Abstract
Importance
The continuum view of the psychosis spectrum (PS) implies that in population-based samples, PS symptoms should be associated with neural abnormalities similar to those found in help-seeking clinical-risk individuals and in schizophrenia. Functional neuroimaging has not previously been applied in large population-based PS samples, and can help understand the neural architecture of psychosis more broadly, and identify brain phenotypes beyond symptomatology that are associated with the extended psychosis phenotype.
Objective
To examine the categorical and dimensional relationships of PS symptoms to prefrontal hypoactivation during working memory and to amygdala hyperactivation during threat emotion processing.
Design
The Philadelphia Neurodevelopmental Cohort (PNC) is a genotyped prospectively accrued population-based sample of nearly 10,000 youths, who received a structured psychiatric evaluation and a computerized neurocognitive battery. A subsample of 1,445 subjects underwent neuroimaging including functional magnetic resonance imaging (fMRI) tasks examined here.
Setting
The PNC is a collaboration between The Children’s Hospital of Philadelphia and the Hospital of the University of Pennsylvania.
Participants
Youths ages 11–22 years identified through structured interview as having psychosis-spectrum features (PS, n=260), and typically developing comparison subjects without significant psychopathology (TD, n=220).
Main Outcomes and Measures
Two fMRI paradigms were utilized, a fractal n-back working memory task probing executive system function, and an emotion identification task probing amygdala responses to threatening faces.
Results
In the n-back task, PS showed reduced activation in executive control circuitry, which correlated with cognitive deficits. During emotion identification, PS demonstrated elevated amygdala responses to threatening facial expressions, which correlated with positive symptom severity.
Conclusions and Relevance
The pattern of functional abnormalities observed in PS participants is similar to that previously found in schizophrenia and help-seeking risk samples. Specific circuit dysfunction during cognitive and emotion-processing tasks is present early in the development of psychopathology, and here cannot be attributed to chronic illness or medication confounds. Hypoactivation in executive circuitry and limbic hyperactivation to threat could reflect partly independent risk factors for psychosis-spectrum symptoms; the former relating to cognitive deficits that increase risk for developing psychotic symptoms, the latter contributing directly to positive psychotic symptoms.
Major efforts are underway to identify neural circuit abnormalities linked to the emergence of psychosis, typically manifesting during adolescence.1,2,3 Functional brain abnormalities previously identified in schizophrenia provide candidates for imaging markers associated with psychosis across the spectrum of age and clinical severity. These include activation abnormalities in prefrontal cortex during working memory4 and in amygdala during processing of threatening facial expressions.5–12
Functional imaging studies examining psychosis risk have included relatively small samples of individuals at genetic risk due to family history, or help-seeking individuals at clinical risk based on attenuated psychosis-spectrum symptoms. These studies have identified various abnormalities, but there are few confirmed findings.
Furthermore, prior fMRI studies of psychosis risk have typically examined only categorical effects, lacking adequate power to identify correlates of illness dimensions. Thus, it remains unclear to what extent reported abnormalities relate to the positive psychotic symptoms used to select the samples, versus negative symptoms or cognitive deficits that are likely also present.
Population-based studies have identified psychosis-spectrum symptoms in non-help seeking youth.13 While rates of transition to clinically diagnosed psychosis are likely to be reduced in such samples, early sub-clinical psychotic symptoms are of clinical relevance even in individuals who do not transition to frank psychosis.14,15 As noted by Kelleher and Cannon,16 the non-clinical psychosis-spectrum represents a valid and valuable population for investigating the early neurodevelopmental etiology of psychosis. However, application of functional neuroimaging in this context has been very limited especially at younger ages,17–23 and it remains unknown whether psychosis-spectrum symptoms in this population have similar neural underpinnings as those in clinical risk samples and schizophrenia, as predicted by the continuum view of the extended psychosis phenotype.16,24,25 Such population-based imaging studies are needed to understand the neural architecture of psychosis more broadly, and could provide imaging markers that enhance the specificity and predictive value of early psychotic-like symptoms that may otherwise be relatively non-specific.
We examined executive and emotion-processing neural circuit function in a community sample of youths with psychosis-spectrum (PS) symptoms, compared to typically developing (TD) youths, in the Philadelphia Neurodevelopmental Cohort. PS subjects in this cohort exhibit significant distress as well as neurocognitive and functional impairment,26,27 consistent with findings in other community samples15,28 and supporting the validity of our PS identification. The large sample enables identification of imaging features that differentiate PS from TD, and examination of heterogeneity among PS subjects relating to dimensional measures of psychosis symptom severity and cognitive deficits.
We hypothesized that psychosis risk subjects would show both hypofunction of executive networks during a working memory task and hyper-reactivity of amygdala in response to threatening faces in an emotion identification task. We further hypothesized that PS would perform more poorly than TD and that, within PS, executive hypofunction would correlate with cognitive deficits while amygdala hyper-reactivity would correlate with psychosis severity.
