Abstract
Background
Noninvasive ventilation (NIV) may reduce the need for intubation and mortality associated with chronic obstructive pulmonary disease (COPD) with type II respiratory failure. Early and simple predictors of NIV outcome could improve clinical management. This study aimed to assess whether nutritional risk screening 2002 (NRS2002) is a useful outcome predictor in COPD patients with type II respiratory failure treated by noninvasive positive pressure ventilation (NIPPV).
Material/Methods
This prospective observational study enrolled COPD patients with type II respiratory failure who accepted NIPPV. Patients were submitted to NRS2002 evaluation upon admission. Biochemical tests were performed the next day and blood gas analysis was carried out prior to NIPPV treatment and 4 hours thereafter. Patients were divided into NRS2002 score ≥3 and NRS2002 score <3 groups and NIV failure rates were compared between both groups.
Results
Of the 233 patients, 71 (30.5%) were not successfully treated by NIPPV. The failure rate was significantly higher in the NRS2002 score ≥3 group (35.23%) in comparison with patients with NRS2002 score <3 (15.79%) (p<0.05). Multivariate analysis indicated that PaCO2 (OR 1.25, 95%CI 1.172–1.671, p<0.05) prior to NIPPV treatment and NRS2002 score ≥3 (OR 1.76, 95%CI 1.303–2.374, p<0.05) were independent predictive factors for NIPPV treatment failure.
Conclusions
NRS2002 score ≥3 and PaCO2 values at admission may predict unsuccessful NIPPV treatment of COPD patients with type II respiratory failure and help to adjust therapeutic strategies. NRS2002 is a noninvasive and simple method for predicting NIPPV treatment outcome.
MeSH Keywords: Biological Markers; Lung Diseases, Obstructive; Noninvasive Ventilation; Nutritional Status; Respiratory Insufficiency
Background
Noninvasive ventilation (NIV) is an effective approach widely used for treating early- and mid-stage acute hypercapnic respiratory failure in chronic obstructive pulmonary disease (COPD) [1,2]. However, due to NIV treatment failure, some patients have to undergo endotracheal intubation or tracheostomy. If patients for whom NIV treatment is likely to fail could be identified early enough, it would be possible to provide more advanced life support and decrease mortality [3].
Failed NIV treatment was shown to be associated with pH <7.3 at admission, hypercapnia, hyperglycemia, high Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and unconsciousness [4–7]. However, the studies that identified these factors mainly assessed intensive care unit (ICU) patients. Therefore, it remains unclear whether the same factors cause unsuccessful NIV treatment in early- and mid-stage respiratory failure patients in the general ward. The existing factors for predicting NIV treatment failure, including blood gas and glucose levels, have certain limitations. The method is invasive, and some relatives may even decline, and the evaluations depend on detection using related equipment and are neither fast nor convenient.
Large studies of hospitalized COPD patients with type II respiratory failure have revealed that malnourished COPD patients or those with nutritional risks have poorer outcomes compared with their counterparts with normal nutritional parameters, such as pulmonary function, hospitalization duration, and mortality rate [8,9]. These findings indicate that nutritional risks are associated with poor prognosis of COPD patients. At present, nutritional risks are assessed by various nutritional risk screening tools. The Nutritional Risk Screening 2002 (NRS2002), a nutritional risk-screening tool for hospitalized patients recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN), is the only evidence-based tool widely used internationally [10–12]. The Chinese version of NRS2002 has been shown to function well for Chinese hospitalized patients [13,14]. However, whether nutritional risks can affect the efficacy of NIV treatment in COPD patients with type II respiratory failure is unknown, and the relationship between nutritional risks and other negative predictive factors is unclear. The NRS2002 has the potential to be a simple, effective, and noninvasive method for determining the prognosis of NIV treatment.
Therefore, this prospective study aimed to assess the relationship between nutritional risks and other risk factors in COPD patients with type II respiratory failure who underwent NIV treatment upon hospital admission. Such data should provide evidence on whether the NRS2002 should be used as a predictive tool for the prognosis of NIV treatment in early- and mid-stage acute hypercapnic respiratory failure in COPD.
