Abstract
Among all nonpharmacological treatments, aerobic or resistance training (RT) has been indicated as a significantly important strategy to control hypertension. However, postexercise hypotension responses after intensity alterations in RT are not yet fully understood. The purpose of this study was to compare the outcomes of differing intensities of RT on hypertensive older women. Twenty hypertensive older women participated voluntarily in this study. After a maximum voluntary contraction test (one repetition maximum) and determination of 40% and 80% experimental loads, the protocol (3 sets/90″ interset rest) was performed in a single session with the following exercises: leg press, leg extension, leg curl, chest press, elbow flexion, elbow extension, upper back row, and abdominal flexion. Systolic and diastolic blood pressures were evaluated at rest, during exercise peak, and after 5, 10, 15, 30, 45, and 60 minutes of exercise and compared to the control. Both experimental loads were effective (P<0.01) in promoting postexercise systolic hypotension (mmHg) compared to controls, after 30, 45, and 60 minutes, respectively, at 40% (113±2, 112±4, and 110±3 mmHg) and 80% (111±3, 111±4, and 110±4 mmHg). Both procedures promoted hypotension with similar systolic blood pressures (40%: −11%±1.0% and 80%: −13%±0.5%), mean arterial blood pressures (40%: −12%±5.5% and 80%: −12%±3.4%), and rate-pressure products (40%: −15%±2.1% and 80%: −17%±2.4%) compared to control measures (systolic blood pressure: 1%±1%, mean arterial blood pressure:\ 0.6%±1.5%, rate-pressure product: 0.33%±1.1%). No differences were found in diastolic blood pressure and heart rate measures. In conclusion, hypertensive older women exhibit postexercise hypotension independently of exercise intensity without expressed cardiovascular overload during the session.
Keywords: resistive training, postexercise hypotension, aging, hypertension
Video abstract
Introduction
Currently hypertension had been associated with the development of cardiovascular disease1,2 and presents a strong relationship to a sedentary lifestyle, high sodium intake, high caloric intake, high alcohol consumption, and the ageing process.3,4
Today, in Brazil, approximately 17.6 million older people over the age of 60 years and approximately 85% present with at least one cardiovascular-associated disease.5 By 2025, this number will rise to more than 30 million people. Among these diseases, hypertension will be the most prevalent increasing progressively with age especially among women.6 It is estimated that hypertension affects approximately 55.3% of women between 60 and 74 years and 60.7% in those of more than 75 years.5
The first-line therapy to reduce the impact of hypertension on health is frequently drug therapy; however, lifestyle modifications that include decreasing sodium and fat in the diet, avoidance of smoking and alcohol consumption, and frequent participation in physical activity and/or exercise2,7–9 have been showed as important correlates in preventing hypertension. Among all nonpharmacological strategies to control hypertension, aerobic exercise modalities have been used most frequently to reduce resting blood pressure. Although less widely used, resistance training (RT) is becoming an important exercise modality to control blood pressure.2,7,10
Aging is often associated with reductions in muscle mass that can be contributed to the decline of muscular strength. RT is an exercise modality that imposes loads upon the skeleton and musculature, to training increases in body strength, muscle mass, and decreases in blood pressure have been noted in the eldery.11 Basic exercise guidelines recommended by the American College of Sports Medicine for healthy adults and older people emphasize that training programs consist of resistance, strength, aerobic, and flexibility exercises. In this perspective, the RT appears to be an important strategy to incrementally improve skeletal muscle function and reduce blood pressure.2,7,10,11
Among the major responses to physical exercise, independent of the modality, is postexercise hypotension (PEH). Significant importance has been given to PEH due to the important accompanying chronic adaptations and acute responses within the cardiovascular system. These changes have relevant clinical implications for hypertensive subjects indicating that PEH can act as an important nonpharmacological agent.12 PEH has been very studied in endurance exercises.12–15 It has seldom been explored in RT2,16,17 indicating a void of information with potential clinical significance. Some proposed mechanisms leading to the change in PEH have been attributed to reduced peripheral vascular resistance,18 reduced sympathetic activity,18,19 diminished systolic volume,18 and changes in the sensitivity of adrenergic cardiac and endothelial factors.20
PEH after RT has been observed in normotensive21 and hypertensive22 subjects. However, controversies still exist, especially in reference to exercise intensity. Studies have documented a reduction in blood pressure after sessions with the use of heavy loads,23,24 while others17,25 have shown no effective response in reducing blood pressure. One reason for this divergence is due to the fact of significant methodological variation related to volume, intensity, and the recovery interval used.26 Subject characteristics also affect outcomes as noted with variance in physical condition and trainability of the sample.27
Further, exercise intensity in training of hypertensive older people has been little studied. Low-intensity training has been considered efficient in reducing cardiac work and decreasing blood pressure1,21,28 with a consequent reduction in the risk of muscle damage. It has become the prevalent exercise intensity recommended.11,29 Interestingly and to the best of our knowledge, little is known about the effects of differing exercise intensities (resistance exercise load). In an effort to improve upon the lack of clarity surround postexercise PEH, the objective of this study was to investigate the influence of a RT session of different intensities on PEH in overweight older women with controlled H.
