Abstract
Epithelial-cadherin (Ecad) deregulation affects cell–cell adhesion and results in increased invasiveness of distinct human carcinomas. In gastric cancer, loss of Ecad expression is a common event and is associated with disease aggressiveness and poor prognosis. However, the molecular mechanisms underlying the invasive process associated to Ecad dysfunction are far from understood. We hypothesized that deregulation of cell–matrix interactions could play an important role during this process. Thus, we focussed on LM-332, which is a major matrix component, and in Ecad/LM-332 crosstalk in the process of Ecad-dependent invasion. To verify whether matrix deregulation was triggered by Ecad loss, we used the Drosophila model. To dissect the key molecules involved and unveil their functional significance, we used gastric cancer cell lines. The relevance of this relationship was then confirmed in human primary tumours. In vivo, Ecad knockdown induced apoptosis; nonetheless, at the invasive front, cells ectopically expressed Laminin A and βPS integrin. In vitro, we demonstrated that, in two different gastric cancer cell models, Ecad-defective cells overexpressed Laminin γ2 (LM-γ2), β1 and β4 integrin, when compared with Ecad-competent ones. We showed that LM-γ2 silencing impaired invasion and enhanced cell death, most likely via pSrc and pAkt reduction, and JNK activation. In human gastric carcinomas, we found a concomitant decrease in Ecad and increase in LM-γ2. This is the first evidence that ectopic Laminin expression depends on Ecad loss and allows Ecad-dysfunctional cells to survive and invade. This opens new avenues for using LM-γ2 signalling regulators as molecular targets to impair gastric cancer progression.
Introduction
Epithelial-cadherin (Ecad) is a major contributor to cell–cell adhesion and tissue integrity, playing critical mechanical and signalling functions in epithelial cells (1). In cancer, Ecad aberrant expression and/or function is frequently associated with an increased invasive behaviour (1,2). Particularly, in gastric cancer, deficient Ecad membrane staining is found in more than 80% of all cases (either sporadic or hereditary), affecting cell–cell adhesion and increasing cell invasive potential, which is a key step in cancer progression (3–10). Nevertheless, the mechanisms by which Ecad-defective cells invade the adjacent stroma are still largely unknown.
Recently, it has been suggested that deregulation of cell–cell adhesion could result in abnormal interactions between cells and the extracellular matrix and, in this way, promote cancer invasion and metastization (11). Interestingly, Laminin-332 (LM-332, also named Laminin-5) overexpression has been reported in gastric cancer, particularly in its γ2 monomeric form, and is considered a poor prognostic factor (12–15). This large-molecular-weight glycoprotein is the major basement membrane component, unique to epithelial cells. It consists of α3, β3 and γ2 subunits organized in a cruciform structure and is known to modulate cell migration and invasion, especially in its soluble form (13,16,17).
Therefore, both Ecad and LM-332 deregulation seem to correlate with the invasive process in gastric cancer. However, to our knowledge, a causal relationship between Ecad loss and LM-332 upregulation has not been described. In this work, we aimed to investigate if Ecad silencing results in LM-332 accumulation and to understand how this interplay affects disease progression. To verify whether LM-332 upregulation was triggered by Ecad loss in a wild-type epithelium, we used the Drosophila model.
Fruit flies have been widely used to study human disease given the high degree of preservation in terms of basic mechanisms and signalling pathways between flies and man. Still, the greatest strength of using Drosophila lies in its ability to model cancer hallmarks in a variety of tissues (18). Elegant genetic techniques enable the study of context-dependent tumourigenesis and provide insights into how tumour cells cooperate with their microenvironment to promote tumourigenesis (18–20).
Further, we took advantage of human gastric cancer cell lines to investigate the signalling pathways involved in this Ecad/Laminin crosstalk. Overexpression and inhibition systems were used to modulate Ecad and Laminin. Using the in vitro gastric cancer model, we also unveiled the biological impact of Laminin γ2 (LM-γ2) on Ecad-defective cells. Then, we evaluated and validated the Ecad/Laminin crosstalk in primary tumours.
Herein, we demonstrate that Ecad-dependent invasion results from an imbalance between cell–cell and cell–matrix interactions. Using both in vivo and in vitro models, we were able to unravel the underlying signalling pathway controlling this process.
Results
In vivo DEcad knockdown results in de novo Laminin A expression accumulation
Previous work has demonstrated the usefulness of Drosophila as a model to identify interactors of Ecad (21–23). Herein, we used the fly model to determine the molecular mechanisms activated in vivo upon Ecad loss. We used the Upstream Activating Sequences (UAS) Gal4 system to simultaneously express Drosophila Ecad (DEcad) double-stranded RNA (dsRNA) and GFP in the fly primordium (known as wing disc). The hedgehog-Gal4 (hh-Gal4) driver enabled targeted expression to the posterior compartment, leaving the anterior compartment as internal control (Fig. 1B).
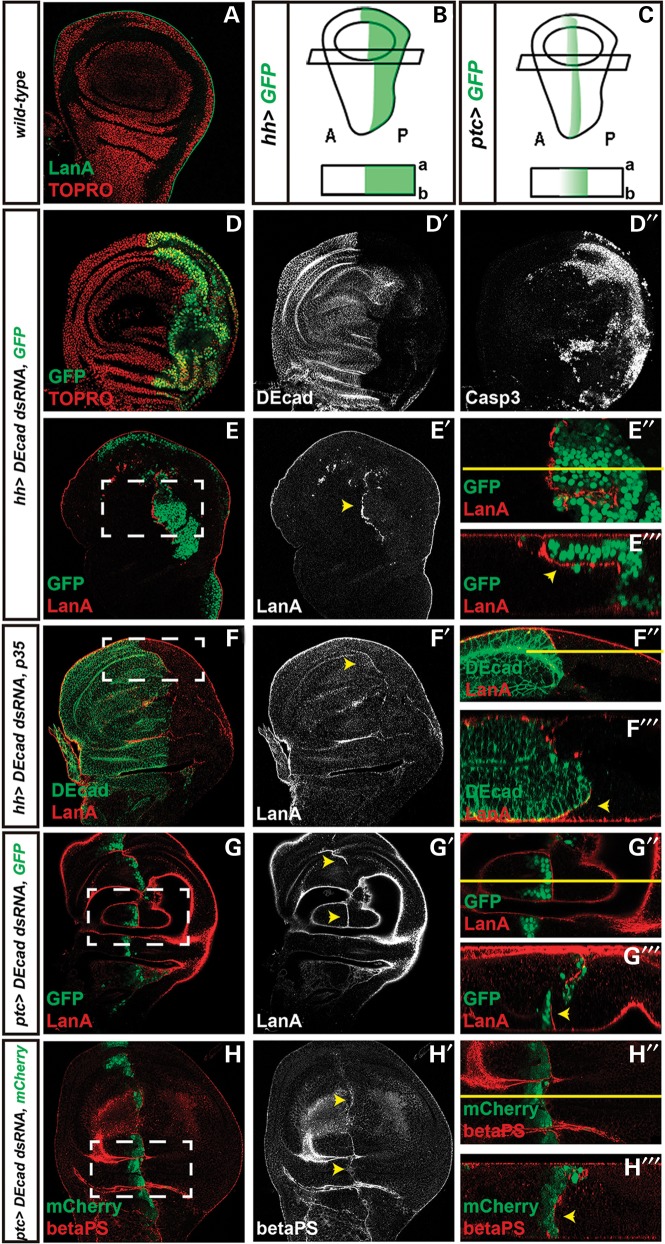
Figure 1.
