Abstract
Formation of male-specific structures and regression of female primordia are regulated in early male embryogenesis by SRY, a single-copy gene on the Y chromosome. Assignment of SRY as the testis-determining factor in eutherian mammals is supported by molecular analysis of cytogenetic sex reversal (i.e., XX males and XY females) and by complementary studies of transgenic murine models. Here we characterize the putative DNA-binding domain of SRY, which contains a conserved sequence motif shared by high-mobility group nuclear proteins and a newly recognized class of transcription factors. The SRY DNA-binding domain specifically recognizes with nanomolar affinity proximal upstream elements (designated SRYe) in the promoters of the sex-specific genes encoding P450 aromatase and Mullerian inhibiting substance (MIS). P450 aromatase catalyzes the conversion of testosterone to estradiol, and in the male embryo its expression is down-regulated. Conversely, MIS is expressed in the male embryo to induce testicular differentiation and regression of female reproductive ducts. SRYe-binding activity is observed in nuclear extracts obtained from embryonic urogenital ridge immediately preceding morphologic testicular differentiation. Our results support the hypothesis that SRY directly controls male development through sequence-specific regulation of target genes.
Full text
PDF

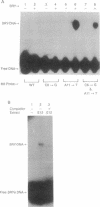
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berta P., Hawkins J. R., Sinclair A. H., Taylor A., Griffiths B. L., Goodfellow P. N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990 Nov 29;348(6300):448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George F. W., Milewich L., Wilson J. D. Oestrogen content of the embryonic rabbit ovary. Nature. 1978 Jul 13;274(5667):172–173. doi: 10.1038/274172a0. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Haqq C., Lee M. M., Tizard R., Wysk M., DeMarinis J., Donahoe P. K., Cate R. L. Isolation of the rat gene for Mullerian inhibiting substance. Genomics. 1992 Apr;12(4):665–669. doi: 10.1016/0888-7543(92)90291-y. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Harrison S. C. A structural taxonomy of DNA-binding domains. Nature. 1991 Oct 24;353(6346):715–719. doi: 10.1038/353715a0. [DOI] [PubMed] [Google Scholar]
- Hickey G. J., Krasnow J. S., Beattie W. G., Richards J. S. Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3',5'-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5' genomic DNA. Mol Endocrinol. 1990 Jan;4(1):3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Melton D. A. Diffusible factors in vertebrate embryonic induction. Cell. 1992 Jan 24;68(2):257–270. doi: 10.1016/0092-8674(92)90469-s. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Koopman P., Münsterberg A., Capel B., Vivian N., Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990 Nov 29;348(6300):450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Kuroda T., Lee M. M., Haqq C. M., Powell D. M., Manganaro T. F., Donahoe P. K. Mullerian inhibiting substance ontogeny and its modulation by follicle-stimulating hormone in the rat testes. Endocrinology. 1990 Oct;127(4):1825–1832. doi: 10.1210/endo-127-4-1825. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Jones C. M., Hogan B. L. The DVR gene family in embryonic development. Trends Genet. 1991 Nov-Dec;7(11-12):408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- Matsumine H., Herbst M. A., Ou S. H., Wilson J. D., McPhaul M. J. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J Biol Chem. 1991 Oct 25;266(30):19900–19907. [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Ner S. S. HMGs everywhere. Curr Biol. 1992 Apr;2(4):208–210. doi: 10.1016/0960-9822(92)90541-h. [DOI] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Parisi M. A., Clayton D. A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991 May 17;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- Picard J. Y., Benarous R., Guerrier D., Josso N., Kahn A. Cloning and expression of cDNA for anti-müllerian hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5464–5468. doi: 10.1073/pnas.83.15.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin R. C., Donahoe P. K., Kenneally M. K., Ahmad M. F., MacLaughlin D. T. Human müllerian inhibiting substance: enhanced purification imparts biochemical stability and restores antiproliferative effects. Protein Expr Purif. 1992 Jun;3(3):236–245. doi: 10.1016/1046-5928(92)90020-w. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Vigier B., Forest M. G., Eychenne B., Bézard J., Garrigou O., Robel P., Josso N. Anti-Müllerian hormone produces endocrine sex reversal of fetal ovaries. Proc Natl Acad Sci U S A. 1989 May;86(10):3684–3688. doi: 10.1073/pnas.86.10.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. L., Jones K. A. Purification of TCF-1 alpha, a T-cell-specific transcription factor that activates the T-cell receptor C alpha gene enhancer in a context-dependent manner. New Biol. 1990 Jul;2(7):621–636. [PubMed] [Google Scholar]
- de la Chapelle A. Analytic review: nature and origin of males with XX sex chromosomes. Am J Hum Genet. 1972 Jan;24(1):71–105. [PMC free article] [PubMed] [Google Scholar]