Abstract
Background and aims
In the last decade, the development of different methods of brain stimulation by electromagnetic fields (EMF) provides a promising therapeutic tool for subjects with impaired cognitive functions. Emisymmetric bilateral stimulation (EBS) is a novel and innovative EMF brain stimulation, whose working principle is to introduce very weak noise-like stimuli through EMF to trigger self-arrangements in the cortex of treated subjects, thereby improving cognitive faculties. The aim of this pilot study was to investigate in patients with cognitive impairment the effectiveness of EBS treatment with respect to global cognitive function, episodic memory, and executive functions.
Methods
Fourteen patients with cognitive decline (six with mild cognitive impairment and eight with Alzheimer’s disease) underwent three EBS applications per week to both the cerebral cortex and auricular-specific sites for a total of 5 weeks. At baseline, after 2 weeks and 5 weeks, a neuropsychological assessment was performed through mini–mental state examination, free and cued selective reminding tests, and trail making test. As secondary outcomes, changes in behavior, functionality, and quality of life were also evaluated.
Results
After 5 weeks of standardized EBS therapy, significant improvements were observed in all neurocognitive assessments. Mini–mental state examination score significantly increased from baseline to end treatment (+3.19, P=0.002). Assessment of episodic memory showed an improvement both in immediate and delayed recalls (immediate recall =+7.57, P=0.003; delayed recall =+4.78, P<0.001). Executive functions significantly improved from baseline to end stimulation (trail making test A −53.35 seconds; P=0.001). Of note, behavioral disorders assessed through neuropsychiatric inventory significantly decreased (−28.78, P<0.001). The analysis concerning the Alzheimer’s disease and mild cognitive impairment group confirmed a significant improvement of cognitive functions and behavior after EBS treatment.
Conclusion
This pilot study has shown EBS to be a promising, effective, and safe tool to treat cognitive impairment, in addition to the drugs presently available. Further investigations and controlled clinical trials are warranted.
Keywords: pulsed electromagnetic fields, cognitive decline, Alzheimer’s disease, Emisymmetric bilateral stimulation
Introduction
The increasing mean age of the population, and as a consequence the aging-related incidence of neurocognitive disorders such as dementia, highlights the need for new, highly effective, therapeutic strategies.
Alzheimer’s disease (AD) is the most prevalent cause of dementia in the elderly, and it is defined both by its clinical features and by its unique pathology. It increases dramatically in both prevalence and incidence after the age of 65, and this doubles approximately every 5 years in individuals between 65 and 95 years of age.1 Based on the pathological findings of loss of cholinergic transmission,2 AD is routinely treated with cholinesterase inhibitors.3 Despite recent advances, currently available drugs for dementia are mostly ineffective against the progression of cognitive and behavioral disorders related to dementia.4,5 This inability to effectively manage disease progression prompts researchers to develop new therapeutic strategies.
In the past few years, the development of different methods of brain stimulation by electromagnetic fields (EMF) has provided a promising therapeutic tool, with potentially beneficial effects on subjects with impaired cognitive functions.6
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are the most widespread noninvasive methods of EMF brain stimulation. Recently, studies revealed that both these neurostimulation techniques might enhance cognitive performances in healthy subjects and patients with AD through modulating cortical excitability and inducing long-lasting neuroplastic changes.7,8
rTMS is based on the principle of electromagnetic induction of an electric field in the brain, which can be of sufficient magnitude and density to modulate cortical excitability. This has been demonstrated to have behavioral consequences and therapeutic potential.9,10
Recent data show that also tDCS is able to induce cognitive changes, specifically improving the memory function in AD patients.11,12 Indeed, it has been shown in these patients that both a single anodal tDCS session, which improved recognition memory, and the application of tDCS over the temporal cortex daily for 5 days brought a memory improvement lasting 4 weeks after neurostimulation therapy ended.12
It has been proposed that the pathophysiological basis of the beneficial effect of EMF in neurodegenerative disorders is connected to several interrelated mechanisms of action, including increased amyloid clearance, neuronal activity, and cerebral blood flow.7 Therefore, EMF exposure could represent a noninvasive therapeutic option against cognitive impairment and could be used as a memory-enhancing approach in the general population.
In this study, we investigate the clinical effects of Emi-symmetric bilateral stimulation (EBS Elkmed 2060), a novel and innovative EMF brain stimulation.
EBS differs from rTMS and tDCS as the power densities used in therapy are much lower (below 100 nW/cm2, where nW =10−9 W). EBS is, in fact, different than the conventional EMF approach and adopts low-power stimulations to cover a wide range of frequency bands, shapes, and durations of pulses of the EMF. The core topic of this study is the utilization of pulsed EMF noise-like stimuli to trigger self-arrangements in the cortex of treated subjects and improve brain faculties. Such a self-organization ability does indeed find its physical basis in quantum field theory interpretation of biological systems: all the stimuli allowed penetrating in, traveling through, and participating in the living dynamics are strictly bound to local supramolecular conditions, in turn depending on the macrostate. This is true because of biological coherence.13–16 Hence, it is interesting to examine what happens relying on a wide broad range in stimulation and on a biological self-selective responsivity, jointly.
In this open-label study, we aim to investigate the cognitive effects of this innovative neurostimulation with pulsed EMF, using EBS in patients with cognitive decline. We investigate the effectiveness of EBS treatment with respect to global cognitive function, episodic memory, and executive functions, as they are the most affected cognitive domains in neurodegenerative dementia.
As a secondary outcome of the study, we aim to describe any changes in behavior, muscular strength, functional status, and quality of life (QoL).
Materials and methods
Study participants
Patients with a clinical diagnosis of cognitive decline were recruited at the Santa Margherita Hospital in Pavia (Italy).
Among them, nine patients were diagnosed as having mild-to-moderate AD according to the National Institute of Neurological and Communicative Disorders and Stroke-The Alzheimer’s Disease and Related Disorders Association Criteria17,18 and the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). According to DSM-IV, to be diagnosed with AD, subjects need to fulfill the following criteria: memory impairment, one or more of the following cognitive disturbances (aphasia, apraxia, agnosia, impaired executive functioning), cognitive deficits causing severe impairment in social and occupational functioning, a gradually progressive disease course, and cognitive deficits not due to other neurologic (cerebrovascular disease, Parkinson’s disease, Huntington’s disease, subdural hematoma, normal pressure hydrocephalus, brain tumor) or systemic conditions (hypothyroidism, vitamin B or folic acid deficiency, niacin deficiency, hypercalcemia, neurosyphilis, HIV infection, substance abuse).