Material and Methods
Hospital and setting
We performed a prospective, observational study to evaluate consecutive adult patients with type II respiratory failure who accepted NIPPV treatment in the general ward of the Department of Respiratory Medicine, West China Hospital of Sichuan University, between December 2010 and May 2012. The study was approved by the Ethics Committee of West China Hospital of Sichuan University. In addition, written informed consent was obtained from each participant.
Study subject selection
The diagnosis of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [15]. Acute-phase COPD was determined by previously described criteria [15] and clinical symptoms, including shortness of breath, cough, fever (>38°C), and abnormal breath sounds. Inclusion criteria were: age ≥18 years, respiratory rates >23 bpm, and partial pressure of CO2 (PaCO2) >50 mmHg. Subjects with the following accompanying conditions were excluded: pregnancy or lactation in women, ventilatory dysfunction due to neuromuscular disorders, acute and chronic thromboembolic disease, and intolerance to the ventilator mask or inability to cooperate with ventilator therapy. Bi-level positive airway pressure (BiPAP) was adopted in the NIPPV.
Intubation criteria
NIV was withdrawn and patients were intubated with SpO2 <85%, venous PaCO2 >65 mmHg, worsened dyspnea despite maximal NIV setting, or the appearance of any exclusion criteria. Maximal NIV settings were considered as inspiratory positive airway pressure (IPAP) ≥25 cmH2O, or expiratory positive airway pressure (EPAP) ≥12 cmH2O, with FiO2 100.
NIV strategy
Bi-level NIV was delivered using a nasal mask or/and a face mask, following GOLD [15] criteria. The initial ventilator EPAP setting was 4–6 cmH2O. IPAP was started at 6–8 cmH2O to achieve tolerance and patient-ventilator synchrony. The fraction of inspired O2 (FiO2) was as low as possible in order to maintain O2 saturation (SpO2) above 90%. Sedation was administered, if required, at the discretion of the attending physician, according to Respiratory Medicine Department protocol.
Data collection
The nutritional risks of all the hospitalized COPD subjects with type II respiratory failure were evaluated on the day of admission by a nutrition nurse with 10 years of experience, using the NRS2002, which includes the following contents: (1) nutritional status, body weight changes in the past 1–3 months, and variations of food intake in the past week; (2) severity of disease; and (3) age ≥70 years. A total score of ≥3 indicates a subject nutritionally at risk [16]. The basic information of the subjects was obtained from the hospital information system (HIS). The decision to carry out the NIPPV treatment was conjointly made by the attending physicians, and the subjects and their relatives; researchers were not involved in the clinical decisions. Before a decision was reached, a research team member was appointed to measure the subject’s respiratory rate. Then, blood gas analysis was performed prior to NIPPV treatment and 4 h thereafter, as part of the clinical pathway, by registered nurses not involved in the research. In addition, biochemical samples were collected as part of the clinical pathway by registered nurses not involved in the research, the next morning after admission.
Follow up
A failed NIPPV treatment was defined by the intubation of the concerned patients. NIV was followed up until the resolution of respiratory acidosis, leading to successful weaning from the ventilator, and no requirement for ventilatory support for at least 48 h. Patients were classified into successful and unsuccessful treatment groups according to the NIPPV treatment results.
Statistical analyses
The SPSS statistical software version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Quantitative data expressed as mean ± standard deviation (SD) and the independent samples t test was used for comparison. Categorical data expressed as frequency and percentage and compared with Pearson Chi-square test. Univariate and multivariate logistic regressions were used to analyze predictive factors for NIV treatment outcome. To enroll the factors associated with NIV treatment as thoroughly as possible, the variables with P<0.1 in univariate analysis were included in the multivariate logistic regression analysis. P<0.05 indicated statistical significance.
