Abstract
During the United States Department of Agriculture (USDA) National Animal Health Monitoring System’s (NAHMS) 2007–2008 beef study, 567 producers from 24 US States were offered the opportunity to collect fecal samples from weaned beef calves and have them evaluated for the presence of parasite eggs (Phase 1). Participating producers were provided with instructions and materials for sample collection. Up to 20 fresh fecal samples were collected from each of the 99 participating operations. Fresh fecal samples were submitted to one of 3 randomly assigned laboratories for evaluation. Upon arrival at the laboratories, all samples were processed for the enumeration of strongyle, Nematodirus, and Trichuris eggs using the modified Wisconsin technique. The presence or absence of coccidian oocysts and tapeworm eggs was also noted. In submissions where the strongyle eggs per gram exceeded 30, aliquots from 2 to 6 animals were pooled for DNA extraction. Extracted DNA was subjected to genus level polymerase chain reaction (PCR) identification for the presence of Ostertagia, Cooperia, Haemonchus, Oesophagostomum, and Trichostrongylus. In this study, 85.6% of the samples had strongyle type, Nematodirus, and Trichuris eggs. Among the samples evaluated, 91% had Cooperia, 79% Ostertagia, 53% Haemonchus, 38% Oesophagostomum, 18% Nematodirus, 7% Trichuris, and 3% Trichostrongylus. The prevalence of coccidia and tapeworm eggs was 59.9% and 13.7%, respectively.
Résumé
Pendant l’étude de 2007–2008 chez les bovins effectuée par le Système national de surveillance des maladies animales (NAHMS) du Département de l’agriculture des États-Unis (USDA), 567 producteurs provenant de 24 états américains se sont vus offrir l’opportunité de prélever des échantillons de fèces de veaux sevrés et de les faire analyser pour la présence d’oeufs de parasite (Phase 1). On a fourni aux producteurs participants les instructions et le matériel pour le prélèvement d’échantillon. Jusqu’à 20 échantillons de fèces fraiches furent prélevés de chacune des 99 opérations participantes. Les échantillons de fèces fraiches furent soumis de manière aléatoire pour évaluation à l’un des trois laboratoires participants. Suite à l’arrivée au laboratoire, tous les échantillons étaient traités pour énumération des strongles, de Nematodirus, et d’oeufs de Trichuris en utilisant la technique de Wisconsin modifiée. La présence ou l’absence d’ookystes de coccidie et d’oeufs de vers plats furent également notées. Dans les échantillons soumis et dont le nombre d’oeufs de strongles par gramme dépassait 30, des aliquots de 2 à 6 animaux étaient regroupés pour extraction de l’ADN. L’ADN extrait était soumis à une réaction d’amplification en chaine par la polymérase (PCR) pour une identification au genre de la présence d’Ostertagia, de Cooperia, d’Haemonchus, d’Oesophagostomum, et de Trichostrongylus. Dans la présente étude, 85,6 % des échantillons avaient des strongles, du Nematodirus, et des oeufs de Trichuris. Parmi les échantillons évalués, 91 % avaient du Cooperia, 79 % de l’Ostertagia, 53 % de l’Haemoncus, 38 % de l’Oesophagostomum, 18 % du Nematodirus, 7 % du Trichuris, et 3 % du Trichostrongylus. Les prévalences de coccidies et d’oeufs de vers plats étaient respectivement de 59,9 % et 13,7 %.
(Traduit par Docteur Serge Messier)
Introduction
Internal parasites, primarily helminths, are common pathogens of the cattle industry and substantially impact the economics of cattle production worldwide. Helminths can reduce the reproductive performance of the herd, reduce weaning weights, and in general, negatively impact animal health. These negative impacts are due to the destructive effects directly on host tissues; indirect responses, such as suppression of the host immune responses; or the loss of appetite (1), which can impact all aspects of animal well-being.
