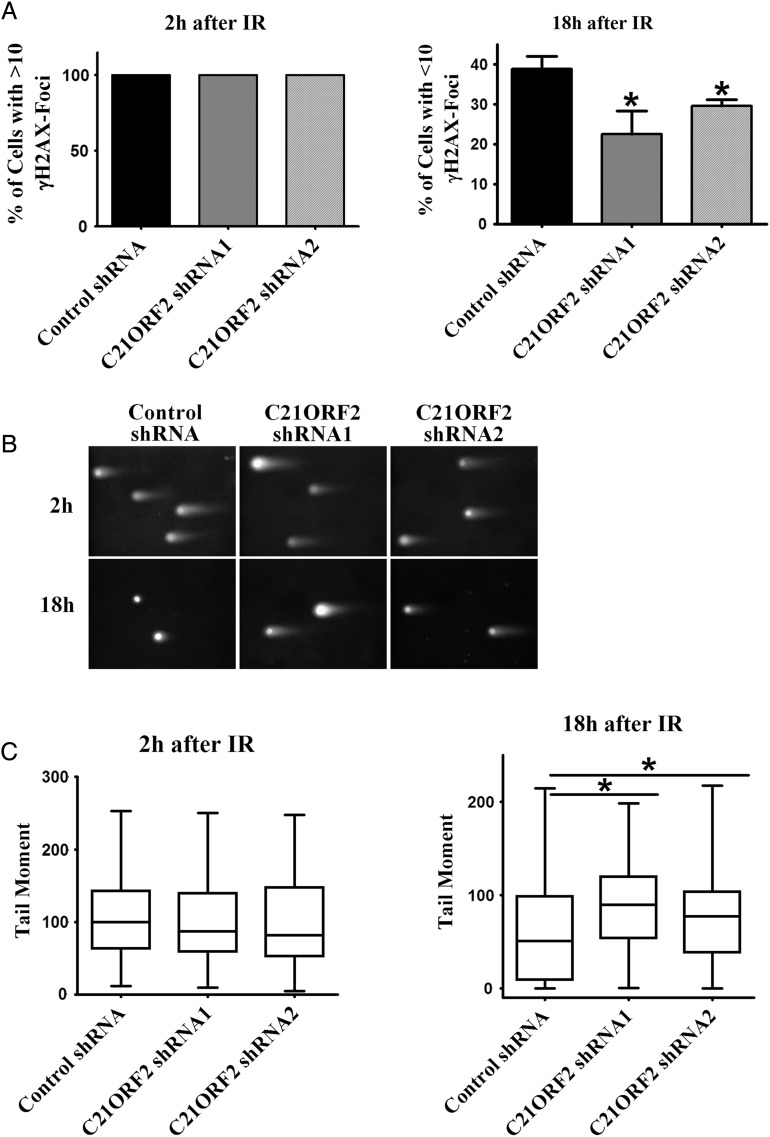
To determine whether C21ORF2 is required for DNA damage repair, the repair ability of control and C21ORF2-knockdown cells is assessed. DNA damage in cells can be determined by the number of γH2AX foci, which form at DNA lesions and serve as a general marker of DNA damage [4]. Cells with >10 γH2AX foci were counted as positive for DNA damage. The cells were synchronized at G2 phase as described previously and subject to IR treatment. Immunostaining for γH2AX was performed to detect DNA damage in the cells after IR treatment (Supplementary Fig. S4). Two hours after IR, 100% of the cells, whether or not C21ORF2 was depleted, were positive for DNA damage. Eighteen hours after IR, however, 38.9% of the control cells were repaired (with <10 γH2AX foci), whereas in the two C21ORF2-knockdown groups, only 22.6% and 29.6% of the cells were repaired, respectively (Fig. 2A). The decreased percentage of repaired cells in C21ORF2-knockdown groups indicated a defect in DNA repair. To further demonstrate the involvement of C21ORF2 in DNA damage repair, the comet assay was performed under neutral conditions to detect double-strand DNA breaks (DSBs). As shown by the measurements of tail moment, C21ORF2-knockdown cells showed more severe DNA damage than control cells at 18 h after IR treatment (Fig. 2B,C). Similar results were obtained from siRNA-mediated depletion of C21ORF2 (Supplementary Fig. S5). Taken together, these results demonstrated that C21ORF2 is required for proficient DSB repairs.