Cellular and molecular effects of DEcad KD in vivo. A and D–H‴ are standard confocal sections with posterior side to the right and ventral side up. E‴, F‴, G‴ and H‴ are optical cross sections (apical side up). E″, F″, G″ and H″ are zooms from E–E′, F–F′, G–G′ and H–H′, respectively (white dashed boxes). (A) Control disc marked with anti-LanA and Topro nuclear staining. B and C are schemes of Drosophila wing disc regions and respective cross sections, in which hh-Gal4 (B) and ptc-Gal4 (C) drive transgene expression. A stands for anterior and P for posterior. (D–F‴) hh-Gal4 is driving UAS-DEcad-dsRNA and UAS-GFP (D–E‴)—green in D, E, E″ and E‴—or UAS-p35 (F–F‴). (G–H‴) ptc-Gal4 is driving UAS-DEcad-dsRNA and UAS-GFP (G–G‴)—green in G, G″ and G‴—or UAS-mCherry (H–H‴)—red in H, H″ and H‴. Discs are stained with (D–D″) anti-DEcad, anti-activated Caspase-3 and Topro, which marks nuclei or (A and E–G‴) anti-LanA (red in A, E, E″, E‴, F, F″, F‴, G, G″, G″ and white in E′, F′, G′) and anti-DEcad (green in F, F″, F‴ and white in D′, F′) or (H–H‴) anti-βPS integrin (green in H, H″, H‴ and white in H′). Yellow arrowheads highlight LanA or βPS accumulation at the DEcad+/DEcad– interface.
We verified that DEcad knockdown (DEcad KD) was effective (Fig. 1D and D′) and induced massive cell death, as observed by an enrichment in Caspase-3 staining (Fig. 1D″), which monitors apoptosis and caspase activation in Drosophila (24). DEcad-defective cells located at the border between the two tissue contexts seem, however, to survive. Remarkably, juxtaposed to the resistant DEcad-defective cells, we detected an upregulation of Laminin A (LanA), which is the Drosophila orthologue of LAMA3, one of the subunits (together with LAMB3 and LAMC2) of LM-332, a crucial basement membrane component (Fig. 1E–E‴) (25). To verify if upregulation of LanA was dependent on caspase activation, we blocked cell death by simultaneous expression of the apoptosis inhibitor p35. As shown in Figure 1F–F‴, apoptosis inhibition did not affect LanA overexpression pattern, suggesting that this particular effect does not depend on the apoptotic stimulus.
Thus, to understand if LanA accumulation is triggered by differences in DEcad levels, we used a distinct driver, patched-Gal4 (ptc-Gal4), that directs expression in a stripe along the anterior–posterior boundary, fading away in cells distant from the border (Fig. 1C) (26,27). In this context, LanA overexpression was only recapitulated at the most posterior edge (Fig. 1G–G‴), where the reduction of DEcad was higher, and occurred concomitantly with an enrichment in βPS integrin (Fig. 1H–H‴), a subunit that is part of the LanA receptor.
Taken together, we demonstrate that in vivo DEcad KD induces the accumulation of LanA and its integrin receptor at the interface between resistant Ecad-defective cells and their wild-type counterparts.
Ecad-defective gastric cancer cells overexpress LM-γ2, β1 and β4 integrin
To support that Ecad modulation leads to ectopic expression of Laminin in a gastric cancer cell context, we used two human gastric cancer cell lines: AGS and MNK28 (28). AGS cells are completely negative for Ecad protein expression due to the presence of a truncating mutation (29) together with loss of heterozygosity (LOH). In addition, AGS represents a reliable in vitro model for Ecad loss of function as it shows invasive properties and loss of cell–cell adhesiveness. MNK28, which expresses wild-type and functional Ecad, was used to address the inverse situation.
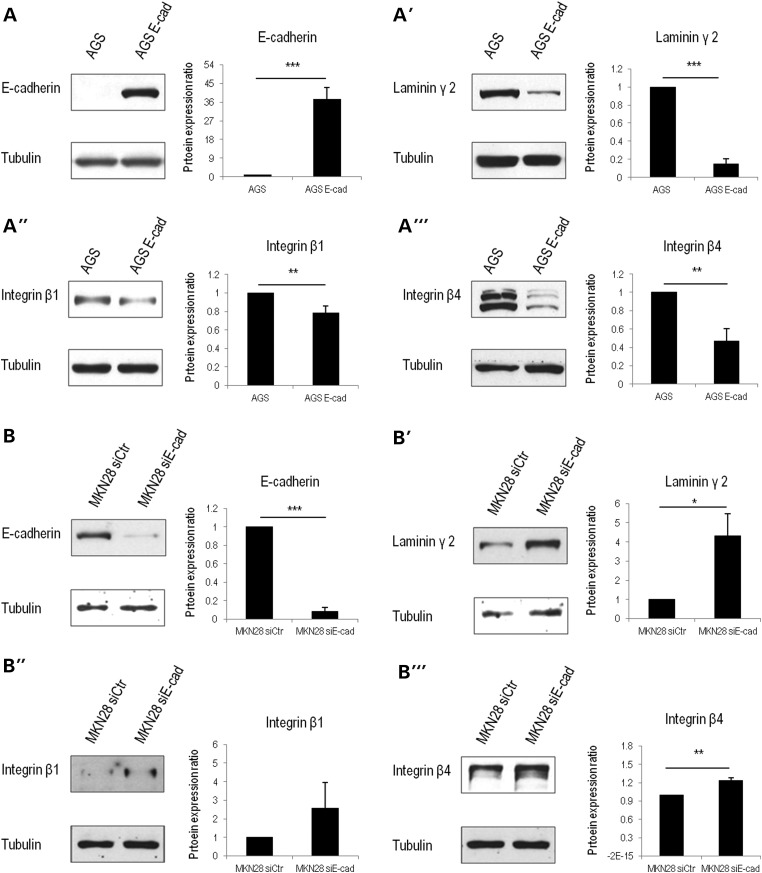
First, we compared AGS parental cells and AGS cells stably transfected with wild-type Ecad (AGS Ecad). We observed that AGS parental cells (Fig. 2A) recapitulated LM-γ2 (the subunit used to identify LM-332) overexpression (Fig. 2A′) and integrin upregulation, particularly β1 (Fig. 2A″) and β4 (Fig. 2A‴) subunits. When compared with AGS Ecad, AGS cells present a 6.8-fold increase in LM-γ2 (P = 2.8E − 5) expression, 1.3-fold in integrin β1 (P = 0.01) and 2.13-fold in integrin β4 (P = 0.009). Soluble LM-γ2 and LM-α3 (Supplementary Material, Fig. S1) were also overexpressed in AGS cells (1.2-fold, P = 0.02 and 1.6-fold, P = 0.02, respectively), but not in AGS Ecad.