The remaining six patients met the diagnostic criteria for mild cognitive impairment (MCI) and were included in the study.19 They were comprehensively assessed by the Rey Complex figure,20 and were shown to have normal general cognitive functioning as assessed by the Raven’s Colored Progressive Matrices21 and tests of short-term memory, naming, and frontal executive functions; a Clinical Dementia Rating score below 0.5;22 no or minimal impairment in activities of daily living (ADL) as assessed by the instrumental activities of daily living (IADL) scale and by the ADL scale; and absence of dementia (score >24 on mini–mental state examination [MMSE]).23
All patients were native Italian speakers and underwent a detailed clinical and neurological evaluation. All patients affected by AD were receiving cholinesterase inhibitor (donepezil hydrochloride or rivastigmine tartrate) therapy and memantine according to standard international guidelines referring to specific cognitive decline. None of the MCI individuals were under cognitive-specific treatments.
For each patient, a structural brain MRI was taken to exclude major causes of cerebrovascular disease and white matter lesions.
Patients with potentially confounding neurological and psychiatric disorders, clinically known hearing or vision impairment, or a history of alcohol abuse, psychosis, or major depression were not included in the study. None of the subjects had implanted metal objects, pacemakers, or a history of seizures. These exclusion criteria were based on the performance of a safe stimulation consensus.9
This open-label study was performed according to the Declaration of Helsinki and was approved by the local institutional review board. Patients and their caregivers signed informed consent before participation.
Experimental protocol
Three applications per week to both the cerebral cortex and specific auricular sites were performed for a total of 5 weeks to the 14 patients included in the study. All subjects enrolled received both stimulations. Side effects and adverse events were regularly assessed during the whole study.
The primary end point of our study was to determine changes in global cognitive function and specific cognitive domains after 5 consecutive weeks (t2) of standardized EBS treatment.
As a part of general comprehensive assessment, we established the following secondary end points: behavioral disorders, functional status, muscular strength, QoL.
Besides, to consider the effectiveness of EBS treatments to changes in specific cognitive impairment, a statistical analysis regarding changes in cognitive functions in patients with AD and MCI from baseline (t0) to end treatment (t2) was performed.
Emisymmetric bilateral stimulation
Each EBS treatment (Elkmed 2060) consists of a stimulation (approximately 20–25 minutes long) during which the patient is exposed to extremely weak EMF (powers in range, 10–100 nW), emitted by two sources: an in-air source placed in the front part of the machine (1–1.5 m away from the patient); and another consisting of a helmet shaped aluminum conductor sheet placed on the patient’s head, connected by four clamps that convey the signal from the machine. Another modality for local stimulation is given by conductive terminal emitters applied to specific points on the skin (typically, acupuncture points according to auricular points by Nogier). The in-air source at a distance of 1 m generates electromagnetic power densities in the range of 50–100 nW/cm2; local emitters generate even lower power densities, between 0.5 and 50 nW/cm2, depending on the terminal emitter used. Owing to the impedance setting associated with the human body at that range of frequency and to emitter conformation, the signal transferred is almost fully capacitive. Substantially, we get an oscillating electric field, whose conformation is in the near-field range.
Electromagnetic signals are pulsed at a frequency that can be varied by preset programs. The carrier wave peaks at 10.5 GHz. Pulsation consists of an amplitude modulation at total index (m =1) and is square shaped so that the carrier wave is switched on/off at a very high rate.
The purpose in designing the EBS apparatus was to obtain the largest frequency band possible at extremely low powers in the emission. So, a square wave intermodulation has been chosen: theoretically it produces an infinite number of harmonics (under- and overtones). The emission from each source together with the production of very low frequency spectral components (below 1 kHz) creates complex interference patterns within the cells and neurons. This is the ideal condition in order to obtain extremely low power electromagnetic noise spread over a wide frequency band, able to stimulate self-feeding, and reenhance traveling wave packets, whose role is involved in biocommunication and homeostasis.23,24
Cognitive assessment and clinical and comprehensive examination
A baseline comprehensive evaluation was performed on admission. A neuropsychological protocol was performed by a trained psychogeriatrist and comprised the following assessments: 1) MMSE for overall multidomains cognitive function and25 2) Free and Cued Reminding Selective Test (FCRST) for episodic memory impairment, including selective reminding and coordinated controlled learning and cued recall.26 The four measures being evaluated here include immediate recall (IR – the cumulative sum of immediate free and cued recall in the three trials; range 0–36), total delayed recall (TDR) (the cumulative sum of delayed free and cued recall, range 0–12), and their relative equivalent scores (relative equivalent free recall – REFR, and relative equivalent delayed recall, – REDR; range 0–4);27 3) trail-making state (TMS), both test A and test B, for information on visual search, scanning, speed of processing, mental flexibility, and executive functions.28
Presence of psychological and behavioral disorders related to dementia was screened through UCLA Neuropsychiatric Inventory (NPI).29 Behavioral disorders represent an open issue in aging care as presence of behavioral disorders related to dementia decreases QoL of both patient and caregivers and increases need for professional care.
Functionality and mobility were determined through a complete battery of physical assessments: 1) Barthel index for evaluating the dependence in ADL;30 2) short physical performance battery status (SPPB) for global mobility;31 3) handgrip performance for muscular strength.32 Handgrip strength (in kilograms) was measured using a dynamometer (Smedley Hand Dynamometer, Stoelting Co, Wood Dale, IL, USA). The mean score of three measures in the dominant hand was used in the analysis.33
QoL perceived by patients was assessed through the administration of short form-12 (SF-12) Health Survey, measuring both physical and mental domains.34
Clinical and comprehensive evaluation was performed by the same trained geriatrist on admission before first EBS treatment (t0), after 2 weeks of EBS treatment (t1), and at the end of the 5-week long standardized EBS treatment (t2).
Statistical analysis
Data are expressed as mean ± standard deviation for continuous variables. Two-tailed paired t-tests were used to assess the differences in cognitive, functional, and QoL markers between individuals at baseline (t0) and after 5 weeks (t2) of treatment (t2–t0), and are presented as mean differences with 95% confidence intervals. Nonnormally distributed data were checked by Shapiro–Wilk test and log transformed for parametric statistics.
Furthermore, a stratified analysis considering the two groups, AD (eight patients) and MCI (six patients), was performed. After accounting for the small stratum sample size, nonparametric Wilcoxon signed ranks tests were used to assess the differences in cognitive, functional, and QoL markers between individuals at baseline (t0) and after 5 weeks (t2) of treatment (t2–t0).
