Abstract
Girdin, an actin-binding protein, is associated with cell migration and is expressed at high levels in glioma cells. However, the association between girdin and the development of glioma remains to be elucidated. In the present study, short-hairpin RNA technology was used to silence the gene expression of girdin. The effects of girdin silencing on glioma cell proliferation, migration and invasion were then assessed using a cell viability assay, wound-healing assay, transwell invasion assay, reverse transcription-quantitative polymerase chain reaction, western blot analysis and gelatin zymography. The results suggested that girdin silencing inhibited the proliferation, migration and invasion of glioma cells. In addition, the expression levels and activity of matrix metalloproteinase (MMP)-2 and MMP-9 were also affected by girdin silencing. Further mechanistic investigation indicated that girdin may regulate glioma cell migration and invasion through the phosphatidylinositol-3-kinase/protein kinase B (PI3K-Akt) signaling pathway. Therefore, the results of the present study provide a theoretical foundation for the development of anticancer drugs.
Keywords: girdin, metastasis, invasion, protein kinase B, glioma, phosphatidylinositol-3-kinase
Introduction
Gliomas are a common type of primary tumor of the central nervous system and account for 45.2% of all intracranial types of tumor (1). The annual incidence of brain glioma ranges between 0.003 and 0.005% (2). Brain gliomas exhibit diffuse and invasive characteristics, and traditional resection has a poor prognosis (3,4). In addition, drug resistance in brain gliomas leads to unsatisfactory radiotherapeutic and chemotherapeutic outcomes and relatively short survival rates (5). Therefore, investigations on the mechanisms underlying brain glioma metastasis and invasion are important for the development of anti-glioma drugs.
The multi-domain signaling molecule girdin, which was discovered by a Japanese group in 2005, acts as a signaling transduction bridge and link (6). Girdin is an actin-binding protein involved in the regulation of the actin cytoskeleton, thus affecting pseudopodia extension (7) and regulating the migration of endothelial, smooth muscle and neural stem cells (8). Girdin also promotes vascular endothelial growth factor (VEGF)-mediated endothelial cell migration and regulates neointimal formation following vascular injury (9,10). Girdin is expressed in various types of cancer, including esophageal cancer, gastric cancer, colon cancer, breast cancer and lung cancer, and its expression is particularly high in invasive tumor cells (11). Girdin is also involved in the regulation of tumor metastasis (11,12), angiogenesis (9,10) and autophagy (13) and has a close association with tumorigenesis and development (12). High expression levels of girdin also correlate closely with poor tumor prognosis (11).
Girdin is expressed at relatively high levels in brain gliomas. A previous study reported an association between the expression of girdin and glioma metastasis (14), and the expression levels of girdin correlate with the degree of malignancy in brain gliomas. However, the role of girdin in brain gliomas remains to be elucidated. The present study aimed to determine the effects of short hairpin (sh)RNA-induced girdin silencing on the proliferation, migration and invasion of glioma cells.
Materials and methods
Cell culture
Human glioma cell lines U251, A172, U87-MG and SHG-44 were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). U373 was purchased from Bioleaf Biotech Co., Ltd (Shanghai, China). U251, U373 and A172 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Sciences, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). U87-MG cells were cultured in minimum essential medium (Gibco Life Sciences) supplemented with 15% FBS. SHG-44 cells were cultured in RPMI-1640 (Gibco Life Sciences) supplemented with 10% FBS. All cells were incubated in a 37°C incubator with 5% CO2.