Data from the United States Department of Agriculture (USDA) National Health Monitoring System’s (NAHMS) 2007–2008 beef study show that cow/calf producers consider parasites to be a major problem in production (Ballweber et al, unpublished data). Previous studies in stocker cattle (2) showed a significant difference in weight gain in the parasite free (drug-treated) groups relative to non-treated groups, ranging from 0.132 to 0.272 kg average daily gain (ADG) for the various treatment groups. In a more confined study involving a beef cow/calf operation over a 2-year period, Stromberg, et al (3) demonstrated a negative effect of gastrointestinal (GI) nematodes on productivity where the calves of treated cows had a mean weaning weight of 18.5 kg over the non-medicated group. There was also a 12% improvement in reproductive performance for the nematode-free cows. As such, the presence of helminth parasites generally equates to a negative impact on production; however, data are lacking that define the magnitude of this economic impact.
In a 1993 cattle parasite survey that sampled over 5500 herds, Myers and Keith (4) found that calves, yearlings, cows, and bulls had > 90% prevalence of helminths in the western, northern, and southern regions of the United States (US). Similar findings have been noted in beef cattle outside of the US. A survey using tracer calves and necropsies in the cerrado region of Brazil (5), culled cows in Ireland (6), and a serological survey of replacement stock in The Netherlands (7) all showed a substantial prevalence of parasites.
Inferences of parasite importance and/or prevalence on animal production are often predicated on studies done on a single farm/ranch. In some cases the parasites are identified and/or quantified from animals using fecal egg counts and coproculture; however, tracer calves grazed with the herd have also been used where adult parasites are recovered and morphologically identified. These narrowly focused studies though important, provide prevalence data that are confined to a particular state or region of the state. The primary objective of this study was to evaluate parasite prevalence in weaned beef calves throughout the major cow-calf producing regions of the US. These data are presented herein.
Materials and methods
Study locations
A stratified random sample of over 4000 operations was chosen by the USDA’s National Agricultural Statistics Service (NASS) for initial contact. The 567 beef cow/calf operations that participated in a national study of animal health and management conducted by the USDA NAHMS were provided an opportunity to collect fecal samples from weaned calves for evaluation of parasite burdens. This population of operations has been described previously (8). Briefly, the eligible production facilities consisted of a stratified random sample of operations in 24 States with the largest beef cow populations that had 1 or more beef cows on October 1, 2007, and agreed to respond to a questionnaire administered during an interview. All participants remained anonymous within the NAHMS reporting system.
Sample collection
Producers interested in participating in the parasite survey were provided with the necessary materials and instructions to collect and ship the samples. Sample collection from weaned calves occurred at the discretion of the producer from March 1 through December 2, 2008. All animals were 6 to 18 mo of age, had grazed on pasture for at least 4 wk prior to the collection, and had not been treated with an anthelmintic in the previous 45 d. Fecal samples were collected from up to 20 calves (9) either directly from the rectum or from fresh fecal pats. Producers were asked to collect “golf ball” sized samples into an unused examination glove, transfer these into individual plastic bags and refrigerate overnight. The chilled samples were shipped with ice packs to one of 3 randomly assigned laboratories participating in the study: Colorado State University, the USDA, ARS, Beltsville, or the University of Minnesota.
Laboratory counting procedures
Upon arrival at the laboratory, samples were logged in and refrigerated. The eggs present in all fecal samples were quantified using the Wisconsin Double Centrifugal Floatation or the Modified Wisconsin technique (10,11). Each laboratory conducted the test according to routine procedures used in that laboratory. Parasite eggs were counted and identified as strongyle type, Nematodirus, or Trichuris. Samples were also evaluated as positive or negative for coccidia oocysts and Moniezia eggs.
Data collection
Information on routine deworming practices for the operation was also available from a previously administered questionnaire. At the time of sample collection, producers were asked to complete a short questionnaire about the group of animals being sampled. The requested information included the number of animals in the group sampled, any prior treatment with anthelmintics, and the anthelmintic used. Egg count data were recorded and transmitted to USDA, NAHMS, for dissemination to the owners and for statistical analysis.
