Abstract
Purpose.
Endogenous anterior uveitis (AU), when untreated, may lead to vision loss. This study compared the safety and efficacy of difluprednate versus prednisolone acetate for the treatment of this condition.
Methods.
This phase III, double-masked, noninferiority study randomized patients with mild to moderate endogenous AU to receive difluprednate 0.05% (n = 56) four times daily, alternating with vehicle four times daily, or prednisolone acetate 1% (n = 54) eight times daily. The 14-day treatment period was followed by a 14-day dose-tapering period and a 14-day observation period. The primary efficacy end point was change in anterior chamber cell grade (range, 0 for ≤1 cell to 4 for >50 cells) from baseline to day 14.
Results.
At day 14, the mean change in anterior chamber cell grade with difluprednate was noninferior to that with prednisolone acetate (−2.2 vs. −2.0, P = 0.16). The proportions of difluprednate-treated patients versus prednisolone acetate–treated patients demonstrating complete clearing of anterior chamber cells at day 3 were 13.0% vs. 2.1% (P = 0.046) and at day 21 were 73.9% vs. 63.8% (P = 0.013). A significant between-group difference in the mean IOP increase was seen at day 3 (2.5 mm Hg for difluprednate-treated patients and 0.1 mm Hg for prednisolone acetate–treated patients, P = 0.0013) but not at other time points. The mean IOP values in both groups remained less than 21 mm Hg throughout the study.
Conclusions.
Difluprednate 0.05% four times daily is well tolerated and is noninferior to prednisolone acetate 1% eight times daily for the treatment of endogenous AU. (ClinicalTrials.gov number, NCT01201798.)
Keywords: acute anterior uveitis, corticosteroid, difluprednate, endogenous anterior uveitis, intraocular pressure, noninfectious uveitis, prednisolone, prednisolone acetate, uveitis
Introduction
Endogenous anterior uveitis (AU) is a form of uveitis that is not directly caused by an infectious pathogen. It is characterized by intraocular inflammation of the uveal structures anterior to the middle of the vitreous cavity, including iritis, iridocyclitis, and anterior cyclitis.1–3 The etiology of endogenous AU is incompletely characterized1,4 but has been associated with systemic diseases such as juvenile idiopathic arthritis, seronegative spondylarthropathies,5 or Behçet disease,1,4 as well as abnormalities of the immune system.2 As with most forms of uveitis, visual morbidity associated with endogenous AU does not usually occur from a single episode; rather, recurrent or prolonged inflammation causes cumulative damage,6 leading to vision-threatening complications, potential vision loss, decreased quality of life (QoL), and increased socioeconomic cost.2 Vision-threatening complications of acute or chronic uveitis include cystoid macular edema,1,6,7 a major cause of vision loss,2,8 as well as cataract, band keratopathy, glaucoma or ocular hypertension, synechiae formation, pupillary membrane, epiretinal membrane,1,6,7 subretinal fibrosis,9 ciliary fibrosis,10 hypotony, vitreous opacification, and optic nerve edema.1,6,7
Given that repeated episodes of uveitis, when untreated or undertreated, may lead to vision impairment,1 timely treatment is critical. Treatment algorithms typically include topical ophthalmic corticosteroids,1 with prednisolone acetate 1% being the chief topical corticosteroid therapy. However, prednisolone acetate 1% usually demands frequent dosing, particularly for severe cases, in which increased administration frequency is required.1,11 As demonstrated with glaucoma medications, frequent dosing may increase the risk of noncompliance,12 which may negatively affect the achievement of therapeutic goals.1 For patients who fail to respond to topical treatment, increased dosage of the topical treatment, periocular (subtenon) or intraocular (intravitreal) corticosteroid injections (triamcinolone), intraocular corticosteroid depot treatment (fluocinolone), or oral corticosteroid therapy may be necessary. However, these alternatives, as well as any prolonged corticosteroid therapy, are often associated with significant adverse effects.13
Difluprednate is a prednisolone acetate derivative14 that is augmented by two fluorinations at carbons 6 and 9, a butyrate group at carbon 17, and an acetic acid group at carbon 21. Relative to its parent molecule, the fluorinations enhance the corticosteroid potency of difluprednate, the butyric acid augments anti-inflammatory activity, and the acetic acid increases penetration.15 Difluprednate 0.05% has been shown to be effective at reducing inflammation and pain in patients undergoing ocular surgery.16,17 This study aimed to test the hypothesis that difluprednate 0.05% dosed four times daily is noninferior to prednisolone acetate 1% dosed eight times daily in patients with endogenous AU.
Methods
Study Design
This was a phase III, multicenter, randomized, double-masked, parallel-group, active-controlled noninferiority study conducted at 21 clinical sites throughout the United States between October 2010 and August 2011 in patients with mild to moderate endogenous AU. Eligible patients were randomized in a 1:1 ratio stratified by center, with the use of a block randomization list generated by a computer program for each site, to receive either difluprednate 0.05% (Durezol ophthalmic emulsion; Alcon Research, Ltd., Fort Worth, TX, USA) four times daily, alternating with vehicle four times daily, or prednisolone acetate 1% (Pred Forte ophthalmic suspension; Allergan, Inc., Irvine, CA, USA) eight times daily for 14 days. To maintain masking of treatment allocation, patients assigned to difluprednate 0.05% were given two bottles, one containing difluprednate 0.05% and the other containing vehicle; patients assigned to prednisolone acetate 1% were also given two bottles, both containing prednisolone acetate 1%. Patients were to alternate instillation from each bottle. Because prednisolone acetate 1% requires shaking before use, all patients were instructed to shake all bottles before instillation. The appearance of difluprednate, prednisolone acetate, and vehicle was indistinguishable from one another on inspection (a white, milky liquid). Patients were tapered off the study medication during days 14 to 27 at the discretion of the investigator. On day 14, the first day after completion of the planned treatment course, individuals who responded satisfactorily began graduated tapering of study drug, successively halving the number of doses per day at each step (steps were at days 14–20, days 21–24, and days 25–27). If further tapering was required after day 28, the study drug was to be discontinued and a suitable drug prescribed as deemed appropriate. All patients were observed until day 42.