Construction of girdin RNA interference vectors and transfection
The girdin short hairpin (sh)RNA sequences and unrelated sequences were designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). The sequences were as follows: Interference sequence, forward 5′-GAT CCC CGT CAA TAA TGA TGC CTC ACT TCA AGA GAG TGA GGC ATC ATT ATT GAC TTTTT-3′ and reverse 5′-AGC TAA AAA GTC AAT AAT GAT GCC TCA CTCTCT TGA AGT GAG GCA TCA TTA TTG ACGGG-3; and unrelated sequence, forward 5′-GAT CCC CTT CTC CGA ACG TGT CAC GTT TCA AGA GAA CGT GAC ACG TTC GGA GAA TTTTT-3′ and reverse 5′-AGC TAA AAA TTC TCC GAA CGT GTC ACG TTC TCT TGA AAC GTG ACA CGT TCG GAG AAGGG-3′. The annealed interference sequences or unrelated sequences were inserted into pGCsi-H1/Neo/GFP vectors via digestion with FastDigest BamHI and FastDigest Hind III (Thermo Fisher Scientific, Pittsburgh, PA, USA), and ligation with T4 DNA Ligase (Thermo Fisher Scientific). The sequencing-confirmed plasmids were termed girdin shRNA and negative control (NC) respectively. The girdin shRNA or NC plasmids were subsequently transfected into the U251 cells using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. At 24 h post-transfection, the culture medium was replaced with medium containing 400 µg/ml G418 (Invitrogen Life Technologies) for the screening of stably transfected cells. Positive clones were identified using quantitative polymerase chain reaction (qPCR) and western blot analysis.
Drug treatment
When the cells transfected with girdin shRNA or NC grew to 70–80% confluency, the LY294002 PI3K inhibitor (20 µM; Beyotime Instutute of Biotechnology, Shanghai, China) or dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) was added to the cells. Following incubation for an additional 24 h, a Transwell invasion assay and western blot analysis were performed.
Analysis of cell viability
Cell viability was examined using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. The cells transfected with girdin shRNA or NC, as well as untreated U251 cells, were seeded into 96-well plates (2×103 cells per well) and incubated at 37°C. MTT solution (0.2 mg/ml, Sigma-Aldrich) was added after 0, 24, 48, 72 or 96 h incubation. Following incubation for an additional 4 h at 37°C, the supernatants were removed and 200 µl DMSO was added to each well to dissolve the formazan crystals. The 96-well plates were placed in a microplate reader (ELX-800; Biotek, Winooski, VT, USA) to measure the absorbance at 490 nm, and growth curves were plotted.
Wound healing assay
The cells were seeded into 6-well plates (1×105 cells per well). When the cells reached 80–90% confluency, scratches were introduced onto the monolayer cell surfaces using 200 µl pipette tips. The cells were subsequently washed with serum-free medium, and then incubated with serum-free DMEM at 37°C. At 0, 12, and 24 h, images of each sample were captured and the data were recorded to calculate the relative cell mobility, based on the following formula: Relative mobility = (1 − distance between the edges of migrated scratches / distance between the edges of initial scratches) ×100%.
Transwell invasion assay
The cells were suspended in serum-free medium at a density of 1×105 cells/ml. Subsequently, 200 µl cell suspension was added to the upper chamber of Transwell chambers (Corning Life Sciences, Tewksbury, MA, USA) pretreated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), and 800 µl DMEM media supplemented with 30% FBS was added to each lower chamber. Following incubation in the incubator for another 24 h at 37°C, the cells on the upper surface of the microporous membrane were removed with cotton swabs, whereas the cells on the lower surface of the membrane were fixed in 4% paraformaldehyde solution (Sinopharm, Shanghai, China) for 20 min at room temperature and subsequently stained with 0.5% crystal violet solution (Amresco, Solon, OH, USA). Images of the stained cells from five selected views were captured under a light microscope (AE31; Motic Incoporation, Ltd., Xiamen, China) at 200× magnification, and the number of cells, which migrated through the micro-porous membranes was calculated.