Thus, linear mixed model (LMM)35 for repeated measures was applied in order to assess the differences in cognitive, functional, and QoL markers, among individuals across weeks. For each outcome, we fit an LMM where time (weeks), the dichotomous cognitive condition (ie, AD or MCI), and their interaction term were “focus” predictors, while age and sex were the adjustment covariates. Because of small sample size, to retain degrees of freedom, we carried out a model selection procedure, using a backward strategy by removing the nonsignificant adjustment covariates. A random effect was used to adjust the models for intrasubject variability produced by three separate measurements (baseline, at 2 and 5 weeks) carried out on same patients (n=14 ×3=42 observations but only 14 independents). The LMM time parameters were interpreted as adjusted week changes (Δ/week), while the interaction parameters as adjusted time difference between dichotomous cognitive conditions (MCI represented the reference category). The parameters associated to dichotomous cognitive condition were interpreted as baseline differences between the two clinical conditions. t-tests (95% confidence interval) were carried out in order to evaluate statistical significance on model parameters. P-values <0.05 were considered significant. Finally, a Pearson correlation analysis was used to assess the relationships among cognitive, functional, and QoL marker changes after 5 weeks (t2–t0) in the overall sample.
The analysis was performed on R 3.0.3 using the R/nlme36 and R/stats packages.37
Results
Thirty-four patients were enrolled, and 16 were found eligible. Of these patients, one refused to participate, and the remaining 15 were recruited (Figure 1). All patients fulfilled the diagnostic criteria for probable AD or MCI. All 15 patients tolerated EBS therapy, and none of them reported any adverse effects. Only one patient with AD left the study because of a hospital discharge. Patient compliance to treatment remained very high throughout the study: participation in 100% of the treatment sessions was registered.
Figure 1.
Flow diagram of the study.
Baseline population demographic and clinical data are shown in Table 1. Mean age was 79.93±6.23 years, mean MMSE score was 22.03±4.53, and average value of NPI was 34.93±17.39. Both episodic memory and executive functions assessed respectively through FCRST and trial making state (TMT) were, on average, impaired (Table 1). With regard to functional profile, the mean Barthel was 82.21±8.95 and ADL, 4.36±0.74.
Table 1.
Statistics across weeks in the overall sample
| n=14 (5 males, age =79.93±6.23 years) | Baseline (t0) mean ± SD | After 2 weeks (t1) mean ± SD | After 5 weeks (t2) mean ± SD | P-value paired t-tests (t2 – t0) |
|---|---|---|---|---|
| Primary outcomes | ||||
| Cognitive functions measurements | ||||
| MMSE | 22.03±4.53 | 24.51±4.46 | 25.22±4.51 | 0.002 |
| Free recall | 14.04±6.48 | 21.81±9.38 | 24.17±11.40 | <0.001 |
| Relative equivalent free recall | 0.43±0.65 | 2.143±1.56 | 2.5±1.70 | <0.001 |
| Immediate recall (free + cued recall) | 21.00±9.50 | 27.21±9.42 | 28.57±10.10 | 0.003 |
| Intrusions at immediate recall | 2.57±0.94 | 1.929±1.00 | 1.43±1.16 | 0.003* |
| Delayed recall | 2.36±2.13 | 5.863±3.70 | 7.15±4.16 | <0.001 |
| Relative equivalent delayed recall | 0.14±0.53 | 1.071±1.49 | 1.93±1.77 | 0.002 |
| Total delayed recall (free + cued recall) | 3.21±3.68 | 6.00±4.57 | 6.93±4.58 | 0.003* |
| Intrusions at delayed recall | 1.92±1.07 | 1.643±1.01 | 0.79±0.70 | 0.007 |
| Trail-making state A (seconds) | 203.4±65.4 | 155.3±52.9 | 150.0±61.7 | 0.001 |
| Secondary outcomes | ||||
| Dementia-related behavioral markers | ||||
| NPI | 34.93±17.39 | 10.43±9.12 | 6.14±6.15 | <0.001 |
| Functional measurements | ||||
| ADL | 4.36±0.74 | 4.714±0.61 | 4.86±0.77 | 0.047 |
| Barthel | 82.21±8.95 | 85.5±8.43 | 86.07±10.37 | 0.209 |
| Hand grip (right) | 17.36±2.90 | 18.05±3.05 | 18.54±2.94 | 0.002 |
| Hand grip (left) | 16.07±3.19 | 16.56±3.19 | 17.09±3.11 | <0.001 |
| SPPB | 6.64±1.39 | 7.07±1.21 | 7.07±1.21 | 0.028 |
| Quality of life variables | ||||
| SF-12 PHS | 27.86±4.17 | 34.77±4.62 | 41.69±5.07 | <0.001 |
| SF-12 MHS | 31.25±4.88 | 40.67±6.86 | 50.08±8.88 | <0.001 |
Note: In bold, the statistically significant evidences (P<0.05).
Log-transformed variables.
Abbreviations: SD, standard deviation; MMSE, mini–mental state examination; NPI, neuropsychiatric inventory; ADL, activities of daily living; SPPB, short physical performance battery status; SF-12 PHS, short form-12 physical health status; SF-12 M HS, short form-12 mental health status.
Other baseline clinical measures, such as handgrip, SPPB, SF-12 MHS (mental health status), and SF-12 PHS (physical health status) are shown in Table 1.
Primary outcomes
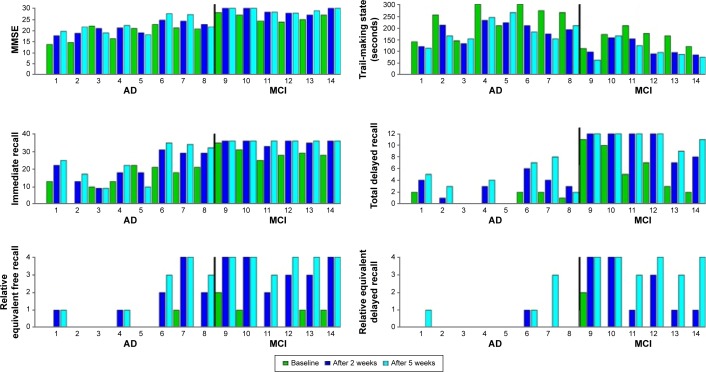
Figure 2 shows all the individual values regarding the primary cognitive outcomes across weeks and divided into cognitive condition (AD and MCI patients).
Figure 2.
Individual values of primary outcomes during protocol treatment.
Note: The black vertical lines in the figures separate the bars of the AD group from MCI group.
Abbreviations: MMSE, mini–mental state examination; AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Concerning cognitive marker changes, it has been found that there is a significant increase in MMSE (+3.19, P=0.002), in immediate and delayed recall (IR: +7.57, P=0.003; DR: +4.79, P<0.001), and relative equivalent free and delayed scores (REFR: +2.07, P<0.001; REDR: +1.79, P=0.002). Table 1 reports mean values across 5 weeks and relative paired t-test significances for all the considered variables in all patients.
