Abstract
Creativity, a multifaceted construct, can be studied in various ways, for example, investigating phases of the creative process, quality of the creative product, or the impact of expertise. Previous neuroimaging studies have assessed these individually. Believing that each of these interacting features must be examined simultaneously to develop a comprehensive understanding of creative behavior, we examined poetry composition, assessing process, product, and expertise in a single experiment. Distinct activation patterns were associated with generation and revision, two major phases of the creative process. Medial prefrontal cortex (MPFC) was active during both phases, yet responses in dorsolateral prefrontal and parietal executive systems (DLPFC/IPS) were phase‐dependent, indicating that while motivation remains unchanged, cognitive control is attenuated during generation and re‐engaged during revision. Experts showed significantly stronger deactivation of DLPFC/IPS during generation, suggesting that they may more effectively suspend cognitive control. Importantly however, similar overall patterns were observed in both groups, indicating the same cognitive resources are available to experts and novices alike. Quality of poetry, assessed by an independent panel, was associated with divergent connectivity patterns in experts and novices, centered upon MPFC (for technical facility) and DLPFC/IPS (for innovation), suggesting a mechanism by which experts produce higher quality poetry. Crucially, each of these three key features can be understood in the context of a single neurocognitive model characterized by dynamic interactions between medial prefrontal areas regulating motivation, dorsolateral prefrontal, and parietal areas regulating cognitive control and the association of these regions with language, sensorimotor, limbic, and subcortical areas distributed throughout the brain. Hum Brain Mapp 36:3351–3372, 2015. © 2015 The Authors. Human Brain Mapping Published by Wiley Periodicals, Inc..
Keywords: creativity, poetry composition, fMRI, neurocognitive model, cognitive disinhibition
INTRODUCTION
While creativity is arguably responsible for the advancement of human civilization and culture, the neurobiology of the creative process remains poorly understood. Over the past decade, improved neuroimaging methods have made it possible to explore the neural correlates of creative behavior, but no clear consensus has emerged. The variability in previous studies is partially due to the fact that creativity is a multifactorial process [Dietrich, 2004], and may manifest in a number of ways—as convergent or divergent thinking [Fink, et al. 2007], deliberate analytical problem solving or sudden insight [Kounios and Beeman, 2014]. This has led to a broad array of experimental paradigms that have generated a diverse set of neuropsychological theories [Jung et al., 2013].
The lack of consensus can also be attributed to the fact that the design of many neuroimaging experiments differs with respect to a number of crucial variables that interact throughout the creative process. Three of these are particularly important: (1) the phase of the creative process being studied; (2) the level of expertise of experimental subjects; and (3) the quality of the creative products that subjects construct—that is, the impact the work has upon an audience. We believe that a comprehensive model of creative activity can be constructed by examining all features together in the context of a single experiment. While a few existing studies have examined some of these elements individually (as discussed below), no study has studied all three simultaneously.
To accomplish this, we have chosen to expand upon a line of research [Limb and Braun, 2008; Liu et al., 2012] focused on artistic creativity. Using an ecologically valid approach—studying the artist during the natural act of creation—allows us to examine creative behavior as a whole and in situ, without the superimposition of unrelated, potentially confounding cognitive task demands. Such paradigms have been used more frequently in recent year [Bengtsson et al., 2007; Berkowitz and Ansari, 2010; Brown et al., 2006; Ellamil et al., 2011; Shah et al., 2013; Villarreal et al., 2013].
The majority of studies in this area, particularly those that have investigated spontaneous artistic creativity or improvisation, have focused on music. We recently reported a set of experiments that characterized lyrical improvisation in a genre that serves as a bridge between music and language [Liu et al., 2012]. Here, we focus exclusively on literary creativity, using a paradigm in which expert and novice poets improvised and then revised poems, which were in turn rated by an independent panel of experts. Importantly, poems can be relatively short in length, which allowed us to study the neural mechanisms that underlie each of the three key features of interest and to characterize the interactions between them within a single experimental session.
The Creative Process
Based on established psychological theories [Campbell, 1960; Finke et al., 1992; Johnson‐Laird, 1988; Wallas, 1926], we work from the assumption that creativity—or at the very least, artistic creativity—is a multistage process, with two principal components: Generation, when novel material is spontaneously produced, and Revision, when previously generated material undergoes focused evaluation and modification. Except for spontaneous performance‐based genres (e.g., jazz improvisation or freestyle rap), both phases typically emerge during the creative process, although they may alternate with one another flexibly rather than proceeding in a linear fashion. As the cognitive processes that characterize these phases are markedly different, both must be studied in order to build a comprehensive model of creative behavior.
To date, research on artistic creativity has focused almost exclusively on the initial generative stage, and due to differences in tasks, methods, and populations, results have varied. Although many regions have been implicated, the most frequent observations have emphasized the frontal lobe [Dietrich and Kanso, 2010]. Activation of the medial prefrontal cortex (MPFC) is perhaps the most consistent finding across studies [Dietrich and Kanso, 2010]. The dorsolateral prefrontal cortex (DLPFC) has also been implicated, albeit in a variety of ways that will be discussed below [Bengtsson et al., 2007; Berkowitz and Ansari, 2008]. Notably, a dissociation between activity in these prefrontal regions has been reported in a series of studies: focal activation of the MPFC was coupled with deactivation of the DLPFC during both melodic and lyrical improvisation [Limb and Braun, 2008; Liu et al., 2012]. These findings suggested that the generative phase of the creative process might be associated both with increases in intrinsic motivation (related to increases in the MPFC) and attenuation of self‐monitoring and top‐down attention (related to decreases in the DLPFC).
To our knowledge, only one neuroimaging study has examined a phase of the creative process other than generation. In a well‐designed experiment [Ellamil et al., 2011] subjects were instructed to alternate between designing and evaluating book cover illustrations (a subcomponent of the revision process we study here). The direct contrast between design and evaluation conditions revealed significant differences in the medial temporal, default mode, and executive regions, underscoring the importance of studying multiple phases of the creative process in a single experiment.
By carrying out a more inclusive revision task (during which material that was previously generated undergoes both evaluation and modification), and utilizing a series of noncreative baseline conditions (discussed below), we sought to characterize the neural underpinnings of both generation and revision phases. In doing so, it was our goal to extend the findings derived from our studies of improvisation into one unified neuroanatomical model accounting for both phases.
Impact of Expertise
It is currently unclear how activity in either phase may be affected by expertise. In a sense, the acquisition and subsequent training of a skill can be viewed as preceding and potentially modulating activity during both generation and revision [Wallas, 1926]. A few neuroimaging studies have explored differences between trained and untrained individuals during the generation phase, as subjects engaged in real [Berkowitz and Ansari, 2010] or imagined artistic tasks [Bhattacharya and Petsche, 2005; Fink et al., 2009; Kowatari et al., 2009]. Although all studies reported differences between experts and novices, the results are subtle and somewhat variable. This may be because most of the differences reported were detected through the direct contrast of experts and novices without the use of control conditions.
Using an interactive model with two factors (experts vs. novices; creative vs. noncreative conditions), we were able to more accurately pinpoint both the differences between groups (via double subtractions), as well as the similarities between them, that is, the extent to which experts and novices utilize a common set of cognitive resources (via conjunction analyses).
Product Quality
As noted above, a comprehensive model of artistic creativity must also consider a third key feature—the aesthetic quality or innovativeness of the end product, distinguishing the product from the process that led to its construction [Finke et al., 1992]. In this study, we evaluate the quality of the creative product by assessing the responses of an audience, in this case an independent panel of experts, to the poems subjects produced. Previous studies rated the complexity or novelty of improvised musical [Bengtsson et al., 2007; Villarreal et al., 2013] or literary material [Liu et al., 2012; Shah et al., 2013] and correlated these measures with the amplitude of brain activity. Other studies utilizing sensorimotor tasks [Baldassarre et al., 2012; Koyama et al., 2011] have suggested that performance is strongly associated with functional connections between brain regions. Such relationships have rarely been examined in the domain of creativity before and their interactions with expertise or with different phases of the creative process are unknown.
In order to carry out these analyses, we acquired a time‐locked digital record of all materials produced by subjects during the scanning sessions and quantified measures associated with the quality of the poems themselves. An independent panel of professional poets blindly rated the poems, assigning a craft score (assessing the use of poetic devices), and a linguistic creativity score (assessing the innovative use of these technical elements). In addition, the raters assessed changes in quality, that is, improvement in the poems from the first to second phase. All of these measures were used in functional network analyses ultimately aimed at linking the phases of the creative process, the quality of the creative product, and the impact of expertise.
In designing this study, we took the opportunity to address several critical issues related to baselines used in studies of creativity, particularly those investigating improvisation [Abraham, 2013]. We utilized rote memorization of previously learned material as our principal baseline condition because it constitutes a direct contrast to the freedom of choice which characterizes the initial phase of the creative process [Johnson‐Laird, 1988]. Importantly however, this baseline fails to control for spontaneous motor activity or spontaneous cognition per se. We therefore used two additional baseline conditions, controlling for these essential, potentially confounding, features in order to identify activity that is more likely related to the creative process itself.
Using this array of baselines and creative tasks, we applied traditional general linear model (GLM)‐based contrasts (conjunction and interaction analyses), as well as more novel independent components (IC) analysis (ICA) based connectivity methods, which allow for a data‐driven examination of the data. The former provides information about which brain regions are involved in a given task and the latter, information about the functional relationships between them.
As we have noted, since all three aspects of creative behavior—process, product, and expertise—are intricately interrelated, we expected that each would be traced to the operation of a common central mechanism. We expected this mechanism to be grounded in the dynamic interaction between brain systems in medial prefrontal, lateral prefrontal, and posterior parietal cortices that play central roles in the regulation of motivation, attention, and cognitive control. Understanding the interactions between these core brain regions and their relationships to other task‐specific brain regions (e.g., the perisylvian areas responsible for linguistic functions that are specifically engaged during in poetry composition) should provide the rudiments of an integrated neuropsychological model of creative cognition.
