Abstract
8-bromo-7-methoxychrysin (BrMC), a novel chrysin analog, was reported to have anti-cancer activities. The aim of the present study was to investigate the molecular mechanism of 8-bromo-7-methoxychrysin (BrMC)-induced apoptosis via the Akt/forkhead box O3a (FOXO3a) pathway in cisplatin (DDP)-sensitive and -resistant ovarian cancer cells. The human ovarian cancer cell lines A2780 and A2780/DDP were cultured in vitro. Various molecular techniques were used to assess the expression of FOXO3a and B cell lymphoma 2 (Bcl-2)-interacting mediator of cell death (Bim) in cisplatin-sensitive and -resistant ovarian cancer cells. Different concentrations of BrMC induced apoptosis in cisplatin-sensitive and -resistant ovarian cancer cells. BrMC-induced apoptotic cell death occurred mainly by the activation of Akt, which was accompanied by the overexpression of transcription factor FOXO3a, with a concomitant increase in the expression levels of Bim. Silencing Bim expression by using small interfering RNA, attenuated the induction of apoptosis by BrMC treatment. The results indicated that BrMC-induced apoptosis in cisplatin-sensitive and -resistant ovarian cancer cells may occur via the regulation of Akt/FOXO3a, leading to Bim transcription.
Keywords: ovarian cancer, 8-bromo-7-methoxychrysin, Akt, forkhead box O3a, Bim, apoptosis
Introduction
Ovarian cancer is a highly lethal gynecological malignancy (1,2). Cisplatin (DDP) is the basal chemotherapeutic agent used to treat ovarian cancer, but due to an increase in resistance to cisplatin (3), there is now an urgent need to explore novel therapeutic interventions to treat ovarian cancer.
Chrysin (ChR), an active natural bioflavonoid found in honey and extracts of numerous plants, has a number of biological activities, including anti-oxidant (4), anti-inflammatory (5,6), and anti-cancer activities (7–10). 8-bromo-7-methoxychrysin (BrMC) a novel ChR derivative, has been reported to have anti-cancer activities with more potent bioactivity than the lead compound (11–14). It has been proposed that BrMC-induced cell cycle arrest and apoptosis may be the mechanisms of its anticancer effects (15,16). However, the precise underlying molecular mechanisms by which BrMC induces apoptosis in ovarian cancer cells are not fully elucidated.
Fork-head box O3a (FOXO3a) is a fork-head transcription factor of the FOXO subfamily characterized by a 'winged-helix' DNA-binding domain. FOXO3a is activated through phosphorylation by several stress kinases such as Akt kinase, which can upregulate multiple genes, of which B cell lymphoma 2 (Bcl-2) interacting mediator of cell death (Bim) is the key promoter of cell apoptosis. Akt has an oncogenic function, initially identified as a proto-oncogene in the mouse leukemia virus Akt8 (17). Phosphorylated Akt isoforms are seen at increased levels in the majority of human tumor types, and in ovarian cancers (18–20). Bim, found in a wide variety of tissues, is a member of the Bcl-2 homology domain 3 (BH3)-only family of pro-apoptotic proteins (21,22). Bim activates pro-apoptotic proteins Bcl-2 associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak), which in-turn exert their apoptotic activities with other pro-apoptotic and anti-apoptotic Bcl-2 family proteins (23). Akt is a key upstream regulator that initiates FOXO3a dephosphorylation and nuclear translocation, thereby enhancing the FOXO3a activity, leading to overexpression of FOXO3a-responsive genes such as Bim (24,25). Hence, FOXO3a links reduced Akt and increased Bim expression levels, which results in the induction of apoptosis in cancer cells (22,26,27).
In the present study, the focus was on understanding the role of BrMC in promoting cycle arrest and apoptosis in ovarian cancer cells. The role of the Akt/FOXO3a/Bim axis as a signaling cascade mediating the anti-apoptotic activity of cisplatin-sensitive and -resistant ovarian cancer was also studied.
Materials and methods
Materials
BrMC was synthesized as described previously (28). BrMC has a molecular weight of 347 g/mol, appeared as yellow crystals and had a purity of 99.0%, which was determined as previously described (14). ChR was purchased from the Sigma Chemical Co. (St Louis, MO, USA). BrMC and ChR were kept in dimethyl sulfoxide (DMSO) and diluted to a final concentration of 0.1% in DMSO. Propidium iodide (PI), MTT, DMSO, a selevtice caspase 3 inhibitor z-DEVD-fmk and LY294002 were also obtained from Sigma. Cell Apoptosis ELISA Detection kit (Roche Applied Sciences, Penzberg, Germany) was purchased. Polyclonal rabbit anti-Bax (cat. no. ab10813), mouse p53-upregulated modulator of apoptosis (PUMA; cat. no. F02210), rabbit anti-NOXA (cat. no. BY-7074R), rabbit anti-Bcl-2 (cat. no. HZ8392123) and rabbit anti-Bcl-extra large (Bcl-XL; cat. no. ab32370), polyclonal rabbit anti-Bim (cat. no. PC-033), rabbit anti-β-actin (cat. no. ab8229), rabbit anti-caspase-3 (cat. no. bs-0081R), and rat anti-caspase-9 (cat. no. C7729) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Caspase-3 specific inhibitor Z-Asp-Glu-Val-Asp-CH2F (Z-DEVD-fmk) was obtained from Calbiochem (La Jolla, CA, USA). Mouse monoclonal antibodies against FOXO3a (cat. no. 04-1007), phospho-FOXO3a-Thr32 (cat. no. JBC1381985), and antibodies against Akt (cat. no. 05-591) and phospho-Akt (Ser473; cat. no. 17-457) were purchased from Millipore (Bedford, MA, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and Lipofectamine™ 2000 were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). The enhanced chemoluminescence (ECL) western blot detection kit was purchased from NENTM Life Science (Dordrecht, Netherlands).