Western blot analysis
The cells were collected via centrifugation and the protein in the cells was extracted using NP-40 lysis buffer (Beyotime Institute of Biotechnology), according to the manufacturer's instructions. The protein concentrations were measured using a BCA Protein Concentration Detection kit (Beyotime Institute of Biotechnology). Subsequently, 40 µg of the protein was separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferring onto polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Bedford, MA, USA). The PVDF membranes containing the transferred proteins were blocked in 5% skim milk or 5% bovine serum albumin (Biosharp, Hefei, China). Following washing with Tris-buffered saline with 0.05% Tween-20 (TBST), the membranes were incubated with the following primary antibodies at 4°C overnight: Rabbit anti-human polyclonal antibody against girdin (1:500 diluted; cat. no. bs-5150R; Bioss, Beijing, China); rabbit anti-human polyclonal antibody against MMP-2 (1:1,000 diluted; cat. no. WL0657); rabbit anti-human polyclonal antibody against MMP-9 (1:1,000 diluted; cat. no. WL0884); rabbit anti-human polyclonal antibody against P85α (1:1,000 diluted; cat. no. WL0191); rabbit anti-human polyclonal antibody against P110α (1:1,000 diluted; cat. no. WL0339); rabbit anti-human polyclonal antibody against AKT (1:1,000 diluted; cat. no. WL0003); rabbit anti-human polyclonal antibody against p-AKT (1:1,000 diluted; cat. no. WLP001). All antibodies were purchased from Wanleibio (Shenyang, China) unless stated. Following washing with TBST, the membranes were subsequently incubated with horseradish peroxidase-linked goat anti-rabbit IgG (1:5,000, Beyotime Institute of Biotechnology) at 37°C for 45 min. Enhanced chemiluminescence solution was added for luminescent image development, and the target protein levels were analyzed using a Gel-Pro-Analyzer (Liuyi, Beijing, China), with β-actin as a reference.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using a Total RNA Extraction kit (Tiangen Biotech, Co., Ltd., Beijing, China) according to the manufacturer's instructions. The extracted RNA from each group was reverse transcribed into cDNA using Super M-MLV reverse transcriptase (BioTeke Corporation, Beijing, China) and oligo (dT)15. The mRNA expression levels of girdin, MMP-2 and MMP-9 were detected by RT-qPCR on an Exicycler TM 96 Real-Time Quantitative PCR instrument (Bioneer Corporation, Daejeon, Korea) using cDNA as a template. The PCR reaction conditions were as follows: 95°C for 10 min; 95°C for 10 sec, 60°C for 20 sec, 72°C for 30 sec (40 cycles); 4°C for 5 min. The relative mRNA levels in each sample were calculated using the 2−ΔΔCt method (15), using β-actin as a reference. The SYBR Green qRT-PCR Master mix was purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). The following primers were used: Girdin, forward CTC CAG GCA TGA AGC GAACA and reverse 5′-TGG CAG AGC GAG CAT CCGA-3′; MMP-2, forward 5′-TGC TGA AGG ACA CAC TAAAG-3′ and reverse, 5′-GTA GCC AAT GAT CCT GTA TGT-3′; MMP-9 forward, GCT ACG TGA CCT ATG ACA TCCT-3′ and reverse 5′-TCC TCC AGA ACA GAA TAC CAGT-3′; and β-actin forward CTT AGT TGC GTT ACA CCC TTT CTTG-3′ and reverse 5′-CTG TCA CCT TCA CCG TTC CAG TTT-3′.
Gelatin zymogram
The glioma cells (1×106 cells/ml) from each treatment group were collected via centrifugation at 112 × g for 3 min at room temperature, suspended in phosphate-buffered saline (PBS) containing 1% phenylmethyl sulfonyl fluoride (PMSF; Beyotime Institute of biotechnology), and homogenized on ice; the samples were subsequently subjected to liquid nitrogen freeze-thaw three times. Following centrifugation, the proteins from the samples were harvested in the supernatant fraction. Following measurement of the protein concentrations using a BCA Protein Concentration Detection kit, equal quantities of protein were separated on an SDS-PAGE gel containing 1 mg/ml gelatin (Sigma-Aldrich). Following electrophoresis, the gel was washed in an eluent solution (5% Triton X-100, 50 mM Tris-HCl, 5 mM CaCl2 and 1 µM ZnCl2; pH 7.6), rinsed in a rinsing solution (50 mM Tris-HCl, 5 mM CaCl2and 1 µM ZnCl2; pH 7.6), incubated in an incubating solution (50 mM Tris-HCl, 5 mM CaCl2, 1 µM ZnCl2, 0.02% Brij, and 0.2 M NaCl), and stained with dye (0.05% Coomassic brilliant blue G-250, 30% methanol and 10% acetic acid; Amresco). The stained gel was then decolorized sequentially in destaining solutions A, B and C (30, 20 and 10% methanol solutions, and 10, 10 and 5% ethanol solutions, respectively). Images of the gels were captured using a gel imaging system, and the optical density values were analyzed.
