Abstract
Although radiation therapy is an important cancer treatment modality, patients may experience adverse effects. The use of a radiation-effect modulator may help improve the outcome and health-related quality of life (HRQOL) of patients undergoing radiation therapy either by enhancing tumor cell killing or by protecting normal tissues. Historically, the successful translation of radiation-effect modulators to the clinic has been hindered due to the lack of focused collaboration between academia, pharmaceutical companies and the clinic, along with limited availability of support for such ventures. The U.S. Government has been developing medical countermeasures against accidental and intentional radiation exposures to mitigate the risk and/or severity of acute radiation syndrome (ARS) and the delayed effects of acute radiation exposures (DEARE), and there is now a drug development pipeline established. Some of these medical countermeasures could potentially be repurposed for improving the outcome of radiation therapy and HRQOL of cancer patients. With the objective of developing radiation-effect modulators to improve radiotherapy, the Small Business Innovation Research (SBIR) Development Center at the National Cancer Institute (NCI), supported by the Radiation Research Program (RRP), provided funding to companies from 2011 to 2014 through the SBIR contracts mechanism. Although radiation-effect modulators collectively refer to radioprotectors, radiomitigators and radiosensitizers, the focus of this article is on radioprotection and mitigation of radiation injury. This specific SBIR contract opportunity strengthened existing partnerships and facilitated new collaborations between academia and industry. In this commentary, we assess the impact of this funding opportunity, outline the review process, highlight the organ/site-specific disease needs in the clinic for the development of radiation-effect modulators, provide a general understanding of a framework for gathering preclinical and clinical evidence to obtain regulatory approval and provide a basis for broader venture capital needs and support from pharmaceutical companies to fully capitalize on the advances made thus far in this field.
INTRODUCTION
Radiation therapy, either alone or in combination with other treatment modalities, is important in the care of millions of patients with malignancies involving different organ sites. However, patients may experience adverse effects due to exposure of normal tissues adjacent to the tumors (1). Physical methods such as intensity modulated radiation therapy and proton radiation therapy have been developed to decrease these adverse effects. While randomized clinical trials are currently in progress for proton vs. photon radiotherapy for comparison of efficacy, there is no level 1 evidence of any decrease in adverse effects by the use of protons to date (2). Even if such studies show positive outcomes, there is potential to further reduce these adverse effects using radioprotectors or radiomitigators. Adverse effects of radiation therapy may be acute (occurring during the first few days or weeks immediately after treatment), intermediate (within weeks to months) or late (months to years after treatment). The availability of agents to prevent (pre-exposure or protection) or mitigate (post exposure) these adverse effects could substantially improve radiation therapy outcomes (3). Furthermore, given the steady increase in the number and longevity of cancer survivors over the last few decades (4), many people would benefit from the improved health-related quality of life (HRQOL) resulting from more effective and less toxic radiation and cancer therapy. In previous publications regarding radiation injury, we have emphasized the importance of countering specific adverse effects of radiation therapy (when used alone or combined with other modality treatment) to improve outcome and HRQOL (1, 2). In addition, we discussed specific examples of acute, intermediate and late-occurring toxicities from radiotherapy and potential strategies for protection, mitigation and treatment (5, 6), and we proposed a general drug development schema to improve radiation therapy (5, 7).
Although radiation-effect modulators collectively refer to radioprotectors, radiomitigators and radiosensitizers, the focus of this article is on radioprotection and mitigation of radiation injury. The use of radiation-effect modulators complements technological and imaging advances. Despite decades of preclinical research, there has been limited translation of radioprotectors and mitigators from bench to bedside with notable successes. Examples of notable successes include amifostine (8), pentoxifylline (9), pentoxifylline combined with tocopherol (10) and ACE inhibitors (11). The limited success in translation is due to the complexity of long-term tissue injury, the need for long-term studies and an established market for such drugs. With new information emerging on tissue injury and repair and the opportunity to repurpose drugs in the clinic, it is an ideal time to enhance collaboration between academia and small businesses in this area.
After the terrorist attacks on September 11, 2001 in the U.S., the development of countermeasures gained importance as a response to a variety of potential scenarios, while global concerns have increased regarding the risks of mass casualties resulting from radiation exposures. The U.S. Government has played a key role in the development of drugs and biologics as medical countermeasures (MCMs) for acute radiation syndrome (ARS) and for delayed effects of acute radiation exposure (DEARE) (12). While the MCM program is developing drugs approved by the U.S. Food and Drug Administration (FDA) that are indicated for use as radiation injury mitigators after a nuclear detonation, the National Cancer Institute’s (NCI) interest is to build on these research and development efforts and investments to explore their potential use for cancer treatment, a concept of “dual utility,” which would encompass newly developed drugs as well as repurposed drugs already in clinical practice for other indications.
With the objective of developing radioprotectors and radiomitigators building from the MCM programs at various government agencies as well as de novo development, the Radiation Research Program (RRP) and the NCI Small Business Innovation Research (SBIR) Development Center issued a new funding opportunity announcement (FOA) in 2010, which was renewed and expanded through 2014. This specific request for proposals (RFP) inspired new collaborations among academia and small businesses and provided funding to such efforts beginning in 2011.
This commentary provides: 1. A summary of the advances made with this funding effort to meet the focused goal of developing radiation-effect modulators for potential applications in the clinic; 2. A description of the application review process; 3. A discussion on the organ/site-specific clinical needs for the development of radioprotectors/mitigators; 4. A proposed framework for collecting preclinical and clinical evidence to obtain regulatory approval; and 5. A discussion on the value of a broader initiative with venture capital and pharmaceutical companies to capitalize on the advances already made in this field.
SBIR FUNDING INITIATIVE
The SBIR program is congressionally mandated and has allocated funding that provides early-stage technology financing to eligible U.S. small business entities (defined as an organized “for profit” entity with a place of business located in the U.S. and less than 500 employees). At the NCI, this program has an annual total budget of approximately $124M to provide funding to small businesses that develop and commercialize novel technologies and products to prevent, diagnose and treat cancer through grants and research contracts. The SBIR program, in collaboration with the RRP, requested proposals from small businesses between 2011 and 2014 to develop radiation-effect modulators (http://sbir.cancer.gov/funding/contracts/pastcontracts). The overall goals of this specific topic were to: 1. Incentivize small businesses to accelerate their research and development by fostering collaborations with academia, contract research organizations and other partners; and 2. Strengthen its translation to radiation oncology clinics to counter the side effects of radiotherapy. While the first year’s RFP solicited proposals exclusively intended to develop radioprotectors and mitigators, RFPs during the subsequent years also included radiosensitizers that are not discussed in this commentary.