Cells and cell culture
The human ovarian cancer cell line A2780 was purchased from the China Center for Type Culture Collection (CCTCC; Wuhan, China). The cells were seeded in DMEM supplemented with 10% FBS (Hyclone), 100 U/ml penicillin and 100 µg/ml streptomycin (Hyclone) and incubated at 37°C in a humidified atmosphere of 5% CO2. Cisplatin-resistant cells were obtained by unremitting treatments with increasing concentrations of cisplatin (1, 5 and 10 µg/ml) (29). In brief, A2780 cells were exposed to cisplatin (5 µg/ml) reproducibly at identical conditions to those described above. Although cell death occured with treatment, after four to six weeks of regular replacement of culture medium (every two days) the drug-surviving cells aquired a normal growth pattern. The concentration of cisplatin was then increased to (7.5–10 µg/ml) and the process was repeated. Following establishment, the chemo-resistant variants were treated with cisplatin every month to maintain their high level of chemo-resistance.
Histone/DNA ELISA
The cisplatin-induced rate of apoptosis in both control and cisplatin-resistant cell lines were detected using an ELISA detection kit (Roche Applied Sciences), according to the manufacturer's instructions. Briefly, cells were cultured in 96-well plates at a density of 1×104 cells/well for 48 h and the test agents were added to the culture medium containing 10% FBS. After 24 h, the cytoplasm of cells in the control (untreated) and treatment (cisplatin-treated) groups was transferred to streptavidin-pre-coated 96-well plates, which had been previously incubated with a biotinylated histone antibody and peroxidase-tagged mouse anti-human DNA for 2 h at room temperature. The absorbance was measured at 405 nm using an ELX-800 ELISA plate reader (Bio-Tek, Winooski, VA, USA). In addition, the apoptosis rate induced by BrMC treatment under identical conditions was estimated using the same method.
MTT assay
Ovarian-cell viability was assessed by the MTT assay (30). Cells were cultured in a 96-well plate at a density of 5,000 cells/well. Following incubation for 24 h to allow for cell attachment, different concentrations of BrMC (1.0, 2.5, 5.0 and 10.0 µmol/l) were added to each well and cultured for 48 h. The medium was removed and then incubated with 5.0 mg/ml MTT for 4 h. Following centrifugation, the supernatant was removed. Finally, DMSO (100 µl) was added and absorbance at 570 nm wavelength (A570) was measured by means of an enzyme-linked immune detector plate reader (ELX-800 type; Bio-Tek, Shanghai, China). The relative cell proliferation inhibition rate was caluculated as follows: Inhibition rate = (1-average A570 of the experimental group/average A570 of the control group)×100%. The IC50 (defined as the drug concentration at which 50% cell viability was inhibited) was assessed from the dose-response curves using GraphPad Prism software (Version 4, GraphPad Software; La Jolla, CA, USA).
Cell cycle analysis by flow cytometry
A2780 and A2780/DDP cells were seeded in six-well plates at a density of 10,000 cells/well for 24 h, and then different concentrations of BrMC (2.5, 5.0 and 10.0 µmol/l) and ChR (50 µmol/l) were added and incubated for 48 h. The cells were harvested and kept at 4°C for 12 h. Then PI (Sigma) was added, and the apoptotic rate was analyzed by using a flow cytometer (EPICSXL; Beckman Coulter, Inc., Brea, CA, USA), with FC500 CXP software (Beckman Coulter, Inc.).
RNA interference
Akt and FOXO3a were purchased from Millipore; Bim and control small interfering RNA (siRNA) were purchased from Santa Cruz Biotechnology Inc. Human ovarian cancer A2780 and A2780/DDP cells were transfected with Akt, FOXO3a, Bim and control siRNA using Lipofectamine™ 2000, according to the manufacturer's instructions (31). The cells were then collected and processed for western blot analysis and histone/DNA ELISA.
Western blot analysis
Total cell extracts for western blot analysis were obtained, as described above for flow cytometry. Cell lysates containing 50 µg protein were run on a 7.5–12.5% SDS-PAGE and blotted onto polyvinylidene fluoride membranes (Millipore). Western blot analysis was carried out as previously described (15) and Anti-Bim, anti-Akt, anti-FOXO3a, anti-phospho-FOXO3a-Thr32 and anti-caspase-3 were used as primary antibodies. The blots were stripped at 37°C for 2 h. After being washed with Tris-buffered saline and Tween 20 for 30 min, the corresponding secondary antibody was added and incubated at room temperature for 1 h. The bound antibody was visualized using chemiluminescent substrate (ECL; GE Healthcare, Arlington Heights, IL, USA) and re-probed with an anti-actin antibody to normalize for differences in protein loading. Changes in the levels of the desired proteins were estimated using GelPro Analyzer® image analysis (Media Cybernetics, Inc., Bethesda, MD, USA) of the immunoreactive bands and corrected for β-actin loading control. Immunoblotting was performed a minimum of two times for each protein using independently prepared lysates to ensure reproducibility of the results.
Statistical analysis
The data were statistically analyzed using the SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA) and presented as the mean ± standard deviation. The means of multiple groups were compared using one-way analysis of variance following the equal check of variance and comparisons between the means were performed using the least significant difference method. Statistical comparison was also performed using a two-tailed t-test when appropriate. P<0.05 was considered to indicate a statistically significant difference between values.