Statistical analysis
Experimental results are presented as the mean ± standard deviations. Differences between groups were analyzed using one-way analysis of variance and Bonferroni's multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of stable girdin shRNA-transfected cell lines
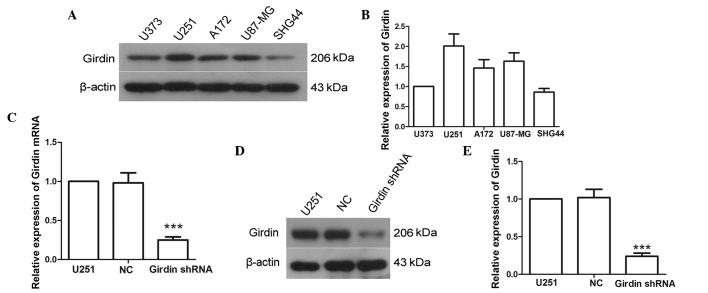
To select the appropriate cell line for the present study, the expression levels of girdin in U373, U251, A172, U87-MG and SHG-44 cell lines were assessed using western blot analysis. As the U251 cell line exhibited the highest expression level of girdin (Fig. 1A and B), the U251 cells were selected for use in the subsequent experiments. Following transfection with girdin shRNA, the expression of girdin was detected using RT-qPCR and western blot analysis. The RT-qPCR results indicated that the mRNA levels of girdin were reduced to 25±4% following transfection with girdin shRNA (Fig. 1C). Similarly, the western blot analysis revealed that the protein expression of girdin was significantly reduced following transfection with girdin shRNA (P<0.001; Fig. 1D and E). These results suggested that the expression of girdin was effectively silenced following transfection with girdin shRNA.
Figure 1.
Girdin shRNA decreases the gene expression of girdin. (A and B) Protein expression levels of girdin in the U373, U251, A172, U87-MG and SHG-44 cell lines were detected using western blot, with β-actin as a reference. (C) Changes in mRNA levels of girdin were detected using reverse transcription-quantitative polymerase chain reaction, following transfection with girdin shRNA. The relative mRNA expression level in each sample was quantified using the 2−∆∆Ct method. (D and E) Expression levels of girdin were measured using western blot analysis following transfection. Each experiment was repeated three times. The experimental results are presented as the mean ± standard deviation. ***P<0.001, compared with the NC group. shRNA, short hairpin RNA; NC, negative control.