Patients with increased IOP during the study were allowed an IOP-lowering agent at the discretion of their physician. Concomitant use of mydriatic or cycloplegic drops (administration to be separated from the study medication by at least 10 minutes) to alleviate photophobia, reduce ciliary spasm pain, or break up synechiae was permitted at the discretion of the investigator.
The study was conducted in accord with the tenets of the Declaration of Helsinki,18 the protocol was approved by all relevant institutional review boards or ethics committees, and all participants or their legal guardians provided written informed consent. The study was registered at clinicaltrials.gov as NCT01201798.
Patient Selection
Male or female patients 2 years or older with mild to moderate endogenous AU in at least one eye were eligible if the diagnosis was made within 2 weeks of study enrollment and they had at least 11 cells in the anterior chamber according to slitlamp microscopy plus a flare grade of 2 or higher in the eligible eye. Patients were excluded from the study if they had intermediate uveitis, posterior uveitis, panuveitis, corneal abrasion, ulceration, or any confirmed or suspected active viral, bacterial, or fungal keratoconjunctival disease in either eye. Other exclusion criteria included the following: pregnancy or lactation, allergy to other corticosteroids, history of corticosteroid-induced increased IOP, any corticosteroid depot within 6 weeks before start of study drug, known human immunodeficiency virus infection or other immunodeficiency conditions, periocular injection of any corticosteroid solution within 1 week before instillation of study drug, history of glaucoma or clinically significant ocular hypertension documenting an IOP of 21 mm Hg or higher in either eye, any introduction of topical corticosteroid or nonsteroidal anti-inflammatory drug in the eligible eyes within 7 days of study drug, and new administration or change in dosage of any corticosteroid or immunosuppressive drug (including inhaled, nasal, or dermatological corticosteroids) within 2 weeks before study enrollment. The use of contact lenses during the study was prohibited.
End Points and Assessments
The primary efficacy end point was the change from baseline to day 14 in anterior chamber cell grade. The secondary efficacy end points included the following: the mean change from baseline for anterior chamber cell grade and flare grade, as well as total symptom and sign score throughout the study; proportions of patients with anterior chamber cell count of 0, anterior chamber cell grades of 0 and 1 or lower, combined anterior chamber cell count of 5 or lower, and flare grade of 0 at all study visits; and discontinuations resulting from lack of efficacy (defined as treatment failure as assessed at the discretion of the investigator or as an adverse event [AE], with a preferred term of iridocyclitis, iritis, uveitis, or vitreitis). Supportive efficacy end points included QoL and optical coherence tomography (OCT) parameters. The safety end points were AEs, IOP, best-corrected visual acuity (BCVA), extent of exposure to study medication, ophthalmoscopic parameters (fundus assessment and ratio of cup to disc), and slitlamp parameters (lid margins, lids, cornea, sclera, lens, capsule, and conjunctiva).
Study assessments were made on eight visits, at baseline (day 0) and seven postbaseline visits (on days 3, 7, and then every 7 days thereafter through day 42). At each study visit, a slitlamp examination was conducted to assess anterior chamber cell count and grade (range, 0 for ≤1 cell to 4 for >50 cells),19 anterior chamber flare (range, 0 for none and 4 for severe), and ocular signs (range, 0 for absent to 3 for severe, for synechiae, peripheral anterior synechiae, hypopyon, keratic precipitates, and limbal injection). The BCVA, IOP, and evaluation of adherence with study medication were documented at each visit. Eye pain, photophobia, blurred vision, and lacrimation were assessed using a visual analog scale (VAS) (range, 0 for absent and 100 for maximal pain and discomfort). The QoL was assessed at baseline and day 42 using the National Eye Institute Visual Function Questionnaire 25 (VFQ-25) and the Work Limitations Questionnaire (WLQ). Optical coherence tomography was repeated only at days 14 and 42. Safety assessments were carried out at all study visits.
Statistical Analysis
The intent-to-treat population comprised all patients who received at least one dose of the allocated study medication. The per-protocol population included patients in the intent-to-treat population who had no major protocol deviation. Major protocol violations were violation of entry criteria, noncompliance (missing ≥24 hours of treatments), and the use of prohibited medications. The per-protocol analyses were performed with visit data excluded when affected by poor compliance or the use of prohibited medications. The per-protocol analyses were performed with last observation carried forward (LOCF) for missing data and for instances when study medication was discontinued or other medication was introduced to manage the condition. The per-protocol population with LOCF was the primary analysis data set for assessing efficacy. Both the per-protocol and intent-to-treat data sets were used for all secondary efficacy end points. Both data sets yielded similar results; for consistency,19 data from the per-protocol with LOCF analyses are reported herein, and results from the intent-to-treat analyses are included as Supplementary Material. The safety population comprised all patients who received at least one dose of the study medication.
Primary Efficacy Analysis.
To demonstrate noninferiority of difluprednate 0.05% compared with prednisolone acetate 1%, the upper boundary of a two-tailed 95% confidence interval for the difference in the mean change in anterior chamber cell grade from baseline to day 14 (difluprednate minus prednisolone acetate) must be less than the proposed margin of 0.5 U (10% of the five-unit scale). This noninferiority margin was selected based on its use in clinical trials of rimexolone.20 Analysis of covariance (ANCOVA), with treatment and investigative site as fixed effects and baseline score as a covariate, was used to compare the change from baseline of continuous variables between the difluprednate and prednisolone acetate groups. With 45 evaluable patients per arm and assuming a noninferiority margin of 0.50 and an SD of 0.75, a treatment difference of −0.07 would yield 94% power to demonstrate that difluprednate was noninferior to prednisolone acetate.19
Secondary Efficacy Analyses.
Statistical analyses for secondary efficacy outcomes used the same ANCOVA model as for the primary analysis for the mean change from baseline outcomes. χ2 test was used to compare proportions between treatment groups for the categorical secondary efficacy end points. Analyses were set to a 5% significance level and were two-sided for all tests.