Figure 2.
Ecad+ versus Ecad– human gastric cancer cells in vitro. (AA‴) By analysing cell lysates, we observe that AGS cells negative for Ecad (A) express higher levels of LM-γ2 (A′), as well as integrin β1 (A″) and β4 (A‴). (B–B‴) In an Ecad inhibition model, Ecad-defective MKN28 cells (B) express higher levels of LM-γ2 (B′), integrin β1 (B″) and integrin β4 (B″) than those transfected with a non-targeting siRNA control. Graphs correspond to the average of protein expression levels obtained by band quantification. SE is represented as the error bar. Values were normalized to tubulin and to the respective control (AGS parental cells or MKN28 siCtr). (*) stands for P ≤ 0.05, (**) for P ≤ 0.01 and (***) for P ≤ 0.001.
To strengthen our results, MKN28 were transfected with siRNAs targeting Ecad. Upon transfection, a significant increase in LM-γ2 (4.3-fold, P = 0.02), integrin β1 (2.6-fold) and β4 (1.23-fold, P = 0.007) expression was clearly observed (Fig. 2B–B‴).
Thus, in human gastric cancer cell lines, Ecad loss induces laminin and integrin overexpression.
Laminin upregulation induces survival and invasion of Ecad-defective cells
In order to address the functional role of LM-γ2, we performed specific inhibition of LM-γ2 in AGS parental cells, negative for Ecad, and thereafter evaluated their invasive behaviour. Matrigel invasion assays were used, given that they better mimic the basement membrane composition in vitro: this matrix contains not only structural proteins such as collagens, fibronectin, laminin (mainly Laminin-111), and proteoglycans, but also growth factors like TGF-β, epidermal growth factor (EGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF) and tissue plasminogen activator (tPA) (30,31).
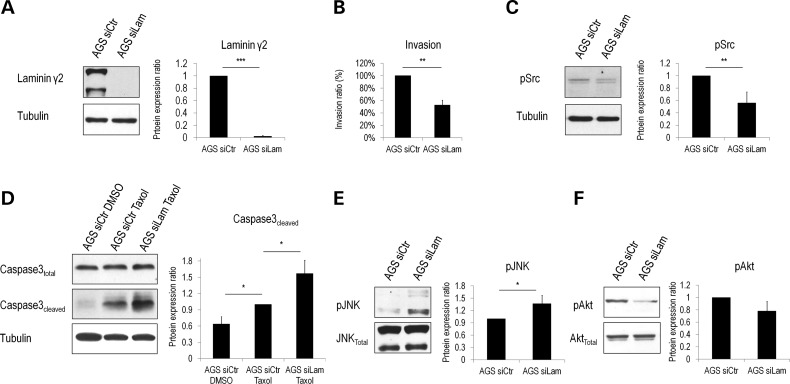
We verified that silencing LM-γ2 (Fig. 3A) significantly suppressed the number of invasive cells (from 100% to 57%, P = 0.0007, Fig. 3B), indicating that LM-γ2 is crucial for cell invasion. Furthermore, this decreased invasiveness, upon LM-γ2 downregulation, occurs concomitantly with an enhancement of cell–cell adhesion (Supplementary Material, Fig. S2A).
Figure 3.
LM-γ2 functional significance. Inhibition of LM-γ2 (A) reduced cell invasion (B) and Src activity (C). (D) LM-γ2 induces resistance to apoptosis, as evidenced by a decrease in Caspase-3 levels in control cells when compared with those transfected with siRNA for LM-γ2. (E) pJNK underexpression and (F) pAkt upregulation in gastric cancer cells expressing LM-γ2 suggest that they are involved in the control of survival. Graphs correspond to protein expression levels obtained by band quantification or percentage of invasive cells. Values were normalized to tubulin and AGS parental cells transfected with non-targeting control siRNA (AGS siCtr). For the experiment in which apoptosis was stimulated, values were normalized by AGS siCtr treated with DMSO. Average ± SE is presented. (*) represents a significant difference of P ≤ 0.05, (**) of P ≤ 0.01 and (***) of P ≤ 0.001.
Given that integrin α3β1 binding to LM-332 has been shown to promote lamellipodia extension via Src activation (32), we investigated pSrc levels in AGS cells silenced for LM-γ2. We showed that cells transfected with siLAMC2 display decreased pSrc levels (from 1 to 0.56, P = 0.04, Fig. 3C), suggesting that Src activation could be involved in regulating LM-γ2 invasive potential. The same effects were demonstrated in AGS Ecad cells (Supplementary Material, Fig. S3).
Interestingly, it is now well established that the α3β1 and α6β4 integrin receptors for LM-332 promote cell survival in several cancer cell lines (33–37). Accordingly, we decided to dissect the role of LM-γ2 in gastric cancer cell survival. We verified that under apoptotic conditions, induced by taxol treatment, AGS cells silenced for LM-γ2 showed a significant increase in Caspase-3 activation (a key apoptotic effector), when compared with AGS control cells (from 1 to 2.48, P = 0.03, Fig. 3D).