Results
BrMC induces apoptosis in cisplatin-sensitive and -resistant ovarian cancer cells
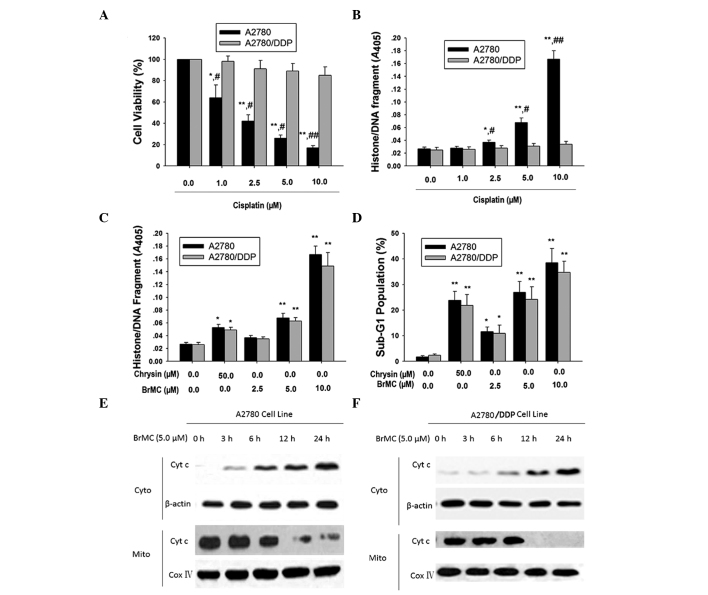
The growth inhibitory effect of cisplatin was determined in various cisplatin-sensitive (A2780) and -resistant (A2780/DDP) human ovarian cell lines. Cell viability was detected by the MTT assay after 48 h of treatment with cisplatin. As shown in Fig. 1A, cisplatin caused a dose-dependent reduction of cell viability in A2780 cells, while A2780/DDP cells exhibited resistance to cisplatin. To examine cisplatin-induced apoptosis, the cells were treated with cisplatin and apoptosis was confirmed by a histone/DNA fragmentation ELISA assay at various concentrations. Cisplatin effectively induced apoptosis in cisplatin-sensitive ovarian cancer cells, but not in cisplatin-resistant cells (Fig. 1B). To investigate the effects of BrMC on apoptosis in both ovarian cancer cell lines, the ovarian cancer cells were treated with BrMC and the cell apoptosis process was analyzed by a DNA fragmentation ELISA assay. BrMC induced cell apoptosis in both ovarian cancer cell lines, regardless of their differences in chemosensitivity (Fig. 1C). Flow cytometric (FCM) analysis with PI staining further revealed that there was no difference in BrMC-induced apoptosis between cisplatin-sensitive and -resistant cells (Fig. 1D). To determine whether mitochondria had an important role in BrMC-induced cell apoptosis, the release of cytochrome c was detected by cell fractionation analysis. The results revealed that BrMC induced the release of cytochrome c in a time-dependent manner in both chemo-sensitive and -resistant cells (Fig. 1E and F), suggesting that BrMC initiated apoptotic cell death through mitochondrial dysfunction.
Figure 1.
BrMC induces apoptosis in cisplatin-sensitive and -resistant human ovarian cells. (A and B) Effect of cisplatin on apoptosis in ovarian cancer cell lines in a dose-dependent manner. Cells were treated with the indicated concentrations of cisplatin for 48 h. ELISA was used to determine histone/DNA fragmentation. Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01 vs. 0.1% DMSO; #P<0.05, ##P<0.01 vs. treatment with 2.5 µmol/l BrMC. (C and D) Effect of BrMC on apoptotic death in cisplatin-sensitive and -resistant cells. Apoptosis was detected using propidium iodide and flow cytometric analysis. Cells were cultured in the presence or absence of BrMC (2.5 µM) for different time periods, and apoptosis was measured. All data are depicted graphically as the mean ± standard error of the mean for at least three independent experiments. *P<0.05, **P<0.01. (E and F) Analyses of cyt c release. Following BrMC treatment for different periods of time, cells were subjected to sub-cellular fractionation. The specific antibodies to cyt c, β-actin and Cox IV were used. Densitometric analysis of the western blots was performed and the amount of cyt c was compared with that of the loading control. Data are representative of at least three independent experiments. BrMC, 8-bromo-7-methoxychrysin; SD, standard deviation; DMSO, dimethyl sulfoxide; DDP, cisplatin; cyt c, cytochrome c; Cox IV, cytochrome c oxidase IV; Cyto, cytosol; Mito, mitochondria.