METHODS
Participants
We report task-based functional magnetic resonance imaging (fMRI) results from youths age 11–22 identified as having psychosis-spectrum features (PS, n=260) and typically developing (TD, n=220) comparison subjects with no significant psychopathology (Table 1). Participants were part of the Philadelphia Neurodevelopmental Cohort (PNC), a previously genotyped prospectively accrued community sample of nearly 10,000 youths,29 of which a subsample of 1,445 subjects underwent neuroimaging.30 Details of clinical and cognitive assessment have been previously reported, 26,27 and are summarized in eMethods in Supplement. PS subjects were identified as endorsing subthreshold symptoms (positive or negative/disorganized) on any of the following measures: 1) age-deviant PRIME Screen-Revised (PS-R) total score >= 2SD above age-matched peers, or >=1 PS-R items rated Definitely Agree or >=3 PS-R items rated Somewhat Agree; 2) endorsed definite or possible hallucinations or delusions on the K-SADS psychosis screen; or 3) had an age-deviant total negative/disorganized SOPS score >=2SD above age-matched peers. TD subjects lacked any significant psychopathology, and any history of psychoactive medication use or inpatient psychiatric treatment. Additional exclusion criteria are described in eMethods and eFigure 1. All study procedures were approved by the Institutional Review Boards of the University of Pennsylvania and Children’s Hospital of Philadelphia. All subjects, and for minors their parent or guardian, provided informed consent, and minors provided assent.
Table 1.
Demographic and clinical variables
| Variable | TD (n=220) | PS (n=260) | p-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Percentage | Proportion | Percentage | Proportion | ||
|
|
|||||
| Gender (% Female) | 52.7% | 116F/104M | 52.7% | 137F/123M | 0.99a,b |
| Race (% Caucasian) | 61.4% | 135C/85NC | 33.5% | 87C/173NC | <0.001 |
| Handed (% Right) | 83.6% | 184R/36L | 85.0% | 221R/39L | 0.71 |
| Mean (SD) | Range | Mean (SD) | Range | ||
|
|
|||||
| Age at scan (yrs) | 16.6 (3.0) | 11.2–22.6 | 15.7 (2.7) | 11.3–21.8 | 0.003c |
| Education (yrs) | 9.5 (2.9) | 4–16 | 8.3 (2.6) | 3–14 | <0.001 |
| Parental Education | 14.6 (2.4) | 7–20 | 13.5 (2.1) | 8–20 | <0.001 |
| Positive Symptomsd | −0.56 (0.31) | −0.86–0.97 | 1.16 (1.23) | −0.86–5.07 | <0.001 |
| Neg./Disorg.Symptomse | −0.54 (0.36) | −0.82–0.95 | 0.65 (1.41) | −0.82–6.49 | <0.001 |
| Global Cognitionf | 0.21 (0.48) | −2.13–1.09 | −0.17 (0.60) | −2.78–1.08 | <0.001 |
All p-values in the table are 2-tailed, uncorrected
Fisher’s Exact Test, two-tailed, was used to compare proportions for categorical variables
Student’s t test used for comparing group means
Age-normed Total score from Prime Screen-Revised
Age-normed sum of negative/disorganized items from Scale of Prodromal Symptoms
Age-normed accuracy score across Penn CNB Domains
Functional Imaging
Two fMRI paradigms were utilized, a fractal n-back working memory task probing executive system function, and an emotion identification task probing amygdala responses to threatening faces (Figure 1A&B). The n-back task 31,32 employed fractals and a robust block design to measure activation of the executive system across three levels of working memory load. To examine amygdala responses to threat, we applied a validated emotion identification paradigm, 33,34 grouping expressions into threatening (anger, fear) and non-threatening (happy, sad) emotions for event-related analysis, as in prior work 12,34–36. This grouping is based on prior theoretical and empirical work (see Satterthwaite et al.34 pp. 354–355 for detailed rationale); it is worth noting, however, that interpretations of this grouping not directly related to Threat are possible (e.g., negative and positive emotions), and subjective responses to particular emotional categories may vary according to individual subject characteristics including psychiatric symptomatology.
Figure 1. fMRI paradigms.
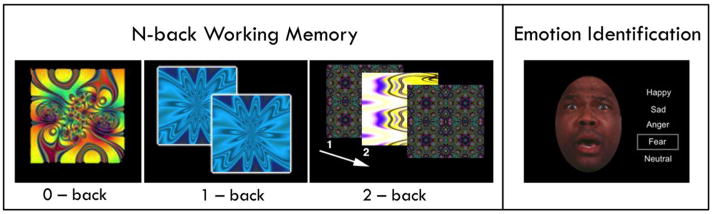
Two fMRI tasks were employed, counterbalancing order across subjects. The fractal n-back task is shown on the left. Working memory was tested with complex geometric figures (fractals), with three levels of memory load (0-back, 1-back, 2-back) across blocks. In the 0-back condition, participants responded with a button press to a specified target fractal. For the 1-back condition, participants responded if the current fractal was identical to the previous one; in the 2-back condition, participants responded if the current fractal was identical to the item presented two trials previously. Each condition consisted of a 20-trial block (60 s); each level was repeated over three blocks. The target-foil ratio was 1:3 in all blocks with 45 targets and 135 foils overall. Each fractal was presented for 500ms, followed by an inter-stimulus interval of 2500ms. The emotion identification task is shown on the right. Faces expressing one of five emotional categories (happy, sad, anger, fear, neutral) were displayed in a pseudorandomized event-related design; there were 12 faces in each category. Subjects decided which emotion was expressed on each face. Emotional expressions were grouped into threatening (anger, fear) and non-threatening (happy, sad) emotions for analysis. Faces were displayed for 5.5 seconds, followed by a variable (0.5–18.5s) interval displaying a complex fixation crosshair matched to faces on perceptual qualities.