Genus identification by polymerase chain reaction (PCR)
Samples having strongyle egg counts > 30 were collected and pooled for genus identification. Following zinc sulfate flotation (12), eggs were washed and frozen at −80°C in 0.5 mL PCR tubes in a minimal volume of water; each sample had no less than 100 eggs. The tubes were transferred uncapped and frozen to a thermal cycler (Perkin Elmer DNA Thermal Cycler 480; Waltham, Massachusetts, USA) preheated to 95°C, incubated a minimum of 15 min, and thereafter until the sample volumes were reduced to 20 μL. The eggs were subsequently treated as described in the Tissue and Hair Extraction kit (Promega Biotec; Madison, Wisconsin, USA). Released DNA was subsequently purified using magnetic bead technology as described (DNA IQTM System; Promega Biotec). All DNAs were eluted from the washed magnetic beads in 50 μL of kit provided elution buffer.
Egg DNA samples were amplified in a non-multiplex format using genus specific primer pairs (13) for Cooperia, Ostertagia, Haemonchus, Oesophagostomum, and Trichostrongylus. Approximately 1 to 2 μL of purified DNA were enzymatically amplified in 25 μL containing 1× PCR buffer (50 mM KCl, 10 mM Tris HCl, pH 9.0 at 25°C, 1.5 mM MgCl2, 1% Triton X-100), 6.25 pmol each primer, 0.2 mM each dNTP, 2% DMSO, 0.8 mg/mL bovine serum albumin (Fraction V) and 0.625 U Taq polymerase (GenScript; Piscataway, New Jersey, USA). All samples were subjected to 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 2 min, followed by a 7 min terminal extension. The PCR products were analyzed on a 2% NuSieve® 3:1 agarose gel (Lonza Rockland; Rockland, Maine, USA) subsequently stained with ethidium bromide then photographed. The presence of bands migrating at 151 bp, 257 bp, 176 bp, 329 bp, and 243 bp were scored for the presence of Cooperia, Ostertagia, Haemonchus, Oesophagostomum, and Trichostrongylus, respectively.
Data analysis
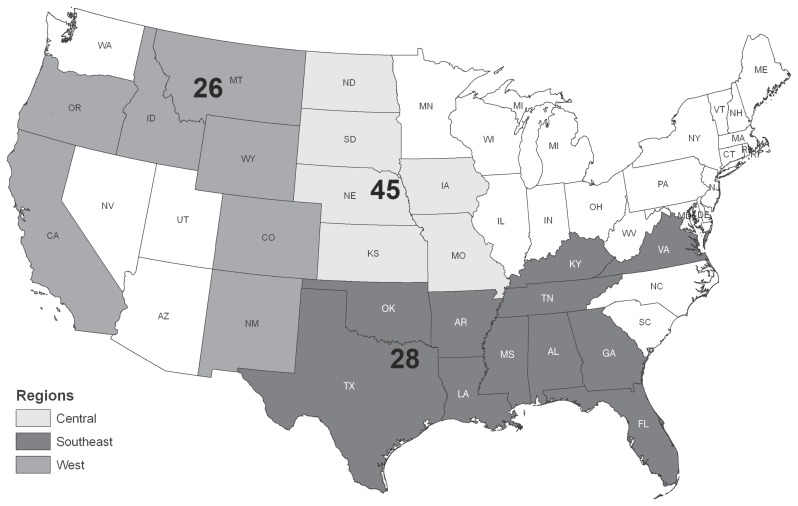
The average eggs per gram (epg) was determined for each operation and parasite species. Operations were grouped into 3 regions for analysis; West (California, Colorado, Idaho, Montana, New Mexico, Oregon, Wyoming), Central (Iowa, Kansas, Missouri, Nebraska, North Dakota, South Dakota), and South (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, Oklahoma, Tennessee, Texas, Virginia) (Figure 1). The number of operations submitting samples is presented by region (Table I) and herd size (Table II) relative to number of farms initially contacted by the NASS. Those groups responding to initial inquiries (Phase IIA) and those agreeing to submit additional survey forms (Phase IIB) are also presented in both tables. Data were also subdivided according to the period of the year in which the samples were taken (Figure 2).
Figure 1.
Number of cow-calf operations submitting samples by region surveyed.
Table I.