Results
Patient Disposition and Demographics
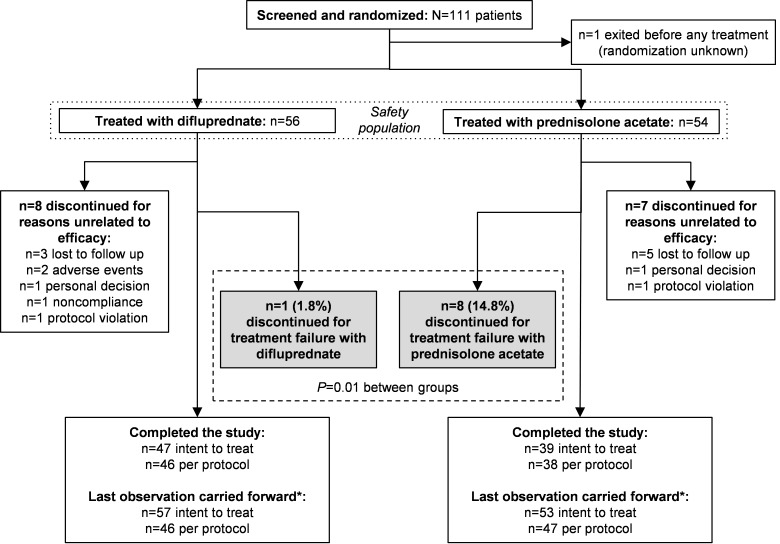
Of 111 patients randomized, 110 patients were treated with either difluprednate 0.05% or prednisolone acetate 1% and were included in the intent-to-treat and safety populations. One patient randomized to receive difluprednate was treated with prednisolone acetate. This patient was included in the difluprednate group for the intent-to-treat population and in the prednisolone acetate group for the safety population (Fig. 1). The per-protocol LOCF analyses included 46 patients and 47 patients receiving difluprednate and prednisolone acetate, respectively. In total, 9 of 56 patients (16.1%) and 15 of 54 patients (27.8%) in the respective groups discontinued study participation. The most common reason for study discontinuation was treatment failure, which was reported for one patient (1.8%) receiving difluprednate and 8 patients (14.8%) receiving prednisolone acetate (P = 0.013).
Figure 1.
Study flow diagram. *One patient was randomized to receive difluprednate 0.05% and was treated with prednisolone acetate 1%. This individual was included in the intent-to-treat population as randomized (difluprednate) and in the safety population as treated (prednisolone acetate) and was excluded from the per-protocol population.
Patient demographic and baseline characteristics were balanced between the two treatment groups (Table 1, Supplementary Table S1). The mean treatment durations were 27.0 days for the difluprednate group and 28.7 days for the prednisolone acetate group (P = 0.25). Baseline anterior chamber cell and flare grades were similar between the two groups.
Table 1.
Demographic Data and Baseline Characteristics (Per-Protocol Population*)
|
Variable |
Difluprednate 0.05%, n
= 46 |
Prednisolone Acetate 1%, n
= 47 |
Overall, N
= 93 |
| Age, y | |||
| Mean (SD) | 49.9 (15.3) | 46.2 (17.7) | 48.0 (16.6) |
| Minimum, maximum | 11, 87 | 12, 76 | 11, 87 |
| Age group, y, n (%) | |||
| 2–11 | 1 (2.2) | 0 | 1 (1.1) |
| 12–17 | 1 (2.2) | 1 (2.1) | 2 (2.2) |
| 18–64 | 39 (84.8) | 35 (74.5) | 74 (79.6) |
| ≥65 | 5 (10.9) | 11 (23.4) | 16 (17.2) |
| Race, n (%) | |||
| White | 28 (60.9) | 25 (53.2) | 53 (57.0) |
| Black | 15 (32.6) | 19 (40.4) | 34 (36.6) |
| Other | 3 (6.5) | 3 (6.4) | 6 (6.5) |
| Sex, n (%) | |||
| Female | 24 (52.2) | 27 (57.4) | 51 (54.8) |
| Treatment duration, d* | |||
| Mean (SD) | 27.0 (7.1) | 28.7 (8.3) | NA |
| Anterior chamber cell grade | |||
| Mean (SD) | 2.6 (0.7) | 2.6 (0.7) | NA |
| Anterior chamber flare grade | |||
| Mean (SD) | 2.2 (0.5) | 2.3 (0.5) | NA |
| VAS total score† | |||
| Mean (SD) | 186.7 (112.6) | 203.2 (110.8) | NA |
| BCVA, logMAR equivalent* | |||
| Mean (SD) | 0.22 (0.29) | 0.32 (0.40) | NA |
| OCT center thickness, μm | |||
| Mean (SD) | 199.8 (38.0) | 201.5 (38.9) | NA |
NA, not applicable.
Safety population was used for treatment duration and baseline BCVA for difluprednate 0.05% (n = 56) and prednisolone acetate 1% (n = 54).
Difluprednate 0.05% (n = 44) and prednisolone acetate 1% (n = 46). The total VAS symptom score was calculated as the sum of four symptom scores (eye pain, photophobia, blurred vision, and lacrimation). Each symptom score was graded by the patient according to a VAS (range, 0–100) using a mark on a 100-mm line (range, 0 for absent to 100 for maximal).
Mean Anterior Chamber Cell Grade Improvement According to the Primary and Secondary Efficacy End Points
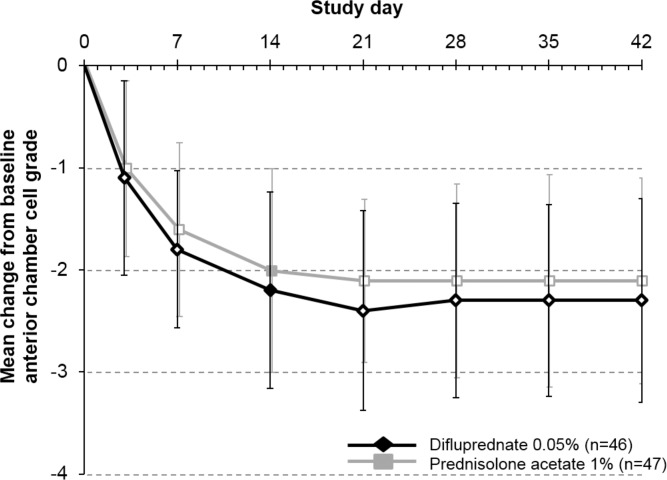
The mean (SD) changes in anterior chamber cell grade from baseline to day 14 were −2.2 (1.0) (a mean decrease of 2.2 grades) for the difluprednate group and −2.0 (1.0) (mean decrease, 2.0 grades) for the prednisolone acetate group (P = 0.16; mean difference, −0.22 [noninferior]). Given that the upper boundary of the 95% confidence interval (−0.53 to 0.09) was less than 0.5, the primary end point of difluprednate 0.05% dosed four times daily being noninferior to prednisolone acetate 1% dosed eight times daily for the treatment of endogenous AU was met. Similar improvements in anterior chamber cell grade were seen in both groups during the study period (Fig. 2, Supplementary Fig. S1).