Consistent with this LM-γ2 pro-survival role, JNK, another master regulator of apoptosis (38,39), was also induced upon LM-γ2 depletion (Fig. 3E). Moreover, the levels of pAkt (a general mediator of the anti-apoptotic response) (40) decreased from 1 to 0.78 in the absence of LM-γ2 (Fig. 3F).
The effect of LM-γ2 silencing on β1 or β4 integrin expression levels was also tested, and no differences were observed upon siRNA treatment (Supplementary Material, Fig. S2B).
All together, these results show that LM-γ2 overexpression promotes survival and invasion of Ecad-defective gastric cancer cells.
Gastric primary tumours lose Ecad and gain LM-γ2 expression
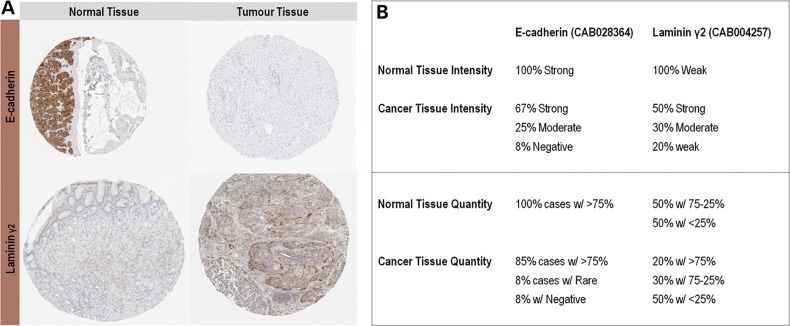
Then, we aimed to investigate the relevance of LM-γ2 in gastric cancer. We used data publicly available on the Human Protein Atlas (41,42) to evaluate Ecad and LM-γ2 expression patterns in human normal stomach tissue and gastric carcinomas. In normal gastric tissue, LM-γ2 was weakly expressed, whereas Ecad was strongly upregulated (Fig. 4A and B, Supplementary Material, Figs S4A and S5A). In contrast, 80% (8 out of 10) of gastric cancer samples showed ‘strong’ to ‘moderate’ LM-γ2 expression levels (Fig. 4A and B and Supplementary Material, Fig. S5B). Despite the fact that only 33% of the tumour samples are annotated to have ‘moderate’ (1787, 2195 and 2959 in Supplementary Material, Fig. S4B) or ‘negative’ (3063 in Supplementary Material, Fig. S4B) Ecad expression, when compared with the ‘strong’ intensity observed in normal tissue (Fig. 4B), this is a clear underestimation, given that in contrast to normal gastric mucosa (Supplementary Material, Fig. S4A), Ecad expression seems to be decreased in most cancer cases (Supplementary Material, Fig. S4B). Regarding the number of cells expressing LM-γ2, it is also increased in 20% (2 out of 10) of gastric cancer cases evaluated, when compared with normal stomach. Concomitantly, the fraction of Ecad positively stained cells diminished in 17% (2 out of 12) of the individuals with the disease. This Ecad/LM-γ2 inverse correlation could not be confirmed at the individual tumour level as the data available in the Human Protein Atlas did not include the same cases for both stainings. Alternatively, we tried to address this issue by performing immunohistochemistry in normal stomach and gastric tumour tissues, but we could not successfully overcome several technical difficulties.
Figure 4.
Ecad and LM-γ2 expression in human gastric cancer samples. (A) Histological samples of normal versus tumour tissue from the Human Protein Atlas database are representative of Ecad downregulation and LM-γ2 overexpression in gastric carcinomas. (B) Data from the Human Protein Atlas database demonstrate an overall and concurrent tendency for Ecad intensity and quantity to decrease and for LM-γ2 to increase in gastric cancer tissues, when compared with normal mucosa. Manual annotation of each sample was performed by a board of certified pathologists or specially educated personnel, using a simplified classification scheme for the immunohistochemical outcome.
These results strongly support a role for LM-γ2 in Ecad-mediated gastric carcinogenesis.
Discussion
This study highlights the value of our integrative/complementary approach to dissect the basic mechanisms regulating Ecad-mediated carcinogenesis. Using both in vivo and in vitro models, we were able to unravel a molecular pathway to invasion, which is dependent on Ecad dysfunction.
Using a Drosophila model, we demonstrated for the first time that, within a normal epithelial tissue, Laminin is overexpressed as a consequence of Ecad loss. Although DEcad downregulation results in massive cell death, as previously reported (43–45), DEcad-negative cells ectopically expressing Laminin could be frequently found in the normal epithelial compartment (data not shown), suggesting an increased capacity to evade cell death and invade the neighbouring tissues. Together with Laminin accumulation, we detected integrin overexpression and showed that this effect depends on the existence of a sharp difference in DEcad levels between DEcad+ and DEcad– cells.
To prove the interdependence of Ecad and Laminin/integrin accumulation in a gastric cancer context, we used two independent in vitro systems: one based on Ecad overexpression and the other dependent on Ecad knockdown. By comparing AGS (Ecad–) and AGS Ecad cell lines, we verified that Ecad-defective cells overexpress LM-γ2 (the subunit used to identify LM-332), as well as its β1 and β4 integrin receptors. The same effects were observed in MKN28 cells upon Ecad silencing. In fact, the crosstalk of Ecad and β1 integrin is currently well known (46,47); nevertheless, it has never been demonstrated that Ecad loss triggers extracellular matrix remodelling through de novo synthesis/secretion of matrix components.
Given that an increase in γ2 staining could reflect protein level differences or cleavage, we evaluated whether the cleaved (soluble) form of LM-γ2 was also increased in Ecad-deficient gastric cancer cells. Our results clearly demonstrate that alterations in both LM-γ2 cleaved and unprocessed forms are Ecad dependent. Further, to verify if Ecad affects LM-332 isoform or a particular laminin subunit, we assessed the expression level of α3 chain. We found that LM-γ2 upregulation was accompanied by LM-α3 overexpression. Still, LM-γ2 levels increase to a higher extent than LM-α3, which might be explained by uncoupled regulation of LM-332 subunits, already described in the literature (48,49).