Number of responding operations by geographic region
| Region | Phase 1: Operations completing 1st questionnaire | Phase IIA: Operations completing 2nd questionnaire | Phase IIB: Operations completing 3rd questionnaire and eligible for sample collection | Phase III: Operations submitting useable sample for evaluation |
|---|---|---|---|---|
| West | 370 | 138 | 105 | 26 |
| Central | 612 | 196 | 175 | 45 |
| South Centrala | 483 | 233 | 190 | 28 |
| Easta | 694 | |||
| Total | 2159 | 567 | 470 | 99 |
Regions were combined for Phases II and III of survey.
Table II.
Number of responding operations by herd size
| Herd size | Phase 1: Operations completing 1st questionnaire | Phase IIA: Operations completing 2nd questionnaire | Phase IIB: Operations completing 3rd questionnaire and eligible for sample collection | Phase III: Operations submitting useable sample for evaluation |
|---|---|---|---|---|
| 1–49 | 819 | 163 | 127 | 18 |
| 50–99 | 386 | 96 | 81 | 17 |
| 100–199 | 381 | 125 | 104 | 26 |
| 200+ | 573 | 183 | 158 | 38 |
| Total | 2159 | 567 | 470 | 99 |
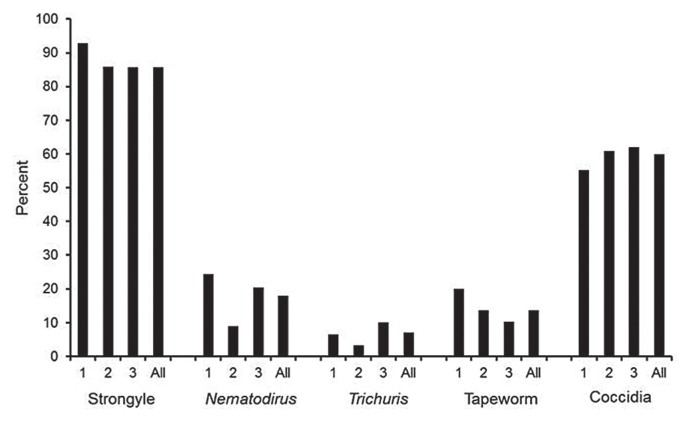
Figure 2.
Percent of positive samples relative to time period of survey: 1 = Mar–May; 2 = Jun–Aug; 3 = Sep–Dec; All = Mar–Dec.
Samples were considered positive for a parasite if any eggs were present in the sample. For sample-level prevalence, the percentage of samples that were positive for each parasite was calculated for each region, each quarter, and overall. Regional and quarterly percentages were compared using Chi-squared tests from logistic models (SAS Proc Genmod; SAS Institute, Cary, North Carolina, USA), accounting for clustering of samples within operations, and presented as arithmetic means. Confidence intervals were calculated from the models and are reported in parentheses.
Mean egg counts per sample as well as regional differences between strongyle eggs were modeled (SAS Proc Genmod) using a negative binomial distribution and accounted for the clustering of samples within operations. Confidence intervals were calculated from the models and regional differences were tested using Wald Chi-squared tests.
For operation-level prevalence, an operation was defined as positive for a particular parasite if it had at least one sample positive for that parasite. The percentage of operations that were positive for each parasite was calculated by region, by quarter, and overall. Regional and quarterly operation-level percentages were compared using Chi-squared tests (SAS Proc Freq; SAS Institute).
Results
Overall 291 producers indicated interest in participating in the parasite prevalence component of the study and ordered sample collection materials. However, only 102 (35%) operations actually submitted samples. Three producers submitted samples that were discarded because they did not follow collection guidelines. These included operations where the animals were too young, where collection took place too soon (< 45 d) after the last anthelmintic treatment, or where the samples were held and not shipped within 24 to 48 h of collection. Thus 99 operations were used in the analysis and accounted for 1772 fecal samples.
Overall 85.6% (95% CI = 80.9, 89.6) of the samples collected had strongyle type eggs, 18.0% (14.2, 22.8) had Nematodirus spp., and 7.1% (5.3, 9.8) had Trichuris spp. eggs (Figure 2). When these data were sub-divided into the months of year in which they were collected (March–May, June–August, and September–December), significant differences were observed between collection periods for Nematodirus (P = 0.031) and Trichuris (P = 0.010). In addition, 59.9% (53.1, 67.0) of the samples contained coccidian oocysts and 13.7% (10.8, 18.4) had tapeworm eggs (Figure 2).