Bim is required for BrMC-induced apoptosis in chemo-sensitive and -resistant ovarian cancer cells
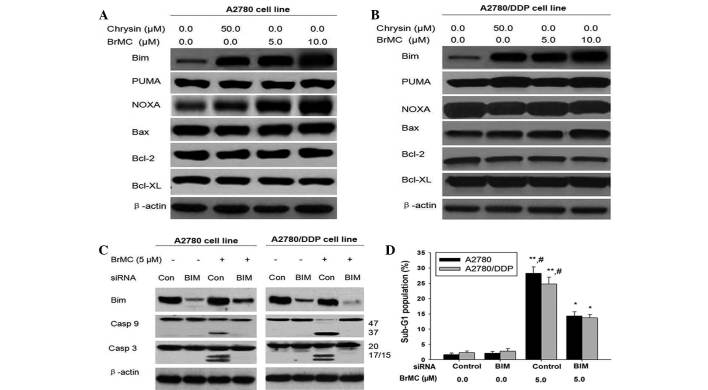
Mitochondrial dysfunction has an important role in apoptosis in ovarian cells (32). Numerous studies have reported that it regulated gene expression of Bcl-2-family proteins involved in chrysin-induced apoptosis (32) and that the BH3-only proteins were necessary for chrysin-induced apoptosis in cancer cells (33). However, it remains elusive whether BH3 proteins have the same function in ovarian cancer cells following BrMC treatment. Therefore, the present study investigated the expression of Bcl-2-family proteins in cisplatin-sensitive and -resistant cells following BrMC treatment. The levels of pro-apoptotic proteins, including Bax, PUMA and NOXA, showed a slight change in A2780 cells at various time-points following BrMC treatment (Fig. 2A). No major changes were recognized in anti-apoptotic Bcl-2 and Bcl-XL proteins. However, the expression levels of Bim showed a marked increase following BrMC treatment, providing evidence that Bim was involved in apoptotic cell death in ovarian cancer cells treated with BrMC. BrMC induced Bim was expressed in the same manner in A2780/DDP cells (Fig. 2B). These results implied that Bim had an important role in BrMC-induced apoptosis in ovarian cancer cells.
Figure 2.
BrMC-induced ovarian cancer cell apoptosis involved in Bim expression. (A and B) The expression levels of Bcl-2 family proteins in chemosensitive and -resistant ovarian cancer cells were examined by the time-dependent analysis and western blot analysis. A2780 and A2780/DDP cells were treated at the indicated concentrations with BrMC (5 and 10 µM) for 24 h. β-Actin was used as a control. (C) Under the same conditions as A, cells were transiently transfected with a control non-specific siRNA or Bim-specific siRNA for 48 h, and treated with or without BrMC for 24 h. Cell lysates were obtained and assayed for Bim, caspase-9 and cleaved caspase-3 by western blot. (D) Effects of Bim on the apoptotic rate in A2780 and A2780/DDP cells. All data were depicted graphically as the mean ± standard error of the mean for at least three independent experiments. *P<0.05, **P<0.01, vs. treatment with siRNA alone; #P<0.05, vs. treatment with BrMC (5 µmol/l) and Bim siRNA. BrMC, 8-bromo-7-methoxychrysin; Bcl-2, B-cell lymphoma 2; DDP, cisplatin; siRNA, small interfering RNA; PUMA, p53-upregulated modulator of apoptosis; Bax, Bcl-2 associated X protein; Bim, Bcl2-interacting mediator of cell death; Bcl-XL, B cell lymphoma extra large; Casp, caspase; Con, control non-specific siRNA; BIM, bim-specific siRNA.
To confirm the effect of Bim on apoptosis in ovarian cancer cells, gene silencing experiments were performed using a small interfering RNA (siRNA) specific for Bim that targeted all known isoforms of its transcript. Similar to the results in Fig. 2A, the expression levels of Bim were high in the control vector-transfected A2780 and A2780/DDP cells following BrMC treatment, while Bim expression levels in cells transfected with Bim siRNA were relatively low (Fig. 2C). Down-regulation of Bim inhibited both caspase-9 and caspase-3 activity induced by BrMC. Similarly, BrMC induced an increase in the percentage of cells in sub-G1 phase in the control vector-transfected A2780 and A2780/DDP cells, but not in Bim siRNA-transfected cells (Fig. 2D). These results provided further evidence that induction of Bim expression is necessary for BrMC-induced apoptosis in ovarian cancer cells.
Akt dephosphorylation is involved in cell apoptotic death in chemo-sensitive and -resistant ovarian cancer cells
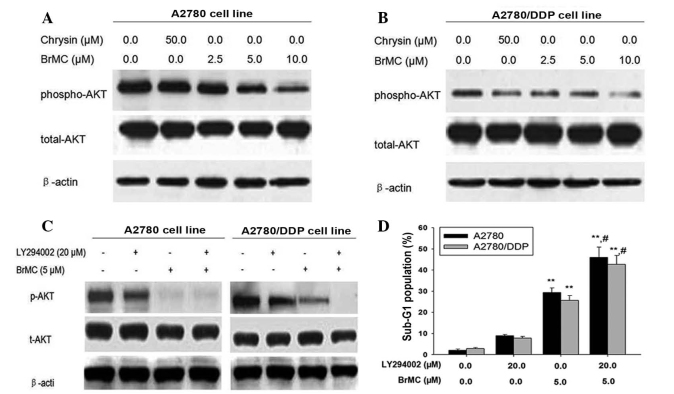
Recent studies have indicated that Akt modulates Bim activation either directly or indirectly (34,35). Moreover, chrysin induced apoptosis in leukemia cells by decreasing Akt activity and expression in cells (36). Based on these observations, it was speculated that Akt may be involved in BrMC-induced apoptosis by regulating Bim expression. Analysis was performed to determine whether Akt was involved in BrMC-induced apoptosis in ovarian cancer cells. As shown in Fig. 3A, BrMC induced the downregulation of Akt phosphorylation at Ser 473 in cisplatin-sensitive A2780 cells in a time-dependent manner. However, expression levels of total Akt protein exhibited no change. Similarly, BrMC induced the reduction of p-Akt in A2780/DDP cells (Fig. 3B), regardless of differences in chemo-sensitivity. To validate the role of Akt in BrMC-induced apoptosis, LY294002, a phosphoinositide 3-kinase inhibitor, was used to inhibit the phosphorylation of Akt by upstream signaling molecules and the pro-apoptotic effect of BrMC in ovarian cancer cells was examined. BrMC alone downregulated p-Akt and induced apoptosis, while LY294002 alone was not sufficient to induce apoptosis, though it partially decreased p-Akt. Further experiments proved that the combination of LY294002 and BrMC completely eliminated p-Akt and enhanced the induction of apoptosis in A2780 and A2780/DDP cells (Fig. 3C and D). This indicated that the effect of the combination of LY294002 and BrMC on p-Akt and apoptosis was more significant than that of LY294002 alone, and that the Akt signaling pathway was involved in BrMC-induced apoptosis in ovarian cancer cells.