Materials and methods
Sample
After approval by the Institutional Research Ethics Committee (391.545), 30 overweight older women (65±3 years old) diagnosed hypertension controlled by antihypertensive medication and without experience in RT were recruited and participated voluntarily in this study. All subjects were physically independent, all specific medications were listed, and researchers utilized specifically exclusion criteria: clinical diagnosis of diabetes mellitus, current smoker status, diagnosed organ damage, musculoskeletal complications, and/or cardiovascular alterations (ST segment depression 1 mm, complex arrhythmias, or when ischemic symptoms) confirmed by physical testing. All procedures were performed according to the Declaration of Helsinki.
Procedures
Anthropometric measurements
Height was measured on a Cardiomed (WCS model) stadiometer, with an accuracy of 115/220 cm. The measurement was performed with the cursor at an angle of 90° with respect to scale, with the patient in the standing position with feet together, and while the subject made contact with the measuring instrument with the posterior surfaces of the heels, occipital bone, and scapula. The subjects were instructed to stay in inspiratory apnea with the top of the head parallel to the ground. Body mass was measured on a Filizola electronic scale (Personal Line Model 150) with a resolution of 100 g and a maximum capacity of 150 kg. Body fat percentage was derived from skinfold thickness, and the body mass index (BMI, kg/m2) was calculated according to the equation BMI = body mass/height.2 The classification of overweight (>30.0 kg/m2) was established according to the previous publication of our group30 utilizing the BMI for this determination.
Blood pressure and heart rate evaluations
Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial blood pressure (MBP) as calculated by the equation MBP = DBP + [SBP − DBP]/3 were measured before, during, and immediately after each RT training session, using an automated, noninvasive BP monitor (Microlife 3AC1-1PC, Microlife, Widnau, Switzerland) as previously described.31,32 Rate-pressure product (RPP) was calculated according to the following equation: RPP = HR × SBP. These measurements were performed after the subjects completed each set (a total of three). The objective of these measurements was to ensure that blood pressure and other indicators did not fall during the exercise session (thus indicating consistent intensity of effort). All SBP and DBP measurements were taken on the left arm. Individual cuffs were labeled to accommodate the ranges of arm circumferences within the subject group. Pre-exercise pressures did not exceed 160 and 100 mmHg for SBP and DBP, respectively. During exercise, HR was continuously measured and recorded on a beat-by-beat basis using a Polar Vantage NV (Polar Electro, Oulu, Finland) HR recorder. To evaluate the occurrence of PEH, SBP, DBP, and HR were also measured at rest, in exercise peak (immediately at the finish of the exercise session), and in the sitting position (resting) after 5, 10, 15, 30, 45, and 60 minutes of postexercise recovery. A control trial was also conducted with subjects seated for 100 minutes, the time necessary to perform the exercise session and PEH study.
One repetition maximum testing and exercise protocol
All subjects performed one repetition maximum (1RM) testing by repeating the methods previously published by our group.33 The following exercises were tested: leg press, leg extension, leg curl, chest press, elbow flexion, elbow extension, upper back row, and abdominal flexion. All exercises were performed on the same day with rest intervals of 5 minutes in between each. All tests were performed with the same examiner present and on the same equipment. The participants were instructed not to perform any other exercise during the period in which the experiment occurred. In each test session and between each exercise, 10 repetitions of the specific exercise were performed to warm-up specific muscles using 50% of the estimated load (approximating 20% and 40% of 1RM). Following a rest of 2–3 minutes, the test began. Four attempts were offered to reach 1RM, a widely accepted indicator of voluntary strength. In the first test set, the subjects were instructed to complete two repetitions. The second test set was performed after a 5-minute rest, with a greater or smaller load than that was applied in the previous test set. If the attempt was successful, weight was increased in the next set. If the attempt was unsuccessful, weight was reduced in the next set. This procedure was repeated during the third and fourth attempts to clearly identify the load corresponding to 1RM. The load corresponding to 1RM was defined as the weight with which the individual could only complete one correct repetition in a set. No more than five attempts were necessary to reach 1RM with any subject.