All operations exhibited cattle shedding strongyle eggs in each collection period and for all regions. Mean strongyle shedding was 32.5 epg (25.3, 43.4) over all samples. Regional differences were observed with 30.3 (22.7, 44.7), 58.1 (36.2, 85.3), and 11.3 (7.5, 18.8) epg for Central, South, and West, respectively (P < 0.0001). The overall average for Nematodirus spp. was 0.8 epg (0.5, 1.2) and there was a significant difference between regions, with the South mean epg less than that of the Central and West regions (P = 0.0003). The mean epg for Trichuris spp. was 0.15 (0.10, 0.23), with no differences between regions. The genera present identified by PCR were Cooperia spp. (91%), Ostertagia (79%), Haemonchus (53%), Oesophagostomum (38%), and Trichostrongylus (3%), and by egg morphology, Nematodirus spp (18%), and Trichuris (7.1%).
Discussion
Anthelmintic use rather than pasture management has become the dominant strategy for controlling GI nematodes of cattle (1). The introduction of thiabendazole followed by other benzimidazoles changed the mindset in the way producers managed parasite infections. This reliance on pharmaceuticals was extended with the introduction and extensive use of the avermectins/milbemycins (1). One would expect that the extensive use of anthelmintics over the past 40 y would have reduced parasite prevalence across the US. However, there have been few large-scale studies evaluating the impact of anthelmintic therapy on the populations of parasites throughout the US.
Historically, most studies have reported parasite prevalence from one or a few farms in a specific state or region. In this study, cow/calf operations in 24 States, representing 87.8% (28.6 million) of the US beef inventory and 79.6% (603 000 operations) of the cow/calf inventory were offered the opportunity to assess parasite burdens and types. Among the 2159 operations initially contacted by NASS, 567 agreed to have their contact information forwarded to APHIS and of these 470 became eligible for the parasite sampling activity. Within this group, 291 ordered kits for parasite sampling and 99 operations from 21 States collectively shipped a total of 1772 samples for analysis. The remaining States were either not a source of cow/calf operations in the US or had no operations wishing to pursue sample analysis. Clearly, the issue of representative sampling and bias comes into play within such a study where approximately 5% of those operations initially contacted, eventually agreed to ship and comply with proper sampling procedures for analysis. Among common reasons for lack of participation were the burden of sample collection/submission/follow through, confidentiality despite assurance of anonymity, and insufficient interest in parasites. As such, this survey represents a convenience sample and really cannot be used as an estimate for the original national population. That being said, the survey still encompassed operations from 21 of the 24 States initially contacted. Further, the operations that agreed to participate spanned the full range of herd size (Table II) with a predilection toward operations greater than 100 head of cattle.
The concept of representative sampling is a relative term. It is clear from the participation levels, that to draw conclusions regarding within state or local dissemination of parasitism would be of limited value because participation at the state level was low; however, the goal of the survey was more holistic in nature and designed to garner information regionally and nationally. To this end, the results showed that even amidst the wide use of anthelmintics and newly developed treatments, infection trends and species dissemination had not changed since Porter (14) had first reported data in 1942.
Among the 1772 samples that were analyzed, 85.6% had one or more strongyle eggs, and 18.0% and 7.1% had Nematodirus spp. or Trichuris spp. eggs, respectively. This is not appreciably different from earlier observations (15) where 75.3% of calves and yearlings were positive for strongyle eggs in Iowa. The Iowa study also reported that 26.8% of the animals were passing Nematodirus eggs. In 1956, a South Carolina study found that 40% of over 2000 fecal samples were positive for strongyle eggs and 0.7% and 1.2% for Nematodirus and Trichuris, respectively (16). In Illinois, Levine and Aves (17) found that all cattle surveyed were shedding strongyle eggs and that counts ranged between 43 to over 600 epg. A Georgia study found 83.9% of all beef cattle were positive for parasites by fecal examination (18). A more recent survey showed high prevalence (> 80%) of strongyle eggs in US beef and dairy operations (4).