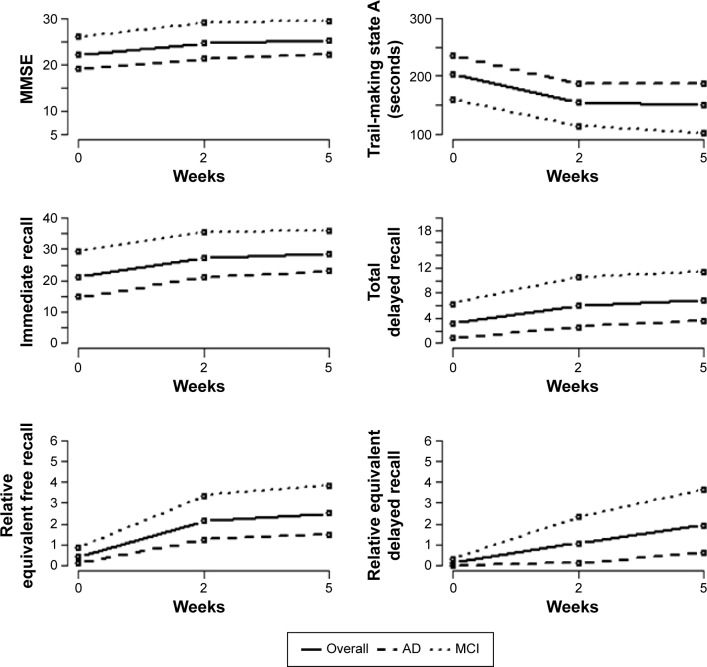
Thus, executive functions significantly improved from baseline after 5-week EBS treatment, as shown by the decrease observed in TMS test A score (−53.36 seconds, P=0.001) (Table 1 and Figure 3).
Figure 3.
Mean plots of primary outcomes during protocol across weeks.
Abbreviations: MMSE, mini–mental state examination; AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Table 2 shows the mean values (± standard deviation) of primary outcome across weeks in AD and MCI group. In particular, all cognitive scores increased in both the AD and MCI group, whereas most significant improvements were remarkably observed in MCI patients.
Table 2.
Statistics over the time period of the study by clinical cognitive condition (AD and MCI)
| Variable | AD (n=8, 2 males, age = 78.12± 6.96 years)
|
MCI (n=6, 3 males, age =82.83 ±4.59 years)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (t0) mean ± SD | After 2 weeks (t1)mean ± SD | After 5 weeks (t2) mean ± SD | P-value Wilcoxon signed rank test (t2–t0) | Baseline (t0) mean ± SD | After 2 weeks (t1)mean ± SD | After 5 weeks (t2) mean ± SD | P-value Wilcoxon signed rank test (t2–t0) | |
| Primary outcomes | ||||||||
| Cognitive functions measurements | ||||||||
| MMSE | 19.07±3.58 | 21.2±2.59 | 22.20±3.57 | 0.072 | 25.97±1.68 | 28.92±1.26 | 29.25±0.89 | 0.031 |
| Free recall | 10.67±6.13 | 16.42±8.55 | 18.04±11.51 | 0.093 | 18.55±3.73 | 29.01±4.20 | 32.34±3.42 | 0.031 |
| Relative equivalent free recall | 0.13±0.35 | 1.25±1.39 | 1.5±1.60 | 0.063 | 0.83±0.75 | 3.33±0.82 | 3.83±0.41 | 0.031 |
| Immediate recall (free + cued recall) | 14.75±7.44 | 21.12±8.06 | 23±10.36 | 0.086 | 29.33±3.39 | 35.33±1.21 | 36±0 | 0.031 |
| Intrusions at immediate recall | 2.88±0.64 | 2.25±0.71 | 2.13±0.99 | 0.148 | 2.17±1.17 | 1.5±1.22 | 0.5±0.55 | 0.031 |
| Delayed recall | 1.24±1.08 | 3.244±2.12 | 4.24±2.96 | 0.031 | 3.86±2.35 | 9.35±1.93 | 11.02±1.09 | 0.031 |
| Relative equivalent delayed recall | 0±0 | 0.125±0.36 | 0.63±1.06 | 0.250 | 0.33±0.82 | 2.33±1.51 | 3.67±0.52 | 0.031 |
| Total delayed recall (free + cued recall) | 0.88±0.99 | 2.625±2.13 | 3.63±2.97 | 0.031 | 6.33±3.67 | 10.5±2.35 | 11.33±1.211 | 0.031 |
| Intrusions at delayed recall | 1.50±0.53 | 1.875±0.83 | 1.13±0.64 | 0.375 | 2.50±1.38 | 1.33±1.21 | 0.33±0.52 | 0.031 |
| Trail-making state A (seconds) | 236.0±64.3 | 187.0±41.5 | 186.4±50.5 | 0.078 | 159.8±36.9 | 113.0±33.2 | 101.5±37.5 | 0.031 |
| Secondary outcomes | ||||||||
| Dementia-related behavioral markers | ||||||||
| NPI | 43.62±16.05 | 15.25±9.13 | 8.5±6.82 | 0.008 | 23.33±11.98 | 4±3.58 | 3.0±3.52 | 0.063 |
| Functional measurements | ||||||||
| ADL | 4.63±0.52 | 4.63±0.74 | 4.75±0.89 | 1 | 4.00±0.89 | 4.83±0.41 | 5±0.63 | 0.063 |
| Barthel | 84.4±8.65 | 86.12±9.13 | 84.12±12.21 | 1 | 79.33±9.27 | 84.67±8.16 | 88.67±7.53 | 0.063 |
| Hand grip (right) | 16.68±2.85 | 16.99±2.58 | 17.39±2.47 | 0.187 | 18.28±2.96 | 19.47±3.26 | 20.07±2.98 | 0.031 |
| Hand grip (left) | 15.50±2.38 | 16.07±2.32 | 16.20±2.31 | 0.047 | 16.83±4.16 | 17.20±4.25 | 18.27±3.84 | 0.031 |
| SPPB | 7.25±1.28 | 7.50±1.31 | 7.50±1.31 | 0.625 | 5.833±1.17 | 6.50±0.84 | 6.50±0.84 | 0.125 |
| Quality of life variables | ||||||||
| SF-12 PHS | 28.64±5.15 | 35.32±6.05 | 42.0±6.95 | 0.016 | 26.83±2.40 | 34.08±2.13 | 41.33±1.86 | 0.031 |
| SF-12 MHS | 29.06±3.88 | 37.25±6.04 | 45.43±8.20 | 0.016 | 34.17±4.79 | 44.84±5.68 | 55.50±6.57 | 0.031 |
Note: In bold, the statistically significant evidences (P<0.05).