Results
Patient characteristics
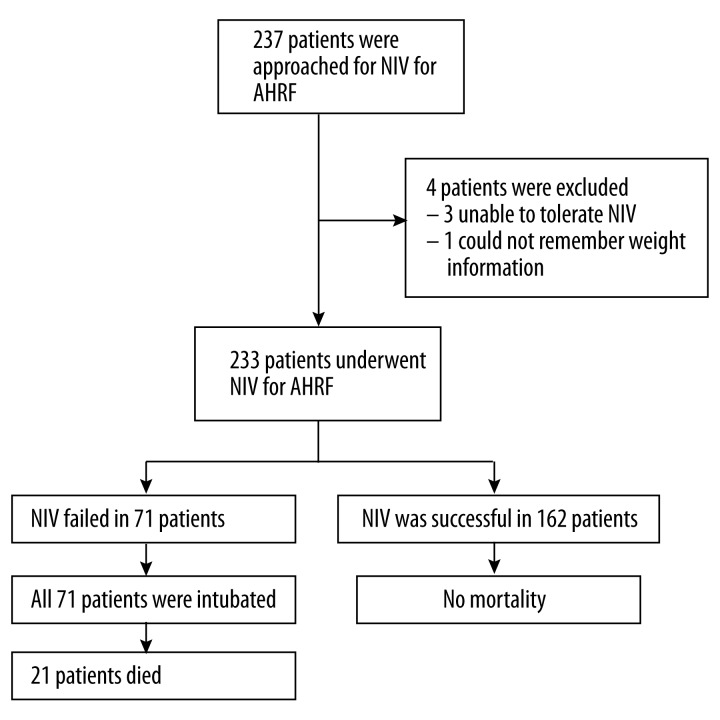
A total of 285 COPD subjects with type II respiratory failure accepted NIV treatment, of which 237 met the enrolment criteria. One subject was excluded for uncertainty of weight changes in the preceding 3 months, and 3 subjects quit the study for inadaptability to masks. Finally, 233 cases were enrolled in the study (Figure 1), including 176 and 57 patients in the NRS2002 score ≥3 and NRS2002 score <3 groups, respectively (Table 1). The 2 groups were similar in most demographic and baseline data. However, the NRS2002 score ≥3 group was composed of older individuals (72±8 vs. 65±8 years, P<0.001) and lower male percentage (65.3 vs. 84.2%, P=0.007) in comparison with the NRS2002 score <3 group; in addition, the time from admission to NIV administration was slightly higher in the NRS2002 score ≥3 group than patients with NRS2002 score <3 (43.49±20.57 vs. 36.12±19.20 h, P=0.018) (Table 1).
Figure 1.
Flow diagram for this study.
Table 1.
Demographic and baseline data of the study population.
| Variables | NRS2002 score ≥3 (n=176) | NRS2002 score <3 (n=57) | P value |
|---|---|---|---|
| Age | 72±8 | 65±8 | <0.001 |
| Male Gender | 115 (65.3%) | 48 (84.2%) | 0.007 |
| FEV1 (liters) | 1.00±0.42 | 1.03±0.41 | 0.690 |
| FVC (liters) | 1.65±0.42 | 1.67±0.39 | 0.751 |
| Respiratory rate prior to NIV initiation (breaths/min) | 25±2.45 | 25±1.37 | 0.154 |
| PaO2 prior to NIV initiation (kPa) | 76.26±27.84 | 73.81±26.81 | 0.560 |
| PaCO2 prior to NIV initiation (kPa) | 65.95±19.16 | 66.26±19.52 | 0.914 |
| Arterial pH prior to NIV initiation | 7.36±0.09 | 7.35±0.07 | 0.415 |
| Time from admission to NIV administration (h) | 43.49±20.57 | 36.12±19.20 | 0.018 |
| IPAP (cm H2O) | 13.35±1.67 | 13.84±1.89 | 0.540 |
| EPAP (cm H2O) | 4.13±0.78 | 4.32±0.85 | 0.558 |
| Total lymphocyte prior to NIV initiation | 0.86±0.60 | 0.98±0.52 | 0.179 |
| Hemoglobin prior to NIV initiation (g/L) | 133.28±25.17 | 138.88±25.36 | 0.148 |
| Serum total protein prior to NIV initiation (g/L) | 64.45±8.47 | 64.81±8.35 | 0.780 |
| Serum albumin prior to NIV initiation (g/L) | 36.83±5.12 | 37.73±4.99 | 0.249 |
| Total cholesterol prior to NIV initiation | 4.03±0.96 | 4.04±1.32 | 0.952 |