METHODS
Participants
Participants consisted of 30 right‐handed, native English‐speakers with no history of neurological or psychiatric illness. Informed written consent was obtained from each participant in accordance with a protocol approved by the NIH Institutional Review Board. Data from three subjects were discarded due to excessive motion artifacts or typing errors. Among the rest 27 subjects (12 male, 15 female), 14 recruited as experts (age: 31.62 ± 10.76 years, education: 18.43 ± 1.34 years; mean ± SD, five male and nine female) had completed at least 1 year of an MFA program and published in poetry journals; 13 novices (age: 32.07 ± 13.19 years, education: 16.92 ± 2.39 years, seven male and six female) had no formal training or experience. No significant differences (P < 0.05) were found between these groups in age or education level (using two sample t‐tests) or in gender (using a Chi‐square test). To evaluate their vocabulary skills, participants were asked to perform two verbal fluency tests—phonemic (generate as many words beginning with a specific letter of F, A, S as possible in one minute each) and categorical (generate as many words belonging to animal as possible in one minute) [Tombaugh et al., 1999]—and a self‐paced rapid picture naming task (name as many and as accurate pictures as possible in two minutes; Missall and McConnell, 2004].
MRI Acquisition
T2*‐weighted BOLD images were acquired on a General Electric Signa HDxt 3.0 Tesla scanner (GE Healthcare, Waukesha, WI) with an 8‐channel High Resolution Brain Coil. Anatomical images were acquired using a magnetization‐prepared rapid gradient‐echo sequence. A single‐shot gradient‐echo echo planar imaging (EPI) sequence was used for functional imaging: the acceleration factor of Array Spatial Sensitivity Encoding Technique = 2, repetition time = 2000 ms, echo time = 30 ms, flip‐angle = 90°, 64 × 64 matrix, field of view = 227 mm, four dummy scans. 40 interleaved sagittal slices with a thickness of 4 mm were used to cover whole brain.
Memorized Materials
One week prior to scanning, subjects were given two 10‐line poems, Marianne Moore's “What Are Years” and Robert Lowell's “Fall 1961,” to memorize. An additional list of 10, single‐sentence facts was assembled by the authors and distributed alongside the poems. A test was given before the experiment to make sure that subjects memorized them. Following the experiment, memorized poems and facts produced by subjects during the scanning sessions were examined and all subjects correctly reproduced the materials required to be memorized.
Experimental Design
Subjects performed six tasks using an MRI‐safe keyboard. These tasks included: recitation of memorized poems (RecMemPoem); generation of new poems (GenNewPoem); revision of poems (RevNewPoem—revision of new poems generated during GenNewPoem); generation of random typing movements (GenRandType); generation of nonmemorized facts(GenNonmemFact); and recitation of memorized facts (RecMemFact). In the RecMemPoem condition, subjects typed memorized poems. In the GenNewPoem condition, subjects were asked to spontaneously generate a novel poem by typing on a keyboard. During RevNewPoem, the subject's output of GenNewPoem was displayed on the screen, allowing them to modify their poems. Subjects were instructed to avoid editing errors in spelling or grammar and to focus solely on revising the aesthetic content. During GenNonmemFact, subjects were asked to spontaneously produce a series of simple facts that were not included on the list of memorized facts (which were produced during RecMemFact). During GenRandType, subjects were instructed to type at a rate comparable to the other conditions, but to make random keystrokes that did not correspond to real words. All participants went through a training session before scanning, in order to make sure they performed all experimental conditions correctly. As shown in the schematic (Supporting Information Fig. 1), subjects performed each task once in a single session. Task order was counterbalanced across sessions. In each session, RevNewPoem tasks always immediately followed the corresponding GenNewPoem tasks. A total of four sessions yielded four blocks (60s per block) for each task. A fixation (+) period of 17s was inserted between tasks to allow the blood oxygen level dependent (BOLD) response to fall back to baseline. The total duration of this experiment was 32 minutes 8 seconds. A program in E‐prime (Psychology Software Tools, Inc., Sharpsburg, PA) controlled the experimental procedures. All materials typed by participants were recorded with time stamps.
Behavioral Measures
Only subjects who reported that they typed every day, both during and outside of work, were included. We assessed typographical accuracy in all materials following the experiments. Two subjects who made excessive typographical errors (typographical error rate > 3%) were excluded. In the remaining subjects, typographical error rate (the number of incorrectly typed letters/the number of total letters in all conditions except GenRandType) was 0.71 ± 0.48% (mean ± s.d.) in experts and 1.0 ± 0.09% in novices, and no significant difference was found between these two groups. We also measured the total numbers of keystrokes and lines in all materials (Supporting Information Table 1). Variations in typing rates were found across conditions (although there were no group differences in any condition other than GenRandTyp). Consequently, the number of keystrokes in each block (which unlike line number is a more direct measure of typing rate) was used as a nuisance variable in the following imaging analyses to minimize the unwanted effects of such variations.
An independent three‐member expert panel was assembled to blindly rate the poems for elements of craft, linguistic creativity, and revision. The three raters were all accomplished poets, and each had won either compensated poetry fellowships or national poetry contests for their work. The rating system used here was designed in collaboration with this panel. Craft was assessed in terms of four primary elements: sound, form, figurative language, and sensory language. Sound includes use of consonance, assonance, alliteration, or rhyme; form includes use of repetition, meter, line enjambment, and stanza; figurative language includes use of simile or metaphor; and sensory language includes words or phrases that relate directly to one of the five senses. Each poem received four positive binary scores, one for the presence/absence of each of these primary elements. Additionally, a binary negative score was given if clichés, that is, overused idiomatic phrases (without the presence of irony, abstraction), or redundancy was identified. A negative score, when present, was subtracted from the sum of the four positive scores to produce an overall craft rating.
While the craft scores reflected application of technical and genre‐specific expertise, Linguistic Creativity assessed the innovative or novel use of craft elements. Linguistic Creativity was assessed on a five‐point Likert scale which ranged from highly uncreative to highly creative. A five‐point Likert scale was also used to assess overall improvement in quality when the revised version of each poem was compared to its original. Unlike the craft score, which utilized a standardized set of technical criteria, the five‐point Likert scale allowed for more flexibility in capturing the more qualitative assessments of novelty, innovation or improvement. Nevertheless, intraclass correlation coefficients among the three raters were calculated to examine the inter‐rater reliability for all three behavioral scores. An average of the scores of all three raters was used for the final score for each rating category.
In addition to the ratings of poetry quality provided by the expert panel, we also measured features of words used in the GenNewPoem, RevNewPoem and GenNonmemFact conditions. Using the Whissel dictionary [Whissell, 2009] we measured pleasantness (1 = unpleasant, 2 = neutral, 3 = pleasant), activation/arousal (1 = passive, 2 = neutral, 3 = active), imagery (1 = hard to imagine, 2 = neutral, 3 = easy to imagine) of each word and calculated the average value for all words per condition and subject. No significant differences were found in these three word‐level measures either across conditions (paired t‐tests) or across expert and novice groups (two‐sample t‐tests) (Supporting Information Table 2). Therefore, these measures were not taken used as regressors in the functional imaging analyses.
Image Preprocessing
In‐plane registration, slice‐time correction and volumetric rigid‐body registration were sequentially applied to the functional images. The structural image was coregistered to the functional images using a mutual‐information based algorithm [Maes et al., 1997]. The structural image was then segmented and normalized into Montreal Neurological Institute (MNI) space using the tissue probability maps in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) [Ashburner and Friston, 2005]. In order to remove susceptibility artifacts generated by motion and physiological noise (blood pulsation, respiration, etc.), which cannot be removed by the conventional coregistration method, we applied the dual‐mask spatial independent component analysis (sICA) to the motion and slice‐time corrected functional data at the individual subject level [Xu et al., 2014]. The denoised functional data were then normalized into MNI space at a voxel size of 3 × 3 × 3 mm by applying the transforms derived from the structural image normalization and smoothed to a target full‐width‐half‐max of 8 mm.
Activation Analysis
At the subject level, the GLM was implemented using SPM8. Separate regressors were constructed by convolving the box‐car function of each condition with the canonical hemodynamic response function. In addition to task regressors, a nuisance covariate of the whole‐brain mean signal was used to account for the global BOLD signal fluctuations induced by changes in PCO2 [Birn et al., 2006; Macey et al., 2004].
For the group analysis, a one‐way voxel‐wise random‐effects analysis of variance (ANOVA) model was used to draw statistical inferences among conditions at the population level without separating experts and novices. A separate two‐way ANOVA model was built to evaluate interaction effects between groups (experts and novices) and conditions. A nuisance covariate of keystrokes (see Supporting Information Table 1) for each block was utilized in both models to account for variances in typing rates across conditions and subjects. Two‐tailed student t‐tests were used to generate contrasts of conditions and groups. All statistical t maps and tables were reported at the threshold of family‐wise error (FWE) < 0.05 based on Monte Carlo simulations to correct for multiple comparisons. The local peaks of t values and the corresponding cluster sizes were calculated and are reported in the Supporting Information tables.
Functional Network Connectivity Analysis
A novel method of functional network connectivity (FNC) analysis has previously been applied to resting‐state data [Allen et al., 2011; Doucet et al., 2011; Jafri et al., 2008]. Here, this FNC method was used in a task‐based study. Unlike the traditional seed‐based functional connectivity analysis applied to the time series derived from individual voxels, FNC is applied to a set of self‐organized networks resulting from decomposition of the time series of all voxels in the brain by group‐level ICA. This data driven method serves as a means of data reduction which makes it possible to effectively investigate functional connections across the entire brain without potential biases in defining seeds.
Preprocessing
A finite impulse response band‐pass filter (0.03–0.08 Hz) was applied to the residual time‐series of the GLM analysis. To account for the delay of hemodynamic response, the data for each condition was shifted by three volumes and concatenated, resulting in 120 data points per subject and condition.
Data reduction using group‐level ICA
To reduce the dimensionality of the search space, the whole‐brain voxel time series were further decomposed into 61 spatially IC by a group‐level sICA [Calhoun et al., 2001], each representing a self‐organized functional unit (or network) with homogenous temporal dynamics. Prior to the ICA, data were preprocessed with two steps of reduction in the time domain using principal component analysis (PCA): one within each subject and condition, and the other at the group level after concatenating the principal components across all subjects and conditions. The group‐level dimensionality for PCA and ICA decompositions (i.e., 61) was set to the minimum order to retain 100% nonzero Eigen values during individual PCA reduction, which is close estimate of the true degree of freedom in the data. The major purpose underlying this procedure was to use a high‐order decomposition [Kiviniemi et al., 2009] to maximize the observable effects while avoiding possible overfitting errors [Sarela and Vigario, 2003].