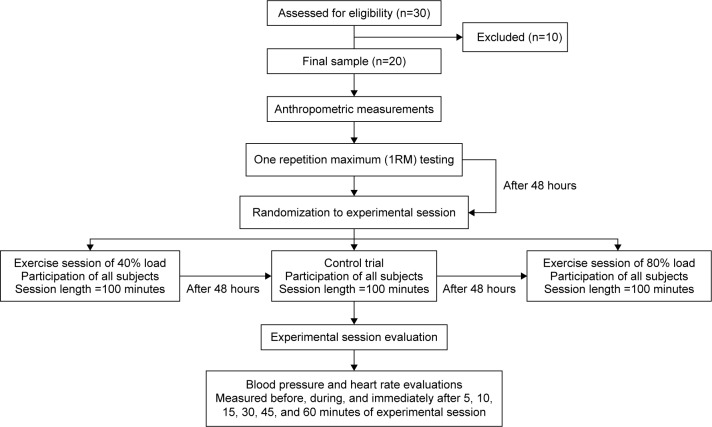
The three experimental sessions were randomized to implemented with subjects. The first session was conducted 48 hours after determining the load to be used in experimental session (control trial, 40% and 80% of 1RM load). Additionally, the experimental session interval was repeated 48 hours after last session conducted (shown in Figure 1). Conforming to previous publication,23 after 1RM testing and determination of loads corresponding to 40% (light load) and 80% (heavy load) of 1RM, subjects were released and told to return to the lab after 48 hours for the first randomly selected experimental exercise session using either the predetermined 40% or 80% loads and 10–12 repetitions.23,33 The order of exercise was leg press, leg extension, leg curl, chest press, elbow flexion, elbow extension, upper back row, and abdominal flexion. Sessions were completed in 40 minutes. Volunteers were also instructed to avoid the Valsalva maneuver during the entire movement, following American College of Sports Medicine guidelines. As the exercises were conducted on resistance machines, there was lumbar and thoracic support present, and the Valsalva was not obligatory for vertebral support and safety. After completion of the first exercise session, individuals were released, and after 48 hours, they returned to the laboratory to perform the remaining session(s). All exercise sessions were performed at the same time of day to minimize the effect of diurnal variation.
Figure 1.
Experimental design (flow diagram).
Statistical analyses
Statistical analyses were assessed by SPSS for Windows software (version 12.0, SPSS Inc., Chicago, IL, USA). The D’Agostino–Pearson test was applied to Gaussian distribution analysis. Analysis of comparisons between groups over the period was performed with two-way analysis of variance with repeated measures, followed by Kruskal–Wallis or Bonferroni’s posthoc test when appropriate. Statistical significance was established at P<0.05.
Results
Twenty women were included in the study. Ten women were excluded from the study for presence of one or more exclusion criteria.
Total work (TW) was calculated by multiplying the total repetitions (first set + second set + third set) by the workload (kg). In this way, the TW of light load protocol (11,856±6,690) was statistically different (P<0.0001) from heavy load (471,915±139,915) protocol, indicating that the light load group performed less work than the heavy load group.
The anthropometric parameters, resting hemodynamics, and medications used are presented in Table 1. No participant had or reported any discomfort during both exercise sessions.
Table 1.
Sample characteristics
| Variables | Results |
|---|---|
| Biometric parameters | |
| Age (years) | 65±3 |
| Weight (kg) | 76±7 |
| Height (m) | 1.58±2 |
| BMI (kg/m2) | 30±5 |
| % body fat | 30±7 |
| Fat mass (kg) | 22±5 |
| Lean mass (kg) | 54±5 |
| Baseline hemodynamic parameters | |
| SBP (mmHg) | 121±7 |
| DBP (mmHg) | 72±6 |
| MAP (mmHg) | 94±6 |
| HR (bpm) | 80±12 |
| HRP (mmHg × bpm) | 8,000±546 |
| Medicine | |
| Period of medicine (years) (mean ± SD) | 13±6 |
| Diuretics (%) | 60 |
| ACE inhibitors (%) | 85 |
| Calcium blocker (%) | 60 |
Note: Values expressed as the mean ± standard error deviation.
Abbreviations: ACE, angiotensin conversing enzyme; BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; HRP, heart rate product; SBP, systolic blood pressure; SD, standard deviation.