Proposals received under these multiyear solicitations (topic 291 in 2011 and 2012, topic 323 in 2013 and topic 332 in 2014) included preclinical and/or early-phase clinical studies investigating safety, efficacy, dose, schedule, pharmacokinetics (PK), pharmacodynamics (PD) and metabolism. The SBIR contract funding mechanism is structured in two phases. The objectives of a phase I SBIR contract are to establish the technical merit and feasibility of the proposed research and to determine the quality of performance of the small business awardee organization prior to providing further federal support in a phase II SBIR contract for full-scale development. The phase I budget ranged from $200K to $300K for nine months of support. Successful SBIR phase I awardees who achieved their key milestones were invited to apply for phase II awards that were typically $1.5–$2M in support for two years. Awardees were required to follow a timeline to meet specific milestones and a logical path towards clinical application, with close monitoring of progress by the NCI program staff at various stages of development. “Fast-track” proposals, in which offerors could submit for phase I and II simultaneously, were also permitted. “Fast-track” funding provides an opportunity for the awardee to transition immediately to phase II upon successful completion of phase I. This is a unique feature of SBIR contracts that is not present in the typical RO1 grant mechanism and is therefore attractive to potential applicants.
PROPOSALS
SBIR phase I proposals were required to include a description of 1. The significance of the problem being addressed; 2. The research design that would be used to gather evidence that the proposed compound protects at least one relevant normal tissue from radiation-induced injury and does not protect relevant cancer cells; 3. The methodologies to evaluate preferential effects on normal tissues in vivo by the compound; 4. The drug’s mechanism of action for efficacy and specificity; 5. Evidence of a lack of in vivo toxicity; 6. A plan to optimize dose and schedule based on preclinical pharmacokinetic and pharmacodynamics studies; and 7. A statistical analysis of the proposed study end points. Early interaction with the FDA was encouraged for developing a plan to obtain regulatory approval of the drug for clinical use.
SBIR phase II proposals for performing advanced preclinical work were required to address the following in detail: 1. The design of in vivo experiments, including statistical analysis of experimental design/sample size, with power calculations as well as Institutional Animal Care and Use Committee (IACUC) approval; 2. The selection of a tumor cell panel and normal tissues for in vitro testing; 3. Demonstration of bioavailability, PK and PD in a suitable rodent model; 4. Demonstration by physiologic testing and histological assessment that irradiated normal tissues are spared from radiation toxicity over a six-month period; 5. The collection of data on efficacy and specificity for normal cells over tumor cells and validation of lack of drug toxicity; and 6. The development of a strong commercialization strategy for advancing these technologies through clinical trials and ultimately to patients.
For proposals advancing to early-phase human trials the offeror needed to: 1. Identify a good manufacturing practice (GMP) drug source and obtain investigational new drug (FDA-IND) approval; 2. Provide evidence of established clinical collaboration and submit a protocol for Institutional Review Board (IRB) approval; 3. Take into consideration, as appropriate, the relevant molecular pathways and targets, and aim to gather PK and PD data confirming the compound’s observed behavior in animal studies; and 4. Describe the approach to assess the safety and efficacy employing, as appropriate, physician-reported end points as well as patient-reported outcomes.
“Fast-Track” proposals were required to outline and pursue a regulatory approval plan, which needed to include filing an IND, and designing and performing phase 0–II clinical trials in preparation for product transition to phase III clinical trials by groups such as NRG Oncology [a nonprofit research organization formed to conduct clinical research, which brings together the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group (RTOG) and the Gynecologic Oncology Group (GOG)]. When cooperation of other partners was critical for implementation of the proposed methodology, applicants were required to show evidence of this cooperation with a partnering arrangement, letters of support, etc.
Table 1 provides a breakdown of the number of proposals received for this topic and funded in the years 2011–2014. The first year’s RFPs (2011) on radiation-effect modulators solicited proposals specifically intended to develop radio-protectors and mitigators. RFPs issued in the subsequent years (2012–2014) also included radiosensitizers. The focus of this commentary is on radioprotectors and mitigators, as progress in this field is more mature compared to radiosensitizer development, for which the RFPs came later.
TABLE 1.
Number of Proposals Received and Funded in Response to Request for Proposals on the Topic Radiation-Effect Modulators
| Year
|
Total | ||||
|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | ||
| Phase I | |||||
| Received | 10 | 5 | 2 | 9 | 26 |
| Funded | 3 | 2 | 1 | 3 | 9 |
| Fast track | |||||
| Received | 1 | 2 | 2 | 2 | 7 |
| Funded | 0 | 2 | 1 | 0 | 3 |
| Transition to phase II from phase I or fast track | 1 | 2a | 1a | Unavailable | 4 |
| Total | |||||
| Received | 11 | 7 | 4 | 11 | 33 |
| Funded | 3 | 4 | 2 | 3 | 12 |
Transitioned from fast track.
REVIEW OF PROPOSALS AND AWARD
The proposals underwent peer review by well-rounded special emphasis review panels organized by the NCI’s Division of Extramural Affairs. These panels consisted of subject matter experts from radiobiology, radiation oncology, biochemistry, pharmacology, biotechnology industry, regulatory affairs and statistics. SBIR phase I proposals were evaluated based on: soundness and technical merit, qualifications of the principal investigator, supporting staff and consultants, potential for technological innovation and commercial application and the facilities and research environment. Review of SBIR phase II proposals included the above with an added emphasis on commercial potential.
A general description of the SBIR program and funding opportunities is available online (http://sbir.cancer.gov/). The goal of this specific RFP is to encourage small businesses to develop novel radiation-effect modulators. Many aspects are unique to the SBIR contract funding mechanism and these are different from the investigator-initiated academic or SBIR grants. Contracts are awarded to companies after the review of proposals as described above. Offerors are required to develop a statement of work (SOW) in coordination with the program staff, taking into consideration reviewers’ comments and providing specific milestones before the awards could be made. An SBIR program official serves as the contracting officer’s technical representative. The SOW and specific milestones ensure close monitoring of the progress towards commercialization. Transition from a phase I to phase II SBIR contract is dependent on successful completion of all phase I milestones and the scientific and technical merit of the phase II proposal. These aspects are novel to the SBIR contract mechanisms of funding.
FRAMEWORK FOR RADIATION EFFECT MODULATOR DEVELOPMENT
The rationale for using radiation-effect modulators in the clinic is to increase therapeutic gain either by reducing normal tissue injury or enhancing tumor control during radiotherapy (3). Figure 1 shows a generalized framework for radiation-effect modulator development. It is a multistep process that may involve acquisition of an innovative drug and/or a therapeutic or a formal collaboration for their potential use in radiation oncology clinics after regulatory approval, which is as described as follows (a–k):
The first step in the process is the discovery or “sourcing” of a potentially useful drug in radiation oncology (i.e., the underlying innovation from academia or some small businesses).
Focused development towards solving an organ/site-based clinical problem by using a mitigator or protector and possibly a molecular target approach for enhancing the radiation effects on a tumor.
Synthesis of the compound in adequate quantities for further development.
Some evidence of organ/site-specific or molecular-target-based activity.
Evaluation of mechanism of action using suitable in vitro cell culture models.
Formulation and dose/schedule optimization and normal tissue toxicity studies demonstrating evidence of efficacy in a drug screen and studies on absorption, distribution, metabolism, excretion and toxicity (ADMET) in a preclinical animal model.
Further evaluation using appropriate assays such as tumor regrowth delay studied in suitable animal models. Such in vivo models may include an orthotopic- or patient-derived xenograft (PDX) or genetically engineered mouse models (GEMM), ideally used in a combined treatment modality (similar to the standard of care (SOC) for a given cancer type) in a fractionated radiotherapy setting, if the intention is to improve SOC.