| Triglycerides prior to NIV initiation | 1.08±0.53 | 1.05±0.79 | 0.729 |
| Creatinine prior to NIV initiation | 84.98±48.10 | 81.67±34.69 | 0.783 |
| Urea prior to NIV initiation, (mmol/L) | 10.15±8.09 | 8.39±4.20 | 0.512 |
| Glucose level prior to NIV initiation (mmol/L) | 7.76±3.21 | 7.07±3.05 | 0.150 |
| Diagnosis of diabetes mellitus | 27 (15.3%) | 3 (5.3%) | 0.081 |
| Oral corticosteroids taken | 69 (39.2%) | 15 (26.3%) | 0.078 |
| 4 h PaO2 | 76.50±23.86 | 83.98±24.49 | 0.131 |
| 4 h PaCO2 | 61.45±18.54 | 57.59±12.76 | 0.145 |
| 4 h pH | 7.36±0.09 | 7.35±0.07 | 0.415 |
Values are given as mean ±SD. NIPPV – noninvasive positive pressure ventilation; F – female; M – male; NIV – noninvasive ventilation FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; Pa – partial pressure; IPAP – inspiratory positive airway pressure; EPAP – expiratory positive airway pressure; OR – odds ratio.
Univariate analysis
Of the 233 subjects, 71 (30.5%) were unsuccessfully treated with NIV. The failure rates were 35.23% and 15.79% in the NRS2002 score ≥3 and NRS2002 score <3 groups, respectively, indicating a statistically significant difference between the 2 groups (p=0.006; Table 2).
Table 2.
Relationship between NRS2002 and outcome of NIV.
| NRS2002 score ≥3 (n=176) | NRS2002 score <3 (n=57) | P-value | |
|---|---|---|---|
| NIV success | 114 (64.77%) | 48 (84.21%) | 0.006 |
| NIV failure | 62 (35.23%) | 9 (15.79%) |
NIV – non-invasive ventilation; NRS2002 – Nutritional Risk Screening 2002.
Most parameters assessed did not significantly predict NIV treatment outcome. For example, 30 subjects (12.9%) were diagnosed with diabetes before admission (Table 1), but diabetes was not a significant prognosis for NIV treatment in univariate analysis (OR=1.63, 95%CI 0.74–3.59, P=0.23). In addition, sex appeared to marginally affect the NIV treatment outcome, but the correlation was not statistically significant (OR=1.41, 95%CI 0.78–2.57, P=0.26). Interestingly, nutrition risk (NRS2002 score) prior to NIV initiation was a significant predictor of NIV treatment outcome (OR=0.35, 95%CI 0.16–0.75, P=0.007), as shown in Table 3.
Table 3.
Relationships between variables and outcome of NIPPV: univariate analysis.