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; SD, standard deviation; MMSE, mini–mental state examination; NPI, neuropsychiatric inventory; ADL, activities of daily living; SPPB, short physical performance battery status; SF-12 PHS, short form-12 physical health status; SF-12 MHS, short form-12 mental health status.
Table 3 reports LMM analysis results. Notably, all parameters (Δ/week), except TDR (P=0.087), were statistically significant. Remarkably, MMSE weekly increased by 0.613 (P=0.006), REFR and REDR by 0.565 (P<0.001) and 0.649 (P<0.001), and the TMS test A score decreased by 11.05 seconds (P=0.004).
Table 3.
Linear mixed models
| Variable (n=14×3=42 observations) | Δ/week P-value 95% CI |
dccb P-value 95% CI |
Time × dcc interaction P-value 95% CI |
|---|---|---|---|
| Primary outcomes | |||
| Cognitive functions measurements | |||
| MMSE | 0.613 | −7.192 | −0.011 |
| 0.006 | <0.001 | 0.966 | |
| 0.191; 1.036 | −10.186; −4.198 | −0.571; 0.548 | |
| Free recall | 2.629 | −9.790 | −1.168 |
| <0.001 | 0.026 | 0.068 | |
| 1.673; 3.586 | −18.194; −1.385 | −2.430; 0.093 | |
| Relative equivalent free recall | 0.565 | −1.029 | −0.302 |
| <0.001 | 0.084 | 0.023 | |
| 0.370; 0.762 | −2.217; 0.159 | −0.559; −0.044 | |
| Immediate recall (free + cued recall) | 1.245 | −15.088 | 0.356 |
| 0.018 | <0.001 | 0.588 | |
| 0.233; 2.259 | −22.460; −7.716 | −0.978; 1.691 | |
| Intrusions at immediate recalla | −0.356 | 0.555 | 0.272 |
| <0.001 | 0.227 | 0.006 | |
| −0.500; −0.213 | −0.391; 1.502 | 0.083; 0.461 | |
| Delayed recall | 1.364 | −3.468 | −0.768 |
| <0.001 | 0.007 | 0.006 | |
| 0.960; 1.769 | −5.791; −1.145 | −1.300; −0.236 | |
| Relative equivalent delayed recall | 0.649 | −0.641 | −0.521 |
| <0.001 | 0.131 | <0.001 | |
| 0.480; 0.818 | −1.502; 0.219 | −0.744; −0.299 | |
| Total delayed recalla (free + cued recall) | 0.137 | −2.316 | 0.120 |
| 0.087 | 0.005 | 0.249 | |
| −0.021; 0.297 | −3.782; −0.850 | −0.090; 0.331 | |
| Intrusions at delayed recall | −0.425 | −0.548 | 0.321 |
| <0.001 | 0.242 | 0.005 | |
| −0.587; −0.264 | −1.513; 0.417 | 0.108; 0.534 | |
| Trail-making state A (seconds) | −11.048 | 73.936 | 1.890 |
| 0.004 | 0.011 | 0.689 | |
| −18.293; −3.804 | 20.268; 127.605 | −7.693; 11.474 | |
| Secondary outcomes | |||
| Dementia-related behavioral markers | |||
| NPI | −3.771 | 19.058 | −2.876 |
| 0.004 | 0.006 | 0.075 | |
| −6.182; −1.361 | 6.606; 31.510 | −6.065; 0.313 | |
| Functional measurements | |||
| ADL | 0.188 | 0.434 | −0.162 |
| 0.002 | 0.257 | 0.033 | |
| 0.076; 0.301 | −0.361; 1.230 | −0.311; −0.014 | |
| Barthel | 1.824 | 4.088 | −1.861 |
| 0.005 | 0.408 | 0.024 | |
| 0.616; 3.033 | −6.241; 14.418 | −3.455; −0.267 | |
| Hand grip (right)c | 0.344 | −0.848 | −0.204 |
| <0.001 | 0.499 | 0.054 | |
| 0.187; 0.501 | −3.496; 1.801 | −0.411; 0.004 | |
| Hand grip (left)c | 0.292 | −0.167 | −0.160 |
| <0.001 | 0.911 | 0.058 | |
| 0.167; 0.417 | −3.397; 3.063 | −0.325; 0.006 | |
| SPPB | 0.123 | 1.393 | −0.078 |
| 0.011 | 0.036 | 0.197 | |
| 0.031; 0.215 | 0.102; 2.683 | −0.200; 0.043 | |
| Quality of life variables | |||
| SF-12 PHS | 2.900 | 1.804 | −0.283 |
| <0.001 | 0.496 | 0.586 | |
| 2.078; 3.722 | −3.790; 7.398 | −1.396; 0.829 | |
| SF-12 MHS | 4.266 | −5.104 | −1.023 |
| <0.001 | 0.142 | 0.150 | |
| 3.190; 5.343 | −12.178; 1.970 | −2.480; 0.434 | |
Notes: In bold, the statistically significant evidences (P<0.05).
Log-transformed variables;
dichotomous cognitive condition (MCI was the reference category);
estimates adjusted for sex.
Abbreviations: CI, confidence interval; dcc, dichotomous cognitive condition; MMSE, mini–mental state examination; NPI, neuropsychiatric inventory; ADL, activities of daily living; SPPB, short physical performance battery status; SF-12 PHS, short form-12 physical health status; SF-12 MHS, short form-12 mental health status; MCI, mild cognitive impairment.
Finally, the weekly improvements were lower in the AD than in the MCI group. In particular, this dynamic is highlighted in REFR, REDR, and DR changes, where improvement reductions are equal to 0.302 (P=0.023), 0.521 (P<0.001), and 0.768 (P=0.006), respectively. Table 2 and Figure 3 show the significant interaction effect in primary outcomes.
In the model selection procedure, age and sex were deleted from the predictor set because they were nonsignificant.
Secondary outcomes
Table 1 shows mean values in secondary outcomes across weeks. NPI average scores significantly decreased by 28.79 at the end of 5 weeks (P<0.001).
After 5-weeks EBS treatment, measures regarding functional outcomes were found to be improved. Handgrip scores are significantly increased from baseline (right: +1.18 kg, P=0.002, left: +1.02 kg, P<0.001). Significant mean changes from baseline were observed in other functional measures (ADL: +0.5, P=0.047; SPPB: +0.43, P=0.028). Finally, the assessments of QoL evaluated by SF-12 showed significant improvements in both physical and mental domains (SF-12 PHS: +13.61, P<0.001; SF-12 MHS: +18.5; P<0.001).
Table 2 shows the mean values (± standard deviation) of secondary outcomes in the time points, divided into AD and MCI group.