After group ICA decomposition, eight artifactual components with spatial patterns clearly localized in major cerebral arteries, ventricles, or dural vein sinuses were excluded from further analysis. The time courses of remaining 53 components for each subject and condition, which were computed from the group ICA time courses by a PCA‐based back‐reconstruction method [Erhardt et al., 2011], were used for the following FNC analyses [Allen et al., 2011; Doucet et al., 2011; Jafri et al., 2008].
Comparative analysis between conditions
Hierarchical Clustering was applied to these 53 ICs in order to search for the common and different connectivity patterns between GenNewPoem and RevNewPoem conditions (Matlab, TheMathWorks Inc., Natick, Massachusetts). Pearson's correlation coefficients and their Fisher's z’ transformations were computed from each pair of ICs for all subjects, indicating the strength of connectivity between functional networks represented by these two ICs. A mean correlation matrix was computed across subjects separately for both GenNewPoem and RevNewPoem. The correlation matrix, R, was converted to a distance or linkage matrix, D = 1−R, indicating dissimilarity between each component pair. Agglomerative hierarchical clustering on these distance values was done to sort the components in a data‐driven fashion, so that those with similar temporal dynamics were placed together in a cluster [Doucet et al., 2011]. The distance between two clusters was the average distance between all pairs of their elements. Based on the averaged distance matrix across GenNewPoem and RevNewPoem conditions, a dendrogram plot was generated to illustrate the hierarchical, binary cluster tree, in which leaves represent components and the height of paths between leaves represents the distances between components (See Fig. 3A for an example). Using the average of GenNewPoem and RevNewPoem instead of a single condition reveals the systematic neural architecture that underlies the two‐phases of poetry composition and allows an objective comparison of cluster interactions between these two conditions. Using a threshold of 70% of the maximum linkage, which is the default value in the Matlab (TheMathWorks Inc., Natick, Massachusetts) function to plot the dendrogram, five clusters were defined in this data‐driven analysis. Two sets of brain regions playing important and distinctive roles in GenNewPoem and RevNewPoem self‐segregated into Cluster 2 and 4. To further understand how these two clusters interact with each other during both phases, the correlation between Cluster 2 and 4 was computed for GenNewPoem and RevNewPoem, by calculating the temporal correlations between the average time series of all components in Cluster 2 and 4.
Correlation analysis with quality measures
Finally, to examine if poetry quality scores correlate with functional connectivity strengths among different ICs under the corresponding condition (GenNewPoem, RevNewPoem) in experts and novices, we built separate analysis of covariance models with group (experts and novices) as an independent factor interacting with covariates of interest (each poetry quality score of craft, linguistic creativity, or revision separated in separate models), and Fisher's z’ transformed correlation coefficient of each IC pair as the dependent factor. Expertise and quality effects were studied in a single model because an interaction effect between expertise and quality may exist, as indicated by the significant differences in all quality scores between experts and novices (Fig. 5). The P value of the interaction effect between group and quality factors was calculated from F value for each model. False discovery rate (FDR) was calculated and P <0.05 (corrected) was set as the threshold to correct for multiple comparisons of different IC pairs.
RESULTS
Two groups of subjects, experts (n = 14) and novices (n = 13), performed six tasks using an MRI‐safe keyboard. These tasks included: recitation of memorized poems (RecMemPoem); generation of new poems (GenNewPoem); revision of poems (RevNewPoem—revision of new poems generated during GenNewPoem); generation of random typing movements (GenRandType); generation of nonmemorized facts (GenNonmemFact); and recitation of memorized facts (RecMemFact).
Experts performed slightly better than novices in both phonological (mean + s.d., experts vs. novices, 53 ± 11 vs. 50 ± 12 words starting with F, A, S in total) and semantic fluency tasks (29 ± 8 vs. 26 ± 4 words belonging to animal), and much better in a self‐paced rapid picture naming task (98 + 12 vs. 86 ± 13 words/pictures), all of which were assessed outside of the scanner. However, none of these differences reached a significant level (P <0.05, two sample t‐tests), indicating that vocabulary size and word retrieval capability are matched between experts and novices.
Brain Activations During Generation of New Poems in All Subjects
We first examined the generation phase, by comparing the generation of new poems and recitation of memorized poems conditions directly using GLM, in all subjects (Fig. 1; Supporting Information Table 3). The generation phase was characterized by a dissociated pattern: increases in the MPFC, broadly extending from the frontal pole into the presupplementary motor area, were accompanied by decreases in the DLPFC, intraparietal sulcus (IPS), and precuneus bilaterally. The generation phase was also associated with increased activity in perisylvian areas, including the bilateral inferior front gyrus (IFG), left middle temporal gyrus (MTG), and superior temporal sulcus (STS) and other language‐related areas in the left hemisphere, including the supramarginal, angular and fusiform gyri. Generation of new poems was also associated with increases in mesial temporal areas, including the parahippocampal gyrus, hippocampus, and amygdala bilaterally and subcortical areas in the left hemisphere, including the body of the caudate nucleus, posterior putamen, and anterior, medial dorsal, and pulvinar thalamic nuclei.
Figure 1.
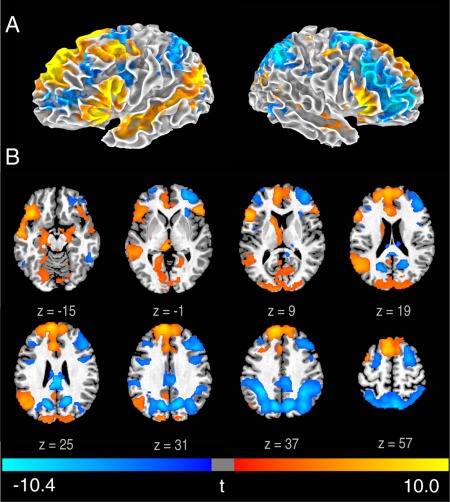
Brain activity associated with the generation phase.
Statistical t map of GenNewPoem‐RecMemPoem on a 3D brain surface (A) and axial slices (B) (FWE < 0.05). t‐scores are rendered in colors ranging from negative (violet) to positive (yellow) as indicated by the accompanying color bar.
To impose a greater degree of experimental control, we performed contrasts using two baseline conditions in addition to RecMemPoem. We used them to test the reliability of results identified in the above contrast and more clearly specify the functional roles of the brain regions that it highlighted. First, when generation of new poems was contrasted with random typing movements (GenNewPoem‐GenRandType; Supporting Information Fig. 2), controlling for spontaneous motor activity per se, we detected activation patterns that were essentially identical to those described above (including decreases in DLPFC and IPS) suggesting that these are inherent features of creative improvisation and do not just reflect recalling memorized materials in the baseline condition.
Second, generation of nonmemorized facts (GenNonmemFact‐RecMemFact) reflects the spontaneous retrieval of semantically meaningful information and generation of language, which is shared with our principal contrast (GenNewPoem‐RecMemPoem), but does not involve production of novel, imagined material, which is only reflected in (GenNewPoem‐RecMemPoem). The double subtraction [(GenNewPoem‐RecMemPoem)‐(GenNonmemFact‐RecMemFact), Supporting Information Fig. 3A] makes it possible to control for spontaneous cognition and highlight brain activity related to production of novel imagined materials that may be specifically associated with verbal creativity.
The double subtraction approach was utilized here to identify differences that could be fully quantified and statistically tested. Accordingly, we found that deactivation of the DLPFC and IPS was significantly greater during poetry generation (significant in the contrast of GenNewPoem‐RecMemPoem, absent in GenNonmemFact‐RecMemFact and significantly greater in [GenNewPoem‐RecMemPoem]‐[GenNonmemFact‐RecMemFact], Supporting Information Fig. 3B). Meanwhile, only a small portion of the dorsal MPFC showed greater activation in GenNewPoem‐RecMemPoem than in GenNonmemFact‐RecMemFact, and no differences were found in the rest areas of the MPFC, indicating both generation of novel poems and nonmemorized facts were associated with activation in most sections the MPFC. We attribute this to the fact that both tasks are characterized by spontaneous cognitive activity.
Brain Activations During Revision of New Poems in All Subjects
To identify differences between the generation and revision phases (Fig. 2; Supporting Information Table 4), we directly subtracted GenNewPoem from RevNewPoem. In contrast to significant deactivations of the DLPFC and IPS in both hemispheres during the generation phase (Fig. 1), activity in these areas was significantly greater during the revision phase.
Figure 2.
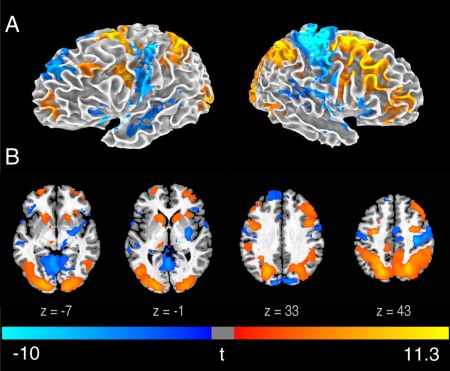
Brain activity associated with the revision phase.
Statistical t map of RevNewPoem‐GenNewPoem on a 3D brain surface (A) and axial slices (B) (FWE < 0.05). t‐scores are rendered in colors ranging from negative (violet) to positive (yellow) as indicated by the accompanying color bar.
Unlike the uniform and robust reciprocal pattern observed in the DLPFC and IPS, differences in the MPFC were sparse and heterogeneous—focal increases in the dorsal and decreases in the ventral portions of this region during the revision phase.
Functional Connectivity during Generation and Revision Phases in All Subjects
To explore the interactions between brain regions, we applied group ICA‐based functional network connectivity (FNC) analyses to the generation and revision phases together. In doing so, we were able to quantitatively compare the connectivity patterns of these two phases in a data‐driven way. The FNC method permits an unbiased examination of functional connections throughout the whole brain (the advantages of this approach are explained in the Methods section and the legend to Fig. 3A). In the dendrogram summarizing these results (Fig. 3A), the brain regions with high temporal correlations are grouped into one cluster. Interestingly, the MPFC and DLPFC/IPS, highlighted in the GLM analysis, naturally fell into two separate clusters, consistent with the central roles played by these two components in both generation and revision phases, as outlined above. Moreover, the MPFC was tightly coupled to many language related areas and the caudate nucleus (Cluster 2 in Fig. 3B), while the DLPFC/IPS operated in a more isolated mode (Cluster 4 in Fig. 3B).