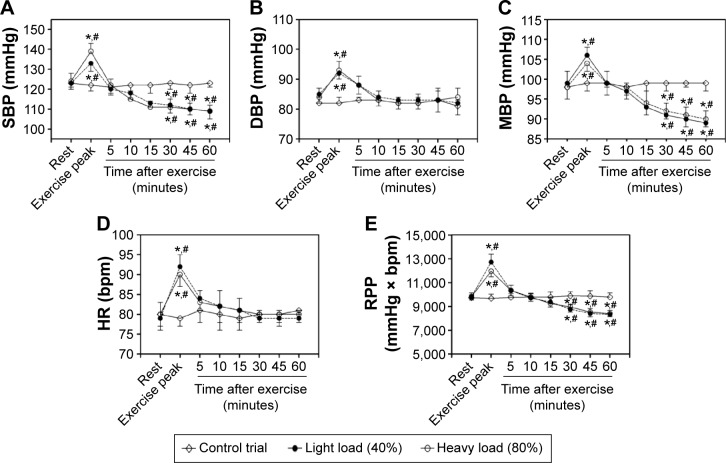
No differences were found between the control measures. However, significant differences were found between exercise conditions immediately after exercise, compared to C (P<0.01). Regarding both (40% and 80% load) training protocols, significant differences were found (P<0.001) between the values at rest and those immediately after the exercise session as illustrated in Figure 2. Differences (P<0.02) were found in SBP (rest: 123±3 mmHg and immediately after exercise: 133±4 mmHg), MBP (rest: 99±3 mmHg and immediately after exercise: 106±2 mmHg), HR (rest: 80±4 bpm and immediately after exercise: 90±3 bpm), and RPP (rest: 9,840±300 mmHg × bpm and immediately after exercise: 11,970±478 mmHg × bpm) at 40% 1RM.
Figure 2.
Values expressed as the mean ± standard error deviation of postexercise hypotension at control trial.
Notes: (A) Systolic blood pressure (SBP); (B) diastolic blood pressure (DBP); (C) mean blood pressure (MBP); (D) heart rate (HR); and (E) rate-pressure product (RPP). *P<0.01 indicates statistically significant differences to rest. #Indicates statistically significant differences to control trial.
Similar results were found at 80% SBP (rest: 124±4 mmHg and immediately after exercise: 139±4 mmHg), MBP (rest: 98±3 mmHg and immediately after exercise: 103±3 mmHg), HR (rest: 79±4 bpm and immediately after exercise: 92±3 bpm), and RPP (rest: 9,796±350 mmHg × bpm and immediately after exercise: 12,788±672 mmHg × bpm). Differences were found in the DBP to 40% (rest: 85±3 mmHg and immediately after exercise: 92±2 mmHg) and 80% of 1RM (rest: 84±3 mmHg and immediately after exercise: 93±3 mmHg).
When evaluating PEH, both protocols used in this study were effective in reducing (P<0.001) DBP after 5, 15, and 30 minutes, respectively (40%: 88±3 mmHg, 84±3 mmHg, and 83±3 mmHg and 80%: 88±3 mmHg, 83±3 mmHg, and 82±2 mmHg).
Regarding MAP, differences were found at all time-points for the 40% (5′: 99±3 mmHg, 10′: 97±2 mmHg, 15′: 95±3 mmHg, 30′: 93±4 mmHg, 45′: 93±4 mmHg, and 60′: 90±3 mmHg) and 80% (5′ 99±3 mmHg, 10′: 98±2 mmHg, 15′: 94±3 mmHg, 30′: 92±45 mmHg, 2′: 92±2 mmHg, and 60′: 91±3 mmHg) trials. Additionally, decreases of 10%±4% and 8%±3% were found when comparing resting values to both protocols (40% and 80% load), respectively.
In respect to SBP and RPP, there were significant differences (P<0.001) after 30, 45, and 60 minutes at 40% 1RM loads (SBP: 30′: 113±2 mmHg, 45′: 112±4 mmHg, and 60′: 110±3 mmHg and HRP: 30′: 9,153±298 mmHg × bpm, 45′: 8,960±423 mmHg × bpm, and 60′: 9,130±398 mmHg × bpm) and 80% 1RM loads (SBP: 30′: 111±3 mmHg, 45′: 111±4 mmHg, and 60′: 110±4 mmHg and HRP: 30′: 8,769±368 mmHg × bpm, 45′: 8,769±401 mmHg × bpm, and 60′: 9,020±397 mmHg × bpm). This corresponded to a decrease of 12%±3% in SBP and an 8%±5% reduction in RPP at 40%; in relationship to 80% 1RM loads, significant (P<0.001) decreases of 13%±2% in SBP and 9%±5% in RPP were found. An important piece of information found, after statistical analyses, was that there were no differences identified between exercise protocols. Additionally, no differences were found on DBP on PEH in relationship to rest to both loads.