Table 3 reports LMM analysis results. Notably, all parameters (Δ/week) were significant. Remarkably, the NPI score decreased weekly by 3.771 (P=0.004). In the model selection procedure, the age variable was deleted from predictor set because it was nonsignificant; whereas the sex variable was retained for handgrip measures because they turned out to be a significant parameter.
Post hoc power analysis
A post hoc power analysis (1 minus Type II error probability) on MMSE mean difference across 5 weeks gave a result equal to 0.68: the power has been computed on MMSE mean difference equal to 3.193 (from the overall sample), considering a type I error probability equal to 0.05 and a standard deviation of MMSE equal to 4.53.
Pearson correlation analysis
Table 4 reports Pearson correlation (ρ) analysis results of changes (t2–t0) of cognitive, behavioral, and functional markers in the overall samples: MMSE improvements are positively and significantly associated to recall ones, such as free recall (ρ =0.64, P=0.013), IR (ρ =0.86, P<0.001), and total delayed recall (ρ =0.68, P=0.008). In addition, the MMSE improvements are associated also with TMSA improvements (ρ =−0.76, P=0.002) and with functional ones, such as right handgrip (ρ =0.70, P=0.005), Barthel index (ρ =0.66, P=0.011), and ADL (ρ =0.58, P=0.028). These findings are explained in the “Discussion” section.
Table 4.
Pearson correlations between changes (t2–t0) following 5-week EBS treatment
| MMSE | FR | REFR | IR | Int IRa | DR | REDR | TDRa | Int DR | TMSA | NPI | ADL | Barthel | r-HG | l-HG | SPPB | SF-12PHS | SF-12MHS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | 1 | 0.64 | 0.30 | 0.86 | −0.20 | 0.50 | 0.24 | 0.68 | −0.33 | −0.76 | 0.08 | 0.58 | 0.66 | 0.70 | 0.34 | 0.37 | 0.44 | 0.16 |
| (P=0.013) | (0.305) | (<0.001) | (0.498) | (0.067) | (0.412) | (0.008) | (0.246) | (0.002) | (0.793) | (0.028) | (0.011) | (0.005) | (0.235) | (0.196) | (0.130) | (0.604) | ||
| FR | 1 | 0.81 | 0.76 | −0.55 | 0.68 | 0.62 | 0.14 | −0.49 | −0.79 | 0.46 | 0.65 | 0.72 | 0.81 | 0.64 | 0.69 | 0.51 | 0.58 | |
| (<0.001) | (0.002) | (0.041) | (0.008) | (0.018) | (0.633) | (0.075) | (<0.001) | (0.097) | (0.011) | (0.003) | (<0.001) | (0.014) | (0.007) | (0.078) | (0.040) | |||
| REFR | 1 | 0.38 | −0.58 | 0.65 | 0.77 | −0.22 | −0.57 | −0.56 | 0.61 | 0.42 | 0.53 | 0.50 | 0.49 | 0.48 | 0.49 | 0.62 | ||
| (0.186) | (0.030) | (0.011) | (0.001) | (0.459) | (0.033) | (0.039) | (0.020) | (0.132) | (0.054) | (0.066) | (0.073) | (0.083) | (0.089) | (0.024) | ||||
| IR | 1 | −0.08 | 0.37 | 0.15 | 0.57 | −0.22 | −0.85 | −0.04 | 0.44 | 0.54 | 0.68 | 0.31 | 0.57 | 0.46 | 0.34 | |||
| (0.785) | (0.198) | (0.610) | (0.034) | (0.443) | (<0.001) | (0.899) | (0.118) | (0.048) | (0.008) | (0.273) | (0.032) | (0.110) | (0.262) | |||||
| Int IRa | 1 | −0.60 | −0.69 | 0.16 | 0.44 | 0.20 | −0.57 | −0.48 | −0.45 | −0.56 | −0.50 | −0.43 | −0.30 | −0.57 | ||||
| (0.024) | (0.006) | (0.574) | (0.115) | (0.493) | (0.033) | (0.086) | (0.110) | (0.038) | (0.071) | (0.124) | (0.318) | (0.043) | ||||||
| DR | 1 | 0.86 | 0.05 | −0.85 | −0.33 | 0.38 | 0.75 | 0.61 | 0.74 | 0.66 | 0.24 | 0.41 | 0.41 | |||||
| (<0.001) | (0.858) | (<0.001) | (0.251) | (0.179) | (0.002) | (0.021) | (0.002) | (0.011) | (0.416) | (0.168) | (0.162) | |||||||
| REDR | 1 | −0.25 | −0.73 | −0.28 | 0.52 | 0.46 | 0.37 | 0.52 | 0.42 | 0.23 | 0.24 | 0.42 | ||||||
| (0.391) | (0.003) | (0.324) | (0.060) | (0.100) | (0.194) | (0.057) | (0.138) | (0.421) | (0.422) | (0.157) | ||||||||
| TDRa | 1 | 0.00 | −0.44 | −0.26 | 0.36 | 0.37 | 0.39 | −0.12 | 0.18 | 0.41 | −0.08 | |||||||
| (0.996) | (0.116) | (0.375) | (0.213) | (0.196) | (0.164) | (0.677) | (0.543) | (0.165) | (0.791) | |||||||||
| IntDR | 1 | 0.22 | −0.46 | −0.67 | −0.61 | −0.69 | −0.59 | −0.28 | −0.46 | −0.53 | ||||||||
| (0.451) | (0.097) | (0.009) | (0.021) | (0.007) | (0.027) | (0.338) | (0.118) | (0.062) | ||||||||||
| TMSA | 1 | −0.26 | −0.42 | −0.57 | −0.61 | −0.25 | −0.64 | −0.61 | −0.24 | |||||||||
| (0.371) | (0.132) | (0.035) | (0.021) | (0.380) | (0.014) | (0.027) | (0.439) | |||||||||||
| NPI | 1 | 0.43 | 0.62 | 0.43 | 0.53 | 0.37 | 0.06 | 0.30 | ||||||||||
| (0.127) | (0.018) | (0.125) | (0.054) | (0.187) | (0.842) | (0.313) | ||||||||||||
| ADL | 1 | 0.90 | 0.85 | 0.79 | 0.42 | 0.53 | 0.29 | |||||||||||
| (<0.001) | (<0.001) | (<0.001) | (0.137) | (0.059) | (0.340) | |||||||||||||
| Barthel | 1 | 0.85 | 0.75 | 0.57 | 0.57 | 0.47 | ||||||||||||
| (<0.001) | (0.002) | (0.035) | (0.041) | (0.102) | ||||||||||||||
| r-HG | 1 | 0.67 | 0.71 | 0.53 | 0.52 | |||||||||||||
| (0.009) | (0.004) | (0.058) | (0.070) | |||||||||||||||
| l-HG | 1 | 0.32 | 0.27 | 0.40 | ||||||||||||||
| (0.265) | (0.379) | (0.175) | ||||||||||||||||
| SPPB | 1 | 0.46 | 0.47 | |||||||||||||||
| (0.113) | (0.103) | |||||||||||||||||
| SF-12 PHS | 1 | 0.44 | ||||||||||||||||
| (0.130) | ||||||||||||||||||
| SF-12 MHS | 1 |
Notes: Pearson correlations are in the upper triangular matrix with P values (in bold: P<0.05). aLog-transformed variables.