Figure 3.
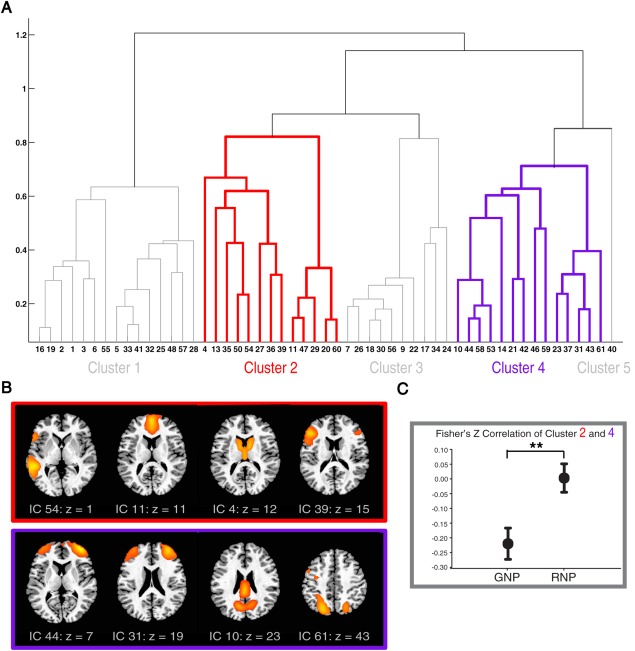
Brain network connections associated with generation and revision phases.
(A) In functional network connectivity (FNC) analyses, the group ICA decomposition was first used to divide the whole brain into 53 spatially independent components (ICs), each representing a self‐organized functional network with homogenous temporal dynamics. In this data‐driven way, we are able to examine the whole brain systematically while avoiding the random nature of seed selection. The hierarchical clustering of these ICs yielded the dendrogram displayed here. On the basis of statistical similarities in temporal dynamics, ICs were organized into the 5 clusters shown. Cluster 2 (red) and cluster 4 (purple) were respectively centered on the MPFC and the DLPFC/IPS. Clusters 1, 3, and 5 include ICs representing auditory‐somatosensory‐motor, visual and retrosplenial areas, respectively (all five clusters are depicted in Supporting Information Fig. 3). In this dendrogram, the x axis represents ICs and the y axis indicates distance between two linked objects, that is, either ICs or sub‐clusters (see Methods for detail). (B) Selected ICs from clusters 2 (red) and 4 (purple) are displayed. The MPFC was grouped together with an extensive set of regions including perisylvian cortices and the caudate nucleus. Only paramedian areas of the precuneus and posterior cingulate cortex (PCC) were grouped with the DLPFC/IPS. (C) Inter‐cluster correlation was calculated between the averaged time series of all ICs in cluster 2 and 4. Significant anticorrelation between cluster 2 and 4 during the generation phase was reversed in the revision phase (N = 27, mean ± standard error, ** indicates P < 0.01).
To understand how these two clusters interact with each other in each phase, we calculated the inter‐cluster correlations between clusters that included the MPFC and DLPFC/IPS during generation and revision phases (Fig. 3C). In accordance with the GLM results, the two clusters were significantly anti‐correlated during the generation phase; this relationship was markedly attenuated during the revision phase.
In addition to the MPFC and DLPFC/IPS, other brain regions including visual, somatosensory, auditory, motor related areas fell separately into the other clusters (cluster 1, 3 and 5 in Fig. 3B). The spatial distributions of all five clusters can be found in Supporting Information Figure 4.
Figure 4.
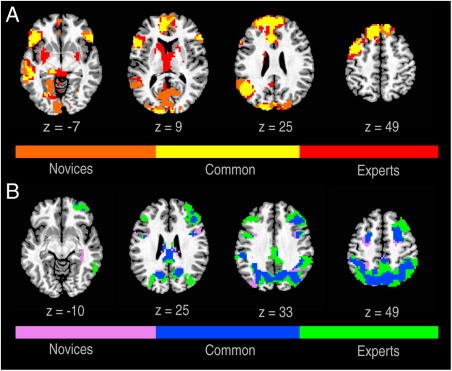
Conjunction of brain activity associated with generation of new poems in experts and novices.
Conjunction of statistical t maps of (GenNewPoem‐RecMemPoem) between experts and novices on axial slices: (A) activations (B) deactivations. Colors indicate activations/deactivations unique to experts, novices, or shared by both (FWE < 0.05).
Brain Activation During Generation and Revision Phases in Experts and Novices
When brain activity in experts and novices was compared using GLM conjunction and contrast analyses, the commonalities were striking. The conjunction of GenNewPoem‐RecMemPoem contrasts between groups revealed overlapping activation patterns in the same set of association areas in both groups (Fig. 4): increased activity in the dorsal MPFC, decreased activity in the DLPFC and IPS, and concomitant activation of the left hemisphere perisylvian areas. The double subtraction (GenNewPoem‐RecMemPoem)experts‐novices (Supporting Information Table 5) revealed significantly greater deactivations of the DLPFC and IPS in expert poets during poetry composition. However, these differences were just in the spatial extent and magnitude of responses in these regions as shown in Figure 4. Only subcortical areas including the body of the caudate, anterior and medial dorsal nuclei of the thalamus were greater in experts and not significantly activated in novices.
Similarly, the RevNewPoem‐GenNewPoem conjunction analysis indicated that activation patterns were comparable in experts and novices during the revision phase as well (Supporting Information Fig. 5). The left DLPFC and lingual gyrus were significantly activated in both groups (Supporting Information Fig. 5) and were greater in novices (Supporting Information Table 6).
Ratings of Poems’ Quality in Experts and Novices
To quantify differences in the quality of poems produced in the scanner, a group of three independent experts blindly rated the poems produced by each subject; measures of quality included ratings for craft (incorporating elements of sound, form, figurative and sensory language), linguistic creativity (LC—the innovative use of craft elements) and improvement following revision. Measures of inter‐rater reliability among three raters were strong for both craft (intraclass correlation coefficient ICC = 0.81) and LC (ICC = 0.76) and moderate for revision (ICC = 0.67). Using two sample t‐tests, we found that experts scored significantly higher in all three measures, especially in measures of craft and LC (P <0.0001), that is, those that were derived from the initial poems, prior to revision (Fig. 5). Although craft and LC scores both reflect the superior performance of experts, scores within each group are not significantly correlated with one another (Pearson's correlation coefficients: r = −0.16, P = 0.59 in experts; r = 0.23, P = 0.45 in novices), indicating that craft and LC in fact reflect two distinct aspects of quality. Experts also scored significantly higher than novices in improvement related to revision, but at a lower significance level (P <0.05). Examples of poems produced by experts and novices in both phases, and how these were scored are illustrated at the end of Supporting Information Results.
Figure 5.
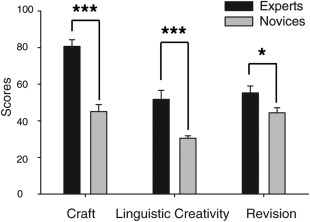
Measures of craft, linguistic creativity, and improvement by revision in experts and novices.
Two sample t‐tests showed experts scored significantly higher in all measures than novices (N = 27, mean ± standard error, *** indicates P < 0.001, * indicates P < 0.05).
In addition to measures of poetry quality provided by the panel of experts, we also used Whissell's dictionary [Whissell, 2009] to measure features of words (pleasantness, activation/arousal, imagery) produced in the GenNewPoem, RevNewPoem, and GenNewFacts conditions by experts and novices. No significant differences were found in these three word‐level measures either across conditions or between expert and novice groups (Supporting Information Table 2).
Interaction Effects of Expertise and Product Quality on Functional Connectivity
Since significant group differences were found for all quality measures rated by the experts, a single model considering group and quality together was used to examine how functional connections between brain regions differed between groups, and might on this basis explain differences in their performance in expert and novice poets. The same FNC methods described above were used in these analyses.
The results highlighted the importance of functional connections of the MPFC and DLPFC identified in the above analyses. During generation, we found that connectivity patterns within two sets of brain regions centered upon these areas were differentially correlated with performance in experts and novices, that is, with the quality of the poems they produced, as measured by craft and LC scores.
Figure 6 shows that when poems with high craft scores were generated, the MPFC was more strongly coupled to a set of language‐related brain regions in experts than in novices; on the other hand, the MPFC was more weakly correlated to the posterior parietal areas and pars opercularis.
Figure 6.
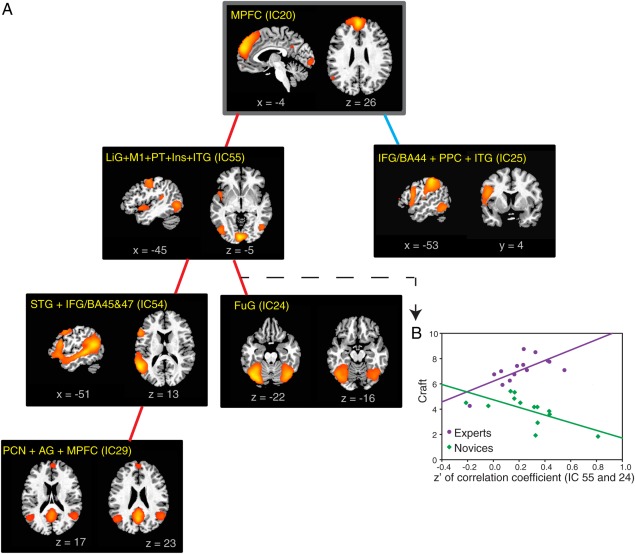
Distinct associations between craft ratings and connectivity patterns in experts and novices.