Figure 3.
Akt is involved in BrMC-induced apoptosis. (A and B) Expression levels of t-Akt and p-Akt (Ser473) were detected by western blot analysis in A2780cells and A2780/DDP cells. Cells were treated with BrMC (2.5 µM) for 24 h, then lysed and assayed for individual protein levels by western blot. β-Actin was used as a control. p-Akt and Akt levels were measured by densitometric analysis of the western blots and compared to actin levels. (C) Detection of the role of LY294002 on apoptosis of cells. Cells were treated with BrMC and/or 20 µM LY294002 for 24 h, and then individual protein levels were detected by western blotting. (D) Apoptotic rate of the A2780 and A2780/DDP cells. All data are depicted graphically as the mean ± standard error of the mean for at least three independent experiments. **P<0.01; #P<0.05, vs. treatment with BrMC (5 µm) and LY294002 (20 µm). p/t-Akt, phosphorylated/total Akt; BrMC, 8-bromo-7-methoxychrysin; DDP, cisplatin.
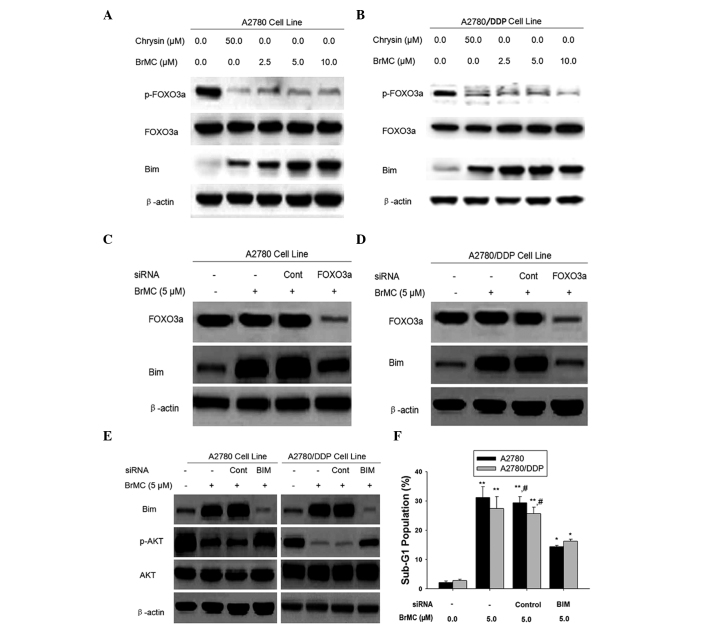
FOXO3a mediates upregulation of Bim during BrMC-induced apoptosis in chemosensitive and -resistant ovarian cancer cells
Akt mediates Bim activation via two primary pathways: (1) Akt phosphorylates Bim directly and inhibits Bim activation (34,35); (2) Akt phosphorylates FOXO3A, leading to its cytoplasmic retention by 14-3-3 proteins (11,37). This prevents FOXO3a translocation to the nucleus to induce Bim transcription, demonstrating that Akt indirectly regulates Bim activation. Therefore, the effect of BrMC on FOXO3a phosphorylation was examined. Fig. 4A and B, demonstrate the dose-dependent inhibition of FOXO3a phosphorylation by BrMC. As phosphorylation suppresses the transcriptional activity of FOXO3a, the expression of Bim was subsequently examined. As shown in Fig. 4A and B, in parallel with reduced FOXO phosphorylation, Bim was upregulated in response to BrMC treatment in A2780 and A2780/DDP cells.
Figure 4.
FOXO3a protein mediates Bim activation in ovarian cancer cells. Western blot analysis of (A) A2780 and (B) A2780/DDP cells treated with BRMC for 24 h. The expression of p-FOXO3a, t-FOXO3a and Bim was analyzed and β-actin was used as a loading control. (C and D) Cells were transiently transfected with a control non-specific siRNA or FOXO3a-specific siRNA for 48 h and treated with or without BrMC for 24 h. Cell lysates were obtained and assayed for FOXO3a and Bim by western blot. (E) Effect of Bim-siRNA on Akt phosphorylation. Cells were transfected with a control non-specific siRNA or Bim-specific siRNA for 48 h and were exposed to BrMC for 24 h. All data are representative of three independent experiments. (F) Effects of Bim-siRNA on the apoptotic rate of the A2780 and A2780/DDP cells. All data are depicted graphically as the mean ± standard error of the mean for at least three independent experiments. *P<0.05, **P<0.01, vs. apoptotic rate of the A2780 and A2780/DDP cells treated with siRNA alone; #P<0.05, vs. treatment with BrMC (5 µm) and Bim siRNA.. p/t-FOXO3a, phosphorylated/total forkhead box O3a; BrMC, 8-bromo-7-methoxychrysin; Bim, Bcl2-interacting mediator of cell death; siRNA, small interfering RNA; DDP, cisplatin; Cont, control; BIM, bim-specific siRNA; z-DEVD-fmk, Caspase-3 specific inhibitor Z-Asp-Glu-Val-Asp-CH2F.