Phase I clinical trials to address safety involving a relatively small cohort of patients (3–6 patients), although, in some cases, a safety expansion cohort (10–20 patients) may be more appropriate.
Phase II clinical trials to address efficacy. Phase II clinical trials are performed on a larger group of patients to determine if the new treatment is effective. Although, generally the number of patients in a phase II clinical trial is in the range of 30–50, given the lower prevalence of radiotherapy-induced adverse effects likely to be observed in the clinic, the number of patients to be studied may be much larger to demonstrate efficacy for a given indication.
With sufficient demonstration of efficacy in phase II trials, a phase III trial will likely be needed to test the efficacy of the experimental treatment with a radiation-effect modulator over SOC. This might involve head-to-head comparison of one treatment with the other involving a larger number of patients in a randomized comparative trial (RCT) with accepted SOC, or a singlearm confirmatory trial with radiotherapy or chemo-radiotherapy as a control. The design of a clinical trial will require a pre-IND meeting with the Center for Drug Evaluation and Research (CDER) and the Oncology Drug Advisory Committee (ODAC).
k. Routine use in radiation oncology clinics after regulatory approval.
FIG. 1.
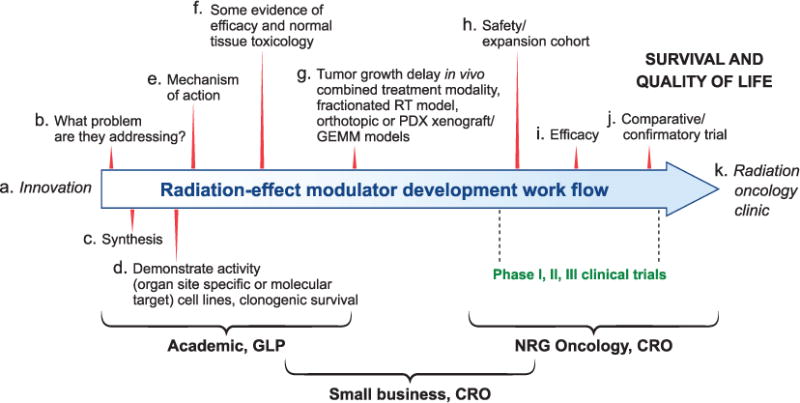
A generalized framework for radiation-effect modulator development including both radioprotectors/mitigators as well as radiation sensitizers. The development of a radiation-effect modulator is a multi-step process and may involve acquisition of an innovation from an academic laboratory for development and ultimately potential use in radiation oncology clinic after regulatory approval (proceeding in order from step a–k). CRO=contract research organization; GLP=good laboratory practice; NRG Oncology: a nonprofit research organization formed to conduct clinical research, which brings together the National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group (RTOG) and the Gynecologic Oncology Group (GOG).
A close interaction among academia, small businesses and clinical trial work groups such as NRG Oncology is necessary for a successful translation of a radiation-effect modulator for clinical use. Three important resources exist within the NCI to facilitate clinical trials. First, any company, an academic institute or a not-for-profit organization can propose an agent for the NCI’s Experimental Therapeutics (NexT) program irrespective of its developmental stage (Fig. 1a–j). If selected, NCI will provide assistance in the further development of the agent at the NCI or the Frederick National Laboratory for Cancer Research. Clinical trials will be conducted at the Center for Cancer Research (CCR) at the main NIH campus or through NCI-supported clinical trial network groups. Second, any company, an academic institute or a not-for-profit organization can directly submit an unsolicited letter of intent (LOI) to the Cancer Therapy Evaluation Program (CTEP) of NCI on a clinical trial concept where it will undergo a formal review. However, this opportunity is limited to phase II/III clinical trial proposals. Third, any company, an academic institute or a not-for-profit organization can directly present their proposal to the CTEP and discuss the possibility of bringing the agent to the CTEP portfolio through a cooperative research development agreement. This mechanism will be open for phase I trials that can be conducted within Experimental Therapeutics Clinical Trial Network (ETCTN) institutions.
In addition to the above resources, the Molecular Radiation Therapeutics Branch (MRTB) of the RRP is coordinating the activities of several site-specific disease clinical trial working groups and their work with radiation modifiers. A company, an academic institute or a not-forprofit organization can approach the MRTB for advice on necessary preclinical data to support new clinical trial concepts, recognizing that their opinion is independent of the FDA. These trial concepts can be submitted through clinical trials network groups, such as NRG Oncology, to the CTEP for funding. In summary, for a small business with limited resources, these multiple mechanisms provide potential avenues to support translation of a radiation-effect modulator for clinical use.
REDUCING THE ADVERSE EFFECTS OF RADIOTHERAPY RELATED TO THE TREATMENT OF SPECIFIC ORGAN SITES
We next discuss specific organs/sites where the use of radioprotectors and mitigators in conjunction with radiotherapy might improve treatment outcome and/or HRQOL by reducing the adverse effects. Table 2 summarizes ongoing activities in the radioprotectors/mitigators portfolio. The nonconfidential information on NIH funding provided below and in Table 2 is publicly accessible (http://projectreporter.nih.gov/reporter.cfm). Below we discuss case studies of radioprotectors or radiomitigators for reducing the adverse effects of radiotherapy in specific organs/sites.
TABLE 2.
Summary of Activities in the Radioprotectors and Radiomitigators Portfolio
| Indication | Drug; company | Award type | Year started | Current status | Model | Aims |
|---|---|---|---|---|---|---|
| Enteritis | DFMO (α-difluoromethylornithine); RxBio Inc., Johnson City, TN | Phase I | 2011 | Complete | Mouse model of pelvis irradiation; HCT116 and H29 cell lines; and mouse model of colon cancer. |
|
| Enteritis | ABC294640 (sphingosine kinase inhibitor); Apogee Biotechnology Corp., Hummelstown, PA | Phase I | 2014 | Ongoing | In vitro cell lines; mouse model of gastrointestinal acute radiation syndrome. |
|
| Proctitis | PAAG-ployglucosamine; Synedgen, Claremont, CA | Phase I | 2014 | Ongoing | Rat, acute radiation-induced proctitis. |
|
| Mucositis | CBLB502 (TLR-5 agonist); Buffalo Biolabs, Buffalo, NY | Phase I | 2012 | Complete | Mouse model of head and neck cancer. |
|
| Mucositis | RLIP76 (proteoliposome); Terapio Inc., Austin, TX | Phase I | 2013 | Complete | Hamster cheek. |
|
| Mucositis | JVRSOD (gene therapeutic); Colby Pharmaceuticals, Menlo Park, CA | Fast track | 2013 | Ongoing | Mouse model and patients with head and neck cancer. |
|
| Mucositis | BMX-001a (metalloporphyrin antioxidant); BioMimetix JV, Engelwood, CO | Phase I | 2014 | Ongoing | Mouse model of head and neck squamous cell carcinoma. |
|
| Lung injury | UTL-5g (TNF-α modulator); 21st Century Therapeutics Inc., Detroit, MI | Phase I | 2011 | Complete | Cell lines and mouse model of lung injury. |
|
| Lung injury | BIO300 (synthetic genistein); Humanetics Corp., Minneapolis, MN | Fast track | 2012 | Ongoing | Mouse xenograft of NSCLC tumor model and NSCLC patients. |
|
| Brain injury | TP508 (biotherapeutic, 23 amino acid peptide); Chrysalis Biotherapeutics, Galveston, TX | Phase I | 2011 | Transitioned to phase II | Cell lines and mouse xenograft orthotopic model. |
|
| Brain injury | TP508 (biotherapeutic, 23 amino acid peptide); Chrysalis Biotherapeutics, Galveston, TX | Phase II | 2013 | Ongoing | Mouse orthotopic xenograft model. |
|
| Brain injury | Fullerene-based radioprotectors; Luna Innovations Inc., Roanoke, VA | Phase I | 2014 | Ongoing | Cell lines and animals. |
|
| Thrombocytopenia | CLT009, human allogenic megakaryocyte progenitors | Fast track | 2012 | Ongoing | Ex vivo cell culture expansion. |
|
All information provided here and in the text is publicly accessible at http://projectreporter.nih.gov/reporter.cfm, except for funds distributed in 2015. While this information is accessible on the website, http://projectreporter.nih.gov/reporter.cfm, at the end of FY 2015, the company BioMimetix has kindly agreed to publicly release this information in this article.