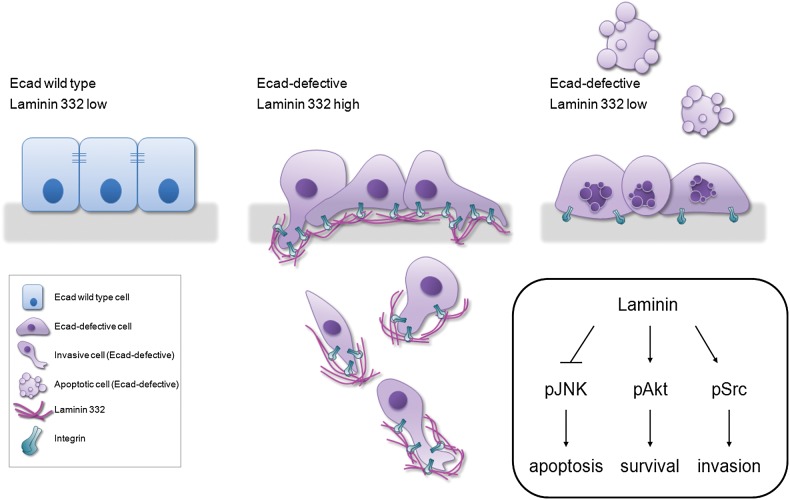
To unravel the functional significance of LM-γ2 accumulation and disclose the signalling pathways affected in Ecad-defective cells, once again we took advantage of our in vitro cancer cell model. We found that LM-γ2 promotes cell survival via Akt activation and JNK downregulation and induces invasion by increasing Src activity (Fig. 5). Indeed, AKT and Src overactivation are commonly found in human malignancies such as gastric cancer and are associated with the development, progression, metastization and poor prognosis of the disease (50,51). Pharmacological inhibitors of these molecules are currently under study and, as suggested by our results, can be promising therapies for cancers with Ecad loss (51).
Figure 5.
Working model for the events that follow Ecad loss in wild-type epithelia. Cells with reduced Ecad are committed to die. However, ectopic expression of Laminin and its integrin receptors enables Ecad-defective cells to evade cell death/elimination from the epithelia (through Akt activation and JNK inhibition) and invade adjacent tissues (via pSrc phosphorylation).
How LM-γ2 affects Src, Akt and JNK activity remains to be determined. These effects may be mediated via ligation of LM-332 to integrin signalling receptors, since gastric cancer cells, like fly tissues that lost Ecad, also accumulate β1 and β4 integrin subunits (independently of LM-γ2).
In agreement with LM-γ2 oncogenic role, increased cell–cell adhesiveness was also observed upon LM-γ2 inhibition. Indeed, a previous report from Kawano et al., using squamous cell carcinoma cells, already showed that loss of extracellular matrix adhesion induces Ecad-dependent aggregation (52). In line with this, we observed Ecad upregulation in AGS Ecad cells silenced for LM-γ2. Still, in AGS parental cells, other adhesion molecules might be upregulated.
Finally, the analysis of the Human Protein Atlas database allowed us to validate our results in human gastric cancer samples, by showing an overall trend for Ecad to decrease and for LM-γ2 to increase, concomitantly. In the future, this correlation should be addressed at the individual tumour level, what was technically hard to do at this time, given technical limitations and a reduced sample size. Nevertheless, our present findings indicate that LM-γ2 regulation by Ecad is crucial during gastric cancer development.
In conclusion, we unravelled novel biological functions for LM-γ2 in gastric cancer progression and disclosed its molecular mechanism. As illustrated in our model (Fig. 5), we propose that LM-γ2 upregulation may constitute an adaptive stimulus that allows Ecad-defective cells to escape anoikis and invade, contributing to cancer progression. We advance that LM-332 regulators could represent very attractive targets for Ecad-dependent gastric cancer therapeutics.
Materials and Methods
Drosophila strains and genetic manipulations
The Gal4/UAS system was used for targeted gene expression in Drosophila tissues (53). The hh-Gal4 (a gift from T. Tabata (54)) line restricts the expression of UAS-controlled transgenes to the posterior compartment of wing imaginal discs and is described in Flybase (http://flybase.bio.indiana.edu) (54). ptc-Gal4 is expressed in a stripe of wing disc cells, in a gradient, along the anterior–posterior boundary (26,27). UAS-GFP (55) and UAS-mCherry (35 787 from Bloomington Drosophila Stock Center, Indiana University, Bloomington, IN, USA) were used as expression reporters. DEcad KD was performed using an UAS-DEcad-RNAi line from the Vienna Drosophila Resource Center (VDRC), Vienna, Austria (Transformant ID 27081), and UAS-p35 (56) was used to block caspase-dependent cell death (57). For immunostainings (performed as in (58)), the following reagents were used: TO-PRO-3 nuclear stain (1:1000, Molecular Probes, Carlsbad, CA, USA), rat anti-DEcad [1:100, CAD2 from Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA], rabbit anti-cleaved Casp3 (1:150 Cell Signalling, Beverly, MA, USA), mouse anti-βPS (1:20, CF.6G11, DSHB) and rabbit anti-LanA (1:1000 (59)). Both anti-βPS and anti-LanA were a gift from Stefan Baumgartner. Appropriate Alexa-Fluor conjugated secondary antibodies were from Molecular Probes. Imaging of third instar wing imaginal discs was performed on Leica SP2 or SP5 confocal systems (Leica Microsystems GmbH, Wetzlar, Germany) and further processed using Adobe Photoshop CS6 (Adobe Systems, San Jose, CA, USA).
Cell lines and transfection
AGS parental cells from the American Type Culture Collection (ATCC) and AGS Ecad cells (stably transfected with Ecad cDNA using a pLenti6/V5 construct and further selected with 5 µg/ml of blasticidin) were used and maintained in RPMI 1640 (Gibco, Invitrogen, Barcelona, Spain) supplemented with 10% foetal bovine serum (Hyclone, Logan, UT) and 1% penicillin–streptomycin (Pen Strep—Gibco, Invitrogen). AGS Ecad cells were provided by Céu Figueiredo (29). MKN28 cells were maintained in the same medium. All the cell lines were seeded in six-well plates. Twenty-four hours after seeding, cells were transfected using Lipofectamine 2000 (Gibco, Invitrogen) in serum-free Opti-MEM (Gibco, Invitrogen), and siLAMC2, siCDH1 or non-targeting siRNA controls (ON-TARGETplus SMART pool and ON-TARGETplus Non-Targeting siRNAs, both from Dharmacon Lafayette, CO, USA) at a final concentration of 200 nM (this values were optimized to achieve the highest silencing efficiency with the lowest toxicity). To test cell response against an apoptotic stimulus, 24 h upon transfection, AGS cells were treated with the apoptotic reagent taxol (paclitaxel) 100 nM (Sigma) or the control vehicle (dimethylsulphoxide, DMSO, Sigma) for 24 h. Protein from adherent cells and from those in suspension was collected around 24 h after apoptosis induction.