To examine the effect of FOXO3a activation on the expression of Bim, A2780 and A2780/DDP cells were transfected with FOXO3a siRNA followed by treatment with BrMC for 24 h and Bim expression was then measured by western blot analysis (Fig. 4C and D). FOXO3a siRNA attenuated the induction of Bim expression by BrMC treatment (Fig. 4C and D). These data suggested that BrMC may regulate the expression of the FOXO3a transcriptional target, Bim. However, knockdown of Bim inhibited Akt dephosphorylation and apoptosis in chemo-sensitive and -resistant ovarian cancer cells (Fig. 4E and F). These results indicated that BrMC-induced Bim expression prevented the phosphorylation of Akt, indicating that Bim regulated Akt activation during BrMC stimulation in ovarian cancer cells.
Bim regulates Akt dephosphorylation by caspase-3 activity during BrMC-induced apoptosis in chemosensitive and -resistant ovarian cancer cells
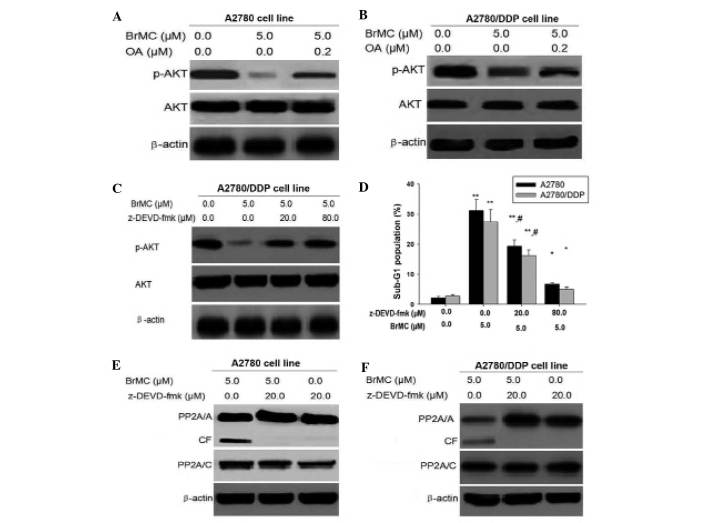
It was reported that caspase-3 regulated the phosphorylation of Akt through the cleavage of the regulatory A subunit of (PP2A/A), increasing PP2A activity (38). In the caspase inhibitor assay, cells were pretreated with caspase inhibitors (20 µM ZVAD-CHO) for 1 h prior to the addition of the agents tested. Results of the present study demonstrated that Bim was involved in the regulation of Akt dephosphorylation (Fig. 4E). Therefore, it was proposed that Bim may regulate Akt phosphorylation by caspase-3-mediated PP2A activation in ovarian cells. To validate this hypothesis, it was first determined whether PP2A mediated Akt phosphorylation. Pretreatment of the cells with okadaic acid (OA), an inhibitor of PP2A, resulted in reversal of BrMC-induced Akt dephosphorylation in A2780 cells (Fig. 5A). At the same time, OA also rescued Akt phosphorylation in A2780/DDP cells during BrMC treatment (Fig. 5B).
Figure 5.
Caspase-3 regulates the phosphorylation of Akt associated with PP2A. (A and B) A2780 and A2780/DDP cells were pre-treated with indicated concentrations of okadaic acid for 1 h and exposed to BrMC for 24 h, then detected for Akt and p-Akt (Ser473) levels by western blot analysis. β-Actin was used as a control. (C) To determine the Akt phosphorylation. A2780/DDP cells were pre-treated with or without various doses of caspase 3 inhibitor z-DEVD-fmk (20 or 80 µM) for 1 h and exposed to BrMC (5 µM) for 24 h, then p-Akt (Ser473) levels were detected using western blot analysis. (D) The apoptotic rate of the A2780 and A2780/DDP cells following pre-treatment with different doses of z-DEVD-fmk. All data are depicted graphically as the mean ± standard error of the mean of at least three independent experiments. *P<0.05, **P<0.01, vs. apoptotic rate of the A2780 and A2780/DDP cells treated with BrMC (5 µm) alone; #P<0.05, vs. treatment with BrMC (5 µm) and z-DEVD-fmk (20 µm). (E and F) Detection of the binding of Akt with PP2A. Cells were incubated with or without BrMC for 48 h, 20 µM of z-DEVD-fmk was added 1 h prior to treatment with the drug. The cells were lysed with lysis buffer for immunoprecipitation with anti-Akt antibody followed by immunoblot assay with anti-PP2A/C and anti-Akt antibodies. Data are representative of at least three independent experiments. p-Akt, phosphorylated Akt; CF, cleaved form of PP2A; PP2A, protein phosphotase 2A; BrMC, 8-bromo-7-methoxychrysin; DDP, cisplatin; z-DEVD-fmk, Caspase-3 specific inhibitor Z-Asp-Glu-Val-Asp-CH2F.
To determine whether caspase-3 mediates Akt phosphory-lation, cells were pre-treated with z-DEVD-fmk, a specific caspase-3 inhibitor. The results indicated that inhibition of caspase-3 activation restored Akt phosphorylation in the presence of BrMC (Fig. 5C and D).