All imaging data were acquired on the same scanner (Siemens Tim Trio 3 Tesla; 32 channel head coil) using the same imaging sequences. Imaging acquisition sequences, procedures, and pre-processing methods have been previously reported 30 and are described in eMethods.
Behavioral Data Analysis
For the n-back, our primary behavioral measure was d′, which summarizes overall task performance accounting for both the number of correct responses and the number of false positives. 32,37 In addition, median response time across correct responses was calculated. For emotion identification, task performance was summarized by percent accuracy and median correct response time. Behavioral measures were analyzed for group differences using t-tests, with a significance criterion of p<0.05, one-tailed to test the hypothesis that the PS group performs more poorly than TD, as expected based on prior study of the full PNC sample.26
Subject-level fMRI Analysis
Subject-level statistical analyses were carried out with a canonical hemodynamic response function in FEAT38. For the n-back, three condition blocks (0-back, 1-back, and 2-back) were modeled. Six motion parameters and the instruction period were included as nuisance covariates, and the rest (fixation) condition provided unmodeled baseline. The contrast of interest was 2-back > 0-back, capturing the effect of increasing working memory load. For emotion identification, events were modeled as 5.5sec boxcar, matching the duration of face presentation. Five individual emotion regressors were included together with their temporal derivatives and six motion parameters. The contrast of interest was Threat (anger + fear > fixation); Nonthreat and Neutral were also examined to assess specificity of effects observed for Threat.
Group-level fMRI Analysis
For each subject, first–level statistical maps for contrasts of interest were entered into second-level group analyses. PS and TD groups were compared using voxelwise whole-brain analyses followed by region of interest (ROI) analyses. Whole-brain group analyses employed voxelwise linear mixed-effects analysis (FLAME1). Type I error was controlled by cluster correction for multiple voxelwise comparisons, using voxel height Z>3.09 and cluster extent significance threshold p<0.05, calculated using Monte-Carlo simulations in AFNI AlphaSim. Hypothesized within-group correlations with cognition and symptom severity (using the measures in Table 1) were examined in relation to first-level regression parameters averaged across voxels within specific ROIs. These ROIs were defined functionally from clusters showing significant abnormalities in the PS group. For the n-back, we used the bilateral DLPFC regions showing TD>PS effects in the 2back>0back contrast (8-mm radius spheres surrounding peak coordinates in middle frontal gyrus regions reported in Table 2, top). For emotion identification, we used the bilateral amygdala clusters showing the PS>TD effect in the Threat contrast (Table 2, bottom). The key ROI results were also examined with confound and sensitivity regression analyses, described in eMethods and eResults. Exploratory whole-brain voxelwise correlations with cognition and positive symptom severity were also conducted with the same multiple-comparison approach described above.
Table 2.
Clusters showing group differences in key fMRI contrasts
| Voxels | Region | Max-Z | Cohen’s d | x | y | z |
|---|---|---|---|---|---|---|
| 2-back>0-back TD>PS | ||||||
| 9208 | dACC/paracingulate/SMA | 6.36 | 0.60 | −4 | 10 | 54 |
| Frontal Pole L | 5.87 | 0.55 | −34 | 48 | 14 | |
| Ant. Insula L | 4.34 | 0.41 | −28 | 28 | −2 | |
| Ant. Insula R | 4.24 | 0.40 | 34 | 26 | −2 | |
| Middle Frontal Gyr L | 4.69 | 0.44 | −46 | 18 | 34 | |
| Sup Frontal Gyr L | 6.30 | 0.59 | −28 | −6 | 62 | |
| Sup Frontal Gyr R | 4.55 | 0.43 | 28 | −2 | 52 | |
| Thalamus/Caudate/Pallidum B | 5.11 | 0.48 | −10 | −18 | 14 | |
| Midbrain R | 4.21 | 0.40 | 8 | −14 | −10 | |
| 4596 | Superior Lateral Occipital R | 6.33 | 0.60 | 10 | −70 | 62 |
| Superior Lateral Occipital L | 4.86 | 0.46 | −20 | −74 | 60 | |
| Precuneus | 5.20 | 0.49 | 4 | −68 | 52 | |
| Superior Parietal Lobule L | 4.43 | 0.42 | −26 | −56 | 44 | |
| Superior Parietal Lobule R | 4.51 | 0.43 | 28 | −56 | 44 | |
| 2361 | Cerebellum L | 5.32 | 0.50 | −8 | −72 | −26 |
| Cerebellum R | 4.80 | 0.45 | 32 | −62 | −30 | |
| 1304 | Frontal Pole R | 5.29 | 0.50 | 34 | 42 | 22 |
| Middle Frontal Gyr R | 4.00 | 0.38 | 40 | 28 | 38 | |
| 209 | Pons | 4.44 | 0.42 | 2 | −38 | −40 |
| 160 | Occipital Pole R | 3.73 | 0.35 | 24 | −84 | −10 |
| 116 | Cerebellum L | 4.08 | 0.38 | −28 | −40 | −40 |
| 86 | Occipital Pole L | 4.02 | 0.38 | −24 | −92 | −2 |
| 2-back>0-back PS>TD | ||||||
| 129 | Superior Frontal Gyr | 4.77 | 0.45 | −12 | 36 | 58 |
| Threat PS>TD | ||||||
| 470 | Superior Frontal Gyr R | 4.27 | 0.40 | 26 | 20 | 64 |
| 195 | Amygdala R | 4.44 | 0.42 | 28 | −4 | −18 |
| 146 | Superior Frontal Gyr L | 4.40 | 0.41 | −26 | 22 | 48 |
| 58 | Fusiform Cortex L | 3.43 | 0.32 | −44 | −70 | −8 |
| 54 | Middle Frontal Gyr L | 3.77 | 0.35 | −34 | 6 | 62 |
| 50 | Amygdala L | 3.70 | 0.35 | −24 | −4 | −18 |
| Threat TD>PS | ||||||
| 619 | Supramarginal Gyr R | 5.31 | 0.50 | 64 | −28 | 28 |
| 198 | Supramarginal Gyr L | 4.00 | 0.37 | −64 | −18 | 28 |
| 55 | Insula L | 4.03 | 0.38 | −40 | 4 | 2 |
Statistical threshold Z>3.09, cluster extent p<.05 (>41 vox).