| Variables | NIV success (n=162) | NIV failure (n=71) | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Age* | 70±9.06 | 74±8.19 | 1.05 | 1.019–1.090 | 0.002 |
| Gender** | M=117; F=45 | M=46; F=25 | 1.41 | 0.78–2.57 | 0.26 |
| FEV1 (liters)* | 1.02±0.39 | 0.98±0.49 | 0.81 | 0.40–1.61 | 0.54 |
| FVC (liters)* | 1.66±0.37 | 1.62±0.51 | 0.80 | 0.40–1.58 | 0.51 |
| Respiratory rate prior to NIV initiation (breaths/min)* | 25±2.15 | 27±2.46 | 1.05 | 0.92–1.20 | 0.50 |
| PaO2 prior to NIV initiation (kPa)* | 80.86±20.08 | 73.43±27.53 | 1.01 | 1.00–1.02 | 0.06 |
| PaCO2 prior to NIV initiation (kPa)* | 56.55±16.80 | 70.21±18.75 | 1.16 | 1.04–1.97 | <0.001 |
| Arterial pH prior to NIV initiation* | 7.40±0.08 | 7.34±0.08 | 1.21 | 0.15–3.20 | <0.001 |
| Time from admission to NIV administration (h) | 42.68±20.65 | 39.42±19.96 | 0.99 | 0.98–1.01 | 0.26 |
| IPAP (cm H2O)* | 13.48±1.79 | 13.85±1.91 | 1.12 | 0.78–1.61 | 0.54 |
| EPAP (cm H2O)* | 4.31±0.89 | 4.15±0.56 | 0.74 | 0.26–2.07 | 0.56 |
| Total lymphocyte prior to NIV initiation* | 0.91±0.59 | 0.84±0.56 | 0.78 | 0.47–1.32 | 0.36 |
| Hemoglobin prior to NIV initiation (g/L)* | 137.11±25.45 | 128.85±24.07 | 0.99 | 0.98–0.99 | 0.03 |
| Serum total protein prior to NIV initiation (g/L)* | 64.12±8.14 | 65.52±9.03 | 1.02 | 0.99–1.06 | 0.25 |
| Serum albumin prior to NIV initiation (g/L)* | 37.13±4.82 | 36.89±5.70 | 0.99 | 0.94–1.05 | 0.75 |
| Total cholesterol prior to NIV initiation* | 4.03±1.00 | 4.03±1.21 | 1.01 | 0.74–1.37 | 0.96 |
| Triglycerides prior to NIV initiation* | 1.11±0.70 | 0.98±0.33 | 0.62 | 0.31–1.24 | 0.08 |
| Creatinine prior to NIV initiation* | 76.65±30.73 | 101.44±33.48 | 1.01 | 0.99–1.01 | 0.12 |
| Urea prior to NIV initiation, (mmol/L)* | 10.14±20.80 | 8.73±4.82 | 0.99 | 0.97–1.02 | 0.59 |
| Glucose level prior to NIV initiation (mmol/L)* | 7.26±2.85 | 8.36±3.73 | 1.11 | 1.02–1.21 | 0.02 |
| Diagnosis of diabetes mellitus** | 18 | 12 | 1.63 | 0.74–3.59 | 0.23 |
| Oral corticosteroids taken** | 61 | 23 | 0.79 | 0.44–1.43 | 0.44 |
| Nutrition risk prior to NIV initiation** | 114 | 62 | 0.35 | 0.16–0.75 | 0.007 |
| 4 h PaO2* | 79.58±25.13 | 76.06±22.13 | 0.99 | 0.98–1.01 | 0.44 |
| 4 h PaCO2* | 54.83±10.35 | 72.01±23.63 | 1.08 | (1.05–1.11) | <0.001 |
| 4 h pH* | 7.38±0.46 | 7.34±0.08 | 0.00 | (0.000–0.003) | <0.001 |
Values are given as mean (SD).
t-test;
Chi-square test.
NIPPV – noninvasive positive pressure ventilation; F – female; M – male; NIV – noninvasive ventilation FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; Pa – partial pressure; IPAP – inspiratory positive airway pressure; EPAP – expiratory positive airway pressure; OR – odds ratio.
The blood gas data prior to NIV treatment and 4 h thereafter are shown in Table 3. Interestingly, PaCO2 (OR=1.16, 95%CI 1.04–1.97, P<0.001), and arterial pH values (OR=1.21, 95%CI 0.15–3.20, P<0.001) prior to NIV treatment were significantly different between the successfully and unsuccessfully treated individuals (Table 3). Four hours after NIV treatment, the same parameters (PaCO2 and arterial pH values) were significantly different between successfully and unsuccessfully treated individuals (P<0.001).