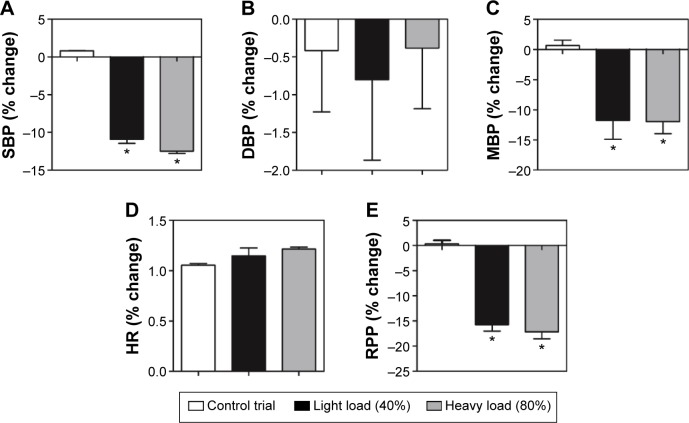
In addition, the hypotension perceptual effects after 60 minutes comparing to rest were analyzed between efforts exercise and around groups (Figure 3). The hypotensive effects were similar to effort at 40% (SBP: −11%±1.0%, MBP: 12%±5.5%, and RPP: 15%±2.1%) and 80% (SBP: 13%±0.5%, MBP: 12%±3.4%, and RPP: 17%±2.4%) but effective (P<0.01) as compared to control parameters (SBP: 1%±1%, MBP: 0.6%±1.5%, and RPP: 0.33%±1.1%). In relation to DBP (C: 0.41%±1.5%, 40%: 1%±1.8%, and 80%: 1%±0.80%) and HR (C: 1.0%±0.13%, 40%: 1.1%±0.13%, and 80%: 1.2%±0.10%), no differences were found around efforts.
Figure 3.
Relative change (%) expressed as the mean ± standard error deviation of systolic blood pressure (A), diastolic blood pressure (B), mean blood pressure (C), heart rate (D), and rate-pressure product (E) to control trial, light load (40%), and heavy load (80%).
Note: *P<0.01 indicates statistically significant differences to control.
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MBP, mean arterial blood pressure; RPP, rate-pressure product; SBP, systolic blood pressure.
Discussion
The blood pressure reduction after physical exercise has been considered a major nonpharmacological intervention for blood pressure control, especially beneficial in hypertensive subjects.14 The magnitude and duration of PEH are important factors to consider as indicators and controls of applied cardiovascular overload. Similarly, the work of MacDonald34 shows that the frequency of PEH after exertion has a chronic effect and beneficial on blood pressure at rest, a significant reduction compared to pre-exercise baseline measures.
Findings regarding the presence and timing of PEH are seemingly consistent. Hardy and Tucker28 found a reduction in SBP and DBP that persisted for at least 1 hour after a RT session. Fisher21 studied normotensive and hypertensive women after exercises at 50% of 1RM and found significant SBP reduction for 60 minutes. Our data were similar to these low-intensity resistance, showing significant SBP reductions to 60 minutes postexercise after low-intensity exercise (40% 1RM). The present study also demonstrated a significant reduction over the same duration after higher intensity exercise (80% 1RM). However, there did not appear to be a dose response as both intensities produced similar magnitudes of PEH. The absence of a dose response here supports the findings of Roltsh et al35 who have shown that PEH occurs regardless of the exercise methodology used.
Moraes et al36 also analyzed the hypotensive effect resistance exercise in 15 hypertensive middle-aged men. The protocol included 3×12 reps. with loads of 60% of 1RM. After 12 weeks of training, conventional RE significantly reduced SBP, DBP, and MBP, respectively, by an average of 16, 12, and 13 mmHg to prehypertensive values. In addition, the BP values remained stable during the 4-week detraining period in the study. Moreover, the authors point out that increased muscle strength is very important for hypertensive individuals because it may lead to less cardiovascular effort when there is need to mobilize a certain weight.