Peak-Z x, y, z coordinates are in MNI space.
Peak Cohen’s d effect size estimated from peak Z value using formula d=2Z/N^0.5
dACC=dorsal anterior cingulate; SMA = supplementary motor area
RESULTS
Demographic and Clinical Variables
As expected, PS subjects had significantly higher levels of psychosis-spectrum symptoms (Table 1). Overall cognitive performance on the CNB was lower in PS, consistent with findings in the full PNC sample.26 In addition, the PS group was significantly younger, achieved fewer years of education (even after accounting for age), and had lower average parental education; groups did not differ in gender ratio.
Behavioral Performance
In the n-back task, the PS group exhibited worse discrimination accuracy (d′) than the TD group (t=6.13, p<0.001); response times (RT) did not differ (t=0.43, p=0.66). As memory load increased, the expected decrease in accuracy was seen in both groups. The group accuracy difference was strongest at the highest (2-back) level of difficulty (eTable 1). In the emotion identification task (eTable 2), PS responded more slowly (t=1.81, p=0.035) and trended to have lower accuracy (t=−1.51, p=0.065) in identifying Threat emotions, with more robust performance impairment evident for Non-threat emotions and the task overall but no significant performance difference for neutral faces.
Task Activation
Both TD and PS groups demonstrated expected regional task activation patterns. For the n-back, the primary contrast of interest captured the effect of working memory load (2-back > 0-back). This contrast revealed robust activation of multiple brain regions in the executive network (Figure 2A), as well as the task-deactivated default mode network, both of which contribute to working memory performance.32 For the emotion identification task, the primary contrast of interest examined activation to threatening faces (anger and fear, “Threat”) relative to baseline. This contrast revealed robust activation in bilateral amygdala (Figure 3A), our primary region of interest in this task, as well as in multiple regions previously shown to activate in facial emotion tasks including the fusiform and orbitofrontal cortex.39
Figure 2. Executive system hypofunction during the N-back Task.
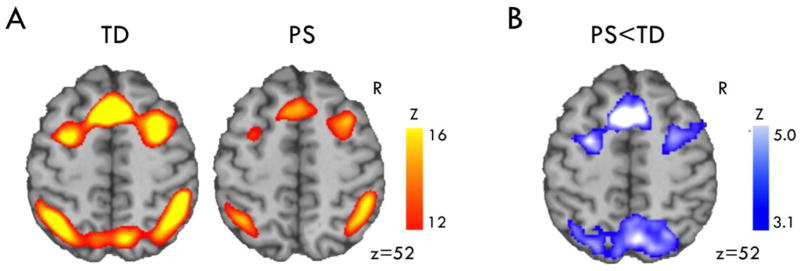
A) The contrast evaluating the effect of working memory load (2-back > 0-back) robustly recruited executive function regions including dACC and DLPFC, with a qualitatively similar regional pattern in both TD and PS groups (thresholded Z 12–16 for display). B) PS showed significantly reduced activation in these regions (significant clusters shown in blue, Z>3.09 p<.05).
Figure 3. Amygdala hyperactivation to Threat in the Emotion Processing Task.
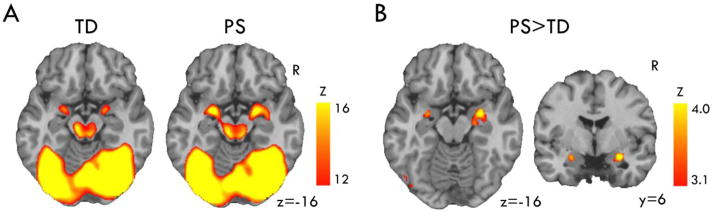
A) Facial emotion processing activated amygdala and other regions implicated in emotion and face processing, with a qualitatively similar regional pattern in both TD and PS groups (thresholded Z 12–16 for display). B) PS showed significantly greater activation in bilateral amygdala (significant clusters shown in red, Z>3.09 p<.05).
Task- and Region-specific Activation Abnormalities
Group differences were present in both tasks, but the direction and location of abnormalities in PS subjects differed by task. In the n-back task, PS showed reduced activation in prefrontal regions as hypothesized, including dorsolateral PFC bilaterally, paracingulate/cingulate, and bilateral frontal pole (Figure 2B; Table 2; eFigure 2). Beyond the PFC, PS also showed reduced activation bilaterally in multiple regions previously linked to executive function and working memory. Except for a small region of right superior frontal gyrus where PS showed reduced load-dependent deactivation, no group differences were observed outside of task-activated and load-sensitive regions, and there were no significant regions where PS activated more than TD. Exploratory analysis of each level of working memory load showed few group differences at 0-back or 1-back levels, and these were limited to task-deactivated regions. At 2-back, group differences were similar to those seen in the 2-back>0-back contrast, although not as strong (eTable 3). Thus, group differences were only seen within the executive network at higher levels of working memory load.