Western blotting
Cells were lysed in cold catenin lysis buffer (1% Triton X-100, 1% Nonidet P-40, both from Sigma, in phosphate-buffered solution, PBS) enriched with a protease and phosphatase inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany and Sigma, respectively). Protein samples (25 µg) were separated by sodium dodecyl sulphate (SDS) 10% polyacrylamide gel electrophoresis (PAGE) and electroblotted onto a Hybond ECL membrane (Amersham Biosciences). Primary antibodies included mouse anti-Ecad (1:1000, BD Biosciences, San Jose, CA, USA), mouse anti-α-tubulin (1:10 000, Sigma), mouse anti-laminin γ2, clone D4B5 (1:250, Merck Millipore, Darmstadt, Germany), mouse anti-laminin α3, clone 12C4 (1:500, Merck Millipore), mouse anti-integrin β1 (1:1000, BD Biosciences), rabbit anti-integrin β4 (1:1000, Cell Signalling), rabbit anti-Phospho SAPK/JNK(Thr183/Tyr185) (1:1000, Cell Signalling), rabbit anti-SAPK/JNK (1:1000, Cell Signalling), rabbit anti-Phospho Akt(Thr308) (1:1000, Cell Signalling), rabbit anti-Phospho Src(Tyr416) (1:1000, Cell Signalling) and mouse anti-Casp3 (1:1000, Cell Signalling). Donkey anti-rabbit (1:4000, Amersham Biosciences) and sheep anti-mouse (1:4000, Amersham Biosciences) HRP-conjugated secondary antibodies were used, followed by ECL detection (Amersham Biosciences). Bands were quantified using Quantity One 4.6.8 Software (Bio-Rad, Amadora, Portugal).
Matrigel invasion assay
Invasion assays were performed using Matrigel Invasion Chambers (BD Biocoat, Erembodegem, Belgium). Twenty-four hours after transfection, inserts containing a polyethylene terephthalate (PET) membrane with 8 µm pores and coated with matrigel basement membrane matrix were placed inside a 24-well plate. Five hundred microliters of a culture medium suspension with 2 × 105 cells/ml were added to the top of the chambers, whereas 750 µl of complete medium was placed under the insert. After 24 h at 37°C (corresponding to 48 h after transfection), in a 5% CO2 atmosphere, cells on the upper side of the membrane (non-invasive) were removed by scraping with a cotton bud, and invasive cells were fixed for 20 min in ice-cold methanol. Inserts were then washed in PBS. The membranes were removed and mounted on a slide in Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Invasive cells were counted under Leica DM2000 (Leica, Cambridge, UK) fluorescence microscope.
Slow aggregation assay
Fifty microliters of an agar solution (100 mg of Bacto-Agar in 15 ml of PBS) were used to coat 96-well plates (60). Adherent cells were treated with trypsin and resuspended in culture medium to a final concentration of 1 × 105 cells/ml. Two hundred microliters of this suspension were added to each well (60). After 48 h at 37°C, in a 5% CO2 atmosphere, aggregation was assessed under an inverted microscope. Images were captured with a Nikon (Tokyo, Japan) digital camera.
Immunohistochemistry
Stomach tissue samples examined are part of a large Tissue Microarray analysis platform, annotated in the Human Protein Atlas database (41,42). Each sample, collected from surgical specimens, with approval from the local ethics committee (Stockholm, Sweden), is represented by a 1 mm circular core. We evaluated normal tissue from 6 individuals and cancer tissue from 22 patients. All antibodies were previously validated, and the protocols for immunohistochemistry and tissue digital imaging had already been described (42). Representative Ecad and Laminin γ2 images were chosen from normal (Patients 1469 and 2326) and stomach cancer (Patients 3063 and 2645) samples, and the antibodies CAB028364 and CAB004257 were chosen for Ecad and Laminin γ2 staining, respectively. We selected them because they are monoclonal and, as so, more specific. In addition, HPA024638 antibody against Laminin γ2 showed a very unspecific cytoplasmic pattern in normal tissue (Supplementary Material, Fig. S6) and not the expected basement membrane staining (12). For that, we excluded it from the analysis. Concerning Ecad antibodies, two of the available ones were monoclonal, but we chose the one for which the antigen was known. Still, a similar trend, concerning Ecad expression loss in tumours when compared with normal tissue, was observed for the three of them (Supplementary Material, Figs. S4 and S7–8). Further details on Human Protein Atlas data methods (antibodies, assays and annotation, data quality and scoring) and usage policies are available at http://www.proteinatlas.org.
Statistical analysis
Statistical analyses were performed using Student's t-test. Results (from at least three independent biological replicates) are expressed as mean ± standard error (SE). Values from P ≤ 0.05 were considered statistically significant, although, in some cases, higher levels of significance are noted and described in the figure legends where applicable: *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
Supplementary Material
Funding
This work was supported by FEDER funds through the Operational Programme for Competitiveness Factors (COMPETE) and National Funds through the Portuguese Foundation for Science and Technology (FCT), under the projects PEst-C/SAU/LA0003/2013, PTDC/SAU-ONC/110294/2009 and PTDC/BIM-ONC/0171/2012; Post-Doc grants: SFRH/BPD/78187/2011-JC, SFRH/BPD/87705/2012-JF and SFRH/BPD/46983/2008-CBP; and Contract: IF/01031/2012-FJ. IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science and Higher Education, integrated the i3S Research Unit, which is partially supported by FCT. We also thank the financial support of the Spanish Ministry for Science and Innovation (MICINN/MINECO) and FEDER Funds through the grant BFU2012-34324 to F.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
Acknowledgements
We acknowledge Céu Figueiredo (IPATIMUP, Porto, Portugal) for providing access to the stable AGS line transfected with Ecad, Marta Pinto for the technical support regarding antibody optimization for immunohistochemistry and Mário Seixas for slide digitalization. We thank TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks used in this study. Further, we acknowledge Bloomington Drosophila Stock Center, the VDRC (http://stockcenter.vdrc.at/) and the DSHB for fly stocks and reagents.
Conflict of Interest statement: The authors declare no conflict of interest.