(A) An analysis of covariance (ANCOVA) model was used to examine group differences in the way craft scores were correlated with functional connections in experts and novices. In experts, craft scores were more tightly correlated with the strength of connections from the MPFC to a set of ICs including perisylvian areas, fusiform and angular gyri, precuneus and a mixture of motor and sensory areas, in a cascading fashion (indicated by red lines, see an example in Fig. 6B). On the other hand, in experts craft scores were more weakly correlated with the strength of connection between the MPFC and a component containing the dorsal portions of the IFG (BA44) and posterior parietal areas than they were in novices (indicated by the blue line) (FDR < 0.05 in each instance). (B) The relationship between ICs 55 and 24 is used to illustrate an instance in which craft score and functional connections were more strongly correlated in experts than in novices. The correlation (slope of the linear fit) between craft score and Fisher's z’ transformed correlation coefficient (of IC 55 and 24) is significantly greater (P = 0.0002, FDR = 0.008) in experts (purple: y = 6.21 × x + 4.10, P = 0.005) than in novices (green: y = −3.02 × x + 4.74, P = 0.01). AG, angular gyrus; FuG, fusiform gyrus; Ins, Insula; ITG, inferior temporal gyrus; LiG, lingual gyrus; M1, primary motor cortex; PCN, precuneus; PPC, posterior parietal cortex; PT, planum temporale; STG, superior temporal gyrus.
Figure 7 illustrates that when poems with high LC scores were produced, functional connections between the DLPFC and sensorimotor (somatosensory, premotor, and auditory) areas were weaker in experts than in novices while the correlation between sensorimotor areas and the left orbitofrontal cortex was stronger.
Figure 7.
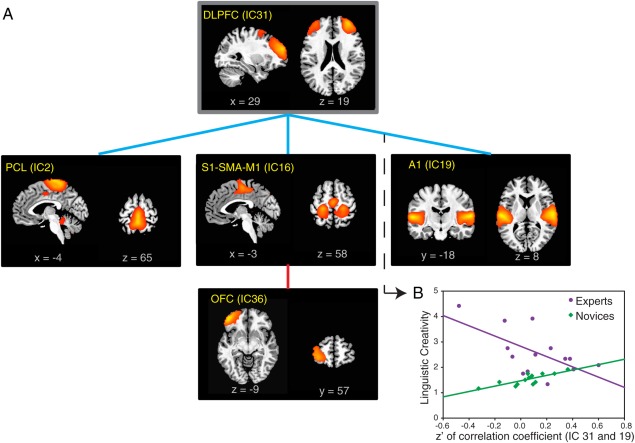
Distinct associations between linguistic creativity ratings and connectivity patterns in experts and novices.
(A) An analysis of covariance (ANCOVA) model was used to examine group differences in the way linguistic creativity scores were correlated with functional connections in experts and novices. In experts, linguistic creativity scores were more weakly correlated with the strength of functional connections between sensorimotor (somatosensory, premotor and auditory) areas and the DLPFC (indicated by blue lines, see an example in Fig. 7B) while these scores were more strongly correlated with the strength of functional connection between sensorimotor area and the left orbitofrontal cortex (indicated by the red line) (FDR < 0.05 in each instance). (B) The relationship between ICs 31 and 19 is used to illustrate an instance in which linguistic creativity scores and functional connections between ICs were more weakly correlated in experts than in novices. The correlation (slope of the linear fit) between LC and Fisher's z’ transformed correlation coefficient (of IC 31 and 19) is significantly less (P = 0.002, FDR = 0.04) in experts (purple: y = −2.02 × x + 2.82, P = 0.02) than in novices (green: y = 1.05 × x + 1.47, P = 0.0009). A1, primary auditory cortex; OFC, orbitofrontal cortex; PCL, paracentral lobule; SMA, supplementary motor area; S1, primary somatosensory cortex.
When the same analysis was carried out using the revision score, only a single functional connection between precuneus and the left orbitofrontal cortex differentiated experts and novices (Supporting Information Fig. 6).
DISCUSSION
In this study, we used fMRI to investigate the neural correlates of poetry composition, a canonical example of artistic creativity that has not been studied before. Using an ecologically valid paradigm, we simultaneously examined three key but poorly understood features of creativity—process, product, and expertise. The results obtained, using carefully selected baseline conditions and strict data‐driven analyses, provide a clearer understanding of each of these features: (1) the two principal phases of the creative process were clearly separated by reciprocal changes in activity in lateral prefrontal and posterior parietal cortices and the functional interaction between these regions and the MPFC; (2) in both phases, although differences in magnitude of BOLD changes in these regions were seen in expert and novice poets, overall activity patterns were dramatically similar; (3) crucially however, distinct connectivity patterns were found in experts and novices that correlated with their performance and were once again centered upon the medial and lateral frontal cortices. More importantly, these results may be integrated into a comprehensive neuropsychological model of creativity, with a common functional neuroanatomical basis, which is urgently needed in this field [Abraham, 2013]. Following a detailed discussion of each of the three key features and the relationships between them, this preliminary model is summarized below.
The Creative Process
The Generation Phase
Creative behaviors are thought to unfold through a multistage process [Finke et al., 1992; Johnson‐Laird, 1988; Wallas, 1926]. The cognitive model proposed by Johnson‐Laird suggests there are two principal phases, one in which a work is generated and a second in which the work is modified in order to correct or improve upon any flaws [Johnson‐Laird, 1988]. Crucially, both phases are nondeterministic; while there are rules or constraints specific to the genre at hand, the creator is still free to make choices within this set of constraints (e.g., a musician selecting the desired note or a poet the appropriate word).
Consistent with previous studies [Limb and Braun, 2008; Liu et al., 2012], we find that the generation phase is marked by a dissociated pattern of activity in the prefrontal cortex: the generation of novel poems elicited increased activity throughout the MPFC but decreased activity in the DLPFC.
The MPFC, a highly heterogeneous area, has been associated with a wide variety of functions ranging from motivation [Kouneiher et al., 2009], drive [Stuss and Alexander, 2005] intentionality underlying self‐generated action [Passingham et al., 2010], to unconscious decision making [Soon et al., 2008], and the integration of multidimensional information [Burgess et al., 2007]. Each of these cognitive functions must be engaged during this initial phase of the creative process. Indeed, a role for the MPFC during spontaneous creative activity has been confirmed by many neuroimaging studies using different paradigms and imaging modalities [Dietrich and Kanso, 2010].
The DLPFC, on the other hand, is thought to mediate high‐order cognitive control: self‐monitoring, planning, maintenance, and manipulation of information in working memory, suppression of irrelevant stimuli, and selection among competing responses [Frith, 2000; Petrides, 2005; Suzuki and Gottlieb, 2013]. The deactivation of the DLPFC observed here suggests that the generative phase may be associated with a suspension of those aspects of cognitive control—for example, consciously monitored step‐by‐step execution of behavior—that could impede the creative process.
It should be noted that the deactivation we observe may emerge only under ecologically valid conditions that do not superimpose additional demands upon this phase of the creative process [Berkowitz, 2010]. This may explain some discrepancies between our observations and those reported in several previous studies. For example, in one early study of musical improvisation [Bengtsson et al., 2007], pianists were asked to memorize the music which they improvised in order to equate task and control conditions with respect to their motor and sensory features. To help subjects memorize their output, they were asked to modify a previously learned eight‐bar melody. It is possible that this paradigm placed demands upon memory and attention that may have resulted in activation rather than deactivation of the DLPFC. Another musical improvisation study [Berkowitz and Ansari, 2008] employed a more restricted paradigm (using a keyboard limited to five keys) that did not simulate the naturalistic context under which improvisation is usually carried out. These authors also reported DLPFC activation.
In both cases, DLPFC activity might be attributed to the use of paradigms that require a significant degree of cognitive control. Indeed, when seen in this way, the results of a number of previous studies might be interpreted in the context of the model we propose below. That is, the improvisation conditions in the earlier studies may have engaged top‐down executive processes to the point that they may be more closely related to the revision condition in our study. We believe, as suggested by Berkowitz [Berkowitz, 2010], that while the various paradigmatic approaches that emphasize strict experimental control or ecological validity may produce different results, they may complement one another rather than compete.
Crucially, our results indicate that deactivation of the DLPFC does not occur in isolation. The IPS was concomitantly deactivated in the generation phase, consistent with our previous study [Liu et al., 2012]. Together the DLPFC and the IPS constitute elements of the so‐called dorsal attention network (DAN) proposed by [Corbetta and Shulman, 2002], a frontoparietal network thought to play a central role in conscious, top‐down attentional control [Bor and Seth, 2012]. On this basis, we suggest that the overall pattern associated with the generative phase of creative activity reflects a state in which spontaneous, self‐generated behaviors (mediated by activation of the MPFC) can unfold in the absence of conscious, attentional control (mediated by deactivation of the DAN. This interpretation is consistent with a longstanding notion that spontaneous creative behavior takes place in a state of cognitive disinhibition or defocused attention that permits lateral thinking and the formation of remote associations [Martindale, 1999; Runco and Sakamoto, 1999].
Empirical evidence appears to support this account as well. For example, a recent transcranial direct current stimulation (tDCS) study [Chrysikou et al., 2013] also showed that cathodal (inhibitory) tDCS over the left prefrontal cortex facilitates performance in an alternative uses task, supporting the idea that deactivation of the DLPFC enhances cognitive flexibility. In addition, patients with lesions in the MPFC had significant impairments in measures of originality on the Torrance test of creative thinking [Shamay‐Tsoory et al., 2011]. At the same time, patients (n = 17) with lesions in the lateral frontal cortex performed better than healthy controls in an insight‐based problem solving task [Reverberi et al., 2005]. Another clinical lesion study, however, provided conflicting evidence [Abraham et al., 2012]. These authors reported that nine patients with lesions in the lateral frontal cortex performed worse than healthy controls on an Alternative Uses task. This discrepancy could be due to variations in the location of lateral frontal lesions in these two cohorts or to differences in the behavioral tasks used. Abraham and coworkers also administered a problem solving task to the same group of patients, which may be more comparable to the task used in the Reverberi et al. study. In this case the performance of patients with lateral frontal lesions did not differ significantly from that of controls. A number of additional brain regions were specifically associated with the generation phase as well. Perhaps unsurprisingly, activations in left hemisphere perisylvian regions (IFG, MTG/STS, and fusiform gyri) emerged during the generation phase in this language‐based genre. Importantly, these activations are above and beyond those seen during the recitation of memorized poems (which also engages the language system) suggesting that constructing novel material imposes additional demands on language areas (e.g., in selecting words that contribute to building meaning, sound and imagery within the poem). Interestingly, activated portions of the dorsal anterior cingulate cortex included the cingulate motor area, which—consistent with observations from a previous study [Liu et al., 2012]—may represent an alternative motor pathway that is engaged during the generation of improvised material. Other activated brain regions included the amygdala (which may contribute to emotional expression) as well as the hippocampus, parahippocampal gyrus, and retrosplenial cortex (which could play a role in retrieval and incorporation of autobiographical material or visuospatial imagery into new poems).