Girdin silencing inhibits the proliferation, migration and invasion of glioma cells
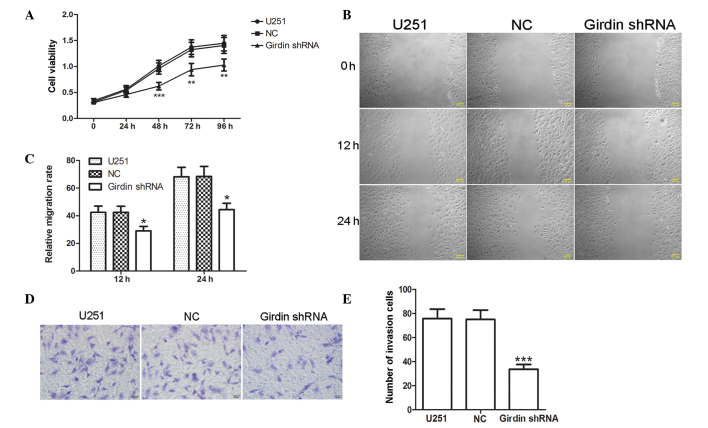
To evaluate the effect of girdin silencing on glioma proliferation, migration and invasion, an MTT assay was used to determine the changes in cell viability following transfection with girdin shRNA. The MTT assay results demonstrated that girdin silencing significantly reduced the proliferation of glioma cells (P<0.01; Fig. 2A). A wound-healing assay was subsequently performed to detect changes in migration capacity of the cells. The results of the wound-healing assay demonstrated that the relative mobility of the glioma cells was significantly lower following girdin silencing, compared with that of the cells transfected with NC (P<0.05; Fig. 2B and C), suggesting that girdin silencing may impede cell migration. In addition, Transwell invasion assays were also performed to evaluate the effect of girdin silencing on glioma cell invasion. The results revealed that fewer cells passed through the microporous membrane following transfection with girdin shRNA (P<0.001; Fig. 2D and E), indicating that girdin silencing significantly reduced glioma cell invasion. The above results suggested that girdin silencing suppressed glioma cell proliferation, migration and invasion.
Figure 2.
Girdin silencing inhibits the proliferation, migration and invasion of glioma cells. (A) Changes of cell viability were detected using a 3-(4,5-dimethy -2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide assay following transfection. (B and C) Following transfection, a wound-healing assay was performed to investigate changes in migration capacity (scale bar, 100 µm). (D and E) A Transwell invasion assay was performed to detect cell invasion capacity following transfection. Images were captured under a light microscope (magnification, ×200). Each experiment was repeated three times. The experimental results are presented as the means ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001, compared with the NC group. shRNA, short hairpin RNA; NC, negative control.
Girdin silencing inhibits the expression and activity levels of MMP-2 and MMP-9
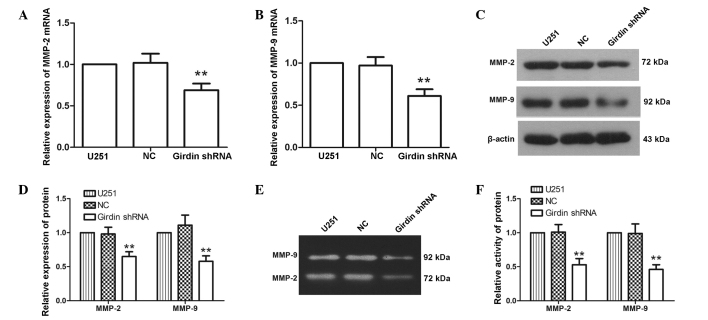
MMP-2 and MMP-9 are closely associated with cell migration and invasion. To further investigate the effect of girdin silencing on glioma cell proliferation, migration and invasion, RT-qPCR and western blot analyses were performed to detect changes in the expression levels of MMP-2 and MMP-9 following transfection with girdin shRNA. The RT-qPCR results demonstrated that the mRNA levels of MMP-2 and MMP-9 were reduced to 69±8 and 61±8%, respectively, following transfection with girdin shRNA (Fig. 3A and B). Similarly, western blot analysis revealed that the protein expression levels of MMP-2 and MMP-9 were decreased to 65±7 and 58±8% following transfection with girdin shRNA (Fig. 3C and D), which was consistent with the results of the RT-qPCR. As the activities of MMP-2 and MMP-9 are important for their biological functions, gelatin zymography was used to characterize the activities of MMP-2 and MMP-9. The results of the gelatin zymogram revealed that the activities of MMP-2 and MMP-9 were significantly reduced following transfection with girdin shRNA (P<0.01; Fig. 3E and F). These results indicated that girdin silencing inhibited the expression levels and activities of MMP-2 and MMP-9.
Figure 3.