Abbreviations: MMSE, mini–mental state examination; FR, free recall; REFR, relative equivalent free recall; IR, immediate recall (free + cued recall); Int IR, intrusions at immediate recall; REDR, relative equivalent delay recall; TDR, total delayed recall (free + cued recall); Int DR, intrusions at delayed recall; TMSA, trail-making state A; NPI, neuropsychiatric inventory; ADL, activities of daily living; r-HG, right hand grip; l-HG, left hand grip; SPPB, short physical performance battery status; SF-12 PHS, short form-12 physical health status; SF-12 MHS, short form-12 mental health status; EBS, Emisymmetric bilateral stimulation.
Discussion
This open-label pilot study evaluated the effectiveness and the safety of a standardized EBS 5-week treatment in 14 patients with cognitive decline.
Regarding the primary objectives of our study, we observed that after 5 weeks of standardized EBS treatment, there was a significant improvement in all neuropsychological protocol assessments related to cognitive functions in all patients. In addition, cognitive improvements remained still significant after stratified analysis concerning AD and MCI groups.
Since each patient’s MMSE range included in the study was on average 22.03±4.53 points, this cognitive improvement of approximately 3 points (+0.61 by week) is of important clinical relevance.38
Notably, both groups, AD and MCI patients, achieved an improvement in cognitive functions, as determined by positive changes in MMSE, assessed at the end of the treatment.
Remarkably, EBS stimulation also improved episodic memory, as shown by FCRST scores. In both free immediate and TDR, a clinically relevant increase was observed. Executive functions have also been improved by EBS stimulation, with a significant decrease in the time spent to complete the TMS test A.
It is important to note that the results obtained in our open-label study were in AD patients receiving standard medication during the trial (AD patients were treated with cholinesterase inhibitors at the time of recruitment, and three AD patients were treated with cholinesterase inhibitors and memantine combined). We hypothesized that EBS therapy may stimulate and exploit a “cognitive reserve” pool through some biological effects, which could provide additional benefit in patients with cognitive decline, when associated with the currently available therapy.
Notably, our results demonstrate not only a direct effect on cognitive functions, but also improvements in secondary outcomes. In particular, of primary importance in dementia, substantial improvements in behavior were noted, and the effects were immediate after a few EBS treatment procedures.
Our data also show significant improvements in functional aspects as well as those related to subjective QoL assessments. Statistical analysis has shown significant correlations between cognitive, behavioral, and functional 5-week improvements. This evidence supports the hypothesis of systemic biological benefits provided by EBS stimulation, which determines global neuromediated biooptimization, with consequent increase in cognition, muscular strength, behavior disorders, and QoL.
Trends in cognitive and behavioral changes show that in both AD and MCI group, EBS stimulation could effort a rapid improvement in all functions, more dominant in the behavioral aspect, with subsequent stabilizing effect until the end of treatment.
Recently, Rabey et al39 showed a synergistic, long-lasting, posttreatment effect of rTMS-COG (repetitive transcranial magnetic stimulus with cognitive training) for patients with mild-to-moderate AD. The randomized, double-blind, controlled study evaluated the effect of rTMS-COG therapy for patients with mild-to-moderate AD compared with a matched placebo group. Following both 6 weeks of intensive daily treatment and an additional 3 months of maintenance treatment, there was a significant improvement in the Alzheimer’s Disease Assessment Scale-cognitive subscale scores of the treatment group as compared with the placebo group.39 Moreover studies on animal models showed that rTMS modifies mechanisms that play a part in the formation of memories.40 Recent reviews about this topic have shown growing evidence of the benefit of rTMS and EMF stimulation in treating patients with AD.7,41
One of the interesting points still not completely resolved is the mechanism of action of electromagnetic variables on the brain of patients with cognitive decline. EMF neurostimulation can induce lasting modulation of brain activities in targeted brain regions and across brain networks through transcranial induction of electric currents in the brain.42
In a quantum field theory approach to living dynamics, it is possible to move ahead toward a deeper comprehension of such sophisticated interactions.14,16 In fact growing evidence suggest that the effects of weak EMF on cells could be explained by the presence of electromagnetic conformations in the over-molecular water structure.43–45
Recently, Montagnier et al46 have been able to detect experimentally the presence of electromagnetic signals originating in the water surrounding biomolecules. To us, this should be the key point of EMF stimulation technique: the stimuli involved in the interaction between neurons and extremely weak electromagnetic signals are not energetic, but are potential and phase based actors able to produce a phase shift in domains of cells and water structure.
It is still underdetermined at which stage of cognitive decline EMF would be optimally applied. Group differences suggest a more consistent positive trend in cognitive functions in MCI patients rather than in those with AD; taking into account the small size of the sample, the higher therapeutic potential of EBS seems to be suitable when brain neurodegenerative damage is at earlier stage of cognitive decline. This is consistent with data coming from other trails about EMF brain stimulation.47,48
This open-label study obviously has several limitations and critical issues. The small size of sample and the absence of a placebo-controlled group are definitely primary limitations. Even if our results are mostly statistically significant, placebo-controlled larger clinical trials are needed to better elucidate the efficacy of this innovative neurostimulation technique. Basic research may further clarify the mechanisms of action of the different stimulation techniques and increase their potential efficacy.
Another limitation is the lack of knowledge of the potential long-term benefits of EBS treatments after the 5-week procedure. From our clinical experience, cognitive and behavioral benefits are maintained for almost 6 months after the 5-week treatment; however, this clinical observation should be supported by data from a further long-term trial.
Conclusion
In conclusion, the results of this pilot study are promising and provide a new nonpharmacological tool, EBS therapy, to treat patients with cognitive decline in addition to the drugs presently available.
Further investigations into the potential clinical effects of EBS for cognitive impairment and AD are warranted.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The study was completed independently with no funding. The authors report no conflicts of interest in this work.