A recent study [Ellamil et al., 2011] used an innovative design in which experienced graphic artists were asked to alternate between designing and evaluating book cover illustrations. The design phase in that study showed both similarities and differences when compared with the analogous generation phase in our study. For example, Ellamil and coworkers reported relative increases in activity of the mesial temporal cortices similar to those that we observed during the generation phase, which may reflect mnemonic and visuospatial processing in both instances.
On the other hand, differences were observed that might be attributed to distinct features of the genres being examined. For example, activation of superior and inferior parietal lobules reported by Ellemil et al. may reflect visuospatial processing required in generation of drawings. In contrast, activation of the left perisylvian areas observed in the present study may reflect language processing demands specifically associated with the generation of poems.
Use of Additional Baselines
Since fMRI results are typically derived from a comparison of two conditions, the selection of appropriate baselines is crucial, particularly in research on creativity [Abraham, 2013]. We attempted to keep our experimental paradigm as naturalistic as possible, but under these conditions it becomes particularly important to maintain an effective degree of experimental control. Therefore, we adopted two additional baseline conditions, providing controls that made it possible to test the reliability of the activity pattern reported above and explore the details of this pattern in a more fine‐grained fashion. Random typing (production of random keystrokes at a rate close to the generation of novel poetry) served to control for spontaneous low level motor activity. When this was used as a baseline, generation of novel poetry showed the same pattern of activations and deactivations that was seen when the memorized condition was used as a baseline, suggesting that our results were not biased in this fashion.
Furthermore, when free generation of factual information was used to control for spontaneous but noncreative cognitive activity and language production, we found minimal differences in the MPFC (i.e., this region was activated during the spontaneous generation of both facts and poems), whereas activity in the DAN was more strongly deactivated during the generation of poetry. This suggests that the MPFC may support general execution of goal‐oriented, spontaneous cognitive processes that are shared by both fact and poetry generation. Deactivation of the DAN, on the other hand, appears to be more specifically associated with creative improvisation typified by the generation of novel poetry.
A related fMRI study [Shah et al., 2013] used a conceptually different set of tasks and baseline conditions to evaluate creative writing. In this experiment, the portion of the process that we refer to as generation was broken down into two phases according to the theory of Flower and Hayes [Flower and Hayes, 1981]. Planning a story (brainstorming) and writing out what was planned (creative writing) were separated in time and scanned independently, along with two control tasks (copying and reading). The contrast between the creative writing condition and copying showed activation of the hippocampus, anterior temporal lobe, and posterior cingulate cortex, consistent with what we observed during generation of novel poetry. On the other hand, brainstorming—that is, planning the story prior to its transcription—may be the condition most closely associated with the process of improvisation. This condition in itself was associated with activation of the same inferior frontal, temporal, and parietal areas that we observed during poetry generation. However, Shah et al. did not report contrasts between the brainstorming condition and either of their lower level baseline tasks, and no deactivations were reported for any of the contrasts performed. In the absence of these, it is not possible to know whether deactivation of the frontoparietal attention system, which our data suggest may be the sine qua non of the generation phase, might have been detected in the Shah et al. study as well.
The Revision Phase
How then do the patterns of brain activity observed during the generation of poems change during the revision phase? We found that revision was associated with relative increases in activity within the DLPFC, extending from its ventral portions to the frontal eye fields, as well as increases in the IPS and precuneus. This suggests that self‐monitoring and top‐down attentional processes, suspended during the generation phase, are robustly reengaged during the revision phase. This is consistent with the idea that during the revision phase, instead of maintaining a state of defocused attention that promotes freedom of association and the generation of novel ideas, poets are explicitly exercising aesthetic judgments and critically monitoring their own output, attending to perceived flaws and selecting from among a series of alternative possibilities in order to correct them. The MPFC, on the other hand, showed very few differences when revision and generation of poems were compared. This is not unexpected as self‐initiated behavior, sustained motivation, and integration of multidimensional information should be required during both phases.
This pattern is similar to one reported by Ellamil et al. [2011] in the only other neuroimaging study to examine a phase of the creative process beyond generation. In that study, Ellamil and coworkers looked at evaluation of previously designed materials, a component of the revision process we study here.
A number of crucial findings were in fact common to the evaluation phase in the Ellamil et al. study and the revision phase in ours. The direct contrast between design and evaluation conditions was characterized by joint recruitment of MPFC and other elements of the default mode network as well as executive regions including the DLPFC. This pattern is not inconsistent with what we observed during the revision phase—sustained activation of the MPFC (at levels established during the generation phase) accompanied by robust increases in activity in the attention network. This supports the existence of a mental state suggested by Christoff et al. [Christoff et al., 2009] in which the default mode and executive systems, which are frequently reported to be anti‐correlated, are in fact coactivated during certain cognitive activities, which in this case may include the process of evaluating and/or revising drawings as well as poetry.
Reciprocal Patterns of Connectivity During Generation and Revision Phases
After determining that the MPFC and DAN/DLPFC play central roles in each of the two phases based upon the contrasts outlined above, we carried out a data‐driven connectivity analysis, which provides critical information about phase‐specific functional interactions between these regions. Importantly, hierarchical clustering of the IC (described in the methods section) showed that these regions naturally segregated into two separate clusters.
In one, the MPFC was clustered together with perisylvian language areas, supporting the notion that the MPFC may be responsible for motivation and/or integration of the linguistic information. The second cluster was comprised entirely of components that make up the DAN, indicating that this attention system may function as a relatively independent module, so that the cognitive processes it supports (e.g., self‐monitoring or top‐down attention to what is formulated) might be flexibly imposed or suspended as necessary.
Supporting this account, inter‐cluster correlation analyses demonstrated that while the MPFC/Perisylvian and DAN clusters were significantly anti‐correlated during poetry generation, activity in these two clusters was uncoupled during the revision phase. In other words, the antagonistic relationship between these two systems that may be the hallmark of spontaneous generation is attenuated as poems are revised.
The notion that the two phases may be characterized by reciprocal interactions between systems supporting extemporaneous generation and cognitive control is consistent with previous models [Jung et al., 2013] that have built upon the notion that creative work represents a phase of blind variation followed by selective retention [Campbell, 1960].
It should also be reiterated that in the real world, these two phases do not alternate with one another in a linear or periodic fashion, a condition that was imposed by our experimental design. During the natural evolution of the creative process, spontaneous improvisation and top‐down evaluation are more flexibly interwoven and will alternate in a less predictable, more adaptive manner. Even under this experimental setting, we know from the typing records that subjects performed generation and revision conditions as we instructed, but we should not fully exclude the possibility that subjects occasionally thought of revision during the condition of generation or vice versa in an unconscious manner, which would reduce our chance to observe differences between these two phases.
Nevertheless, our results indicate that both activity and connectivity patterns are able to clearly differentiate generation and revision. Crucially, these differences are characterized by a reorganization of functional relationships within the same set of frontal and parietal regions, and these relationships appear to constitute the defining features of each phase.
Expertise
Are differences between experts and novices also associated with these frontal and parietal regions, or do experts have privileged access to a unique neuronal architecture during creative activity? In order to examine this, we performed both contrast and conjunction analyses of activity patterns accompanying both phases. Our results argue against a unique architecture and implicate the same set of regions that defined the creative process per se. This implies that a single neuroanatomical model may be used to explain both process and the impact of expertise.
Differences between experts and novices were observed in the magnitude of activation during both the generation and revision phases. Importantly, these differences were again found within the same set of frontal and parietal regions described above. The contrast analysis revealed that deactivation of the DLPFC and IPS was significantly greater in experts during the generation phase. This suggests that experts may be able to more readily suspend cognitive control and enter into a state of defocused attention that may enable the production of more innovative and original work [Martindale, 1999].
In a related finding, experts showed activation of subcortical structures including the dorsal caudate and dorsomedial thalamus during the generation phase. Together with the DLPFC, these subcortical areas represent elements of the dorsolateral corticostriatal circuit [Alexander et al., 1986] which has been implicated in a previous study of creativity [Jung et al., 2010]. The reciprocal pattern of activity within this system—increases in the striatum, decreases in the DLPFC—is consistent with the possibility that in experts, generation of novel poetry engages automatic and routinized behaviors mediated by the striatum [Saling and Phillips, 2007], rather than conscious, attentionally driven processes mediated by the cortex. Some behaviors involved in generating poems—processing meter, establishing rhythm, etc.—may shift to these subcortical structures as they became less effortful for experts over time, resulting in attenuated activity in prefrontal regions relied upon by novices. This account is supported by the observation that the dorsal striatum modulates the excitatory status of the DLPFC [Balleine et al., 2007; Grahn et al., 2008].
The DLPFC was also significantly less active in experts than novices during revision of their poems, suggesting that the top‐down cognitive operations engaged during this phase may be less effortful and more automatic in experts as well.
Crucially however, our conjunction analyses revealed both generation and revision phases were dominated by patterns of activity that were the same in trained individuals as they were in laypersons. This suggests that the creative process may be grounded in a common neural architecture that is available to experts and novices alike. This interpretation—that novices already have access to the necessary neural resources ‐may have implications for educational approaches that seek to augment creative thinking or performance. Indeed, previous studies have suggested that insights derived from neurocientific research can increase the effectiveness of creativity training [Onarheim and Friis‐Olivarius, 2013; Scott et al., 2004].
The Creative Product
In spite of the overall similarities in activity patterns between groups, when we examined the poems produced by experts and novices, we found striking differences related to product quality assessed from the readers’ point of view.