Figure 2.
The mean change from baseline in anterior chamber cell grade in patients receiving difluprednate 0.05% dosed four times daily (n = 46) or prednisolone acetate 1% dosed eight times daily (n = 47) (per-protocol population with LOCF). Filled data labels represent the results for the primary efficacy end point. The mean (SD) changes in anterior chamber cell grade from baseline to day 14 were −2.2 (1.0) with difluprednate and −2.0 (1.0) with prednisolone acetate (P = 0.16; mean difference, −0.22 [favoring difluprednate]). Hollow data labels represent the secondary efficacy outcomes. Error bars denote SD. The dosing schedule does not include placebo doses, which were interspersed with difluprednate drops to maintain masking. Anterior chamber cell grade was based on a five-point scale ranging from 0 (≤1 cell) to 4 (≥50 cells).
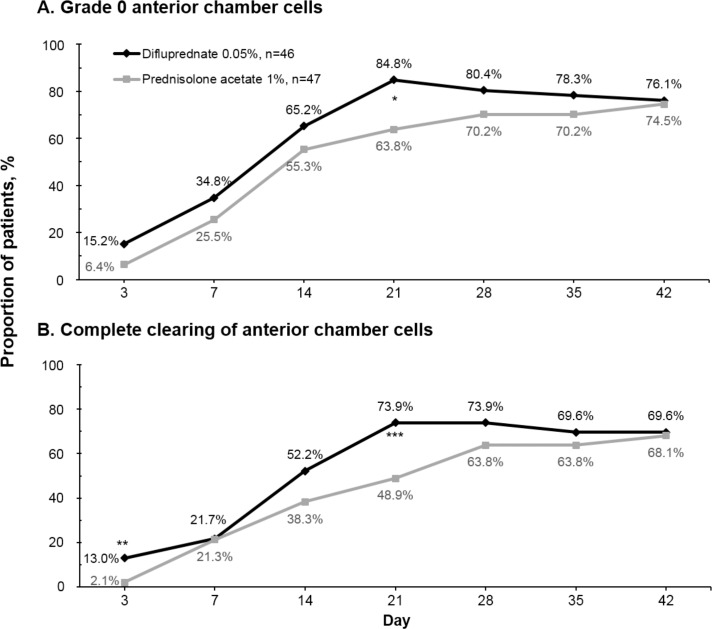
The proportions of difluprednate-treated patients versus prednisolone acetate–treated patients achieving grade 0 for anterior chamber cells were comparable at most study visits (P ≥ 0.17) except for the day 21 visit (84.8% for the difluprednate group and 63.8% for the prednisolone acetate group, P = 0.021) (Fig. 3A, Supplementary Fig. S2A). The proportions of patients whose anterior chamber cell grade improved to grade 1 or lower (better) were also similar for both treatment groups at all study visits (P ≥ 0.19).
Figure 3.
Proportion of patients achieving grade 0 anterior chamber cells (≤1 cell per high-power field) (A) and with complete clearing of anterior chamber cells (i.e., achieving zero cells) (B) during the study (per-protocol population with LOCF). *P = 0.021; **P = 0.046; ***P = 0.013.
Other Secondary Efficacy End Points
Anterior Chamber Cell Improvements.
The proportions of patients whose anterior chamber had been completely cleared of cells were comparable between the difluprednate group and the prednisolone acetate group at most study visits (P ≥ 0.18) (Fig. 3B, Supplementary Fig. S2B). The exceptions were for the visits at day 3 (13.0% vs. 2.1%, P = 0.046) and during the dose-tapering period at day 21 (73.9% vs. 48.9%, P = 0.013).
Anterior Chamber Flare.
Improvements in flare grades from baseline were seen in both groups from day 3 (mean change, −1.1 for the difluprednate group and −1.2 for the prednisolone acetate group) through day 14 (mean change, −2.0 for the difluprednate group and −1.9 for the prednisolone acetate group) and remained stable thereafter. The improvements seen in the two treatment groups were comparable at all visits (P ≥ 0.25). The proportions of patients who had both anterior chamber flare grade of 0 and cell count of 5 or lower were also similar between the difluprednate and prednisolone acetate groups, with 78.3% and 61.7% of patients achieving this status on day 14 and 80.4% and 78.7% on day 42, respectively (P ≥ 0.082 at all study visits).
Symptom and Sign Scores.
Improvements from baseline in VAS total symptom score for eye pain, photophobia, blurred vision, and lacrimation were observed in both treatment groups. At day 3, the mean change from baseline in total symptom score was −88.4 for both treatment groups. Further improvements were observed at each subsequent visit, with mean changes from baseline in total symptom score (eye pain, photophobia, blurred vision, and lacrimation) of −146.2 and −155.5 at day 42 for the difluprednate and prednisolone acetate groups, respectively (P = 0.71).
Supportive Efficacy End Points
On a scale of 0 to 100, the mean baseline VFQ-25 composite scores were 79.4 and 79.1 for the difluprednate and prednisolone acetate groups, respectively. At day 42, the mean improvement in the VFQ-25 composite score was 7.8 for the difluprednate group versus 5.5 for the prednisolone acetate group (P = 0.671) (subcategory data not shown). Similar improvements on the WLQ subscales (time management, physical, mental-interpersonal, and output) and in the loss index and the productivity loss score of the WLQ were also observed for both groups (P > 0.087) (Table 2, Supplementary Table S2). The mean foveal thickness and retinal volume according to OCT remained largely unchanged throughout the study in both groups.