Head and Neck Cancers
Among patients undergoing radiation treatment for head and neck cancers, oral mucositis is a frequent and potentially serious symptomatic toxicity. Its prevalence, patient-associated variables, pathobiology, risk factors, impact and current management approaches have been previously reviewed (5, 13, 14). Currently available treatment options could be augmented by innovative compounds that target underlying biological pathways associated with mucositis (15). Three specific examples of novel developmental efforts of radioprotectors and radiomitigators against radiation-induced mucositis funded under this topic are discussed below.
Gene therapy
Gene therapy strategies are seen as promising and novel approaches for radioprotection (16, 17). Plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene has been shown to prevent radiation-induced oral mucositis with no detectable compromise in the therapeutic response of orthotopically transplanted tumors in mouse models (18). Oral administration of the genetically engineered therapeutic plasmid DNA/liposome (MnSOD-PL) containing the human SOD transgene was shown to be safe in a phase I clinical trial in patients with unresectable, stage III, non-small cell lung cancer (NSCLC) (19).
Colby Pharmaceutical (Menlo Park, CA), via a fast-track contract, is formulating MnSOD as a mouthwash (JVRSOD) to prevent or reduce the severity of oral mucositis for patients with head and neck squamous cell carcinoma (HNSCCa) who are undergoing chemoradiation treatment. This therapeutic DNA/liposome formulation consists of a bacterial plasmid engineered to overexpress MNSOD 2 upon targeted local delivery. During the course of the SBIR phase I portion of the contract, Colby proposed to optimize dosage and schedule of administration of JVRSOD in a mouse model for protection from radiation-induced oral mucositis and select a GMP manufacturing site. After successful transition from the phase I SBIR contract, under the phase II portion of the fast-track contract, Colby proposed to submit an IND application for JVRSOD to receive regulatory approval, manufacture JVRSOD and test in humans for safety and efficacy in clinical trials with head and neck cancer patients undergoing SOC radiation treatment.
Protein therapeutics
Ral-binding GTPase activating protein, RLIP76, is a multifunctional membrane protein that effluxes glutathione conjugates of electrophilic compounds and other xenobiotics (20, 21). RLIP76 has been implicated in cellular defense mechanisms against oxidative injury (22) and is viewed as a major determinant of radiation sensitivity (23). Cells transfected with RLIP76 acquire resistance to doxorubicin because of increased efflux of doxorubicin, suggesting its possible role in the mechanisms of drug resistance (22).
Terapio Corporation (Austin, TX) is developing RLIP76-proteoliposome as a radiation countermeasure with support from the National Institute for Allergies and Infectious Diseases (NIAID) SBIR program. RLIP76 delivered in a liposome showed significant effects in protecting mice exposed to whole-body irradiation. Currently, the company is performing IND-enabling studies under NIAID’s phase II SBIR award, which is different from the activities performed under NCI’s RRP-SBIR initiative. These studies include verifying the mechanism of action and performing animal safety and toxicokinetic studies in two species as required for approval of radiation countermeasures under the FDA’s animal rule (http://1.usa.gov/1Jyzhhz).
With funding from the NCI RRP-SBIR initiative, Terapio is now modifying the current formulation of RLIP-PL to increase its efficacy as a local oral topical agent to prevent oral mucositis in a proof-of-concept study. Terapio proposed to demonstrate in a radiation-induced oral mucositis hamster model that topical formulation of RLIP76 significantly reduces both the severity and duration of mucositis. Studies are also proposed to demonstrate a lack of tumor protection from the drug during the course of radiation treatment.
TLR5 agonist
CBLB502, a polypeptide drug derived from Salmonella flagellin that binds to Toll-like receptor 5 (TLR5) and activates NF-κB signaling, protects mice from radiation-induced gastrointestinal and hematopoietic syndromes and improves survival without significantly decreasing tumor radiosensitivity in mouse models (24). The treatment of mice with CBLB502 (before irradiation) results in a significant decrease in DNA damage, which seems to be regulated via expression of Gadd45b, Sod2 and Rad21 (25). CBLB502 reduces the severity of dermatitis and mucositis after single and fractionated irradiation of the head and neck area and accelerates tissue recovery. Radioprotection of normal epithelium does not affect the radiosensitivity of syngeneic squamous cell carcinoma (SCCVII) grown orthotopically in mice (26).
In a phase I SBIR contract, Buffalo BioLabs (Buffalo, NY) proposed to demonstrate a protective effect of CBLB502 against radiation-induced damage to the normal mouth epithelium of mice in close-to-clinical radiation regimens. Buffalo BioLabs also proposed to demonstrate the efficacy and safety of CBLB502 in combination with radiation therapy in a mouse head and neck cancer model.
Lung Cancers
More than 60% of the patients with non-small cell lung cancer (NSCLC) are treated with radiation therapy (27). Stone et al. reviewed radiation-induced damage to the lung and described mechanisms of its onset, development and contributing factors (28). Radiation-induced lung damage is an intermediate- to late-occurring side effect of radiation therapy in some patients after thoracic irradiation. The damage initially manifests as pneumonitis at about 1–3 months and then leads to fibrosis months to years after treatment. Fibrogenesis, excessive extracellular matrix and collagen deposition and transforming growth factor-β (TGF-β) also are involved in the development and expression of lung and other forms of tissue fibrosis. As its activation, signaling pathway and downstream effects are somewhat understood, it offers a number of potential targets for mitigation (29).
Genistein
Genistein, an isoflavonoid derived from soy products, inhibits tumor growth by enhancing apoptosis, inducing cell cycle arrest and modulating intracellular signaling pathways. In mice at nontoxic doses, it protects against acute radiation injury (30) and radiation-induced lung damage (31). Genistein inhibits the growth of H460 (NSCLC) cells in vitro via cell cycle arrest (32) and apoptosis through a p53-independent pathway (33). Treatment with phytoestrogen-containing standardized soy extract reduces the growth of NSCLC xenografts (A549) in mice (34), which was attributed to the modulation of the phosphorylated protein kinase (p-Akt) pathway (35). Recent progress on mechanistic studies, absorption, distribution, metabolism and excretion (ADME) of genistein has been reviewed by Yang et al. (36).