The current study found that the average sample contained 32.5 strongyle epg across all regions surveyed. However, regional variation was observed, where the average epg were 30.3, 58.1, and 11.3 for the Central, South, and West regions, respectively. Interestingly, the lowest egg shedding was observed in the West and the highest in the South. This observation is consistent with the warm and moist, parasite friendly environment of the South.
Coccidia oocysts were found in 59.9% of all the fecal samples in this study with little variation between regions. The numbers presented here differ from those published from 2 Wisconsin studies (10,19) in which a prevalence of 84% compared to 58.0% was observed in the Central region defined by the current study. Samples in both studies were from animals of similar age. In a third study conducted in South Carolina (16), the prevalence was 21.5% compared with 63.1% in the region defined as South in the current study. The South Carolina samples were from primarily adult animals suggesting that acquired immunity may have played a factor in the lower prevalence values.
The prevalence of tapeworm eggs (Moniezia) was relatively low at 13.7% with few differences observed among regions. In the 1962 Wisconsin study (10), only 5.1% of cattle were found infected with tapeworms compared with 11.9% found in the Central region of the US in this study. These data suggest little has changed over the past 50 y.
Numerous surveys have identified the presence of internal parasite species based on necropsy findings. Porter (14) found an overall prevalence of 91% for Cooperia punctata in cattle in the southeastern US (Alabama, Florida, Georgia, Louisiana, Mississippi). Other nematode species identified were Haemonchus contortus (83%), Ostertagia ostertagi (74%), Bunostomum sp. (62%), Oesophagostomum radiatum (59%), Trichostrongylus axei (47%), Cooperia pectinata (32%), and Strongyloides papillosus (21%). These data are similar to our identifications based on PCR; Cooperia spp. (91.2%), Ostertagia sp. (79.4%), Haemonchus spp. (52.9%), and Oesophagostomum (38%). Likewise, the prevalence of Nematodirus helvetianus (15%) was similar to the 18.3% prevalence of Nematodirus sp. in the current study. The Trichostrongylus (3%) observation was considerably lower than the 47% prevalence observed by Porter (14).
Surprisingly there appears to have been little change in parasite prevalence (regardless of diagnostic technique) and numbers of eggs shed in the past 40 to 60 y, even with the extensive use of benzimidazoles and macrocyclic lactones, and less reliance on pasture management. It is interesting to postulate that in the early years of parasite control (40s, 50s, and 60s), parasitism was at a steady state as demonstrated by Porter (14). With few large-scale studies to detail prevalences in the intervening years, parasite numbers may have waned. A smaller more limited report supports that hypothesis where a tracer calf study in Minnesota found Ostertagia ostertagi (37.5%), Trichostrongylus spp. (36.6%), Cooperia spp. (20.1%), and Haemonchus sp. (4.9%) (3). Now, as resistance continues to emerge and production systems have become more high intensity, we are once again approaching that “steady state” in dissemination of GI nematodes observed back in the 40s, 50s, and 60s. Presented another way, would a similar study, done several years after the introduction of the macrocyclic lactones and before the appearance of resistance have displayed a different level of parasitism? Perhaps a joint role for animal management and anthelmintic therapy must be considered rather than simply relying on the use of anthelmintics to effectively control parasitic nematodes.
Acknowledgments
This study was carried out as part of the USDA APHIS National Animal Health Monitoring System and, as such, the collection of data and samples was funded by the USDA. Supplemental funding was provided by the USDA, Agricultural Research Service to support the evaluation of the fecal samples.