References
- 1.van Roy F., Berx G. (2008) The cell-cell adhesion molecule E-cadherin. Cell Mol. Life Sci., 65, 3756–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paredes J., Figueiredo J., Albergaria A., Oliveira P., Carvalho J., Ribeiro A.S., Caldeira J., Costa A.M., Simoes-Correia J., Oliveira M.J. et al. (2012) Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim. Biophys. Acta, 1826, 297–311. [DOI] [PubMed] [Google Scholar]
- 3.Nottingham J. (1994) Signet-ring carcinoma of stomach in a child. Histopathology, 24, 490–491. [PubMed] [Google Scholar]
- 4.Corso G., Carvalho J., Marrelli D., Vindigni C., Carvalho B., Seruca R., Roviello F., Oliveira C. (2013) Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J. Clin. Oncol., 31, 868–875. [DOI] [PubMed] [Google Scholar]
- 5.Takeichi M. (1993) Cadherins in cancer: implications for invasion and metastasis. Curr. Opin. Cell Biol., 5, 806–811. [DOI] [PubMed] [Google Scholar]
- 6.Birchmeier W., Behrens J. (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta, 1198, 11–26. [DOI] [PubMed] [Google Scholar]
- 7.Christofori G., Semb H. (1999) The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci., 24, 73–76. [DOI] [PubMed] [Google Scholar]
- 8.Suriano G., Oliveira C., Ferreira P., Machado J.C., Bordin M.C., De Wever O., Bruyneel E.A., Moguilevsky N., Grehan N., Porter T.R. et al. (2003) Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum. Mol. Genet., 12, 575–582. [DOI] [PubMed] [Google Scholar]
- 9.Huntsman D.G., Carneiro F., Lewis F.R., MacLeod P.M., Hayashi A., Monaghan K.G., Maung R., Seruca R., Jackson C.E., Caldas C. (2001) Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N. Engl. J. Med., 344, 1904–1909. [DOI] [PubMed] [Google Scholar]
- 10.Guilford P., Hopkins J., Harraway J., McLeod M., McLeod N., Harawira P., Taite H., Scoular R., Miller A., Reeve A.E. (1998) E-cadherin germline mutations in familial gastric cancer. Nature, 392, 402–405. [DOI] [PubMed] [Google Scholar]
- 11.Lu P., Takai K., Weaver V.M., Werb Z. (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol., 3, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshikawa N., Moriyama K., Takamura H., Mizushima H., Nagashima Y., Yanoma S., Miyazaki K. (1999) Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res., 59, 5596–5601. [PubMed] [Google Scholar]
- 13.Miyazaki K. (2006) Laminin-5 (laminin-332): unique biological activity and role in tumor growth and invasion. Cancer Sci., 97, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sentani K., Matsuda M., Oue N., Uraoka N., Naito Y., Sakamoto N., Yasui W. (2014) Clinicopathological significance of MMP-7, laminin gamma2 and EGFR expression at the invasive front of gastric carcinoma. Gastric Cancer, 17, 412–422. [DOI] [PubMed] [Google Scholar]
- 15.Katayama M., Sekiguchi K. (2004) Laminin-5 in epithelial tumour invasion. J. Mol. Histol., 35, 277–286. [DOI] [PubMed] [Google Scholar]
- 16.Navdaev A., Eble J.A. (2011) Components of cell-matrix linkage as potential new markers for prostate cancer. Cancers (Basel), 3, 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinkovich M.P. (2007) Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer, 7, 370–380. [DOI] [PubMed] [Google Scholar]
- 18.Tipping M., Perrimon N. (2014) Drosophila as a model for context-dependent tumorigenesis. J Cell Physiol., 229, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudrapatna V.A., Cagan R.L., Das T.K. (2012) Drosophila cancer models. Dev. Dyn., 241, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles W.O., Dyson N.J., Walker J.A. (2011) Modeling tumor invasion and metastasis in Drosophila. Dis. Model Mech., 4, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira P.S., Teixeira A., Pinho S., Ferreira P., Fernandes J., Oliveira C., Seruca R., Suriano G., Casares F. (2006) E-cadherin missense mutations, associated with hereditary diffuse gastric cancer (HDGC) syndrome, display distinct invasive behaviors and genetic interactions with the Wnt and Notch pathways in Drosophila epithelia. Hum. Mol. Genet., 15, 1704–1712. [DOI] [PubMed] [Google Scholar]
- 22.Caldeira J., Simoes-Correia J., Paredes J., Pinto M.T., Sousa S., Corso G., Marrelli D., Roviello F., Pereira P.S., Weil D. et al. (2012) CPEB1, a novel gene silenced in gastric cancer: a Drosophila approach. Gut, 61, 1115–1123. [DOI] [PubMed] [Google Scholar]
- 23.Simoes-Correia J., Silva D.I., Melo S., Figueiredo J., Caldeira J., Pinto M.T., Girao H., Pereira P., Seruca R. (2014) DNAJB4 molecular chaperone distinguishes WT from mutant E-cadherin, determining their fate in vitro and in vivo. Hum. Mol. Genet., 23, 2094–2105. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S. (2007) Caspase function in programmed cell death. Cell Death Differ., 14, 32–43. [DOI] [PubMed] [Google Scholar]
- 25.St Pierre S.E., Ponting L., Stefancsik R., McQuilton P., FlyBase C. (2014) FlyBase 102—advanced approaches to interrogating FlyBase. Nucleic Acids Res., 42, D780–D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C.Y., Sun Y.H. (2002) Use of mini-white as a reporter gene to screen for GAL4 insertions with spatially restricted expression pattern in the developing eye in drosophila. Genesis, 34, 39–45. [DOI] [PubMed] [Google Scholar]
- 27.Sun X., Artavanis-Tsakonas S. (1997) Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development, 124, 3439–3448. [DOI] [PubMed] [Google Scholar]
- 28.Barranco S.C., Townsend C.M. Jr., Casartelli C., Macik B.G., Burger N.L., Boerwinkle W.R., Gourley W.K. (1983) Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res., 43, 1703–1709. [PubMed] [Google Scholar]
- 29.Oliveira M.J., Costa A.M., Costa A.C., Ferreira R.M., Sampaio P., Machado J.C., Seruca R., Mareel M., Figueiredo C. (2009) CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J. Infect. Dis., 200, 745–755. [DOI] [PubMed] [Google Scholar]
- 30.Kleinman H.K., McGarvey M.L., Liotta L.A., Robey P.G., Tryggvason K., Martin G.R. (1982) Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry, 21, 6188–6193. [DOI] [PubMed] [Google Scholar]