Humanetics Corporation (Minneapolis, MN) is developing BIO300, a patented, nanoparticle formulation of genistein for medical radiation countermeasure applications with funding from the U.S. Department of Defense (DOD) and Biomedical Advanced Research and Development Authority (BARDA), to pursue it as a postirradiation treatment option to prevent pneumonitis and lung fibrosis. BIO300 is now in a fairly advanced developmental stage with completed efficacy and toxicology studies in animal models (http://www.humaneticscorp.com). The safety and PK of BIO300 capsules at doses expected to provide radioprotective or therapeutic benefit in humans is being evaluated in a phase I safety and pharmacokinetic trial (https://clinicaltrials.gov; NCT 00504335).
In phase I of this fast-track SBIR contract, Humanetics proposed to demonstrate that mitigation of radiation-induced damage to normal lung tissue is feasible in a mouse xenograft model of NSCLC BIO300. In the phase II SBIR contract, Humanetics has proposed to use the results of phase I experiments along with the existing safety data to file an IND for the use of BIO300 in patients receiving radiotherapy for NSCLC.
Brain Tumors
Radiation exposure is a core component in the treatment of brain tumors. Advances in SOC, including integrating molecularly targeted therapy into precision medicine, is likely to further increase survival, resulting in more people at risk for developing neurocognitive dysfunction long after treatment (6). While increased survival is the goal, so is sustaining and improving HRQOL. Thus, development of mitigators for this delayed effect for use among survivors is imperative. Literature on radiation-induced normal tissue injury in the context of treatment of primary and metastatic brain tumors with a focus on mechanisms of injury, approaches to prevention and mitigation and other potential opportunities to improve treatment outcome and HRQOL has been reviewed (5, 6).
Proangiogenic compound
Rusalatide acetate (TP508) is a 23-amino acid peptide from a portion of highly conserved, noncatalytic, receptor-binding domain in the native thrombin molecule (37) that can interact with alphaVbeta 3 (αVβ3) integrin, a high-affinity binding site found on endothelial cells (38). TP508 plays an active role in the healing of dermal wounds (39), stimulates angiogenesis (40) and counters chronic hypoxia (41).
Promotion of normal vascularization is likely to mitigate chronic hypoxia and help overcome chronic oxidative stress and thus radiation-induced neurocognitive dysfunction. TP508 has demonstrated preclinical safety and potential efficacy in phase I and II clinical studies for diabetic foot ulcers (42) and phase II and III clinical trials for fracture repairs with no drug-related adverse effects (43). Chrysalis Biotherapeutics (Galveston, TX; formerly RADIX Therapeutics) proposed to accelerate the development of TP508 (Chrysalin®) by repurposing it for mitigating the radiation effects on brain tissue. In a phase I SBIR contract, Chrysalis proposed to determine: 1. The optimal dose and timeline for vascular protection; 2. Whether TP508 protects brain tissue from adverse effects of radiotherapy; and 3. Whether TP508 protection is selective to normal tissue without altering radiation killing of cancer cells. Encouraging results obtained in phase I, demonstrating protection of neuroprogenitor cells from the adverse effects of radiotherapy and the restoration of neuronal integrity and stimulation of neurogenesis, led to a successful phase II SBIR contract application, independent of the fast-track application process. In the phase II SBIR, studies are being conducted in a mouse model to demonstrate that TP508: 1. Does not protect cancer stem cells and that protection is specific for neuroprogenitor cells; and 2. reduces radiotherapy-induced neuronal atrophy and cognitive impairment. These studies will also elucidate the mechanisms of TP508 protection to neuroprogenitor cells and generation of new neurons.
OTHER INDICATIONS
Radiation-Induced Thrombocytopenia
Thrombocytopenia is a treatment interrupting, common complication that increases the risk of infection and bleeding from radiation therapy. This condition mostly occurs during combined modality treatment involving whole-abdominal radiotherapy for a variety of tumor types. Thrombocytopenia typically occurs during the initial cycles of high-dose therapy (44) and is observed 6–14 days after treatment is started (45). Unscheduled treatment interruptions can reduce the probability of local tumor control due to the repopulation of tumor cells (46). Platelet transfusion is one option to mitigate thrombocytopenia, but it is labor intensive and expensive. Allogenic cellular therapy may be a superior alternative to fulfill this unmet need to control this treatment interruption.
Megakaryocyte progenitor (MKP) cells
Cellerant Therapeutics, Inc. (San Carlos, CA) is developing allogeneic MKP cells as cell therapeutics to treat radiation-induced thrombocytopenia in cancer patients. Cellerant has been developing cell culture methods for the expansion, characterization and production of sufficient quality and quantity MKP cells to initiate and complete FDA IND-enabling studies. The company has been successful in production of MKP at the research level and they are able to produce human platelets in irradiated rodent xenograft models in vivo with functionality similar to normal human platelets in ex vivo platelet activation assays. In the phase I contract, Cellerant has proposed to develop and demonstrate a clinical cell culture process to generate MKP from CD34+ hematopoietic stem cells using starting material from a wide range of donors. In the phase II SBIR contract, Cellerant has proposed to demonstrate the feasibility of scaling up the process developed in phase I for large-scale clinical manufacturing and has also proposed to develop assays for product characterization and release. Cellerant plans to optimize media formulations, growth and culturing conditions and scalability of MKP production for preclinical efficacy, safety and related IND studies, ultimately leading to enablement of GMP manufacture of CLT-009.
SYNERGY AND SUSTAINABILITY: AN EVOLVING PARADIGM IN THE DEVELOPMENT OF RADIOPROTECTORS AND MITIGATORS
Figure 2 illustrates how different entities – 1. Academia, various components of the Department of Health and Human Services (DHHS; Radiation Research Program, SBIR Development Center and NIAID’s Medical Counter-measure Development Program and BARDA); 2. The small business research community; and 3. The pharmaceutical industry – serve different niches along the development pathways in research and development. As these unique niches complement each other’s efforts in research and development, there is potential synergy in investment to facilitate commercialization of radioprotectors and mitigators. Historically, development of radioprotectors and radiomitigators for clinical use has been a challenge. This was mainly due to the lack of an established market of patients with cancer undergoing radiation therapy who would need such a drug to prevent or mitigate adverse effects, the high-risk nature of product development in this field (and examples of late-stage failures during product development) and the high cost of drug development for FDA label indication.
FIG. 2.