Figure 2.
Depletion of C21ORF2 leads to less-efficient DNA repair (A) Quantitation of γH2AX focus staining. Control and C21ORF2-knockdown cells were synchronized at G1/S phase by double-thymidine (2.5 mM) treatment and released into G2 phase as described previously. After synchronization, the cells were treated with IR at 5 Gy, collected at indicated time points, and prepared for immunostaining. (B) Comet assay (single cell electrophoresis) on cells from (A). (C) Quantitation of the results of (B). *P < 0.05.
C21ORF2 is involved in HR, but not in NHEJ-mediated DSB repair
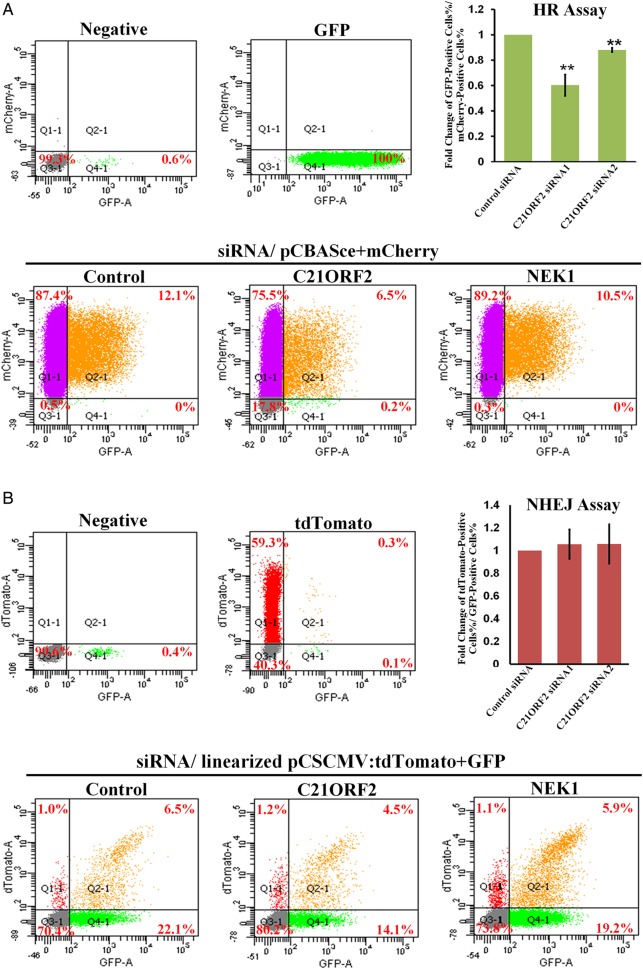
DSBs are repaired through two major pathways: HR and NHEJ [8]. The choice of repair pathway is determined by a number of factors, among which the cell cycle phase is the chief one [8]. Given the defects of repairing DSBs in C21ORF2-depleted cells, it is interesting to know which repair pathway is affected by the C21ORF2 deficiency. Therefore, HR and NHEJ DNA repair assays were performed. Knockdown of both C21ORF2 and NEK1 resulted in defective HR repair, as shown by significantly decreased percentages of GFP-positive (HR-repaired) cells (Fig. 3A), whereas no significant difference in the percentages of tdTomato-positive (NHEJ-repaired) cells was observed among control, C21ORF2-knockdown, and NEK1-knockdown cells (Fig. 3B).
Figure 3.
C21ORF2 is required for proficient HR repair C21ORF2 and NEK1 were knocked down by siRNA in U2OS cells. (A) FACS analysis of GFP- and mCherry-positive U2OS cells in HR assay. The upper right panel showed statistics of the results. (B) FACS analysis of tdTomato- and GFP-positive U2OS cells in the NHEJ assay. The upper right panel showed statistical results of the NHEJ assay. **P < 0.01.