Girdin silencing inhibits the expression and activity of MMP-2 and MMP-9. (A and B) Changes in the mRNA levels of MMP-2 and MMP-9 were measured using reverse transcription-quantitative polymerase chain reaction following transfection. The relative mRNA expression levels were calculated using the 2−ΔΔCt method. (C and D) Following transfection, changes in the protein levels of MMP-2 and MMP-9 were detected using western blot analysis. (E and F) Following transfection, gelatin zymography was performed to detect changes in the activities of MMP-2 and MMP-9. Each experiment was repeated three times. The experimental results are presented as the mean ± standard deviation. **P<0.01, compared with the NC group. shRNA, short hairpin RNA; NC, negative control.
Girdin regulates glioma cell migration and invasion via the PI3K-Akt signaling pathway
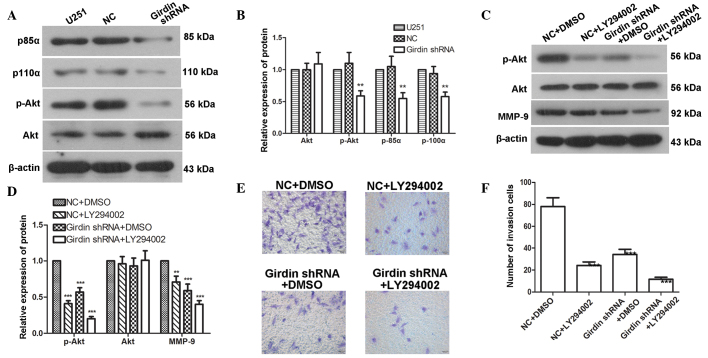
To further investigate how girdin affects glioma cell migration and invasion, western blotting was used to detect the protein levels of p85α, p110α, Akt and p-Akt following transfection with girdin shRNA. The results demonstrated that the protein levels of p85α, p110α and p-Akt were reduced to 55±9, 58±7 and 59±8%, respectively; however, no significant changes were detected in the expression of Akt (Fig. 4A and B). These results indicated that girdin silencing affected the activation of the PI3K-Akt signaling pathway. The LY294002 PI3K signaling pathway inhibitor was used to inhibit the PI3K-Akt pathway, followed by western blot analysis to detect changes in the levels of Akt, p-Akt and MMP-9. The results revealed that the levels of p-Akt and MMP-9 were significantly reduced following girdin silencing. In addition, the LY294002-mediated PI3K-Akt signaling pathway inhibition yielded the same results as girdin silencing. The combined application of LY294002 with girdin silencing further enhanced the reductions of p-Akt and MMP-9 (Fig. 4C and D). A Transwell invasion assay, which was used to detect the effect of LY294002 on glioma cell invasion, also demonstrated that LY294002 further enhanced the inhibitory effect of girdin silencing on glioma cell invasion (Fig. 4E and F). These results suggested that girdin silencing may affect glioma cell migration and invasion by regulating the PI3K-Akt signaling pathway.
Figure 4.
Girdin regulates glioma cell migration and invasion via the PI3K-Akt signaling pathway. (A and B) Western blot analysis was performed to detect changes in the levels of p85α, p110α, p-Akt and Akt following transfection with girdin shRNA. (C and D) Western blot analysis was performed to detect the effects of girdin silencing on the protein levels of p-Akt, Akt and MMP-9 following treatment with LY294002. (E and F) A Transwell invasion assay was performed to detect the effect of girdin silencing on cell invasion following treatment with LY294002. Images were captured under a light microscope (magnification, ×200). Each experiment was repeated three times. The experimental results are presented as the mean ± standard deviation. **P<0.01, compared with the negative control. shRNA, short hairpin RNA; NC, negative control; DMSO, dimethyl sulfoxide; MMP, matrix metalloproteinase; Akt, protein kinase B; p-, phosphorylated.
Discussion
Glioma is a common type of brain tumor and poses a serious threat to human health. Girdin is expressed at high levels in glioma cells and is closely associated with glioma development. The present study focused on the effects of girdin silencing on glioma cell proliferation, migration and invasion, as well as investigating the underlying mechanism. The results of the present study suggested that girdin silencing may affect glioma cell migration and invasion by regulating the PI3K-Akt signaling pathway.