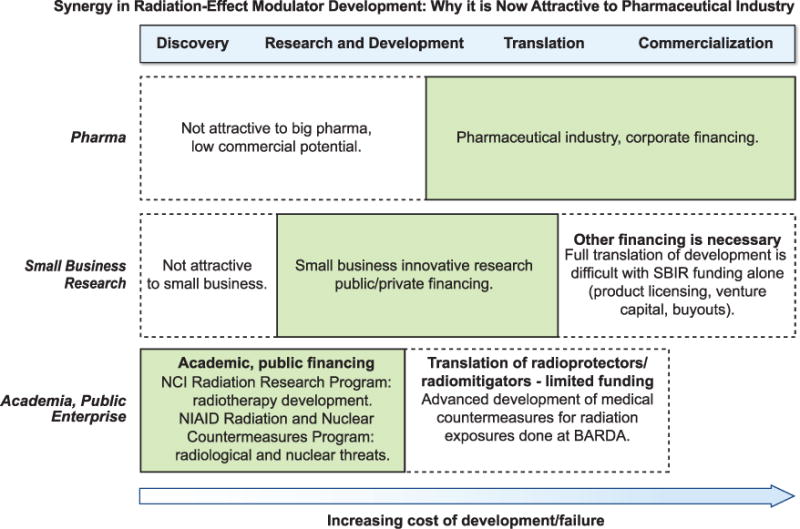
Illustration of how different entities: academia, various components of the DHHS (Radiation Research Program); SBIR Development Center; the NIAID’s Medical Countermeasure Development Program and BARDA; the small business research community; and the pharmaceutical industry, serve different niches along the development pathways in research and development and the concept of potential synergy in investment to facilitate the commercialization of radioprotectors and radiomitigators.
NCI’s RRP has been supporting radiation therapy development for several decades. Establishment of the Public Health Emergency Countermeasures Enterprise (PHEMCE), which includes research and development efforts in the medical countermeasure arena at NIAID as well as advanced development at BARDA to deal with national security threats, is approximately a decade old (12). These programs have supported basic research and have enhanced the understanding of the molecular and systemic mechanisms of normal tissue injury from ionizing radiation for which investment has been limited. These programs have also supported the translation of that knowledge into potential radioprotectors and mitigators for use in oncology.
Adaptation of products that are already under advanced development for a public health mandate after accidental radiation exposures provides a great opportunity to capitalize on the MCM investment by NIAID and BARDA for use in cancer care. An agent approved solely as a countermeasure may (one hopes) never be used for its “intended use.” The product’s stockpile must be discarded and replenished after the expiration date, a cycle that is unattractive from the perspectives of the purchaser, viz. the government and taxpayers. On the other hand, if an agent is in routine use in the radiation oncology clinic, its stockpile and inventory can be far better managed in the event of an emergency. The agent will always be available and there will be familiarity in managing and using such a product (47). Therefore, at the NCI, the RRP and the SBIR Development Center partnered to fill a funding gap to bring in a much desired synergy among academia, government, small businesses and potentially the pharmaceutical industry to facilitate the development of agents for preventing and/or mitigating the adverse effects of radiation therapy in cancer patients through this specific FOA.
As much of the developmental cost is already borne by academia and the government, clinical translation of these products to decrease or mitigate adverse effects of radiation therapy seems to be much more attractive to pharma since the cost of development and risk of late-stage failure is likely to be reduced. Thus, such a cooperative “win-win” model that enhances the value of early-stage research and reduces late-stage failure should be an excellent model for repurposing drugs in general.
CHALLENGES AND OPPORTUNITIES
This collaborative initiative successfully stimulated the radiation research community through the provision of incentives for commercialization of their innovations, by encouraging collaborations with small businesses and/or giving them the means to start businesses on their own. This was evident by the number of proposals received and a significant number of proposals funded (Table 1). This initiative also provided an alternative-funding source to some academic investigators involved in radioprotector and radiomitigator research as they no longer had to solely rely on “investigator-initiated grants”. This specific contract topic has also provided funding to a number of companies who did not have any prior experience with the SBIR funding mechanism. This was particularly useful for capitalizing on new discoveries because the research funding for radiation biology has been of late diminishing.
One of the goals of the NCI’s SBIR contract program is to stimulate growth in research and development in specified areas, in this case, to improve radiation therapy. Prior to this special RFP, the number of applications received under the broad grant omnibus funding opportunity announcement in radiation-effect modulators was relatively low. A significantly higher number of phase I SBIR/STTR (Small Business Technology Transfer) applications were received after the release of this specific FOA in 2011, which suggests an enhanced interest in the clinical translation of radiation-effect modulators (data not shown). Thus, this specific RFP was successful in reinvigorating the radiation-effect modulator field.
This specific RFP was discontinued in FY2015. However, the SBIR Development Center continues to accept applications for funding via the SBIR/STTR grant mechanism (omnibus funding opportunity for investigator-initiated applications). These submissions demonstrate continued interest among small businesses in the technology and clinical translation efforts related to the development of radiation therapy.
Considering the variety of complex issues involved for academia and industry, this initiative was fairly successful in invigorating several technology transfer efforts stemming from academia to industry. There are a number of challenges to incentivizing academic and industry collaborations due to cultural differences between these two organization types. These differences include the following: 1. An academic investigator’s own career goals that emphasize discovery over more routine development work; 2. Obligatory financial and time commitments to a contract funding mechanism that may be considered less attractive in a competitive academic environment; 3. The means of obtaining funding, which are considerably different for a startup or a new business compared to an academic research team; 4. Different skills needed in a business environment and to manage a company; 5. Use of the animal rule for drug approval (http://1.usa.gov/1Jyzhhz); and 6. Discomfort or inexperience of principal investigators in allowing “outside business experts” to manage their work and develop their technologies into products (48). Such routine challenges in the context of university/industry collaborations also relate to the complex relationship, or even conflict between intellectual property protection and the public release and publication of new discoveries (49, 50). Further challenges in academic/industry partnerships include budgetary strategies, regulatory processes and technology transfer and commercialization strategies that vary widely among universities (50). These are all navigable and the SBIR process can clearly be mutually beneficial on a number of levels.
As discussed by Boccanfuso, successful translation of academic research to the clinic depends on the “depth” of academic/industry collaboration (50). However, it appeared to us in some instances that this academic/industry partnership could have been stronger if both partners had fully realized the value of a long-term relationship rather than an “opportunistic onetime partnership” only to obtain limited government funding. In our opinion, such “opportunistic onetime partnerships” will be of limited duration and value. In contrast to these “opportunistic onetime partnerships”, there could be high rewards for those academic investigators with potent intellectual property or individuals with experience at big pharma who start their own small businesses.
Some applicants and their academic partners were less than optimally aware of the variety of challenges likely to be encountered during focused development of a radioprotector, radiomitigator or a therapeutic for clinical use. These include the following: 1. A difficulty in understanding the rigorous requirements of contracts and encapsulating their novel ideas into the framework of a product development requirement for clinical use, including making timely “go/no go decisions”, anticipating and identifying pitfalls early on, adopting alternative approaches when failure is imminent and outlining deliverables; 2. The significance of the problem they intended to address (e.g., repurposing of a radiomitigator developed as a medical countermeasure against radiation exposures requires defining its “intended use” in the clinic and obtaining a new FDA label indication); 3. Type, quantity and quality of the preclinical data essential for successful translation; 4. Understanding of currently used SOC for a given type of cancer and integrating the new treatment paradigm within the SOC; and 5. Working efficiently within the regulatory requirements.