Multivariate analysis
All variables with a P value of ≤0.1 in baseline comparison and in the univariate analysis were selected as candidates for the multivariate analysis model. The multivariate analysis indicated that PaCO2 value prior to the NIV treatment (OR 1.25, 95% CI 1.172 to 1.671, P<0.001), and NRS2002 score (OR 1.76, 95% CI 1.303 to 2.374, P=0.015) could predict the NIV prognoses (Table 4).
Table 4.
Multivariate analysis of NIPPV outcome.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| NRS2002 | 1.759 | 1.303–2.374 | 0.015 |
| PaCO2 prior to NIV | 1.251 | 1.172–1.671 | <0.001 |
NIPPV – noninvasive positive pressure ventilation.
Discussion
This study aimed to assess whether NRS2002 could be used to predict the outcome of patients treated with NIPPV for type II respiratory failure. Such information would help the early clinical management of COPD. Indeed, nutrition risk (NRS2002 score) and PaCO2 prior to NIV initiation were found to be significant predictors of NIV treatment outcome.
NIV is an important approach for treating early- and mid-stage COPD patients with type II respiratory failure. This prospective study showed improvement in 69.5% of COPD patients with hypercapnia after NIV treatment, a rate lower than previously reported [17,18]. Of note, patients in those studies accepted NIV treatment within 24 h of admission, while the patients assessed here accepted treatment with greater delay (about 40 h after admission); this might have resulted in poorer overall conditions and increased treatment failure. Also, our patients were from a developing country with low economic index. Indeed, the mortality rate of COPD patients decreases with improvements in economics [19]. For example, excessive financial burdens may make patients delay the decision for expensive therapies [20].
Patients nutritionally at risk include not only malnourished individuals but also those with clinical prognoses that would be affected by the incoming nutritional issues caused by factors such as surgeries or infections [21]. To the best of our knowledge, this study is the first to introduce NRS2002 into nutritional risk evaluations of NIV treatment in COPD patients.
The nutritional risks of the patients evaluated here were higher compared with other reports [13,22,23], possibly due to patient ages, which averaged 71 years, indicating that they were generally older than those assessed previously. Indeed, after 70 years of age, the NRS2002 score increases by 1 point. NRS2002 is the ESPEN-recommended nutritional risk-screening tool for hospitalized patients, and it is more convenient and less time consuming than other available methods [24]. According to the univariate analysis, nutritional risk rates between the successfully and unsuccessfully treated groups were significantly different.
Serum albumin level was not able to predict NIV treatment outcome in this study, in contrast to the results of Wu et al. [25], who reported low serum albumin levels as a positive predictor for failure in withdrawing ventilators from patients on long-term invasive mechanical ventilators. Serum albumin and prealbumin levels decrease in an inflammatory state; therefore, low serum albumin levels may not accurately reflect the nutritional status [26].
Multivariate analysis indicated that 2 factors – PaCO2 value and NRS2002 score prior to NIV treatment – were independent predictive factors for NIPPV treatment failure. The higher the admission PaCO2 value, the more likely the NIV treatment would fail, in agreement with previous studies [18,27]. Four hours after NIV treatment, PaCO2 values of successfully treated patients decreased, while those of unsuccessfully treated patients increased, as shown above.
We also found that the higher the admission NRS2002 score, the more likely the NIV treatment of COPD patients will fail. Considering the lack of a relationship between low serum albumin levels and NIV treatment failure, the NIV treatment failure was probably not caused by an existing malnourished status, but rather by the potential nutritional risks. Even with normal serum albumin levels, the likelihood of failure of patient NIV treatment will still increase because of high nutritional risks. Compared with monitoring serum albumin, evaluation of NRS2002 for nutritional risks is convenient and noninvasive, and can effectively ensure patient safety.