Table 2.
Work Limitations Questionnaire QoL Measures (Per-Protocol Population With LOCF)*
|
Variable |
Difluprednate 0.05% |
Prednisolone Acetate 1% |
P
Value |
| Time management subscale score | |||
| Baseline, mean | 25.7 (n = 33) | 30.7 (n = 34) | |
| Follow-up, mean | 11.2 (n = 25) | 8.6 (n = 24) | |
| Mean change | −16.6 (n = 23) | −25.1 (n = 21) | 0.240 |
| Physical subscale score | |||
| Baseline, mean | 15.6 (n = 36) | 18.2 (n = 35) | |
| Follow-up, mean | 5.8 (n = 26) | 18.0 (n = 24) | |
| Mean change | −8.8 (n = 25) | 3.0 (n = 22) | 0.087 |
| Mental-interpersonal subscale score | |||
| Baseline, mean | 19.5 (n = 34) | 23.2 (n = 34) | |
| Follow-up, mean | 12.0 (n = 25) | 7.1 (n = 25) | |
| Mean change | −8.7 (n = 23) | −12.1 (n = 21) | 0.326 |
| Output subscale score | |||
| Baseline, mean | 17.6 (n = 34) | 23.8 (n = 34) | |
| Follow-up, mean | 12.3 (n = 25) | 8.8 (n = 25) | |
| Mean change | −4.1 (n = 23) | −12.1 (n = 21) | 0.312 |
| Loss index | |||
| Baseline, mean | 0.054 (n = 31) | 0.068 (n = 32) | |
| Follow-up, mean | 0.032 (n = 25) | 0.027 (n = 23) | |
| Mean change | −0.023 (n = 22) | −0.039 (n = 19) | 0.449 |
| Percentage productivity score | |||
| Baseline, mean | 5.11 (n = 31) | 6.33 (n = 32) | |
| Follow-up, mean | 3.03 (n = 25) | 2.65 (n = 23) | |
| Mean change | −2.20 (n = 22) | −3.61 (n = 19) | 0.476 |
The WLQ resulted in four composite subscores on time management, physical, mental-interpersonal, and output subscales. Subscale scores range from 0 (limited none of the time) to 100 (limited all of the time), and they indicate the percentage of time in the prior 2 weeks that the respondent was limited in performing the specific dimension of a job. The WLQ index is a weighted sum of scores from the four WLQ subscales. The WQL productivity loss score was based on the index score and indicates the percentage decrement in work output because of health problems.
Safety
Overall, 44 of 110 patients in the safety population reported 76 AEs, including 25 of 56 patients (44.6%) in the difluprednate group and 19 of 54 patients (35.2%) in the prednisolone acetate group. In both groups, most AEs were ocular related and were mild in intensity (Table 3). Eight AEs were deemed to be treatment related, including two cases of mild punctate keratitis (both in the difluprednate group), which resolved without treatment discontinuation, and six cases of elevated IOP (five mild cases in the difluprednate group and one moderate case in the prednisolone acetate group). Of five difluprednate-treated patients who reported the treatment-related AE of increased IOP, four had ophthalmic and medical histories indicating a predisposition for increased IOP. No patient discontinued difluprednate or prednisolone acetate as a result of increased IOP. A total of five AEs led to the discontinuation of study drug for two patients in the difluprednate group (nausea, asthenia, malaise, and sinusitis in one patient and necrotizing retinitis in the other). Of these, three AEs were nonserious, mild, and assessed as not related to treatment, and one AE was serious (necrotizing retinitis), severe, and also assessed as not related to treatment. One patient in the prednisolone acetate group reported an AE of moderate recurrent iritis; the patient discontinued the study medication and withdrew from study participation because of treatment failure. There were no deaths reported during the study. Two serious AEs were reported in two patients randomized to difluprednate treatment (one case of severe necrotizing retinitis and one case of moderate systemic hypertension), but neither event was assessed as related to the study drug. The event of severe necrotizing retinitis resolved with appropriate treatment, but unfortunately the patient had sustained loss of VA after resolution. The patient with systemic hypertension had a history of hypertension, and the AE was resolved without the patient discontinuing the study drug.
Table 3.
Adverse Events Occurring in More Than 3% in the Difluprednate 0.05% Group or in the Prednisolone Acetate 1% Group (Safety Population)*
|
Variable |
Patients,
n
(%) |
|
|
Difluprednate 0.05%, n
= 56 |
Prednisolone Acetate 1%, n
= 54 |
|
| General | ||
| Any AE | 25 (44.6) | 19 (35.2) |
| Any serious AE | 2 (3.6) | 0 |
| AE leading to withdrawal of study medication | 2 (3.6) | 1 (1.9)† |
| Death | 0 | 0 |
| Most common (>3%) AE in any group | ||
| Eye disorder | ||
| Iridocyclitis | 3 (5.4) | 2 (3.7) |
| Punctate keratitis | 3 (5.4) | 0 |
| Uveitis | 2 (3.6) | 1 (1.9) |
| Infections and infestations | ||
| Nasopharyngitis | 2 (3.5) | 1 (1.9) |
| Sinusitis | 2 (3.6) | 0 |
| Investigations | ||
| IOP increased | 5 (8.9) | 2 (3.7) |
| Nervous system disorders | ||
| Headache | 3 (5.4) | 4 (7.4) |
An individual reporting more than one event within a preferred term (according to codes used in version 13 of the Medical Dictionary for Regulatory Activities18) was counted only once. Ocular events in an untreated eye were excluded.
This patient reported iritis as an AE but discontinued the study because of treatment failure.
Visual Acuity.