In addition, as shown in Fig. 5E and F, a low but detectable quantity of the catalytic C subunit of PP2A (PP2A/C) was co-precipitated with Akt in untreated cells, indicating the occurrence of a physiological association between these two proteins. Of note, the PP2A-Akt association was greatly improved after 24 h treatment with BrMC, whereas this interaction was significantly decreased by the inhibition of caspase-3 and PP2A activation. This suggested that the dephos-phorylation of Akt was a consequence of caspase-mediated PP2A activation and that the caspase-3-PP2A-Akt pathway provided a positive feedback loop for apoptosis. These results also indicated that Bim-caspase-Akt may represent a novel pathway that regulates BrMC-induced apoptosis in ovarian cancer cells.
Discussion
The present study found that BrMC had a stronger effect than ChR on the inhibition of apoptosis in the colon cancer cell line HT-29 and gastric cancer cell line SGC-7901. Understanding the mechanism by which BrMC induces apop-tosis may identify potential novel targets for cancer therapies. The present study demonstrated that BrMC induced apoptotic cell death of cisplatin-sensitive and -resistant ovarian cancer cells in a dose-dependent manner and induced the release of cytochrome c in a time-dependent manner. The apoptotic effect of BrMC was found to be greater than that of ChR, regardless of their differences in chemosensitivity. Furthermore, it was shown that BrMC induced apoptosis of cisplatin-sensitive and -resistant ovarian cancer cells accompanied with the upregulation of Bim. Conversely, the downregulation of Bim inhibited caspase-9 and caspase-3 activities induced by BrMC. It was also found that the downregulation of p-Akt at Ser 473 in BrMC-treated cells occurred in a time-dependent manner. In addition, it was indicated that BrMC eliminated p-Akt and enhanced induction of apoptosis. Moreover, BrMC inhibited the expression of p-FOXO3a in cells, which in parallel increased Bim expression with increasing BrMC dose, therefore demonstrating that the FOXO3a transcriptional target was upregulated. Furthermore, Bim may regulate Akt phosphorylation by caspase-3-mediated PP2A activation in ovarian cells (Fig. 5).
It was demonstrated that Bim had a very important role in cisplatin-sensitive and -resistant ovarian cancer cells during BrMC-induced apoptosis through detecting the expression levels of Bcl-2-family proteins. It has been reported that upregulation of Bim expression contributes to nitric oxide synthatse releasing compound-induced apoptosis in MDA-MB-453 cells (39). Therefore, results of the present study confirmed that the upregulation of Bim prompted apop-tosis induced by BrMC treatment. Furthermore, the present study showed that downregulation of Bim by siRNA inhibited BrMC-induced cell apoptosis.
Akt fuctioning as a stress kinase can upregulate multiple genes that promote cell apoptosis (40). It has been reported by several studies that Akt modulates Bim activation either directly or indirectly, resulting in cell death (22,34,35). The present study showed that pre-treatment with LY294002 to inhibit the phosphorylation of Akt enhanced BrMC-induced apoptosis in chemosensitive cells. These activities were accompanied by a decrease in p-Akt, but not total Akt. Furthermore, the data indicated that knockdown of Bim inhibited Akt dephosphorylation and apoptosis in chemo-sensitive and -resistant ovarian cancer cells. The FOXO3a transcription factor is a tumor suppressor that is inactivated in the majority of human cancers (40). FOXO3a can directly up-regulate Bim expression levels and induce apoptosis in cells. Akt-dependent phosphorylation of FOXO3A leads to its cytoplasmic retention by 14-3-3 proteins and loss of target gene activation (11,37). Consequently, FOXO3a cannot translocate into the nucleus and induce Bim transcription, which therefore indicates indirect Akt regulation of Bim activation. In the present study, it was demonstrated that BrMC inhibited FOXO3a phosphorylation. Moreover, knockdown of FOXO3a by transfection with siRNA blocked the BrMC-induced downregulation of Bim expression and inhibited ovarian cancer cell apoptosis. These findings indicated that FOXO3a was a key regulator of BrMC-induced apoptosis in ovarian cancer cells.
Conversely, observation of the mechanism of p-Akt showed that Bim siRNA prevented caspase-3 cleavage. In addition, a PP2A-linked pathway to determine Bim-dependent phosphorylation of Akt during BrMC-induced ovarian cancer cell apoptosis was detected as well as the factors that mediate Akt/FOXO3a expression during cell apoptosis.
In conclusion, the present study indicated that BrMC regulated the Akt/FOXO3a signal cascade pathway through mediating the expression of its target genes, including Bim, caspase-3 and PP2A, leading to apoptosis in ovarian cancer cells. A thorough understanding is required to assess the anticancer activities of BrMC in vivo. The mechanisms of BrMC-induced apoptosis may lead to the identification and development of novel therapeutic compounds for the treatment and prevention of ovarian cancer.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr Jianguo Cao for instructing the experiments and providing the laboratory facilities. The authors would also like to thank Mrs Kaiqun Ren for analyzing the data.