Although PEH is considered beneficial to hypertensive groups, they are not the only groups to respond to RT in the same manner. Among young people, reductions of SBP25 and DBP37,38 have been noted after RT sessions. To our knowledge, there is only one study29 that showed a reduction in SBP, DBP, and MBP for 60 minutes simultaneously.
The physiological mechanisms involved in post-RT decreases in BP were investigated in only two studies: one using individuals’ normotensive subjects23 and hypertensive subjects.16 These studies suggested that kallikrein release and reduction in peripheral vascular resistance with concomitant reduction in cardiac output are indicators involved in PEH. Thus, a plausible explanation for the reduction of BP after RT is that blood flow increases and shear stress act on vascular endothelial cells by activating a cascade of events leading to production of nitric oxide (an important vasodilator) arising from successive muscle contractions.39 This mechanism seems to occur independent of the intensity employed.10
Some studies have found contradictory results9,15,17 in relation to post-RT BP decrease. The inconsistency in experimental results can be linked to several variables relative to experimental design, such as BP measurement monitoring at differing times postexercise and use of different exercise parameters such as volume, intensity, rest between sets, and training status. However, management of hypertension in the elderly is an important concern, since it can be difficult to achieve a SBP of lower than 140 mmHg.40,41 Therefore, optimizing drug therapy through exercise is a relevant clinical issue treated this study. Data presented in Figure 1 show that the patients had a rest SBP less than 130 mmHg. These findings are in compliance with the recommended target range of American College of Cardiology/American Heart Association – SBP level <140 mmHg for older persons younger than 80 years.42 The main reported finding of this study is that resistance exercise induced a significant reduction in SBP. This result confirms previous research showing that resistance exercise can be used as a nonpharmacological antihypertensive approach.43 In this study, the blood pressure reduction dropped below resting values by up to 60 minutes postexercise. Although our study does not ensure that the induced hypotension persists as a chronic condition adaptation, the acute effect has important clinical implications. This issue has been recently documented by Moreira et al44 in which blood pressure response to a single resistance exercise bout was strongly associated to chronic hypotensive effects of a RT program in hypertensive elderly women. The main clinical implication of this study is how the magnitude of exercise load affects blood pressure level. We demonstrate that a resistance exercise bout with light load produced similar blood pressure reduction to heavy loads in this sample population. Thus, a high intensity workload appears not to be essential to reap the hypotensive effect of resistance exercise. This finding is particularly important for elderly hypertensive patients with contraindication to performing high-intensity exercise.
Our study does have some limitations that should be mentioned. First, the mechanisms of hypotension29 were not investigated in the present study. We did not assess the exercise effects on peripheral vascular resistance and sympathetic activity, systolic volume, beta-adrenergic receptors, or endothelial factors. Second, all subjects were prescribed antihypertensive medications by their personal physician. As such there was a lack of control over the drugs and doses used. It is conceivable that the variance in medications may have affected the outcomes of the experiment as not all antihypertensives work via the same biochemical mechanisms. A third limitation is the auscultation method used for assessing blood pressure. This method, while universally used, has limitations compared to invasive methods, such as intra-arterial catheterization. However, all safeguards were taken to ensure that these measures were obtained in a consistent, reliable, and accurate manner. The fourth issue that may have affected the outcome is the temporal distribution of measures. The data presented here demonstrate that RT at 40% and 80% loads is effective in reducing BP significantly for up to 60 minutes postexercise. The low and high intensities might not have had differences due to other durations of training.
Despite these few limitations, we propose that RT is safe for older hypertensive women undergoing treatment and participation in RT should be encouraged as hypertensive peaks (over 160 mmHg SBP) were not seen. This outcome meets relevant American College Sports Medicine recommendations. These findings are also notable in that the breadth of resistance load that produces PEH is quite large indicating that PEH is achievable as a consequence of virtually any RT program purpose and design.