- 31.Kleinman H.K., Martin G.R. (2005) Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol., 15, 378–386. [DOI] [PubMed] [Google Scholar]
- 32.Choma D.P., Pumiglia K., DiPersio C.M. (2004) Integrin alpha3beta1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J. Cell Sci., 117, 3947–3959. [DOI] [PubMed] [Google Scholar]
- 33.Tran M., Rousselle P., Nokelainen P., Tallapragada S., Nguyen N.T., Fincher E.F., Marinkovich M.P. (2008) Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res., 68, 2885–2894. [DOI] [PubMed] [Google Scholar]
- 34.Ryan M.C., Lee K., Miyashita Y., Carter W.G. (1999) Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol., 145, 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim B.G., Gao M.Q., Choi Y.P., Kang S., Park H.R., Kang K.S., Cho N.H. (2012) Invasive breast cancer induces laminin-332 upregulation and integrin beta4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype during tissue remodeling. Breast Cancer Res., 14, R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahir N., Lakins J.N., Russell A., Ming W., Chatterjee C., Rozenberg G.I., Marinkovich M.P., Weaver V.M. (2003) Autocrine laminin-5 ligates alpha6beta4 integrin and activates RAC and NFkappaB to mediate anchorage-independent survival of mammary tumors. J. Cell Biol., 163, 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.G., Lee G., Lee S.K., Lolkema M., Yim J., Hong S.H., Schwartz R.H. (2000) Epithelial cell-specific laminin 5 is required for survival of early thymocytes. J Immunol, 165, 192–201. [DOI] [PubMed] [Google Scholar]
- 38.Chen F. (2012) JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res., 72, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S., Yamashita H., Weidow B., Weaver A.M., Quaranta V. (2010) Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J. Cell Physiol., 223, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta S.R., Brunet A., Greenberg M.E. (1999) Cellular survival: a play in three Akts. Genes Dev., 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- 41.Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S. et al. (2010) Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol., 28, 1248–1250. [DOI] [PubMed] [Google Scholar]
- 42.Uhlen M., Bjorling E., Agaton C., Szigyarto C.A., Amini B., Andersen E., Andersson A.C., Angelidou P., Asplund A., Asplund C. et al. (2005) A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell Proteomics, 4, 1920–1932. [DOI] [PubMed] [Google Scholar]
- 43.Derksen P.W., Liu X., Saridin F., van der Gulden H., Zevenhoven J., Evers B., van Beijnum J.R., Griffioen A.W., Vink J., Krimpenfort P. et al. (2006) Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell, 10, 437–449. [DOI] [PubMed] [Google Scholar]
- 44.Boussadia O., Kutsch S., Hierholzer A., Delmas V., Kemler R. (2002) E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev., 115, 53–62. [DOI] [PubMed] [Google Scholar]
- 45.Fouquet S., Lugo-Martinez V.H., Faussat A.M., Renaud F., Cardot P., Chambaz J., Pincon-Raymond M., Thenet S. (2004) Early loss of E-cadherin from cell-cell contacts is involved in the onset of anoikis in enterocytes. J. Biol. Chem., 279, 43061–43069. [DOI] [PubMed] [Google Scholar]
- 46.Canel M., Serrels A., Miller D., Timpson P., Serrels B., Frame M.C., Brunton V.G. (2010) Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res., 70, 9413–9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber G.F., Bjerke M.A., DeSimone D.W. (2011) Integrins and cadherins join forces to form adhesive networks. J. Cell Sci., 124, 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zboralski D., Bockmann M., Zapatka M., Hoppe S., Schoneck A., Hahn S.A., Schmiegel W., Schwarte-Waldhoff I. (2008) Divergent mechanisms underlie Smad4-mediated positive regulation of the three genes encoding the basement membrane component laminin-332 (laminin-5). BMC Cancer, 8, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korang K., Christiano A.M., Uitto J., Mauviel A. (1995) Differential cytokine modulation of the genes LAMA3, LAMB3, and LAMC2, encoding the constitutive polypeptides, alpha 3, beta 3, and gamma 2, of human laminin 5 in epidermal keratinocytes. FEBS Lett., 368, 556–558. [DOI] [PubMed] [Google Scholar]
- 50.Almhanna K., Strosberg J., Malafa M. (2011) Targeting AKT protein kinase in gastric cancer. Anticancer Res., 31, 4387–4392. [PubMed] [Google Scholar]
- 51.Tapia O., Riquelme I., Leal P., Sandoval A., Aedo S., Weber H., Letelier P., Bellolio E., Villaseca M., Garcia P., Roa J.C. (2014) The PI3 K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch., 465, 25–33. [DOI] [PubMed] [Google Scholar]
- 52.Kawano K., Kantak S.S., Murai M., Yao C.C., Kramer R.H. (2001) Integrin alpha3beta1 engagement disrupts intercellular adhesion. Exp. Cell Res., 262, 180–196. [DOI] [PubMed] [Google Scholar]
- 53.Brand A.H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 54.Tanimoto H., Itoh S., ten Dijke P., Tabata T. (2000) Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell., 5, 59–71. [DOI] [PubMed] [Google Scholar]
- 55.Kaltschmidt J.A., Davidson C.M., Brown N.H., Brand A.H. (2000) Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat. Cell. Biol., 2, 7–12. [DOI] [PubMed] [Google Scholar]
- 56.Hay B.A., Wolff T., Rubin G.M. (1994) Expression of baculovirus P35 prevents cell death in Drosophila. Development, 120, 2121–2129. [DOI] [PubMed] [Google Scholar]
- 57.Dietzl G., Chen D., Schnorrer F., Su K.C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature, 448, 151–156. [DOI] [PubMed] [Google Scholar]
- 58.Dichtel-Danjoy M.L., Caldeira J., Casares F. (2009) SoxF is part of a novel negative-feedback loop in the wingless pathway that controls proliferation in the Drosophila wing disc. Development, 136, 761–769. [DOI] [PubMed] [Google Scholar]
- 59.Kumagai C., Kadowaki T., Kitagawa Y. (1997) Disulfide-bonding between Drosophila laminin beta and gamma chains is essential for alpha chain to form alpha betagamma trimer. FEBS Lett., 412, 211–216. [DOI] [PubMed] [Google Scholar]
- 60.Boterberg T., Bracke M.E., Bruyneel E.A., Mareel M.M. (2001) Cell aggregation assays. Methods Mol. Med., 58, 33–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.