C21ORF2 might participate in the DNA repair process via interaction with NEK1
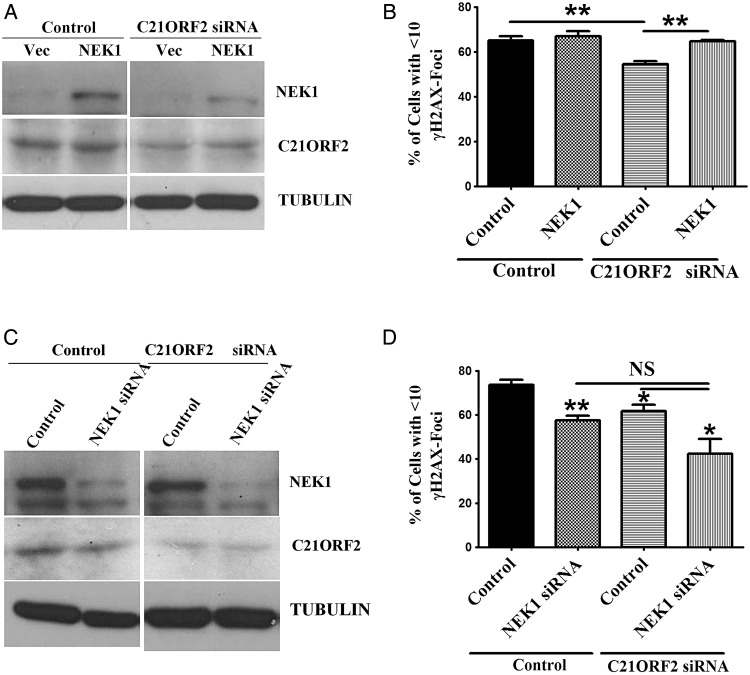
The fact that C21ORF2 interacts with NEK1 biochemically predicts that they interact functionally as well. Indeed, our aforementioned results showed that both these proteins were involved in the HR repair of DSBs, suggesting that they work together. Given that NEK1 is a large protein kinase, it is very likely that C21ORF2 assists NEK1 to function. Thus, the absence of C21ORF2 may simply be overcome with extra expression of NEK1. To that end, NEK1 was overexpressed in C21ORF2-depleted cells, and the ability of these cells to repair DSBs was examined. By the analysis of γH2AX foci formation, overexpression of NEK1 was found to significantly rescue DNA damage repair defects in C21ORF2-knockdown cells (Fig. 4A,B). Furthermore, the efficiency of DNA repair in C21ORF2 and NEK1 double-knockdown cells was examined. As expected, double-knockdown cells did not show more significant worsening of DNA repair than single-knockdown cells (Fig. 4C,D). Taken together, these results indicated that C21ORF2 and NEK1 function together in the repair of DSBs.
Figure 4.
C21ORF2 and NEK1 work together in DNA damage repair (A) Overexpression of NEK1 in C21ORF2-depleted cells. C21ORF2 was knocked down in HeLa cells by siRNA. Twenty-four hours later, C21ORF2-knockdown cells were transfected with HA-NEK1. Endogenous NEK1 cannot be visualized in the western blot analysis because of the short exposure time. (B) DNA damage repair assay. The cells in (A) were synchronized at G2 phase as described previously, treated with IR at 2 Gy, released into fresh medium, and collected for immunostaining of γH2AX at 24 h after IR. (C) Depletion of both C21ORF2 and NEK1. C21ORF2 siRNA was transfected into HeLa cells. Twenty-four hours after transfection, the cells were transfected with NEK1 siRNA. (D) DNA damage repair assay. The cells in (C) were synchronized in G2, treated with IR at 2 Gy, released into fresh medium, and collected for immunostaining of γH2AX at 24 h after IR. *P < 0.05 and **P < 0.01.
Discussion
The DDR is critical for genome integrity. Aberrant DDR is observed in cancers and other human diseases [9]. DDR invokes two cellular processes: checkpoint activation and damage repair. Upon DNA damage, the DNA damage checkpoint is activated and leads to cell cycle arrest, allowing time for DNA repair. Once DNA lesions are repaired, the checkpoint is released and cells can re-enter mitosis. However, if the damage is too severe, cells undergo senescence or apoptosis [10,11]. In this study, C21ORF2 was found to be required for efficient DNA damage repair, but not for G2-phase checkpoint activation. As a result, decreased proliferation was observed in C21ORF2-knockdown cells after DNA damage because of delayed mitotic entry (Fig. 1D and Supplementary Fig. S3). No obvious apoptosis was caused by DNA damage in C21ORF2-depleted cells, as indicated by (i) no increase of floating or senescent cells in the cell culture and (ii) no increase of apoptotic cells by nuclear staining with DAPI when observed under the microscope (Supplementary Figs. S4 and S6). However, whether aging is another cause of cell growth deficiency remains to be addressed. Moreover, asynchronized cells were arrested at different DNA damage checkpoints throughout the cell cycle, which might interfere with the observation at a specific cell cycle phase. Therefore, cells were synchronized at G2 phase in this study to determine whether G2-phase checkpoint was affected by C21ORF2 depletion. The effect of C21ORF2 depletion on G1-phase checkpoint will be examined in the future to further understand the function of C21ORF2 in DDR.