It has been previously indicated [17] that hyperglycemia prior to NIV treatment could predict its outcome, in contrast to our findings; the effect of plasma glucose on NIV treatment failure in this study might have been shielded by other factors.
The simplicity, feasibility, and noninvasiveness of NRS2002 for prediction NIV treatment outcome is an important benefit of this method of risk evaluation. It seems unlikely that the 2 factors identified in this study can totally explain NIV treatment outcome. There is a possibility that other factors affecting the results were not included. For example, body mass index (BMI) [28], state of consciousness [29], and infectious complications [30] were all proposed to be associated with NIV treatment outcome. We did not include these factors in the present study for the following reasons: BMI could not be accurately measured in the general ward, with patients unable to stand; all enrolled patients were conscious and able to answer the questions accurately and there were no unconscious patients; and although some potential accompanying diseases that might affect the nutritional status were considered (diabetes and pneumonia), others might not have been included.
Limitations of this study should be mentioned. Although this was a prospective study, the patients were evaluated by the NRS2002 only upon admission, and their nutritional risks might change as the disease develops; therefore, a single evaluation might not reflect their nutritional status thoroughly. In addition, all subjects were from the general ward and conscious when admitted; therefore, they could successfully complete the assessments. However, when patients were unconscious or could not accurately answer the questions asked by the assessors, the assessment was discontinued, which constitutes a limitation for the application of NRS2002. Furthermore, all subjects were from the same center; the homogeneity of the study population and therapeutic environment may have precluded the identification of certain factors affecting the NIV treatment outcome. Finally, treatment failure was still high in the NRS score <3 group. However, among the studied variables, no other variable could be identified to improve the predictive model. A prospective study in multiple centers assessing many time points is necessity to confirm and improve our findings.
Conclusions
The risk of NIV failure in treating COPD patients with type II respiratory failure in a general ward can be predicted by admission NRS2002 score and PaCO2 value. Then, the therapeutic strategy could be adjusted according to the NRS2002 and PaCO2 results by using advanced life support, decreasing the risk of treatment failure, and decreasing mortality. NRS2002 is a noninvasive and convenient way to predict the failure of NIV treatment.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: This study was supported by a grant from the Science and Technology Department of Sichuan Province (2013SZ0001), P. R. China
References
- 1.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326:185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nava S, Grassi M, Fanfulla F, et al. Non-invasive ventilation in elderly patients with acute hypercapnic respiratory failure: a randomised controlled trial. Age Ageing. 2011;40:444–50. doi: 10.1093/ageing/afr003. [DOI] [PubMed] [Google Scholar]
- 3.Wood KA, Lewis L, Von Harz B, Kollef MH. The use of noninvasive positive pressure ventilation in the emergency department: results of a randomized clinical trial. Chest. 1998;113:1339–46. doi: 10.1378/chest.113.5.1339. [DOI] [PubMed] [Google Scholar]
- 4.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–35. doi: 10.1016/s0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 5.Moretti M, Cilione C, Tampieri A, et al. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–25. doi: 10.1136/thorax.55.10.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scala R, Bartolucci S, Naldi M, et al. Co-morbidity and acute decompensations of COPD requiring non-invasive positive-pressure ventilation. Intensive Care Med. 2004;30:1747–54. doi: 10.1007/s00134-004-2368-4. [DOI] [PubMed] [Google Scholar]
- 7.Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25:348–55. doi: 10.1183/09031936.05.00085304. [DOI] [PubMed] [Google Scholar]
- 8.Benedik B, Farkas J, Kosnik M, et al. Mini nutritional assessment, body composition, and hospitalisations in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(Suppl 1):S38–43. doi: 10.1016/S0954-6111(11)70009-9. [DOI] [PubMed] [Google Scholar]