The expanding landscape of biological and technological advances in radiation oncology and the challenges and potential avenues for improvements have been recently reviewed (3, 51). Preclinical research evidence required might vary considerably from one organ site to another for a radiation effect modulator’s possible use in the clinic since protection and mitigation strategies for different organ sites are based on different biology. A major issue in drug development over the last few years has been reproducibility and reliability of the preclinical data (52, 53).
The SOC for different cancer types varies and evolves. In many cases it is a combination therapy in which radiation is used in conjunction with a chemotherapy agent. In such instances, it becomes imperative to have a clear plan of how a radiation-effect modulator will be integrated with the existing SOC or how likely it will be that the new treatment will replace or alter it. Potential replacement of existing SOC will necessitate a “head-to-head comparison” randomized clinical trial to demonstrate superiority of the new treatment approach. An effort to develop a new SOC will invariably attract a higher level of scrutiny and require strong preclinical evidence. This may include testing the new treatment regimen in an animal model simulating the clinical conditions and comprehensive pharmacological and toxicological follow-up evaluation before proceeding to a clinical investigation.
PATH FORWARD: PREDICTIVE BIOMARKERS
In the era of precision medicine, as molecularly targeted therapy is being integrated into radiotherapy and chemotherapy, selecting the right type of treatment at the right time for a particular patient is critical to improving the outcome (54, 55). Consequently, the application of radiation biomarkers is another rapidly developing area of research, with potential to: 1. Predict individual differences in radiation sensitivity (56); 2. Predict severity of normal tissue injury among patients (57); 3. Assess and monitor tumor response to radiation therapy (58); and 4. Estimate dose to accidentally radiationexposed individuals (59). A biomarker-based test that can predict the risks of developing severe radiotherapy-related complications will not only allow the delivery of alternative treatment modalities and the use of mitigators and protectors, but it may also allow dose escalation to the tumor in less sensitive patients. This might improve the overall therapeutic benefit and also improve outcomes.
A variety of radiation biomarkers have already been explored or are currently under development at different technology readiness levels in the context of their potential application for individual dose assessment in accidental exposures to radiation or malevolent use of radiation (59). In fiscal year 2016, leveraging on the recent advances made in academia and other government agencies, a SBIR contract topic is issued to develop radiation biomarkers to specifically identify and exclude likely “over-responders” to radiation prior to radiation therapy to avoid severe complications (https://sbir.cancer.gov/funding/contracts/nihnci345). However, discovery, development and validation of predictive biomarkers of radiation hypersensitivity are as challenging, if not more challenging, than radiation-effect modulators. This is due to the low prevalence of normal tissue complications in the clinic, a need for long-term studies for predicting late effects (e.g., radiation-induced cognitive damage) (6) and complexity related to the combination of chemotherapy with radiation as SOC for most tumors. Nevertheless, the synergy and sustainable development model presented here for radiation-effect modulators is also applicable for developing predictive biomarkers and presents unique opportunities.
CONCLUSIONS
While the challenges involved in the development of a radiation-effect modulator for clinical use in radiation oncology are quite different from those of a medical countermeasure for accidental or intentional radiation exposures, the early research approaches for both applications in terms of innovation, synthesis and mechanism of action may be similar. Nevertheless, synergy among government agencies in funding ensures our investments are rational, viable, sustainable and cost-effective. The DHHS is committed to the development of MCMs to address national security threats from chemical, biological, radiological, nuclear and explosive (CBRNE) agents. Through the PHEMCE, the DHHS has launched and managed a multiagency, comprehensive effort to develop and manage MCMs. These efforts are leveraged in the NCI SBIR program to bring some of the countermeasures to radiation oncology clinics so that such products have “dual utility”, greatly reducing developmental costs and risks of failure, while enhancing the value of research.
The current RRP-SBIR effort for advanced development of radiation-effect modulators serves as a model for a pipeline involving basic research (R01), targeted solutions for specific societal needs (MCMs and diagnostics) and reducing risk for small businesses by providing government support that can be targeted to a topic when needed and aiming for dual-utility, thereby enhancing the likelihood of commercial success (47).
Acknowledgments
This work was supported by the NCI Radiation Research Program and Small Business Innovation Research Development Center. The views expressed are those of the authors; no endorsement by NCI or other agencies has been given or inferred. The authors gratefully acknowledge the helpful suggestions of the reviewers. Editorial assistance provided by Ms. Erica Butler, RRP, program specialist and Brittany Connors, scientific editorial assistant in the SBIR Development Center, is also appreciated.
References
- 1.Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 2. State of the science by anatomic site. Oncol. 2009;23:380–5. [PubMed] [Google Scholar]
- 2.Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 1. Challenges and resources. Oncol. 2009;23:279–83. [PubMed] [Google Scholar]
- 3.Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Trans Med. 2013;5:173sr2. doi: 10.1126/scitranslmed.3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantley LC, Dalton WS, DuBois RN, Finn OJ, Futreal PA, Golub TR, et al. AACR Cancer Progress Report 2012. Clin Can Res. 2012;18:S1–100. doi: 10.1158/1078-0432.CCR-12-2891. [DOI] [PubMed] [Google Scholar]
- 5.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: Where are the gaps? Trans Can Res. 2012;1:35–48. [PMC free article] [PubMed] [Google Scholar]
- 6.Prasanna PG, Ahmed MM, Stone HB, Vikram B, Mehta MP, Coleman CN. Radiation-induced brain damage, impact of Michael Robbins’ work and the need for predictive biomarkers. Int J Radiat Biol. 2014;90:742–52. doi: 10.3109/09553002.2014.925607. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JL, Krishnan S, Movsas B, Coleman CN, Vikram B, Yoo SS. Decreasing the adverse effects of cancer therapy: an NCI Workshop on the preclinical development of radiation injury mitigators/protectors. Radiat Res. 2011;176:688–91. doi: 10.1667/rr2704.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care in Cancer. 2010;18:1061–79. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 9.Okunieff P, Augustine E, Hicks JE, Cornelison TL, Altemus RM, Naydich BG, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207–13. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 10.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–9. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 11.Johnke RM, Sattler, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol. 2014;10:2345–57. doi: 10.2217/fon.14.175. [DOI] [PubMed] [Google Scholar]
- 12.Coleman CN, Sullivan JM, Bader JL, Murrain-Hill P, Koerner JF, Garrett AL, et al. Public health and medical preparedness for a nuclear detonation: the nuclear incident medical enterprise. Health Phys. 2015;108:149–60. doi: 10.1097/HP.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonis ST. The pathobiology of mucositis. Nature Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 14.Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452–6. doi: 10.1016/j.oraloncology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Yuan A, Sonis S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin Emerg Drugs. 2014;19:343–51. doi: 10.1517/14728214.2014.946403. [DOI] [PubMed] [Google Scholar]