References
- 1.Rafii MS, Ellis RJ, Corey-Bloom J. Clinical Adult Neurology. New York, NY: Demos Medical; 2009. Dementing and degenerative disorders; pp. 395–417. [Google Scholar]
- 2.Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 3.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;1:CD005593. 58. doi: 10.1002/146518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can J Psychiatr. 2014;59(12):618–623. doi: 10.1177/070674371405901202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley JS, Salpeter SR. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32(6):453–467. doi: 10.1007/s40266-015-0266-9. [DOI] [PubMed] [Google Scholar]
- 6.Freitas C, Mondrago'n-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: systematic review and perspectives for the future. Exp Gerontol. 2011;46:611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardone R, Holler Y, Tezzon F, et al. Neurostimulation in Alzheimer’s disease: from basic research to clinical applications. Neurol Sci. 2015;36(5):689–700. doi: 10.1007/s10072-015-2120-6. [DOI] [PubMed] [Google Scholar]
- 8.Cotelli M, Manenti R, Cappa SF, et al. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol. 2006;63:1602–1604. doi: 10.1001/archneur.63.11.1602. [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, The Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotelli M, Calabria M, Manenti R, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatr. 2011;82:794–797. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 11.Boggio PS, Khoury LP, Martins DC, Martins OE, De Macedo EC, Fregni F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatr. 2009;80:444–447. doi: 10.1136/jnnp.2007.141853. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci R, Mameli F, Guidi I, et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71:493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 13.Arani R, Bono I, Del Giudice E, Preparata G. QED coherence and the thermodynamics of water. Int J Mod Phys B. 1995;9:1813–1841. [Google Scholar]
- 14.Del Giudice E, Doglia S, Milani M. A quantum field theoretical approach to the collective behaviour of biological systems. Nucl Phys. 1985;251(13):375–400. [Google Scholar]
- 15.Smith CW, Endler PC, Shculte J. Ultra High Dilution. Dordrecht, Netherland: Kluwer Academic; 1994. Electromagnetic and magnetic vector potential bio-information and water; pp. 187–201. [Google Scholar]
- 16.Ho MW, French A, Haffegee J. Bioelectrodynamics and Biocommunication. Singapore: World Scientific; 1994. Can weak magnetic fields affect pattern formation; pp. 195–212. [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- 19.Petersen RC, Smith GE, Waring SC, Ivnick RJ, Tangalos EG, Komen E. Mild cognitive impairment, clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Carlesimo GA, Buccione I, Fadda L, et al. Standardizzazione di due testi di memoria per uso clinico: breve racconto e figura di Rev. Nuova Rivista di Neurologia. 2002;12:2–13. [Google Scholar]
- 21.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analysis of cognitive impairment. The Group of the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CP, Verg L, Danziger WL, Coben LA, Matin RL. A new clinical scale for the staging of dementia. Br J Psychiatr. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 23.Brizhik L, Del Giudice E, Jorgensen SE, Marchettini N, Tiezzi E. The role of electromagnetic potentials in the evolutionary dynamics of ecosystems. Ecol Model. 2009;220:1865–1869. [Google Scholar]
- 24.Bischof M, Del Giudice E. Communication and the emergence of collective behavior in living organisms: a quantum approach. Mol Biol Int. 2013;2013:987549. doi: 10.1155/2013/987549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Frasson P, Ghiretti R, Catricalà E, et al. Free and cued selective reminding test: an Italian normative study. Neurol Sci. 2011;32(6):1057–1062. doi: 10.1007/s10072-011-0607-3. [DOI] [PubMed] [Google Scholar]
- 27.Lemos R, Simoes MR, Santiago B, Santana I. The free and cued selective reminding test: validation for mild cognitive impairment and Alzheimer’s disease. J Neuropsychol. 2014 Jun 3; doi: 10.1111/jnp.12048. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsy. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 29.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney FI, Barthel DW. Functional evalutation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 31.Miller DK, Wolinsky FD, Andresen EM, Malmstrom TK, Miller JP. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. J Gerontol A Biol Sci Med Sci. 2008;63(5):487–494. doi: 10.1093/gerona/63.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 33.Cheung C-L, Tan KCB, Bow CH, Soong CSS, Loong CHN, Kung AW-C. Low handgrip strength is a predictor of osteoporotic fractures: cross-sectional and prospective evidence from the Hong Kong Osteoporosis Study. Age. 2012;34(5):1239–1248. doi: 10.1007/s11357-011-9297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York, NY: Wiley-Interscience; 2004. [Google Scholar]
- 36.Pinheiro J, Bates D, Debroy S, Sarkar DR, Development Core Team nlme: Linear and nonlinear mixed effects models. R package version 3.1–111 [Google Scholar]
- 37.Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 38.Hensel A, Angermeyer MC, Riedel, Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini Mental State Examination. J Neurol Neurosurg Psychiatr. 2007;78(12):1298–1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm. 2013;120:813–819. doi: 10.1007/s00702-012-0902-z. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed Z, Wieraszko A. Modulation of learning and hippocampal, neuronal plasticity by repetitive transcranial magnetic stimulation. Bioelectromagnetics. 2006;27:288–294. doi: 10.1002/bem.20211. [DOI] [PubMed] [Google Scholar]
- 41.Nardone R, Bergmann J, Christova M, et al. Effect of transcranial brain stimulation for the treatment of Alzheimer disease: a review. Int J Alzheimers Dis. 2012:687909. doi: 10.1155/2012/687909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner T, Valero-Cabre A, Pascual-Leone A. Non invasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 43.Del Giudice E, Preparata G. Microscopic Quantum Coherence. Hackensack, NJ: World Scientific; 1998. A new QED picture of water: understanding a few fascinating phenomena; pp. 108–129. [Google Scholar]
- 44.Pollack GH. Water, energy and life: fresh views from water’s edge. Int J Des Nat Ecodyn. 2010;5:27–29. doi: 10.2495/DNE-V5-N1-27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clegg JS. Biophysics of Water. New York, NY: John Wiley and Sons; 1982. Alternative views of role of water in cell function; pp. 365–385. [Google Scholar]
- 46.Montagnier L, Aissa J, Ferris S, Montagnier JL, Lavallée C. Electromagnetic signals are produced by aqueous nanostructures derived from DNA bacterial sequences. Interdisciplin Sci Comput Life Sci. 2009;1:81–90. doi: 10.1007/s12539-009-0036-7. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed MA, Darwish ES, Khedr EM, El Serogy YM, Ali AM. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation and functional excitability in Alzheimer’s dementia. J Neurol. 2012;259:83–92. doi: 10.1007/s00415-011-6128-4. [DOI] [PubMed] [Google Scholar]
- 48.Eliasova I, Anderkova L, Marecek R, Rektorova I. Noninvasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci. 2014;346:318–322. doi: 10.1016/j.jns.2014.08.036. [DOI] [PubMed] [Google Scholar]