During emotion identification, PS had elevated BOLD responses to threatening expressions in amygdala (Figure 3B, Table 2; eFigure 3). PS also showed greater activation in left fusiform cortex and right middle frontal gyrus, less deactivation in bilateral superior frontal gyrus, and reduced activation in bilateral supramarginal gyrus and left insula. We examined the non-threat and neutral condition for comparison, and did not find significant group differences within amygdala (eTables 4 & 5).
Correlations with Cognition and Psychotic Symptoms
The PS group is heterogeneous in the severity of core clinical dimensions. We examined whether activation in key regions showing group differences was selectively linked to cognitive deficits, positive psychotic symptoms, or negative/disorganized symptoms.
Within the PS group, there was a significant correlation between global cognitive ability and bilateral dorsolateral prefrontal cortex (DLPFC) activation in the 2-back>0-back contrast (r=0.32, p<0.001; see scatterplot in eFigure 4). Significant correlations were seen in the other areas of executive function circuitry as well. N-back activation in DLPFC or other regions showing group differences did not correlate with severity of positive symptoms or negative/disorganized symptoms (p’s >0.5).
A different pattern of dimensional correlations was seen for amygdala activation in the emotion identification task. Within the PS group, amygdala activation to Threat did not correlate significantly with cognitive ability (r=−0.02, p=0.82). However, there was a small but significant positive correlation between the dimensional severity of positive psychotic symptoms and bilateral amygdala activation to Threat (r=0.16, p=0.01; see scatterplot in eFigure 5 and individual PS-R item analysis in eTable 6). Effects were similar in left amygdala (r=0.18, p=0.005) and right amygdala (r=0.15, p=0.02). No correlation with negative/disorganized symptoms was found (r=−0.001, p=0.99). The amygdala response to Threat was not associated with DLPFC working memory activation (r=0.10, p=0.15).
Confound and Sensitivity Analyses
We examined the influence of potential confound variables showing significant group differences, the impact of comorbid disorders, or a history of psychiatric treatment. The details of these analyses are reported in the supplement eMethods, eResults, and eTables 7–11. While these analyses identified relationships between these factors and the key imaging outcomes, the key group differences and correlations reported above remained significant and could not be fully attributed to these other factors. However, the group difference in DLPFC n-back activation was strongly attenuated by covarying global cognition or n-back task accuracy, and moderately attenuated by covarying demographic factors that themselves correlated with cognitive performance. This likely reflects substantial shared variance between factors related in complex causal pathways to both cognitive performance and risk for psychosis, rather than indicating the reduced DLPFC activation in PS is an “artifact” of demographic differences.40
Age effects are of particular interest in this developmental PNC cohort. Age did show significant relationships to fMRI activation, increasing DLPFC n-back activation and decreasing amygdala Threat activation (eTables 7–10). However, age effects cannot explain our results as covarying for age only reduced group differences by 10–20% (eTables 7 & 9), and key group differences remained significant in subsamples matched for age (eTable 14). Furthermore, we did not find any significant group x age interaction effects; this stands in contrast to smaller studies in clinical high risk samples that reported age x group effects in amygdala activation during emotion processing41 and in prefrontal cortex and other regions during a working memory task.42
DISCUSSION
We present the largest functional neuroimaging study to date of psychosis-spectrum subjects, and the first such fMRI study to apply a population-based approach to identifying PS youths. We find that psychosis-spectrum symptoms are associated with both executive circuit hypofunction during working memory, and amygdala hyper-reactivity during processing of threatening emotional expressions. Evaluation of these two categorical effects revealed significant within-group heterogeneity: executive hypofunction related to cognitive deficits but not to symptom severity, while amygdala hyperfunction showed the reverse pattern.
PS Hypofunction in Executive System Circuitry During Working Memory
Deficits in recruiting prefrontal executive control circuitry during working-memory tasks are among the most consistent fMRI findings in schizophrenia.4 The same abnormality has also been reported in clinical risk samples, including a recent meta-analysis.43 Our findings in a large independent sample provide a reliable confirmation that executive system hypofunction is found in youth with psychosis-spectrum symptoms.