The measures of craft and linguistic creativity produced by the panel of experts were uncorrelated, strongly suggesting that they reflect two critical, independent features of the creative product. The craft score may reflect the knowledge and application of techniques commonly used for good effect in writing poetry typically acquired as a result of training. The linguistic creativity score, on the other hand, is an index of the innovative use of these techniques and likely reflects innate talent or creative aptitude. In both instances, expert performance significantly exceeded that of novices.
Given the clear behavioral differences, we next investigated the neural correlates of these measures of product quality to see if they more effectively differentiated expert and novice poets. Since it is possible that the most salient group differences may lie not in which regions are activated, but in how these regions are functionally connected we used data‐driven connectivity analyses (as outlined in methods section) to assess regional connectivity and determine how these connections are modulated by the technical and innovative features of the product in both groups.
In light of our previous results, an important question was whether these connections would be instantiated in the same large‐scale systems that played a central role in defining the stages of the creative process and the impact of expertise. The answer appears to be yes. Importantly, our results revealed that experts produced higher quality poems by engaging unique patterns of functional connectivity during the creative process, and that these patterns are centered in the same frontal and parietal systems that were identified in our earlier analyses.
The results, presented as schematics in Figures 6 and 7, indicate that measures of craft and linguistic creativity modulate connectivity in a distinct way in expert poets. Both prefrontal regions identified in the above analyses again appear to play critical roles: the MPFC is implicated in the modulation of connectivity by craft; the DLPFC in the modulation of connectivity by linguistic creativity.
During the generation phase, the effective use of craft is associated with a unique set of cascading interactions in experts: the more successfully craft elements were incorporated into their poems, the tighter the coupling was found between the MPFC and the left perisylvian language regions, as well as the inferior parietal lobule and the precuneus/PCC (elements of the default mode network). The default mode network supports a wide variety of functions [Spreng and Grady, 2010]. Consistent with reports that these include internally generated thinking [Mason et al., 2007] it is possible that the MPFC and other components of this network facilitate the spontaneous, internal generation of novel ideas during the generation of new poems. Encoding these ideas in language must involve interactions with the perisylvian cortices, and enhanced neural synchronization between these two sets of regions may indicate a more efficient transfer of ideas into text which may itself result from years of training that improves processing efficiency [Lewis et al., 2009].
Connections of the DLPFC, on the other hand, were implicated by the rating of linguistic creativity (innovative use of the same craft elements). Experts’ poems that were judged to be of higher quality in this sense were associated with decreased connectivity between the DLPFC and auditory, somatosensory and motor regions and a concomitant increase in connectivity between these same regions and the orbitofrontal cortex. This suggests a shift from regulation by prefrontal regions involved in top‐down control to regulation by regions with stronger connections to the limbic system. The fact that these regions are operating outside of the control normally imposed by the DLPFC and are coupled instead to regions associated with emotional processing [Bechara et al., 2000], could contribute to the spontaneous production of more vivid sensorimotor images or more innovative uses of sound during the generation phase, ultimately resulting in poems that were judged to be more engaging. The fact that this pattern is reversed in novice poets (i.e., that sensorimotor and auditory regions are instead more strongly coupled to the DLPFC) again suggests that composition—even of poems that were judged to be more creative—may be more effortful and subject to more stringent top‐down control in novices.
The same approach was used to examine the relationships between product quality and network connectivity during the revision phase, in this case using the revision score (based on comparisons of the original poem and the revised version). Somewhat surprisingly, this interaction revealed only a single pair of components in which connectivity was modulated differently in experts and novices—the default mode network and the orbitofrontal cortex. The attenuated coupling between these components in experts suggests that successful revision may be in this case result from internally generated thinking that is less driven by emotion (and that the opposite may be true for novices). These results must be interpreted with caution since the revision score (based on more qualitative comparison of the original poem and the revised versions) showed weaker inter‐rater reliability measures than craft and linguistic creativity scores.
A Multidimensional Model for Creative Behavior
Taken together, results of the foregoing analyses suggest that a single neuroanatomical model can account for the stages of the creative process, the impact of talent and experience, and the technical and innovative features of the creative product. This model would be grounded on two brain systems: medial prefrontal (MPFC) regions, representing anterior elements of the default mode network, and dorsolateral prefrontal regions and parietal cortices (DLPFC/DAN) that regulate executive control. The dynamic interactions between these regions and their relationships to other cortical and subcortical areas lie at the heart of the model and represent the central elements of a large‐scale network that regulates all three aspects creative behavior: Activity within and interactions between the MPFC and DLPFC/DAN characterize both phases of the creative process; activity within these same systems defines and differentiates expert and novice poets; interactions between these systems and an array of regions distributed throughout the brain appear to significantly modulate the quality of the poems produced.
The model we propose (Fig. 8), while it extends beyond the present set of findings, successfully incorporates all three of these essential features of creative activity and attempts to characterize the neural mechanisms underlying creative cognition in a way that can account for previous discrepancies in the neuroimaging literature [Abraham, 2013].
Figure 8.
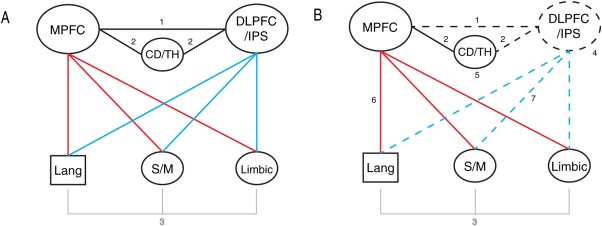
A schematic depicting the proposed multidimensional model of creativity.
The medial prefrontal cortex (MPFC) and dorsal attention network (DLPFC/IPS) are central to the operation of this model. The dynamic interactions between the MPFC and DLPF/IPS and between these regions and other areas of the brain characterize the three essential features of creativity: stages of the creative process, expertise and product quality. Panels (A) and (B) represent two alternating cognitive states that support revision (A) and improvisation/generation (B). As indicated by red lines during both phases, the medial prefrontal cortex (MPFC) motivates and maintains activity in other cortical regions (3) including language related (Lang), sensorimotor (S/M) and limbic areas. Solid blue lines indicate regulatory control imposed by the DLPFC/IPS on these same regions during the revision phase (A). During improvisation (B), such top‐down cognitive control is attenuated (dotted lines), in association with deactivation of the DLPFC/IPS (4). The MPFC and DLPFC/IPS are linked via direct intracortical connections (1), as well as connections (2) mediated by corticostriatal‐thalamocortical circuits, including caudate and thalamus (CD/TH),that regulate their excitability. These connections play a role in regulating activity in the DLPFC/IPS as these changes across the two phases. Compared to novices, experts show greater deactivation of the DLPFC/IPS (4) and activation of CD/TH (5) during the generation phase (B), reflecting their capacity to more readily suspend cognitive control and enter a state conducive to creative improvisation. Nevertheless, experts and novices show strikingly similar patterns of activity during both phases, suggesting that the same cognitive resources that support creative behavior are accessible to everyone, regardless of training or experience. Importantly, however, experts establish unique connections between MPFC and DLPFC/IPS and downstream regions that account for the superior quality of their creative products. Greater technical skills evidenced by experts are associated with stronger connections between the MPFC and downstream regions (6). In contrast, attenuated connections between the DLPFC/IPS and downstream regions (7) may reflect a selective disinhibition that results in the more innovative nature of their poetry.
The MPFC supports intrinsic motivation, initiating and sustaining activity in a wide array of downstream regions (3 in Fig. 8) related to language, sensorimotor and limbic functions. The DLPFC and IPS, which constitute elements of the dorsal attention network, play a countervailing role—consciously monitoring ongoing behavior and exerting cognitive control by modulating activity within the downstream regions targeted by the MPFC. These two systems may interact with each other either by direct intra‐cortical connections between them (1 in Fig. 8) or through interactions with subcortical elements of key corticostriato‐thalamocortical circuits (2 in Fig. 8) that regulate the excitability of these cortical areas.
Ultimately however, it is the collective impact of these systems on activity in language, sensorimotor and limbic regions (3 in Fig. 8) that constitute the final common pathway regulating the creative process. The precise nature of the downstream regions involved will differ depending upon the creative behavior in question. For example, language‐related areas play a prominent role in poetry composition whereas visual cortices may be expected to play a central role in painting.
As noted, the model accounts for each of the three features of creative cognition we have evaluated in these experiments (Fig. 8). In general, it suggests that the initial phase of the creative process, generation is a state in which self‐initiated behaviors unfold in the relative absence of top‐down cognitive control, and that revision is characterized by re‐emergence of executive processes—attention, evaluation, error detection—that characterize this phase. Importantly, this model is able to incorporate both the differences and remarkable similarities in activation patterns seen in experts and novices. And on the basis of connectivity between the core and downstream brain regions, the same model accounts for variations in the technical and innovative qualities of the creative products themselves.
The model we propose not only plausibly accounts for the results we report here, it is in accord with previous studies in performance‐based genres such as jazz [Limb and Braun, 2008] and lyrical improvisation [Liu et al., 2012], has been supported conceptually by other researchers [Abraham, 2013; Berkowitz, 2010] and is consistent with TMS and lesion studies [Chrysikou et al., 2013; Reverberi et al., 2005; Shamay‐Tsoory et al., 2011] as well. (Although another lesion study [Abraham et al., 2012] reported results that were inconsistent with the model proposed here, the discrepancy, as discussed above, may have been due to differences in lesion distribution or in the behavioral tasks that were employed). Importantly, however, our model is consistent with a number of other models that have been previously proposed by Jung [Jung et al., 2013], Campbell [Campbell, 1960], Johnson‐Laird [Johnson‐Laird, 1988], Martindale [Martindale, 1999], and Dietrich [Dietrich, 2004].
It is important to point out that the dynamic changes in prefrontal cortical activity that characterize this model may not only reflect phases of the creative process, but rather reciprocal cognitive states of which creative behaviors are only a subset. This notion is consistent with the matched filter hypothesis recently proposed by Chrysikou and colleagues [Chrysikou et al., in press] in which the level of activity in the prefrontal cortex increases or decreases in a context dependent fashion to optimize performance on any given task. Importantly, our model adds two critical details to this hypothesis: rather than a uniform function performed by the prefrontal cortices, we differentiate specific functions associated with its medial and lateral regions. And while the matched filter hypothesis proposes that top‐down cognitive control or filtering is exerted upon sensory input, we suggest that such control also applies to internally generated, stimulus independent thoughts and emotions that may play a fundamental role in creative activity.