References
- 1.Stromberg BE, Gasbarre LC. Gastrointestinal nematode control programs with an emphasis on cattle. Vet Clin North Am Food Anim Pract. 2006;22:543–565. doi: 10.1016/j.cvfa.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Ballweber LR, Smith L, Stuedemann JA, Yazwinski T, Skogerboe TL. The effectiveness of a single treatment with doramectin or ivermectin in the control of gastrointestinal nematodes in grazing yearling stocker cattle. Vet Parasitol. 1997;72:53–68. doi: 10.1016/s0304-4017(97)00078-2. [DOI] [PubMed] [Google Scholar]
- 3.Stromberg BE, Vatthauer RJ, Schlotthauer JC, et al. Production responses following strategic parasite control in a beef cow/calf herd. Vet Parasitol. 1997;68:315–322. doi: 10.1016/s0304-4017(96)01081-3. [DOI] [PubMed] [Google Scholar]
- 4.Myers GH, Keith EA. Nationwide cattle survey: Zeroing in on parasites. Large Anim Vet. 1993;48:30–32. [Google Scholar]
- 5.Bianchin I, Honer MR. Helminth parasites of beef cattle in the cerrado region of Brazil. Trop Anim Health Prod. 1987;19:39–45. doi: 10.1007/BF02250844. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TM, Fahy KN, McAuliffe A, Forbes AB, Clegg TA, O’Brien DJ. A study of helminth parasites in culled cows from Ireland. Prev Vet Med. 2006;76:1–10. doi: 10.1016/j.prevetmed.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Ploeger HW, Borgsteede FH, Sol J, et al. Cross-sectional serological survey on gastrointestinal and lung nematode infections in first and second-year replacement stock in the netherlands: relation with management practices and use of anthelmintics. Vet Parasitol. 2000;90:285–304. doi: 10.1016/s0304-4017(00)00246-6. [DOI] [PubMed] [Google Scholar]
- 8.USDA. Part IV: Reference of Beef Cow-calf Management Practices in the United States, 2007–08. USDA:APHIS:VS, CEAH; Fort Collins, Colorado, USA: 2010. [Last accessed June 26, 2015]. Beef 2007–08. [Website on the Internet] #523.0210. Available from: http://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef0708/Beef0708_dr_PartIV.pdf. [Google Scholar]
- 9.Gasbarre LC, Leighton EA, Bryant D. Reliability of a single fecal egg per gram determination as a measure of individual and herd values for trichostrongyle nematodes of cattle. Am J Vet Res. 1996;57:168–171. [PubMed] [Google Scholar]
- 10.Cox DD, Todd AC. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J Am Vet Med Assoc. 1962;141:706–709. [PubMed] [Google Scholar]
- 11.Bliss DH, Kvasnicka WG. The fecal examination: A missing link in food animal practice. Compendium on continuing education for the practicing veterinarian. 1997;19(Suppl 4):S104–S109. [Google Scholar]
- 12.Herlich H. Attempts to produce protection against Ostertagia ostertagi in cattle. Am J Vet Res. 1976;37:61–64. [PubMed] [Google Scholar]
- 13.Zarlenga DS, Chute MB, Gasbarre LC, Boyd PC. A multiplex PCR assay for differentiating economically important gastrointestinal nematodes of cattle. Vet Parasitol. 2001;97:199–209. doi: 10.1016/s0304-4017(01)00410-1. [DOI] [PubMed] [Google Scholar]
- 14.Porter DA. Incidence of gastrointestinal nematodes of cattle in the Southeastern United States. Am J Vet Res. 1942;3:304–308. [Google Scholar]
- 15.Zimmermann WJ, Hubbard ED. Gastrointestinal parasitism in Iowa cattle. J Am Vet Med Assoc. 1961;139:555–559. [PubMed] [Google Scholar]
- 16.Hitchcock DJ. A survey of gastrointestinal parasites of cattle of South Carolina. J Am Vet Med Assoc. 1956;129:34–35. [PubMed] [Google Scholar]
- 17.Levine ND, Aves IJ. The incidence of gastrointestinal nematodes in Illinois cattle. J Am Vet Med Assoc. 1956;129:331–332. [PubMed] [Google Scholar]
- 18.Ciordia H. Occurrence of gastrointestinal parasites in Georgia cattle. Am J Vet Res. 1975;36:457–461. [PubMed] [Google Scholar]
- 19.Hasche MR, Todd AC. Prevalence of bovine coccidia in Wisconsin. J Am Vet Med Assoc. 1959;134:449–451. [PubMed] [Google Scholar]