Notably, the largest single clinical risk fMRI study prior to our own, published after the Fusar-Poli meta-analysis, showed increased working-memory related activity in executive circuitry.44 We applied a fractal n-back paradigm in which PS subjects had impaired performance that correlated with activation deficits. In contrast, Yaakub et al. applied a verbal working memory task that did not elicit performance deficits in the psychosis risk group. These divergent findings may be reconciled by DLPFC inefficiency as seen in schizophrenia, where hypoactivation is associated with impaired performance while hyperactivation occurs when performance is equated.45–47 Additionally, Yaakub et al.’s use of letter stimuli may have contributed to increased DLPFC recruitment and spared performance in the risk group by facilitating a verbal rehearsal strategy, which our fractal n-back was designed to minimize.31
PS Hyperactivation in Amygdala During Threat Emotion Processing
Reduced behavioral performance during emotion identification has been repeatedly found in clinical risk and spectrum samples48–53 including the PNC.26 Only two small fMRI studies have examined facial emotion processing in clinical risk. One reported increased right fusiform activation across emotions, and increased amygdala response in the neutral>sad contrast.54 The other did not comment on group differences in amygdala activation, but did note abnormal correlations between amygdala activation and age, and abnormal amygdala-prefrontal connectivity.41 In our large PS sample, we identify amygdala hyper-reactivity in response to threatening emotional expressions during emotion identification. Our hypotheses focused on the amygdala; however other visual and prefrontal regions also showed increased activation in PS, but without the threat-selectivity seen in amygdala. Amygdala activation abnormalities during emotion processing are consistently reported in schizophrenia, particularly in response to potentially threatening emotions.5–7,9–12 In schizophrenia, amygdala hypoactivation is more commonly reported than hyperactivation during emotion processing tasks.5,6,8 However, this hypofunction may relate to hyperactivation to neutral expressions often used as a baseline,6,7,55 to use of implicit paradigms,8 and to nonspecific disease-associated factors such as medication effects.12 Our approach avoided these factors, which may have allowed the positive correlation between symptoms and amgydala activation to also increase average activation across the PS group.
Heterogeneity in Dimensional Relationships
In addition to these categorical group effects, our study is the first to demonstrate dissociable dimensional relationships for these two abnormalities in PS subjects. Executive system hypofunction during working memory related to cognitive ability but not positive psychotic symptom severity, whereas amygdala hyper-reactivity during emotion processing showed the reverse pattern. The correlation we observe between cognition and n-back activation is consistent with findings in schizophrenia as well as healthy controls.32,46 Some studies of amygdala activation during emotion processing in schizophrenia have identified correlations with negative symptoms, while others have found a relationship to positive symptom severity as we observe here.10,56–58
Our findings support two distinct mechanisms of psychosis risk, one linked to executive dysfunction and the other linked to positive symptoms. Speculatively, these two abnormalities could interact to increase psychosis risk, with amygdala hyper-reactivity to threat increasing likelihood of paranoid feelings or ideas, and executive dysfunction reducing ability to cognitively contextualize excessive fears or unusual experiences. Our results suggest that at least within the milder spectrum of psychosis severity, impaired executive system function may constitute a risk factor for the development of psychosis but is not directly related to progression along the severity of positive symptoms; a path consistent with a prior study employing structural equation modeling.59 In contrast, amygdala reactivity to threat appears to relate to psychosis severity but not cognition, and may therefore constitute a subclinical illness phenotype rather than a marker of trait vulnerability. As positive symptom severity has shown the greatest predictive power for transition to frank psychosis,3 this suggests that amygdala responses to threat may be particularly useful in augmenting clinical data for predicting such transition. In contrast, cognitive deficits may appear earlier and be of greater use in efforts to screen for risk prior to the emergence of positive psychotic spectrum symptoms. Clarifying the degree to which reduced cognition and executive circuit function in PS youth reflect baseline differences in IQ versus deterioration associated with PS pathophysiology will require longitudinal study in this population.
Implications of Population-Based Approach
Our study is the first to examine imaging findings in PS identified through a population-based approach. This approach facilitated the identification of a PS sample far larger than fMRI samples in prior studies of help-seeking clinical risk individuals (maximum 60, median <20), with greatly enhanced statistical power and control of type I and type 2 error. Notably, this approach yielded neuroimaging phenotypes similar to those found in help-seeking clinical-risk samples and established schizophrenia, providing strong neurobiological evidence supporting a continuum account of PS pathophysiology.16,24 Our sampling strategy also identified PS subjects younger than those typically studied in high-risk samples. Thus our findings help define patterns of neural dysfunction associated with PS at an earlier age, a critical step toward developing interventions that might “bend the curve” of disease trajectory60 and permit primary prevention of schizophrenia and other adverse long-term outcomes associated with PS.
The present cross-sectional analysis does not define the extent to which observed neuroimaging abnormalities are driven by the subgroup that will transition to frank psychosis. Evidence of continuum effects in PS16,24 make it likely that our findings are not limited to this subgroup, and the proportion of subjects in our population-based approach that transition to schizophrenia is likely to be lower than seen with typical clinical high-risk ascertainment strategies. With planned longitudinal follow up, our large sample size should yield both a sizable transition cohort and a large non-transitioning group, providing statistical power to better identify transition-related abnormalities, and further enhancing the value of our present findings.
The observed correlations and group differences are relatively small in magnitude. This points to the value of a large sample in identifying small effects that may have important mechanistic implications. However, with small effect sizes it is prudent to emphasize that the reported phenotypes by themselves cannot serve to clinically categorize individuals. Future work may identify subgroups showing larger effects, and the current phenotypes may contribute to multivariate approaches with significant predictive utility.
Notably, our criteria did not require help-seeking or recent progression of symptom severity, and independently incorporated negative and disorganized symptoms; our PS sample therefore includes subjects that have been excluded by most high-risk studies. Given the importance of negative and disorganized symptoms in clinical risk,14,61–65 we believe this broader inclusion constitutes a strength, and our follow-up of 300 PS and 200 TD participants will permit reexamination of our results with respect to traditional high-risk criteria.