Changes in BCVA from baseline were similar between the two treatment groups at all study visits (P ≥ 0.08). Losses of at least 0.3 logMAR from baseline were documented for the treated eyes of eight patients, five in the difluprednate group and three in the prednisolone acetate group. Six of these eight patients recovered vision (BCVA loss from baseline, <0.3 logMAR) during the study. Two patients reported sustained loss of VA during the study period, including a difluprednate-treated patient who experienced loss of VA resulting from necrotizing retinitis (presumably of viral etiology) and a prednisolone acetate–treated patient who reported VA loss and an AE of uveitis (in the nonstudy eye). These two patients were discontinued from the trial as a result of the AE (the patient with necrotizing retinitis) or for being lost to follow-up after the day 35 study visit (the patient with uveitis). A review of these data revealed no discernible trend toward a decrease in VA during the course of the clinical trial.
Intraocular Pressure.
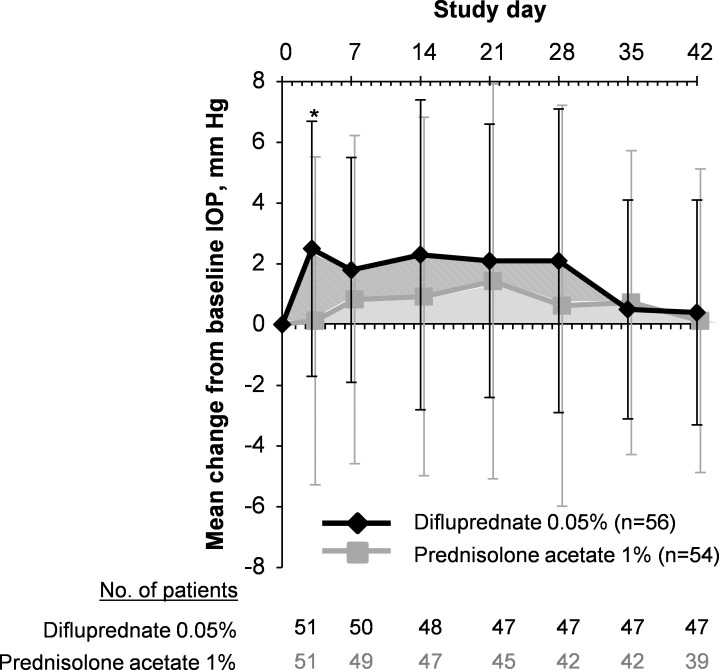
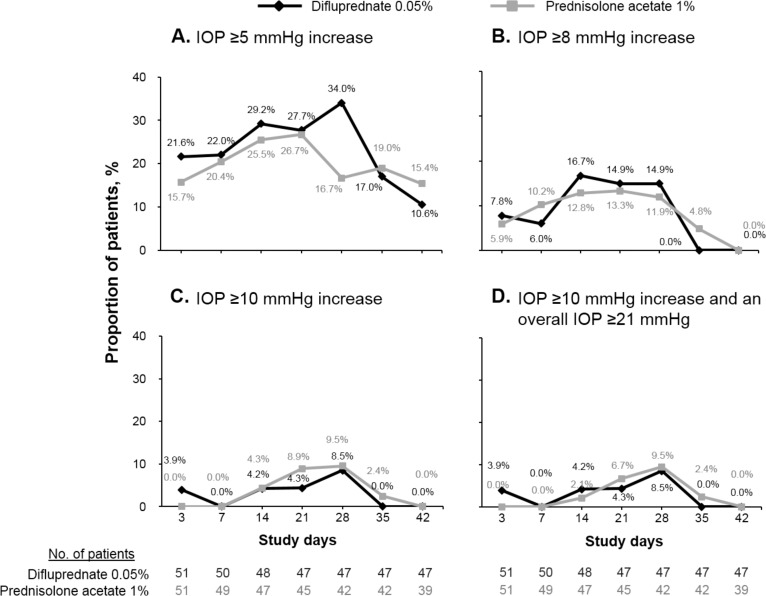
The mean IOP values in both treatment groups remained within normal limits (i.e., <21 mm Hg) throughout the study. At each study visit, the mean increases in IOP from baseline ranged from 0.5 to 2.3 mm Hg for the difluprednate group and from 0.1 to 1.4 mm Hg for the prednisolone acetate group. The mean IOP increases from baseline in the study eye were 2.5 mm Hg for difluprednate-treated patients and 0.1 mm Hg for prednisolone acetate–treated patients at day 3 (P = 0.0013) (Fig. 4); the mean values were not significantly different between groups at all other time points (P ≥ 0.055). Criterion IOP increase (defined as an IOP increase of ≥21 mm Hg and a change of ≥10 mm Hg greater than baseline at the same visit) was reported for nine patients (16.1%) in the difluprednate group and for six patients (11.1%) in the prednisolone acetate group (P ≥ 0.15 at all time points) (Fig. 5).
Figure 4.
Intraocular pressure change from baseline in study eyes (safety population). *P = 0.0013 (difluprednate 0.05% [diamonds] versus prednisolone acetate 1% [squares]). Error bars denote SD.
Figure 5.
Proportion of patients with increased IOP from baseline (safety population). (A) Increase of at least 5 mm Hg. (B) Increase of at least 8 mm Hg. (C) Increase of at least 10 mm Hg. (D) Increase of at least 10 mm Hg and an overall pressure of at least 21 mm Hg.
Other Safety Outcomes.
Overall, slitlamp and ophthalmoscopy data showed no discernible patterns suggesting changes in any other ocular signs parameter during the course of the study in either treatment group. The mean changes from baseline in the ratio of cup to disc were not significantly different between treatment groups at any time point (P ≥ 0.19 for all).
Discussion
The present study demonstrated that difluprednate 0.05% dosed four times daily was noninferior to prednisolone acetate 1% dosed eight times daily in improving the signs of acute endogenous AU. This conclusion is also supported by the secondary efficacy findings, which showed that difluprednate was associated with similar anterior chamber cell scores throughout the study compared with prednisolone acetate. Comparable improvements in anterior chamber flare grades, symptom and sign scores, QoL, OCT parameters, and BCVA outcomes were also seen in both treatment groups. These results suggested that difluprednate 0.05% four times daily was as effective as prednisolone acetate 1% eight times daily in the treatment of endogenous AU.