Girdin is an actin-binding protein and is activated by girdin phosphorylation via the PI3K-Akt signaling pathway. Activated girdin migrates to the pseudopodia at the front edges of migrating cells. At this location, girdin regulates the front-edge actin cytoskeletal structure and promotes cyto-skeletal rearrangement, thereby facilitating cell motility and being important in tumor invasion and metastasis (7). Girdin is expressed at high levels in glioma cells, and the expression level of girdin is associated with tumor metastasis (14). In the present study, the proliferation, migration and invasion of glioma cells were significantly reduced following girdin silencing. Consistent with these findings, Natsume et al (16) reported that genetic girdin knockout promoted glioma stem cell differentiation, but inhibited cell motility, invasion, metastasis and proliferation in vivo. Cao et al (17) demonstrated that girdin knockout reduced esophageal cancer cell proliferation, migration and invasion, which was also similar to the findings of the present study. Girdin deprivation has also been observed to inhibit vascular smooth muscle cell (VSMC) proliferation and to affect actin cytoskeletal rearrangement, resulting the in impaired migration of VSMCs and altered neointimal formation following vascular injury (10). Therefore, girdin is important in the processes of tumor cell proliferation, migration and invasion.
In the present study, girdin silencing inhibited the expression levels and activities of MMP-2 and MMP-9. MMP-2 and MMP-9 exerted certain effects on cell migration and invasion, and inhibition of the expression and activities of MMP-2 and MMP-9 by girdin silencing demonstrated the regulatory effects of girdin on glioma cell migration and invasion at the molecular level. Similar to the findings of the present study, Gu et al (18) reported that girdin silencing inhibits the in vivo and in vitro expression of MMP-2 and MMP-9, and reduces cell migration and invasion. In addition, girdin silencing affects the phosphorylation of integrin β1 and focal adhesion kinase adhesion molecules, suggesting an effect on cell adhesion (18).
The PI3K-Akt signaling pathway is involved in the regulation of various cellular processes and is important in tumor proliferation, invasion and metastasis (19,20). Studies have revealed that Akt knock down inhibits brain glioma invasion and metastasis (19,21). Girdin is an important downstream target of the Akt signaling pathway. This protein enhances PI3K-Akt signaling pathway activity and regulates cell proliferation and apoptosis (7). Girdin can also be activated via phosphorylation by Akt, and can bind and activate Gαi3 to further activate the PI3K-Akt signaling pathway (22). In the present study, activation of PI3K-Akt signaling pathway was suppressed by girdin silencing. In addition, treatment with a PI3K-Akt signaling pathway inhibitor enhanced the inhibitory effects of girdin silencing on glioma cell migration and invasion. These results suggested that girdin may regulate glioma cell migration and invasion through the PI3K-Akt signaling pathway. Similar to these findings, Lin et al (23) reported that, in breast cancer cells, girdin binds to the PI3K regulatory subunit p85α and promotes the phosphorylation of p85α and activation of the PI3K-Akt signaling pathway, regulating breast cancer cell migration.
In the present study, shRNA silencing technology was used to evaluate the effects of girdin on the proliferation, migration and invasion of glioma cells. The results demonstrated that girdin silencing decreased the proliferation, migration and invasion of glioma cells, and subsequent mechanistic investigation indicated that girdin may regulate glioma cell migration and invasion via the PI3K-Akt signaling pathway. The results of the present study provide a theoretical basis for the development of anti-glioma drugs.
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (grant. no. 81300601), the Social Development Project of Department of Science and Technology, Liaoning Province (grant. no. 2013225049) and the Nature Science Foundation of Liaoning Province (grant. no. 2013022025).