Conclusion
In summary, the data presented in this study show that overweight older women with pharmacologically controlled hypertension exhibit a hypotensive response after participation in RT. Further, the cardiovascular overload during produced the session fell within recommended health and safety parameter, and this finding was independent of exercise intensity (40% or 80%). While these data are quite promising, more research involving hypertensive subjects of both sexes is necessary to confirm this hypothesis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Costa JBY, Gerage AM, Gonçalves CGS, Pina FLC, Polito MD. Influence of the training status on the blood pressure behavior after a resistance training session in hypertensive older females. Rev Bras Med Esporte. 2010;16(2):103–106. [Google Scholar]
- 2.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sport Exerc. 2004;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Hipertensão; Sociedade Brasileira de Nefrologia VI Brazilian Guidelines on Hypertension. Arq Bras Cardiol. 2010;95(4):553. [PubMed] [Google Scholar]
- 5.BRASIL. Ministério da Saúde . Política Nacional de Atenção Básica [National Policy of Primary Care] Brasília: Ministério da Saúde; 2012. Portuguese. [Google Scholar]
- 6.De Oliveira SMJV, Santos JLF, Lebrão ML, Duarte YAO, Pierin AMG. Reported hypertension in elderly women: prevalence and associated factors. Texto Contexto Enferm. 2008;17(2):241–249. [Google Scholar]
- 7.American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 8.American College of Sports Medicine Position Stand. Physical activity, physical fitness, and hypertension. Med Sci Sports Exerc. 1993;25(10):i–x. [PubMed] [Google Scholar]
- 9.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109(16):2031–2041. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 10.Bentes CM, Costa PB, Neto GR, et al. Hypotensive effects and performance responses between different resistance training intensities and exercise orders in apparently health women. Clin Physiol Funct Imaging. 2014 doi: 10.1111/cpf.12144. [DOI] [PubMed] [Google Scholar]
- 11.Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health. 2009;21(3):519–527. doi: 10.1177/0898264309332839. [DOI] [PubMed] [Google Scholar]
- 12.Casonatto J, Polito MD. Post-exercise hypotension: a systematic review. Rev Bras Med Esporte. 2009;15(2):151–157. [Google Scholar]
- 13.Brown SP, Clemons JM, He Q, Liu S. Effects of resistance exercise and cycling on recovery blood pressure. J Sports Sci. 1994;12(5):463–468. doi: 10.1080/02640419408732196. [DOI] [PubMed] [Google Scholar]
- 14.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sports Sci Rev. 2001;29(2):65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Polito MD, Simão R, Senna GW, Farinatti PTV. Hypotensive effects of resistance exercises performed at different intensities and same work volumes. Rev Bras Med Esporte. 2003;9(2):69–73. [Google Scholar]
- 16.Moraes MR, Bacurau RF, Ramalho JD, et al. Increase in kinins on post-exercise hypotension in normotensive and hypertensive volunteers. Biol Chem. 2007;388(5):533–540. doi: 10.1515/BC.2007.055. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor PJ, Bryant CX, Veltri JP, Gebhardt SM. State anxiety and ambulatory blood pressure following resistance exercise in females. Med Sci Sports Exerc. 1993;25(4):516–521. [PubMed] [Google Scholar]
- 18.Brandão Rondon UM, Alves MJ, Braga AM, et al. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39(4):676–682. doi: 10.1016/s0735-1097(01)01789-2. [DOI] [PubMed] [Google Scholar]
- 19.Kulics JM, Collins HL, DiCarlo SE. Postexercise hypotension is mediated by reductions in sympathetic nerve activity. Am J Physiol. 1999;276(1):H27–H32. doi: 10.1152/ajpheart.1999.276.1.H27. [DOI] [PubMed] [Google Scholar]
- 20.Eysmann SB, Gervino E, Vatner DE, Katz SE, Decker L, Douglas PS. Prolonged exercise alters beta-adrenergic responsiveness in healthy sedentary humans. J Appl Physiol. 1996;80(2):616–622. doi: 10.1152/jappl.1996.80.2.616. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MM. The effect of resistance exercise on recovery blood pressure in normotensive and borderline hypertensive women. J Strength Cond Res. 2001;15(2):210–216. [PubMed] [Google Scholar]
- 22.Melo CM, Alencar Filho AC, Tinucci T, Mion D, Jr, Forjaz CL. Postexercise hypotension induced by low-intensity resistance exercise in hypertensive women receiving captopril. Blood Press Monit. 2006;11(4):183–189. doi: 10.1097/01.mbp.0000218000.42710.91. [DOI] [PubMed] [Google Scholar]
- 23.Rezk CC, Marrache RC, Tinucci T, Mion D, Jr, Forjaz CL. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: influence of exercise intensity. Eur J Appl Physiol. 2006;98(1):105–112. doi: 10.1007/s00421-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 24.Simão R, Fleck SJ, Polito M, Monteiro W, Farinatti P. Effects of resistance training intensity, volume, and session format on the postexercise hypotensive response. J Strength Cond Res. 2005;19(4):853–858. doi: 10.1519/R-16494.1. [DOI] [PubMed] [Google Scholar]