Consistent with the results from a previous similarly designed trial by Foster et al.,19 1.8% of patients in the difluprednate group discontinued the present study owing to treatment failure compared with 14.8% of patients in the prednisolone acetate group (P = 0.01 in both studies). This finding is important because patients not responding to topical treatment commonly require systemic corticosteroid therapy, which is associated with undesirable effects, including hyperglycemia21; furthermore, untreated or undertreated AU may lead to vision-threatening complications and blindness.1 However, it is acknowledged that treatment failure herein was assessed at the discretion of the investigator.
Ophthalmic medications with a less frequent dosing requirement have been associated with better compliance as demonstrated in therapies for glaucoma12,22 or allergic conjunctivitis.23 Similar benefits can be expected with difluprednate 0.05%, which requires dosing at a substantially lower frequency than prednisolone acetate 1%. The formulation differences between difluprednate and prednisolone acetate may also influence the effectiveness of the two drugs. Notably, prednisolone acetate is a suspension that requires shaking before use,11 whereas difluprednate emulsion does not.24 One study25 found that even with shaking (using a wrist-action mechanical shaker at six cycles per second) only 40% of the prednisolone acetate concentrations were within 15% of the declared concentration compared with 100% for the difluprednate concentrations. Furthermore, prednisolone acetate contains benzalkonium chloride, a preservative that has been associated with allergies, tear film instability, disruption of the corneal epithelium barrier, and damage to deeper ocular tissues in clinical or preclinical investigations.26 The sorbitol-based preservative in difluprednate has been shown to be less toxic to the corneal epithelium than benzalkonium chloride.27
Safety outcomes in the study were within expectations in accord with the package inserts for both study medications.11,24 Increases in the mean IOP occurred in both groups, which were expected19,28; for example, difluprednate treatment has been shown to be associated with IOP spikes 1 and 7 days after surgery in patients with uncomplicated postoperative cataract.28 Because inflammation involving the ciliary body may reduce aqueous secretion, resolution of inflammation tends to normalize ciliary function, restoring aqueous secretory capacity and leading to an increase in IOP.29 Differences between treatment groups in the mean change from baseline IOP were small in magnitude, but five patients in the difluprednate group reported an AE of IOP increase compared with three patients in the prednisolone acetate group. One hypothesis is that difluprednate may be associated with faster recovery of aqueous secretion than prednisolone acetate, thereby explaining at least early differences. It is reassuring that the potentially clinically important criterion increase in IOP (an increase of ≥10 mm Hg that yielded an IOP of ≥21 mm Hg) in this study was infrequent, with similar incidences reported for both groups at any time point. Nevertheless, judicious IOP monitoring with the use of topical corticosteroids (including difluprednate and prednisolone acetate) in patients with uveitis, particularly in those with a history of glaucoma, is recommended. When clinically indicated, prescription of an IOP-lowering topical medication may be appropriate.30,31 Long-term use of ophthalmic corticosteroids should be also avoided or minimized when possible because it may result in glaucoma, with damage to the optic nerve, visual field defects, VA loss, or cataract formation.11,24,32,33
The present study has several limitations. To maintain study masking, the difluprednate group received eight doses per day, four of which were placebo, which may not reflect the real-world use of difluprednate. The study did not include a dosing schedule of prednisolone acetate that is more frequent than every 2 hours, as is sometimes prescribed for severe cases. Owing to the study duration, this study did not include a remission end point, which is commonly defined as uveitis inactivity for a 90-day interval after discontinuing all treatments.3 Studies with a longer follow-up period may be useful in clarifying between-treatment differences in the duration of remission and other long-term outcomes. Another limitation of this study is that the causes of uveitis among patients, outside of the exclusion criteria, were not addressed. Furthermore, treatment failure was not defined as per protocol and was determined at the discretion of the investigator. Finally, given that the mean baseline anterior chamber cell grade was 2.6 out of a maximum of 4, the difference in the efficacy between difluprednate and prednisolone acetate in the most severe endogenous AU cases is unclear.
In summary, this randomized trial confirmed that difluprednate 0.05% dosed four times daily was noninferior to prednisolone acetate 1% dosed eight times daily for the treatment of endogenous AU during a 42-day observation period; both therapies had comparable safety profiles. Results from this study, together with those reported by Foster et al.,19 suggest that difluprednate is a reasonable alternative approach to prednisolone acetate for resolving and controlling ocular inflammation in patients with endogenous AU.
Acknowledgments
Medical writing funded by Alcon Research, Ltd., was provided by Magdalene Chu of DJE Science. Anthony Realini of Hypotony Holdings, LLC, provided writing assistance (funded by Alcon Research, Ltd.) on an early draft.
Supported by Alcon Research, Ltd., research funding from the National Institutes of Health and the Virginia Eye Foundation (JDS), investigator-initiated grants from the National Eye Institute and the Food and Drug Administration (JHK), and research funding from Research to Prevent Blindness and the Mackall Foundation (JHK).