References
- 1.Lan C, Chenggang W, Yulan B, Xiaohui D, Junhui Z, Xiao W. Aberrant expression of WWOX protein in epithelial ovarian cancer: a clinicopathologic and immunohistochemical study. Int J Gynecol Pathol. 2012;31:125–132. doi: 10.1097/PGP.0b013e3182297fd2. [DOI] [PubMed] [Google Scholar]
- 2.Vlahovic G, Meadows KL, Uronis HE, et al. A phase I study of bevacizumab, everolimus and panitumumab in advanced solid tumors. Cancer Chemother Pharmacol. 2012;70:95–102. doi: 10.1007/s00280-012-1889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egawa-Takata T, Endo H, Fujita M, et al. Early reduction of glucose uptake after cisplatin treatment is a marker of cisplatin sensitivity in ovarian cancer. Cancer Sci. 2010;101:2171–2178. doi: 10.1111/j.1349-7006.2010.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SS, Bae I, Lee YJ. Flavonoids-induced accumulation of hypoxia-inducible factor (HIF)-1alpha/2alpha is mediated through chelation of iron. J Cell Biochem. 2008;103:1989–1998. doi: 10.1002/jcb.21588. [DOI] [PubMed] [Google Scholar]
- 5.Shin EK, Kwon HS, Kim YH, Shin HK, Kim JK. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun. 2009;381:502–507. doi: 10.1016/j.bbrc.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 6.Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281:37102–37110. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Yoon JH, Song KS. Chrysin inhibited stem cell factor (SCF)/c-Kit complex-induced cell proliferation in human myeloid leukemia cells. Biochem Pharmacol. 2007;74:215–225. doi: 10.1016/j.bcp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12:6097–6105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Monasterio A, Urdaci MC, Pinchuk IV, López-Moratalla N, Martínez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 10.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881–884. doi: 10.1016/S0960-894X(02)01081-8. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Cao JG, Liao DF, Zhu BY, Liu HT. Synthesis and anticancer effect of gem-difluoromethylenated chrysin derivatives. Chin Chem Lett. 2006;11:1431–1442. In Chinese. [Google Scholar]
- 13.Xiang H, Zheng X, Cao J. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 8-bromo-7-methoxychrysin. Zhongguo Yaolixue Tongbao. 2008;24:1370–1373. [Google Scholar]
- 14.Ai XH, Zheng X, Tang XQ, et al. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol. 2007;13:3824–3828. doi: 10.3748/wjg.v13.i28.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XH, Zheng X, Cao JG, Xiang HL, Liu F, Lv Y. 8-Bromo-7-methoxychrysin-induced apoptosis of hepatocellular carcinoma cells involves ROS and JNK. World J Gastroenterol. 2010;16:3385–3393. doi: 10.3748/wjg.v16.i27.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao G, Tang X, Yao C, Wang C. Potentiation of arsenic trioxide-induced apoptosis by 8-bromo-7-methoxychrysin in human leukemia cells involves depletion of intracellular reduced glutathione. Acta Biochim Biophys Sin (Shanghai) 2011;43:712–721. doi: 10.1093/abbs/gmr065. [DOI] [PubMed] [Google Scholar]
- 17.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher JI, Huang DC. Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 24.van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 2006;66:10760–10769. doi: 10.1158/0008-5472.CAN-06-1111. [DOI] [PubMed] [Google Scholar]
- 25.Essafi A, Fernàndez de Mattos S, Hassen YA, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 26.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 27.Sunters A, Fernàndez de Mattos S, Stahl M, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 28.Simonin K, Brotin E, Dufort S, et al. Mcl-1 is an important determinant of the apoptotic response to the BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian carcinoma cells. Mol Cancer Ther. 2009;8:3162–3170. doi: 10.1158/1535-7163.MCT-09-0493. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Wu ZM, McGowan E, et al. Arsenic trioxide and cisplatin synergism increase cytotoxicity in human ovarian cancer cells: therapeutic potential for ovarian cancer. Cancer Sci. 2009;100:2459–2464. doi: 10.1111/j.1349-7006.2009.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Ahmad A, Banerjee S, et al. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharm Res. 2010;27:1159–1168. doi: 10.1007/s11095-010-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning Y, Li Q, Xiang H, Liu F, Cao J. Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep. 2012;27:1857–1864. doi: 10.3892/or.2012.1739. [DOI] [PubMed] [Google Scholar]
- 32.Polier G, Ding J, Konkimalla BV, et al. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011;2:e182. doi: 10.1038/cddis.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao XC, Cao XC, Liu F, et al. Regulation of the FOXO3a/Bim signaling pathway by 5,7-dihydroxy-8-nitrochrysin in MDA-MB-453 breast cancer cells. Oncol Lett. 2013;5:929–934. doi: 10.3892/ol.2012.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Z, Wang F, Zhao Z, et al. BIM-mediated Akt phosphorylation is a key modulator of arsenic trioxide-induced apoptosis in cisplatin-sensitive and -resistant ovarian cancer cells. PLoS One. 2011;6:e20586. doi: 10.1371/journal.pone.0020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen KF, Yeh PY, Yeh KH, Lu YS, Huang SY, Cheng AL. Down-regulation of phospho-Akt is a major molecular determinant of bortezomib-induced apoptosis in hepatocellular carcinoma cells. Cancer Res. 2008;68:6698–6707. doi: 10.1158/0008-5472.CAN-08-0257. [DOI] [PubMed] [Google Scholar]
- 36.Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325:1215–1222. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 37.Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int J Cancer. 2008;122:534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Akhand AA, Takeda K, et al. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death Differ. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 39.Ramos AM, Fernández C, Amràn D, Sancho P, de Blas E, Aller P. Pharmacologic inhibitors of PI3K/Akt potentiate the apoptotic action of the antileukemic drug arsenic trioxide via glutathione depletion and increased peroxide accumulation in myeloid leukemia cells. Blood. 2005;105:4013–4020. doi: 10.1182/blood-2004-07-2802. [DOI] [PubMed] [Google Scholar]
- 40.Kornblau SM, Singh N, Qiu Y, et al. Highly phosphorylated FOX03A is an adverse prognostic factor in acute myeloid leukemia. Clin Cancer Res. 2010;16:1865–1874. doi: 10.1158/1078-0432.CCR-09-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]