- 25.Focht BC, Koltyn KF. Influence of resistance exercise of different intensities on state anxiety and blood pressure. Med Sci Sports Exerc. 1999;31(3):456–463. doi: 10.1097/00005768-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 26.de Matos DG, Aidar FJ, Manzini Filho ML, et al. Analysis of hemodynamic responses to resistance exercise performed with different intensities and recovery intervals. Health. 2013;5(2):159–165. [Google Scholar]
- 27.Tibana RA, Boullosa DA, Leicht AS, Prestes J. Women with metabolic syndrome present different autonomic modulation and blood pressure response to an acute resistance exercise session compared with women without metabolic syndrome. Clin Physiol Funct Imaging. 2013;33(5):364–372. doi: 10.1111/cpf.12038. [DOI] [PubMed] [Google Scholar]
- 28.Hardy DO, Tucker LA. The effects of a single bout of strength training on ambulatory blood pressure levels in 24 mildly hypertensive men. Am J Health Promot. 1998;13(2):69–72. doi: 10.4278/0890-1171-13.2.69. [DOI] [PubMed] [Google Scholar]
- 29.Brandão Rondon MU, Alves MJNN, Braga AMFW, et al. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39(4):676–682. doi: 10.1016/s0735-1097(01)01789-2. [DOI] [PubMed] [Google Scholar]
- 30.Bocalini DS, Lima LS, de Andrade S, et al. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin Interv Aging. 2012;7:551–556. doi: 10.2147/CIA.S33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topouchian JA, El Assaad MA, Orobinskaia LV, El Feghali RN, Asmar RG. Validation of two devices for self-measurement of brachial blood pressure according to the International Protocol of the European Society of Hypertension: the SEINEX SE-9400 and the Microlife BP 3AC1-1. Blood Press Monit. 2005;10(6):325–331. doi: 10.1097/00126097-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Olher Rdos R, Bocalini DS, Bacurau RF, et al. Isometric handgrip does not elicit cardiovascular overload or post-exercise hypotension in hypertensive older women. Clin Interv Aging. 2013;8:649–655. doi: 10.2147/CIA.S40560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bocalini DS, Portes LA, Ribeiro KJ, et al. Insight for learning and stability of one repetition maximum test in subjects with or without experience on resistance training. Gazzeta Medica Italiana. Archivio Per Le Scienze Mediche. 2013;172(11):845–851. [Google Scholar]
- 34.MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16(4):225–236. doi: 10.1038/sj.jhh.1001377. [DOI] [PubMed] [Google Scholar]
- 35.Roltsh MH, Mendez T, Wilund KR, Hagberg JM. Acute resistive exercise does not affect ambulatory blood pressure in young men and women. Med Sci Sports Exerc. 2001;33(6):881–886. doi: 10.1097/00005768-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Moraes MR, Bacurau RF, Simões HG, et al. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J Hum Hypertens. 2012;26(9):533–539. doi: 10.1038/jhh.2011.67. [DOI] [PubMed] [Google Scholar]
- 37.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 38.Saccomani MG, Casonatto J, Christofaro D, et al. Impact of circuit strength training on blood pressure in adolescents. Rev SOCERJ. 2008;21(5):305–310. [Google Scholar]
- 39.Goto C, Higashi Y, Kimura M, et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108(5):530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 40.Fagard RH, Van Den Enden M, Leeman M, Warling X. Survey on treatment of hypertension and implementation of World Health Organization/International Society of Hypertension risk stratification in primary care in Belgium. J Hypertens. 2002;20(7):1297–1302. doi: 10.1097/00004872-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Mancia G, Grassi G. Systolic and diastolic blood pressure control in antihypertensive drug trials. J Hypertens. 2002;20(8):1461–1464. doi: 10.1097/00004872-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Bielecka-Dabrowa A, Aronow WS, Rysz J, Banach M. The rise and fall of hypertension: lessons learned from Eastern Europe. Curr Cardiovasc Risk Rep. 2011;5(2):174–179. doi: 10.1007/s12170-010-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brito Ade F, de Oliveira CV, Brasileiro-Santos Mdo S, Santos Ada C. Resistance exercise with different volumes: blood pressure response and forearm blood flow in the hypertensive elderly. Clin Interv Aging. 2014;9:2151–2158. doi: 10.2147/CIA.S53441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreira SR, Cucato GG, Terra DF, Ritti-Dias RM. Acute blood pressure changes are related to chronic effects of resistance exercise in medicated hypertensives elderly women. Clin Physiol Funct Imaging. 2014 doi: 10.1111/cpf.12221. [DOI] [PubMed] [Google Scholar]