Disclosure: J.D. Sheppard, 1-800-Doctors (I), Abbott (C), Abbvie (C), Alcon (C), Alimera (C), Allergan (C), Bausch & Lomb (C), EyeGate (I, C), EyeRx (I), Gerson Lehrman (C), Inspire (C), Ista (C), Lux Biosciences (C), Merck (C), Mimetogen (C), OcuCure (I), Omeros (C), Santen (C), SarCode (C), Science Based Health (C), TearLab (I, C), Vistakon (C); M.M. Toyos, Allergan (C, R), Alcon (F, C), Toyos Clinic (S), Ista (F, C), Bausch & Lomb (F, C), Inspire (C), Pfizer (F), Shire (F), iTherapeutix (F); J.H. Kempen, Alcon (C), Allergan (C), Clearside (C), Can-Fite (C), EyeGate (F), Lux Biosciences (C), Sanofi Pasteur (C), Xoma (F, C); P. Kaur, Alcon (F, E); C.S. Foster, Abbott (F, C), Alcon (F, C, R), Allergan (F, C, R), Bausch & Lomb (R), Eyegate (F, I), Inspire (R), Ista (C, R), Lux Biosciences (F, C, R), Novartis (C, F)
References
- 1. American Optometric Association. Optometric Clinical Practice Guideline: Care of the Patient With Anterior Uveitis. St. Louis, MO: American Optometric Association; 1994. Reviewed 2004. [Google Scholar]
- 2. Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004; 218: 223–236. [DOI] [PubMed] [Google Scholar]
- 3. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mercanti A, Parolini B, Bonora A, Lequaglie Q, Tomazzoli L. Epidemiology of endogenous uveitis in north-eastern Italy: analysis of 655 new cases. Acta Ophthalmol Scand. 2001; 79: 64–68. [DOI] [PubMed] [Google Scholar]
- 5. Ali A, Samson CM. Seronegative spondyloarthropathies and the eye. Curr Opin Ophthalmol. 2007; 18: 476–480. [DOI] [PubMed] [Google Scholar]
- 6. McCluskey PJ, Towler HM, Lightman S. Management of chronic uveitis. BMJ. 2000; 320: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004; 111: 2299–2306. [DOI] [PubMed] [Google Scholar]
- 8. Lardenoye CW., van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006; 113: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 9. Merck. The Merck manual for health care professionals: overview of uveitis. 2008. Available at: http://www.merckmanuals.com/professional/eye_disorders/uveitis_and_related_disorders/overview_of_uveitis.html. Accessed March 17, 2014. [Google Scholar]
- 10. Sen HN, Drye LT, Goldstein DA, et al. Hypotony in patients with uveitis: the Multicenter Uveitis Steroid Treatment (MUST) Trial. Ocul Immunol Inflamm. 2012; 20: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pred Forte (prednisolone acetate ophthalmic suspension, USP), 1% [package insert]. Irvine, CA: Allergan, Inc; 2004. [Google Scholar]
- 12. Vandenbroeck S, De Geest S, Dobbels F, Fieuws S, Stalmans I, Zeyen T. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO). J Glaucoma. 2011; 20: 414–421. [DOI] [PubMed] [Google Scholar]
- 13. American Academy of Ophthalmology. Young ophthalmologists: 10 clinical pearls for treating uveitis. 2013. Available at: http://www.aao.org/yo/newsletter/200808/article05.cfm. Accessed March 17, 2014. [Google Scholar]
- 14. Tajika T, Waki M, Tsuzuki M, Kida T, Sakaki H. Pharmacokinetic features of difluprednate ophthalmic emulsion in rabbits as determined by glucocorticoid receptor-binding bioassay. J Ocul Pharmacol Ther. 2011; 27: 29–34. [DOI] [PubMed] [Google Scholar]
- 15. Bodor N, Harget AJ, Phillips EW. Structure-activity relationships in the antiinflammatory steroids: a pattern-recognition approach. J Med Chem. 1983; 26: 318–328. [DOI] [PubMed] [Google Scholar]
- 16. Donnenfeld ED, Holland EJ, Solomon KD, et al. A multicenter randomized controlled fellow eye trial of pulse-dosed difluprednate 0.05% versus prednisolone acetate 1% in cataract surgery. Am J Ophthalmol. 2011; 152: 609–617. [DOI] [PubMed] [Google Scholar]
- 17. Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009; 35: 26–34. [DOI] [PubMed] [Google Scholar]
- 18. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. 2008. Available at: http://www.wma.net/en/30publications/10policies/b3/. Accessed March 17, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Foster CS, Davanzo R, Flynn TE, McLeod K, Vogel R, Crockett RS. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010; 26: 475–483. [DOI] [PubMed] [Google Scholar]
- 20. Biswas J, Ganeshbabu TM, Raghavendran SR, Raizada S, Mondkar SV, Madhavan HN. Efficacy and safety of 1% rimexolone versus 1% prednisolone acetate in the treatment of anterior uveitis: a randomized triple masked study. Int Ophthalmol. 2004; 25: 147–153. [DOI] [PubMed] [Google Scholar]
- 21. Udoetuk JD, Dai Y, Ying GS, et al. Risk of corticosteroid-induced hyperglycemia requiring medical therapy among patients with inflammatory eye diseases. Ophthalmology. 2012; 119: 1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995; 26: 233–236. [PubMed] [Google Scholar]
- 23. Abelson MB, Gomes PJ. Olopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol. 2008; 4: 453–461. [DOI] [PubMed] [Google Scholar]
- 24. Durezol (difluprednate ophthalmic emulsion 0.05%) [package insert]. Fort Worth, TX: Alcon Research Ltd.; 2012. [Google Scholar]
- 25. Stringer W, Bryant R. Dose uniformity of topical corticosteroid preparations: difluprednate ophthalmic emulsion 0.05% versus branded and generic prednisolone acetate ophthalmic suspension 1%. Clin Ophthalmol. 2010; 4: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29: 312–334. [DOI] [PubMed] [Google Scholar]
- 27. Tripathi BJ, Tripathi RC, Kolli SP. Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res. 1992; 9: 361–375. [PubMed] [Google Scholar]
- 28. Cable MM. Intraocular pressure spikes in phacoemulsification patients using difluprednate ophthalmic emulsion 0.05% postoperatively twice daily and once daily. 2011. Available at: http://ascrs2011.abstractsnet.com/acover.wcs?entryid=000027. Accessed March 17, 2014. [Google Scholar]
- 29. Tran VT, Mermoud A, Herbort CP. Appraisal and management of ocular hypotony and glaucoma associated with uveitis. Int Ophthalmol Clin. 2000; 40: 175–203. [DOI] [PubMed] [Google Scholar]
- 30. Meehan K, Vollmer L, Sowka J. Intraocular pressure elevation from topical difluprednate use. Optometry. 2010; 81: 658–662. [DOI] [PubMed] [Google Scholar]
- 31. Birnbaum AD, Jiang Y, Tessler HH, Goldstein DA. Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011; 129: 667–668. [DOI] [PubMed] [Google Scholar]
- 32. Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012; 1535: 932–938. [DOI] [PubMed] [Google Scholar]
- 33. Kurz PA, Chheda LV, Kurz DE. Effects of twice-daily topical difluprednate 0.05% emulsion in a child with pars planitis. Ocul Immunol Inflamm. 2011; 19: 84–85. [DOI] [PubMed] [Google Scholar]