References
- 1.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii93–iii101. doi: 10.1093/annonc/mdu050. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Tonn JC, Brada M, Pentheroudakis G. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190–v193. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 4.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 5.Mrugala MM, Adair J, Kiem HP. Temozolomide: Expanding its role in brain cancer. Drugs Today (Barc) 2010;46:833–846. doi: 10.1358/dot.2010.46.11.1549024. [DOI] [PubMed] [Google Scholar]
- 6.Anai M, Shojima N, Katagiri H, Ogihara T, Sakoda H, Onishi Y, Ono H, Fujishiro M, Fukushima Y, Horike N, et al. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem. 2005;280:18525–18535. doi: 10.1074/jbc.M500586200. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Ohara K, Enomoto A, Kato T, Hashimoto T, Isotani-Sakakibara M, Asai N, Ishida-Takagishi M, Weng L, Nakayama M, Watanabe T, et al. Involvement of Girdin in the determination of cell polarity during cell migration. PLoS One. 2012;7:e36681. doi: 10.1371/journal.pone.0036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10:329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 10.Miyake H, Maeda K, Asai N, Shibata R, Ichimiya H, Isotani-Sakakibara M, Yamamura Y, Kato K, Enomoto A, Takahashi M, Murohara T. The actin-binding protein Girdin and its Akt-mediated phosphorylation regulate neointima formation after vascular injury. Circ Res. 2011;108:1170–1179. doi: 10.1161/CIRCRESAHA.110.236174. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Marcos M, Jung BH, Ear J, Cabrera B, Carethers JM, Ghosh P. Expression of GIV/Girdin, a metastasis-related protein, predicts patient survival in colon cancer. FASEB J. 2011;25:590–599. doi: 10.1096/fj.10-167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P, Enomoto A, Jijiwa M, Kato T, Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y, Takahashi M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008;68:1310–1318. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Marcos M, Ear J, Farquhar MG, Ghosh P. A GDI (AGS3) and a GEF (GIV) regulate autophagy by balancing G protein activity and growth factor signals. Mol Biol Cell. 2011;22:673–686. doi: 10.1091/mbc.E10-08-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Ma S, Liu Q, Liang P. Clinical implications of Girdin protein expression in glioma. ScientificWorldJournal. 2013;2013:986073. doi: 10.1155/2013/986073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Natsume A, Kato T, Kinjo S, Kato T, Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y, Takahashi M, Wakabayashi T. Girdin maintains the stemness of glioblastoma stem cells. Oncogene. 2012;31:2715–2724. doi: 10.1038/onc.2011.466. [DOI] [PubMed] [Google Scholar]
- 17.Cao K, Jiang W, Cao P, Zou Q, Xiao S, Zhou J, Huang C. Talen-mediated girdin knockout downregulates cell proliferation, migration and invasion in human esophageal carcinoma ECA109 cells. Mol Med Rep. 2014;10:848–854. doi: 10.3892/mmr.2014.2268. [DOI] [PubMed] [Google Scholar]
- 18.Gu F, Wang L, He J, Liu X, Zhang H, Li W, Fu L, Ma Y. Girdin, an actin-binding protein, is critical for migration, adhesion and invasion of human glioblastoma cells. J Neurochem. 2014;131:457–469. doi: 10.1111/jnc.12831. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Gu F, She C, Guo H, Li W, Niu R, Fu L, Zhang N, Ma Y. Reduction of Akt2 inhibits migration and invasion of glioma cells. Int J Cancer. 2009;125:585–595. doi: 10.1002/ijc.24314. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Bagaitkar J, Watabe K. Roles of AKT signal in breast cancer. Front Biosci. 2007;12:4011–4019. doi: 10.2741/2367. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh P, Garcia-Marcos M, Bornheimer SJ, Farquhar MG. Activation of Galphai3 triggers cell migration via regulation of GIV. J Cell Biol. 2008;182:381–393. doi: 10.1083/jcb.200712066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C, Ear J, Pavlova Y, Mittal Y, Kufareva I, Ghassemian M, Abagyan R, Garcia-Marcos M, Ghosh P. Tyrosine phosphorylation of the Galpha-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal. 2011;4:ra64. doi: 10.1126/scisignal.2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]