Caveats and Future Directions
It should be noted that although all of the results we report are statistically significant, our sample size is relatively small. An additional concern is that while the experts in this study were clearly talented and highly trained, the group did not contain extraordinarily gifted and accomplished poets. While our results may pertain to the general population, including the smaller percentage that is extremely skilled, true genius might be characterized by a unique and discontinuous neural architecture that may have been missed here. In any case, future studies should use the present paradigms to study this unique population.
It must also be noted that although the naturalistic paradigms we employ here are useful (as we have argued, they were designed to be unambiguously related to the creative behaviors of interest without superimposition of secondary cognitive processes), they are inherently difficult to control. We attempted to strike a balance, imposing control using a number of different baseline conditions as discussed above. It will essential in the future to conduct a systematic comparison between results of studies that use naturalistic paradigms and those using more rigorously controlled experimental conditions, to identify underlying consistencies and, ultimately, a common neural basis of verbal or artistic creativity, if one exists.
Related to this, while many of the neural mechanisms illustrated here may be specific to artistic creativity, verbal creativity, or poetry in particular, some may be relevant for other forms of creative activity, and it will be important to identify any domain general features that characterize this multifaceted process [Dietrich, 2004]. Future studies should explore alternative forms of creative behavior—including technical and scientific creativity [Andreasen and Ramchandran, 2012]—to identify potentially universal features of this model.
Supporting information
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information
ACKNOWLEDGMENTS
The authors thank for Philip Johnson‐Laird and Steven Pinker for their valuable comments on this manuscript. They also thank all expert and novice poets for participating in our studies, and Colin Hoy for assisting in the recruitment of subjects.
REFERENCES
- Abraham A (2013): The promises and perils of the neuroscience of creativity. Front Hum Neurosci 7:246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Beudt S, Ott DV, Yves von Cramon D (2012): Creative cognition and the brain: dissociations between frontal, parietal‐temporal and basal ganglia groups. Brain Res 1482:55–70. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Ramchandran K (2012): Creativity in art and science: Are there two cultures? Dialogues Clin Neurosci 14:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839−851. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, Corbetta M (2012): Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A 109:3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O (2007): The role of the dorsal striatum in reward and decision‐making. J Neurosci 27:8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR (2000): Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10:295–307. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Csikszentmihalyi M, Ullen F (2007): Cortical regions involved in the generation of musical structures during improvisation in pianists. J Cogn Neurosci 19:830–842. [DOI] [PubMed] [Google Scholar]
- Berkowitz AL. (2010): The Neurobiology of Improvisation In: Berkowitz AL, editor. The Improvising Mind. New York: Oxford; pp 131–144. [Google Scholar]
- Berkowitz AL, Ansari D (2008): Generation of novel motor sequences: The neural correlates of musical improvisation. Neuroimage 41:535–543. [DOI] [PubMed] [Google Scholar]
- Berkowitz AL, Ansari D (2010): Expertise‐related deactivation of the right temporoparietal junction during musical improvisation. Neuroimage 49:712–719. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H (2005): Drawing on mind's canvas: Differences in cortical integration patterns between artists and non‐artists. Hum Brain Mapp 26:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA (2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31:1536–1548. [DOI] [PubMed] [Google Scholar]
- Bor D, Seth AK (2012): Consciousness and the prefrontal parietal network: Insights from attention, working memory, and chunking. Front Psychol 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM (2006): Music and language side by side in the brain: A PET study of the generation of melodies and sentences. Eur J Neurosci 23:2791–2803. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ (2007): The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 11:290–298. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DT (1960): Blind Variation and Selective Retentions in Creative Thought as in Other Knowledge Processes, 6th ed. US: American; Psychological Association; pp 380–400. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH, Coslett HB, Datta A, Bikson M, Thompson‐Schill SL (2013): Noninvasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cogn Neurosci 4:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Weber MJ, Thompson‐Schill SL (2014): A matched filter hypothesis for cognitive control. Neuropsychologia 62:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Dietrich A (2004): The cognitive neuroscience of creativity. Psychon Bull Rev 11:1011–1026. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R (2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136:822–848. [DOI] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio‐Mazoyer N, Mazoyer B, Mellet E, Joliot M (2011): Brain activity at rest: A multiscale hierarchical functional organization. J Neurophysiol 105:2753–2763. [DOI] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K (2011): Evaluative and generative modes of thought during the creative process. Neuroimage 59:1783–1794 [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD (2011): Comparison of multi‐subject ICA methods for analysis of fMRI data. Hum Brain Mapp 32:2075–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Benedek M, Grabner RH, Staudt B, Neubauer AC (2007): Creativity meets neuroscience: experimental tasks for the neuroscientific study of creative thinking. Methods 42:68–76. [DOI] [PubMed] [Google Scholar]
- Fink A, Graif B, Neubauer AC (2009): Brain correlates underlying creative thinking: EEG alpha activity in professional vs. Novice dancers. Neuroimage 46:854–62. [DOI] [PubMed] [Google Scholar]
- Finke RA, Ward TB, Smith SM (1992): Creative Cognition: Theory, Research, and Applications. Cambridge, MA: MIT Press. [Google Scholar]
- Flower L, Hayes JR (1981): A cognitive process theory of writing. College Compos Commun 32:365–387. [Google Scholar]
- Frith C (2000): The role of the dorsolateral prefrontal cortex in the selection of action as revealed by functional imaging In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; pp. 549–565. [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM (2008): The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD (2008): A method for functional network connectivity among spatially independent resting‐state components in schizophrenia. Neuroimage 39:1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson‐Laird PN (1988): Freedom and constraint in creativity In: Sternberg RJ, editor. The Nature of Creativity. Cambridge: Cambridge University Press; pp. 202–219. [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ (2010): White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS One 5:e9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA (2013): The structure of creative cognition in the human brain. Front Hum Neurosci 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, Veijola J, Moilanen I, Isohanni M, Zang YF, Tervonen O (2009): Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp 30:3865–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E (2009): Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12:939–945. [DOI] [PubMed] [Google Scholar]
- Kounios J, Beeman M (2014): The cognitive neuroscience of insight. Annu Rev Psychol 65:71–93. [DOI] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M (2009): Neural networks involved in artistic creativity. Hum Brain Mapp 30:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP (2011): Resting‐state functional connectivity indexes reading competence in children and adults. J Neurosci 31:8617–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M (2009): Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Braun AR (2008): Neural substrates of spontaneous musical performance: An FMRI study of jazz improvisation. PLoS One 3:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chow HM, Xu Y, Erkkinen MG, Swett KE, Eagle MW, Rizik‐Baer DA, Braun AR (2012): Neural correlates of lyrical improvisation: An FMRI study of freestyle rap. Sci Rep 2:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM (2004): A method for removal of global effects from fMRI time series. Neuroimage 22:360–366. [DOI] [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P (1997): Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 16:187–198. [DOI] [PubMed] [Google Scholar]
- Martindale C. (1999): Biological bases of creativity In: Sternberg RJ, editor. Handbook of Creativity. Cambridge: Cambridge University Press; pp 137–152. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missall KN, McConnell SR. (2004): Psychometric Characteristics of Individual Growth & Development Indicators: Picture Naming, Rhyming, and Alliteration. Minneapolis, MN: University of Minnesota Center for Early Education and Development. [Google Scholar]
- Onarheim B, Friis‐Olivarius M (2013): Applying the neuroscience of creativity to creativity training. Front Hum Neurosci 7:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC (2010): Medial frontal cortex: From self‐generated action to reflection on one's own performance. Trends Cogn Sci 14:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2005): Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi C, Toraldo A, D'Agostini S, Skrap M (2005): Better without (lateral) frontal cortex? Insight problems solved by frontal patients. Brain 128:2882–2890. [DOI] [PubMed] [Google Scholar]
- Runco MA, Sakamoto SO (1999): Experimental studies of creativity. In: Sternberg RJ, editor. Handbook of Creativity. Cambridge: Cambridge University Press; pp 62–92. [Google Scholar]
- Saling LL, Phillips JG (2007): Automatic behaviour: Efficient not mindless. Brain Res Bull 73:1–20. [DOI] [PubMed] [Google Scholar]
- Sarela J, Vigario R (2003): Overlearning in marginal distribution‐based ICA: Analysis and solutions. J Machine Learning Res 4:1447–1469. [Google Scholar]
- Scott G, Leritz LE, Mumford MD (2004): The effectiveness of creativity training: A quantitative review. Creativity Res J 16:361–388. [Google Scholar]
- Shah C, Erhard K, Ortheil HJ, Kaza E, Kessler C, Lotze M (2013): Neural correlates of creative writing: An fMRI study. Hum Brain Mapp 34:1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG, Adler N, Aharon‐Peretz J, Perry D, Mayseless N (2011): The origins of originality: The neural bases of creative thinking and originality. Neuropsychologia 49:178–85. [DOI] [PubMed] [Google Scholar]
- Soon CS, Brass M, Heinze HJ, Haynes JD (2008): Unconscious determinants of free decisions in the human brain. Nat Neurosci 11:543–545. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL (2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP (2005): Does damage to the frontal lobes produce impairment in memory? Curr Directions Psychol Sci 14:84–88. [Google Scholar]
- Suzuki M, Gottlieb J (2013): Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 16:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L (1999): Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14:167–77. [PubMed] [Google Scholar]
- Villarreal MF, Cerquetti D, Caruso S, Schwarcz Lopez Aranguren V, Gerschcovich ER, Frega AL, Leiguarda RC (2013): Neural correlates of musical creativity: Differences between high and low creative subjects. PLoS One 8:e75427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallas G. (1926): The Art of Thought. London: Jonathan Cape.
- Whissell C (2009): Using the revised dictionary of affect in language to quantify the emotional undertones of samples of natural language. Psychol Rep 105:509–521. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tong Y, Liu S, Chow HM, AbdulSabur NY, Mattay GS, Braun AR (2014): Denoising the speaking brain: Toward a robust technique for correcting artifact‐contaminated fMRI data under severe motion. Neuroimage 103C:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Figure 2.
Supporting Information Figure 3.
Supporting Information Figure 4.
Supporting Information Figure 5.
Supporting Information Figure 6.
Supporting Information