Symptoms such as depression, anxiety, irritability and mania are elevated in PS and could potentially impact the brain phenotypes examined here. Our supplementary analyses indicate the reported findings in PS are not explained by comorbid conditions, although a history of depression was associated with less n-back DLPFC impairment and a history of anxiety was associated with greater amygdala Threat hyperactivation. Future work with the full neuroimaging sample will include a detailed exploration of how other dimensions of psychopathology relate to working memory and emotion processing. In addition, future work incorporating more definitively threatening situations, such as fear conditioning paradigms, will be important to further elucidate the role of amygdala responses to threat in PS.
CONCLUSIONS
Our study demonstrates that early psychosis-spectrum symptoms in a population-based youth sample are associated with a pattern of functional abnormalities similar to that previously found in established schizophrenia. Specific circuit dysfunction during cognitive-emotional tasks emerges at an early age in association with psychosis-spectrum symptoms, and cannot be attributed to chronic illness or medication confounds. Such imaging biomarkers may reveal novel therapeutic targets and mechanisms of treatment efficacy, and could help tailor interventions targeting specific populations and symptom domains. Integrating functional imaging phenotypes with neuroanatomic, genetic, and clinical data can help advance the field beyond sole reliance on symptom-based classification. Such integrated measures may yield more robust quantification of risk,59 which can be tested in longitudinal follow-up and applied to improve diagnosis, prevention, and treatment.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924, as well as P50MH096891 and T32 MH019112. Dr. Wolf was also supported by MH085096, APIRE, and the Sidney R. Baer, Jr. Foundation through the Brain and Behavior Research Foundation. Dr. Satterthwaite was supported by K23MH098130 and the Marc Rapport Family Investigator grant through the Brain and Behavior Foundation. Dr. Calkins was supported by K08MH079364.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the acquisition and recruitment team: Raphael Gerraty, Marisa Riley, Jack Keefe, Nick DeLeo, Elliott Yodh, and Rosetta Chiavacci.
Previous Presentation: Portions of this manuscript were presented in preliminary form at the International Congress on Schizophrenia Research (April 21–25, 2013, Grand Lakes, Florida).
Author Contributions: Drs. Wolf and R.E. Gur had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wolf, Satterthwaite, Calkins, Elliott, Bilker, Hakonarson, R.C. Gur, R.E. Gur. Acquisition of data: Calkins, Ruparel, Elliott, Hopson, Prabhakaran, Hakonarson, R.C. Gur, R.E. Gur. Analysis and interpretation of data: All authors. Drafting of the manuscript: Wolf, Satterthwaite, Calkins, R.C. Gur, R.E. Gur
Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Wolf, Satterthwaite, Calkins, Ruparel, Bilker. Obtained funding: Hakonarson, R.C. Gur, R.E. Gur. Administrative, technical, and material support: All authors. Study supervision: Wolf, Satterthwaite, Calkins, Ruparel, R.C. Gur, R.E. Gur.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication.
References
- 1.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 3.Addington J, Heinssen R. Prediction and prevention of psychosis in youth at clinical high risk. Annu Rev Clin Psychol. 2012;8:269–289. doi: 10.1146/annurev-clinpsy-032511-143146. [DOI] [PubMed] [Google Scholar]
- 4.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36(5):1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38(3):608–621. doi: 10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82(2–3):153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol Psychiatry. 2012;71(2):136–145. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosaka H, Omori M, Murata T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57(1):87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 10.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64(12):1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 11.Pinkham AE, Loughead J, Ruparel K, Overton E, Gur RE, Gur RC. Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am J Psychiatry. 2011;168(3):293–301. doi: 10.1176/appi.ajp.2010.10060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satterthwaite TD, Wolf DH, Loughead J, et al. Association of enhanced limbic response to threat with decreased cortical facial recognition memory response in schizophrenia. Am J Psychiatry. 2010;167(4):418–426. doi: 10.1176/appi.ajp.2009.09060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychological medicine. 2012;42(9):1857–1863. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelleher I, Keeley H, Corcoran P, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201(1):26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41(1):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- 17.Modinos G, Ormel J, Aleman A. Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophr Res. 2010;118(1–3):88–97. doi: 10.1016/j.schres.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Modinos G, Pettersson-Yeo W, Allen P, McGuire PK, Aleman A, Mechelli A. Multivariate pattern classification reveals differential brain activation during emotional processing in individuals with psychosis proneness. Neuroimage. 2012;59(3):3033–3041. doi: 10.1016/j.neuroimage.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25(3):295–305. doi: 10.1037/a0021747. [DOI] [PubMed] [Google Scholar]
- 20.Modinos G, Renken R, Shamay-Tsoory SG, Ormel J, Aleman A. Neurobiological correlates of theory of mind in psychosis proneness. Neuropsychologia. 2010;48(13):3715–3724. doi: 10.1016/j.neuropsychologia.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson McEwen SC, Connolly CG, Kelly AM, et al. Resting-state connectivity deficits associated with impaired inhibitory control in non-treatment-seeking adolescents with psychotic symptoms. Acta Psychiatr Scand. 2013;129(2):134–142. doi: 10.1111/acps.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson S, Kelleher I, Harley M, et al. Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage. 2010;49(2):1875–1885. doi: 10.1016/j.neuroimage.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Orr JM, Turner JA, Mittal VA. Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. Neuroimage Clin. 2014;4:343–351. doi: 10.1016/j.nicl.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 25.Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci Biobehav Rev. 2013;37(3):317–327. doi: 10.1016/j.neubiorev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Gur RC, Calkins ME, Satterthwaite TD, et al. Neurocognitive Growth Charting in Psychosis Spectrum Youths. JAMA Psychiatry. 2014;71(4):366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- 27.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, Cannon M. Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cognitive neuropsychiatry. 2013;18(1–2):9–25. doi: 10.1080/13546805.2012.682363. [DOI] [PubMed] [Google Scholar]