DDR has been studied in many model organisms, and a large number of proteins, including protein kinases, have been identified. NEK1 was initially known to regulate ciliogenesis [12,13] and was later found to play a role in DDR [4,5]. In this study, we reported that C21ORF2 complexes with NEK1 and plays a role in DNA damage repair but not in checkpoint activation. We showed that the repair defects in C21ORF2-depleted cells could be rescued by NEK1 overexpression, suggesting that C21ORF2 helps but is not absolutely required for NEK1's function in DNA damage repair. Interestingly, only HR-dependent DSB repair requires NEK1/C21ORF2, whereas NHEJ-dependent repair does not. Identification of the NEK1 substrate in HR repair will shed light on this important process. Moreover, the foci formation of the critical participants in DSB repair, such as RAD51 and phosphorylated RPA [14], remains to be examined in C21ORF2-depleted cells in the future to clarify the function of C21ORF2 in HR repair.
To better understand the role of C21ORF2 in DDR, the subcellular localization of C21ORF2 was examined by stably expressing HA-tagged C21ORF2 in HeLa cells using lentivirus infection. C21ORF2 was observed to be localized to the cytoplasm in these cells (Supplementary Fig. S6A,B). Furthermore, C21ORF2 was not recruited to DNA lesions after DNA damage, as no irradiation-induced foci formation (IRIF) was observed after the treatment of IR (Supplementary Fig. S6C). In addition, C21ORF2 did not change its localization after IR (Supplementary Fig. S6C), indicating that C21ORF2 might indirectly regulate the DDR. Previous results showed that NEK1 was translocated to the nucleus from the cytoplasm and formed IRIF upon IR treatment [15]. Given the interaction between C21ORF2 and NEK1, it is possible that C21ORF2 mediates translocalization of NEK1 to the nucleus upon DNA damage. However, further studies are still needed to fully understand the role of C21ORF2 in DDR.
Supplementary Data
Funding
This work was supported by the grants from the National Institutes of Health (CA116097 and CA122623).
Supplementary Material
Acknowledgement
We thank Dr Lee Zou at Massachusetts General Hospital and Dr Shiaw-Yih Lin at University of Texas MD Anderson Cancer Center for providing various reagents.
References
- 1.Malovannaya A, Lanz RB, Jung SY, Bulynko Y, Le NT, Chan DW, Ding C et al. Analysis of the human endogenous coregulator complexome. Cell 2011, 145: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shim KS BJ, Furuse M, Ovod V, Krude T, Lubec G. Reduction of chromatin assembly factor 1 p60 and C21orf2 protein, encoded on chromosome 21, in Down syndrome brain. J Neural Transm Suppl 2003, 67: 117–128. [DOI] [PubMed] [Google Scholar]
- 3.Morawiec Z, Janik K, Kowalski M, Stetkiewicz T, Szaflik J, Morawiec-Bajda A, Sobczuk A et al. DNA damage and repair in children with Down’s syndrome. Mutat Res 2008, 637: 118–123. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chen PL, Chen CF, Jiang X, Riley DJ. Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle 2008, 7: 3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Ho CK, Ouyang J, Zou L. Nek1 kinase associates with ATR-ATRIP and primes ATR for efficient DNA damage signaling. Proc Natl Acad Sci USA 2013, 110: 2175–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng G, Yim EK, Dai H, Jackson AP, Burgt I, Pan MR, Hu R et al. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat Cell Biol 2009, 11: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sotiropoulou PA, Candi A, Mascre G, De Clercq S, Youssef KK, Lapouge G, Dahl E et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol 2010, 12: 572–582. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res 2008, 18: 134–147. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009, 461: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411: 366–374. [DOI] [PubMed] [Google Scholar]
- 11.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 2008, 8: 512–522. [DOI] [PubMed] [Google Scholar]
- 12.White MC, Quarmby LM. The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 2008, 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalom O, Shalva N, Altschuler Y, Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 2008, 582: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 14.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010, 40: 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div 2011, 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.