- 16.Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, et al. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys. 1999;43:169–81. doi: 10.1016/s0360-3016(98)00355-1. [DOI] [PubMed] [Google Scholar]
- 17.Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Seixas-Silva JA, Jr, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res. 2003;159:361–70. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Tarhini AA, Belani CP, Luketich JD, Argiris A, Ramalingam SS, Gooding W, et al. A phase I study of concurrent chemotherapy (paclitaxel and carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase plasmid liposome protection in patients with locally advanced stage III non-small-cell lung cancer. Hum Gene Ther. 2011;22:336–42. doi: 10.1089/hum.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, et al. RLIP76 and Cancer. Clin Can Res. 2008;14:4372–7. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awasthi S, Sharma R, Singhal SS, Zimniak P, Awasthi YC. RLIP76, a novel transporter catalyzing ATP-dependent efflux of xenobiotics. Drug Metab Dispos. 2002;30:1300–10. doi: 10.1124/dmd.30.12.1300. [DOI] [PubMed] [Google Scholar]
- 22.Awasthi S, Sharma R, Yang Y, Singhal SS, Pikula S, Bandorowicz-Pikula J, et al. Transport functions and physiological significance of 76 kDa Ral-binding GTPase activating protein (RLIP76) Acta Biochim Pol. 2002;49:855–67. [PubMed] [Google Scholar]
- 23.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, et al. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005;65:6022–8. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 24.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Wang ZD, Chen MS, Zhang XQ, Shen LP, Zhang JX, et al. Activation of toll-like receptors by intestinal microflora reduces radiation-induced DNA damage in mice. Mut Res Genet Toxicol Environ Mutagen. 2014;774:22–8. doi: 10.1016/j.mrgentox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Burdelya LG, Gleiberman AS, Toshkov I, Aygun-Sunar S, Bapardekar M, Manderscheid-Kern P, et al. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:228–34. doi: 10.1016/j.ijrobp.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–85. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 28.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 29.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Rev Can. 2006;6:702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 30.Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379–85. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 31.Day RM, Barshishat-Kupper M, Mog SR, McCart EA, Prasanna PG, Davis TA, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat Res. 2008;49:361–72. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31:184–91. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 33.Lian F, Li Y, Bhuiyan M, Sarkar FH. p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer. 1999;33:125–31. doi: 10.1207/S15327914NC330202. [DOI] [PubMed] [Google Scholar]
- 34.Hillman GG, Singh-Gupta V, Hoogstra DJ, Abernathy L, Rakowski J, Ynker CK, et al. Differential effect of soy isoflavones in enhancing high intensity radiotherapy and protecting lung tissue in a pre-clinical model of lung carcinoma. Radiother Oncol. 2013;109:117–25. doi: 10.1016/j.radonc.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo D, Zannoni GF, De Stefano I, Mosca M, Ferlini C, Mantuano E, et al. Soy phytochemicals decrease nonsmall cell lung cancer growth in female athymic mice. J Nutr. 2008;138:1360–4. doi: 10.1093/jn/138.7.1360. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer Agents Med Chem. 2012;12:1264–80. doi: 10.2174/187152012803833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glenn KC, Frost GH, Bergmann JS, Carney DH. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept Res. 1988;1:65–73. [PubMed] [Google Scholar]
- 38.Tsopanoglou NE, Papaconstantinou ME, Flordellis CS, Maragoudakis ME. On the mode of action of thrombin-induced angiogenesis: thrombin peptide, TP508, mediates effects in endothelial cells via alphavbeta3 integrin. Thromb Haemost. 2004;92:846–57. doi: 10.1160/TH04-04-0208. [DOI] [PubMed] [Google Scholar]
- 39.Stiernberg J, Norfleet AM, Redin WR, Warner WS, Fritz RR, Carney DH. Acceleration of full-thickness wound healing in normal rats by the synthetic thrombin peptide, TP508. Wound Repair Regen. 2000;8:204–15. doi: 10.1046/j.1524-475x.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 40.Vartanian KB, Chen HY, Kennedy J, Beck SK, Ryaby JT, Wang H, et al. The non-proteolytically active thrombin peptide TP508 stimulates angiogenic sprouting. J Cell Physiol. 2006;206:175–80. doi: 10.1002/jcp.20442. [DOI] [PubMed] [Google Scholar]
- 41.Olszewska-Pazdrak B, Carney DH. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. J Vasc Res. 2013;50:186–96. doi: 10.1159/000348250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fife C, Mader JT, Stone J, Brill L, Satterfield K, Norfleet A, et al. Thrombin peptide Chrysalin stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 2007;15:23–34. doi: 10.1111/j.1524-475X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 43.Carney DH, Olszewska-Pazdrak B. Could rusalatide acetate be the future drug of choice for diabetic foot ulcers and fracture repair? Expert Opin Pharmacother. 2008;9:2717–26. doi: 10.1517/14656566.9.15.2717. [DOI] [PubMed] [Google Scholar]
- 44.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457–69. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 45.Kaushansky K. Thrombopoietin: in vitro predictions, in vivo realities. Am J Hematol. 1996;53:188–91. doi: 10.1002/(SICI)1096-8652(199611)53:3<188::AID-AJH7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 46.Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phy. 1992;23:457–67. doi: 10.1016/0360-3016(92)90768-d. [DOI] [PubMed] [Google Scholar]
- 47.Coleman CN, Hrdina C, Casagrande R, Cliffer KD, Mansoura MK, Nystrom S, et al. User-managed inventory: an approach to forward-deployment of urgently needed medical countermeasures for mass-casualty and terrorism incidents. Disaster Med Public Health Prep. 2012;6:408–14. doi: 10.1001/dmp.2012.46a. [DOI] [PubMed] [Google Scholar]
- 48.Smith CD. Your idea and your university: issues in academic technology transfer. J Invest Med. 2011;59:752–7. doi: 10.231/JIM.0b013e31820d0fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kneller R, Mongeon M, Cope J, Garner C, Ternouth P. Industry-university collaborations in Canada, Japan, the UK and USA - with emphasis on publication freedom and managing the intellectual property lock-up problem. PloS One. 2014;9:e90302. doi: 10.1371/journal.pone.0090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boccanfuso AM. Why university-industry partnerships matter. Sci Transl Med. 2010;2:51cm25. doi: 10.1126/scitranslmed.3001066. [DOI] [PubMed] [Google Scholar]
- 51.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nature Rev Cancer. 2011;11:239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 52.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–3. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamburg MA, Collins FS. The path to personalized medicine. New Eng J Med. 2010;363:301–4. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 55.Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nature Rev Drug Disc. 2012;11:201–14. doi: 10.1038/nrd3651. [DOI] [PubMed] [Google Scholar]
- 56.Beaton LA, Marro L, Samiee S, Malone S, Grimes S, Malone K, et al. Investigating chromosome damage using fluorescent in situ hybridization to identify biomarkers of radiosensitivity in prostate cancer patients. Int J Radiat Biol. 2013;89:1087–93. doi: 10.3109/09553002.2013.825060. [DOI] [PubMed] [Google Scholar]
- 57.Chua ML, Rothkamm K. Biomarkers of radiation exposure: can they predict normal tissue radiosensitivity? Clin Oncol. 2013;25:610–6. doi: 10.1016/j.clon.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Torres-Roca JF. A molecular assay of tumor radiosensitivity: a roadmap towards biology-based personalized radiation therapy. Per Med. 2012;9:547–57. doi: 10.2217/pme.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan JM, Prasanna PG, Grace MB, Wathen LK, Wallace RL, Koerner JF, et al. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations. Health Phys. 2013;105:540–54. doi: 10.1097/HP.0b013e31829cf221. [DOI] [PMC free article] [PubMed] [Google Scholar]