- 29.Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragland JD, Turetsky BI, Gur RC, et al. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16(3):370. [PMC free article] [PubMed] [Google Scholar]
- 32.Satterthwaite TD, Wolf DH, Erus G, et al. Functional Maturation of the Executive System during Adolescence. J Neurosci. 2013;33(41):16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf DH, Satterthwaite TD, Loughead J, et al. Amygdala abnormalities in first-degree relatives of individuals with schizophrenia unmasked by benzodiazepine challenge. Psychopharmacology (Berl) 2011;218(3):503–512. doi: 10.1007/s00213-011-2348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterthwaite TD, Wolf DH, Pinkham AE, et al. Opposing amygdala and ventral striatum connectivity during emotion identification. Brain Cogn. 2011;76(3):353–363. doi: 10.1016/j.bandc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satterthwaite TD, Wolf DH, Gur RC, et al. Frontolimbic responses to emotional face memory: the neural correlates of first impressions. Hum Brain Mapp. 2009;30(11):3748–3758. doi: 10.1002/hbm.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loughead J, Gur RC, Elliott M, Gur RE. Neural circuitry for accurate identification of facial emotions. Brain Res. 2008;1194:37–44. doi: 10.1016/j.brainres.2007.10.105. [DOI] [PubMed] [Google Scholar]
- 37.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- 40.Meehl PE. Nuisance variables and the ex post facto design. In: Radner M, Winokur S, editors. Minnesota studies in the philosophy of science. Minneapolis, MN: University of Minnesota Press; 1970. pp. 373–402. [Google Scholar]
- 41.Gee DG, Karlsgodt KH, van Erp TG, et al. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res. 2012;134(1):1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlsgodt KH, van Erp TG, Bearden CE, Cannon TD. Altered relationships between age and functional brain activation in adolescents at clinical high risk for psychosis. Psychiatry Res. 2013;221(1):21–29. doi: 10.1016/j.pscychresns.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusar-Poli P. Voxel-wise meta-analysis of fMRI studies in patients at clinical high risk for psychosis. J Psychiatry Neurosci. 2012;37(2):106–112. doi: 10.1503/jpn.110021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaakub SN, Dorairaj K, Poh JS, et al. Preserved working memory and altered brain activation in persons at risk for psychosis. Am J Psychiatry. 2013;170(11):1297–1307. doi: 10.1176/appi.ajp.2013.12081135. [DOI] [PubMed] [Google Scholar]
- 45.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 46.Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108(1–3):143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35(1):19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amminger GP, Schafer MR, Papageorgiou K, et al. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr Bull. 2012;38(5):1030–1039. doi: 10.1093/schbul/sbr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192(1):67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolwer W, Brinkmeyer J, Stroth S, et al. Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophr Bull. 2012;38(5):1021–1029. doi: 10.1093/schbul/sbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Rijn S, Aleman A, de Sonneville L, et al. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol Med. 2011;41(3):499–508. doi: 10.1017/S0033291710000929. [DOI] [PubMed] [Google Scholar]
- 52.Roddy S, Tiedt L, Kelleher I, et al. Facial emotion recognition in adolescents with psychotic-like experiences: a school-based sample from the general population. Psychol Med. 2012;42(10):2157–2166. doi: 10.1017/S0033291712000311. [DOI] [PubMed] [Google Scholar]
- 53.Dickson H, Calkins ME, Kohler CG, Hodgins S, Laurens KR. Misperceptions of facial emotions among youth aged 9–14 years who present multiple antecedents of schizophrenia. Schizophr Bull. 2014;40(2):460–468. doi: 10.1093/schbul/sbs193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seiferth NY, Pauly K, Habel U, et al. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008;40(1):289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Lakis N, Mendrek A. Individuals diagnosed with schizophrenia assign emotional importance to neutral stimuli: an FMRI study. ISRN Psychiatry. 2013;2013:965428. doi: 10.1155/2013/965428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinkham AE, Gur RE, Gur RC. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Rev Neurother. 2007;7(7):807–816. doi: 10.1586/14737175.7.7.807. [DOI] [PubMed] [Google Scholar]
- 57.Surguladze S, Russell T, Kucharska-Pietura K, et al. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006;60(5):423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58(2–3):159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 59.Shah J, Eack SM, Montrose DM, et al. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophr Res. 2012;141(2–3):189–196. doi: 10.1016/j.schres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66(2):128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- 61.Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, Bechdolf A, Schimmelmann BG, Klosterkotter J. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012;18(4):351–357. doi: 10.2174/138161212799316064. [DOI] [PubMed] [Google Scholar]
- 62.Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr Bull. 2012;38(2):351–359. doi: 10.1093/schbul/sbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol. 2013;122(3):807–815. doi: 10.1037/a0033759. [DOI] [PubMed] [Google Scholar]
- 64.Piskulic D, Addington J, Cadenhead KS, et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196(2–3):220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dominguez MD, Saka MC, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry. 2010;167(9):1075–1082. doi: 10.1176/appi.ajp.2010.09060883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