- 9.Gupta B, Kant S, Mishra R. Subjective global assessment of nutritional status of chronic obstructive pulmonary disease patients on admission. Int J Tuberc Lung Dis. 2010;14:500–5. [PubMed] [Google Scholar]
- 10.Raslan M, Gonzalez MC, Dias MC, et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. 2010;26:721–26. doi: 10.1016/j.nut.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Velasco C, Garcia E, Rodriguez V, et al. Comparison of four nutritional screening tools to detect nutritional risk in hospitalized patients: a multicentre study. Eur J Clin Nutr. 2011;65:269–74. doi: 10.1038/ejcn.2010.243. [DOI] [PubMed] [Google Scholar]
- 12.Almeida AI, Correia M, Camilo M, Ravasco P. Nutritional risk screening in surgery: valid, feasible, easy! Clin Nutr. 2012;31:206–11. doi: 10.1016/j.clnu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Zhang ZF, Cai JJ, et al. NRS2002 assesses nutritional status of leukemia patients undergoing hematopoietic stem cell transplantation. Chin J Cancer Res. 2012;24:299–303. doi: 10.3978/j.issn.1000-9604.2012.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P, Yan X, Wang BS, Xu XD. Three methods assess nutritional status of leukemia patients before hematopoietic stem cell transplantation. Chin Med J (Engl) 2012;125:440–43. [PubMed] [Google Scholar]
- 15.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 16.Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21. doi: 10.1016/s0261-5614(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti B, Angus RM, Agarwal S, et al. Hyperglycaemia as a predictor of outcome during non-invasive ventilation in decompensated COPD. Thorax. 2009;64:857–62. doi: 10.1136/thx.2008.106989. [DOI] [PubMed] [Google Scholar]
- 18.Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. 2001;56:708–12. doi: 10.1136/thorax.56.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Schooling CM, Johnston JM, et al. How does socioeconomic development affect COPD mortality? An age-period-cohort analysis from a recently transitioned population in China. PLoS One. 2011;6:e24348. doi: 10.1371/journal.pone.0024348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou P, Zhu Y, Chen P, et al. Vulnerability, beliefs, treatments and economic burden of chronic obstructive pulmonary disease in rural areas in China: a cross-sectional study. BMC Public Health. 2012;12:287. doi: 10.1186/1471-2458-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 22.Fang S, Long J, Tan R, et al. A multicentre assessment of malnutrition, nutritional risk, and application of nutritional support among hospitalized patients in Guangzhou hospitals. Asia Pac J Clin Nutr. 2013;22:54–59. doi: 10.6133/apjcn.2013.22.1.01. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Xu X, Yan J, Mou Y. Nutritional risk is still a clinical predictor of postoperative outcomes in laparoscopic abdominal surgery. Surg Endosc. 2013;27:2569–74. doi: 10.1007/s00464-013-2790-1. [DOI] [PubMed] [Google Scholar]
- 24.Kyle UG, Kossovsky MP, Karsegard VL, Pichard C. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr. 2006;25:409–17. doi: 10.1016/j.clnu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Wu YK, Kao KC, Hsu KH, et al. Predictors of successful weaning from prolonged mechanical ventilation in Taiwan. Respir Med. 2009;103:1189–95. doi: 10.1016/j.rmed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Doley J, Mallampalli A, Sandberg M. Nutrition management for the patient requiring prolonged mechanical ventilation. Nutr Clin Pract. 2011;26:232–41. doi: 10.1177/0884533611405536. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosino N, Foglio K, Rubini F, et al. Non-invasive mechanical ventilation in acute respiratory failure due to chronic obstructive pulmonary disease: correlates for success. Thorax. 1995;50:755–57. doi: 10.1136/thx.50.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkius J, Sundh J, Nilholm L, et al. What determines immediate use of invasive ventilation in patients with COPD? Acta Anaesthesiol Scand. 2013;57:312–19. doi: 10.1111/aas.12049. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosino N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:471–76. [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolini A, Tonveronachi E, Navalesi P, et al. Effectiveness and predictors of success of noninvasive ventilation during H1N1 pandemics: a multicenter study. Minerva Anestesiol. 2012;78:1333–40. [PubMed] [Google Scholar]