Abstract
Despite the decline in U.S. cancer incidence and mortality rates, cancer remains the number one cause of death for people under the age of 85 and one in four people in the US will die of cancer, mainly due to metastasis1. Recently, interest in mesenchymal stem cell (MSC) tumor-homing has led to inquires into: 1) why MSCs home to tumors, 2) what the inherent pro- and anti-tumor consequences are, and 3) how to best capitalize on MSC tumor-homing for cell-based diagnostics and therapy. Here these questions are reviewed and method for addressing them using animal models and tracking methodologies (or, synonymously, detection methodologies) are discussed. First, MSCs in a regenerative and tumor-homing context are reviewed, followed by MSC delivery and genetic labeling methods for tissue model systems. Lastly, the use of the non-optical methods MRI (magnetic resonance imaging), PET (positron emission tomography) and SPECT (single photon emission computed tomography), along with optical methods, fluorescence imaging and BLI (bioluminescent imaging), are reviewed related to tracking MSCs within disease model settings. The benefits and drawbacks of each detection method in animal models is reviewed along with the utility of each for therapeutic use.
Keywords: Mesenchymal Stem Cells, Diagnostic Imaging, Neoplasm Metastasis, Neoplasms, Disease Models, Animal
Introduction
Over the last decade, non-invasive imaging and cell detection technologies have undergone a revolution. This has allowed for a better grasp of the roles of stroma in cancer progression and improved designs of therapeutic and diagnostic methods based on tumor-host interactions. Tumors hijack normal, healthy cells in a multitude of ways including the recruitment of endothelial cells, utilization of tumor-associated macrophages (TAMs) and osteoclasts to degrade extracellular matrix (ECM) and bone matrix, and, most important to this review, the multifaceted exploitation of MSCs. Understanding and capitalizing on MSC tumor-homing requires accurate tracking methods in model systems and clinical settings where stem cell therapies are increasingly utilized. The accuracy of delivering therapeutic or diagnostic cells, or both (“theragnostic”) cells, to target tumors and the technological ability to assess this accuracy will determine the success of basic science model systems and clinical cell-based anti-cancer therapies.
Tumor-homing is a complex, multistep process used by many cells to travel from a distant location to a tumor. Similar to tumor cells in the metastatic cascade, homing cells may become activated, intravasate, travel through circulation, extravasate, migrate and undergo phenotypic changes. Importantly, much of this process is still unknown in terms of MSC tumor-homing. We have chosen to define “tumor-homing” as any action where cells travel from a distant location to a tumor and reserve the term “migration” to define only active, filopodia-based motion through tumor or surrounding local microenvironment based on local chemoattractants.
MSCs: Definition and Potential in Regenerative Medicine
MSCs are a heterogeneous population of fibroblast-like cells found surrounding blood vessels, similar to pericytes, and are concentrated in the bone marrow and adipose tissue, from where they are often isolated. They can also be isolated from cord blood and placental tissues including umbilical cord2,3. Their heterogeneity may be the key to their diverse therapeutic effects, but the lack of consistent isolation methods for multipotent MSCs often complicates comparisons between studies. The following three criteria are agreed upon to identify MSCs: 1) plastic adherence; 2) expression of CD105, CD90, and CD73 and lack of expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface markers; and 3) ability to differentiate into osteoblasts, adipocytes and chondrocytes in vitro4.
MSCs can also differentiate into ectodermal cells (i.e. neurons), cardiomyocytes, and hepatocytes5. They have potential for repair of diseased or damaged tissues and the regeneration of native tissues due to their differentiation potential, wound-tropism, and production of soluble factors that stimulate healing, angiogenesis, growth, and cell recruitment, and inhibit inflammation5. These stem cell sources are also less prone to ethical debate than other sources, suggesting a more realistic path to therapeutic impact in the shorter term. MSCs may be predisposed to home to different locations and express different marker profiles depending on their source tissue (e.x.: bone marrow, adipose, umbilical cord)5. However, practically all types of MSCs are successful in animal and clinical trials5 and MSCs have been utilized in regeneration or treatment of damaged heart tissue, vascular disease, spinal cord injury, bone injury, cartilage injury, osteogenesis imperfecta (OI), lung injury, kidney disease, diabetes, neurological disorders, and autoimmune-diseases, among others5.
MSCs: Potential in Tumor-Homing and Cancer Treatment
MSCs are inherently tumor-homing and immunosuppressive and can be isolated, cultured, expanded, and transduced, making them viable candidates for cell therapy6. MSCs can also act as universal donor cells due to their immunocompatibility, making them useful for allogeneic transplantation7. MSCs can home specifically to tumors including gliomas8, and breast9,10,11, colon12, ovarian13, and lung carcinomas, among many other primary and metastatic tumors14,15. In these models, MSCs have successfully homed to tumors from a large variety of administration routes including the carotid artery, femur, tail vein, tibia, and trachea.
MSC tumor migration is motivated by many factors including tumor-cell specific receptors and soluble tumor-derived factors such as SDF-1, tumor necrosis factor (TNF)-α, and interleukins (ILs), among other identified and unidentified inflammatory mediators13,16,18. Upon arrival in the tumor, MSCs display many pro-tumor, or tumor-supporting, roles including immune response suppression19, inhibition of tumor apoptosis20, and stimulation of an epithelial-to-mesenchymal transition (EMT)21, angiogenesis12 proliferation10, extravasation22, migration22, and metastasis9. MSCs can also differentiate into supportive stromal cells such as pericytes23 and cancer-associated fibroblasts (CAFs)16 and specifically support cancer stem cell populations10. Some reports find that paracrine signaling from MSCs may affect tumors even without MSC tumor-engraftment19.
In contrast to their pro-tumor effects, MSCs also display a range of anti-tumor properties in sarcomas24 and leukemias20 and can decrease breast cancer cell growth and lung metastasis in vivo25. Certain immortalized MSCs can also inhibit primary tumor growth26 and colony formation27. Fundamentally, MSCs have potential for anti-cancer gene delivery, but innate pro-tumor effects present significant barriers for clinical therapies. The accurate tracking of transplanted stem cells is essential for understanding homing and differentiation patterns and cell clearance and designing effective treatments in terms of cell types used, administration timing and location, co-administered drugs, and side-effects to monitor. Better understanding of cell fate is especially crucial now that stem cell therapy is being used increasingly to treat other diseases, often in patients who may have undetectable micrometastases.
MSC Genetic Modifications
MSCs have been modified into anti-cancer vectors through transduction with genes such as TRAIL (TNF-related apoptosis-inducing ligand)28, IFN-gamma (IFN-γ)29, interferon-beta (IFN-β), and CX3CL1 (fractalkine)15, and soluble decoy receptors such as the type I insulin-like growth factor receptor30. MSCs are commonly transduced with viral vectors including adenoviruses8, lentiviruses and other retroviruses28,30, or adeno-associated viruses (AAVs) for therapeutic and tracking purposes. Transfection allows cells to produce high concentrations of proteins in a spatially and temporally controllable manner using inherent tumor-homing properties and inducible promoters, respectively31. MSC tumor-homing can also be augmented by increasing the cells’ expression of tumor-specific receptors32.
In contrast to lentiviruses or AAVs, which insert genes into the host genome, adenoviruses insert genes that remain epichromosomal (meaning they are not inserted within the host’s genome) and hence are not replicated upon cell division33. Still, adenovirally-transfected MSCs expressing IFN-β have been effective at killing glioma cells in vitro and in vivo using intra-arterial injections8. Adenoviruses cause a large immune response, but they have the largest cloning capacity, up to 7.5 kb, compared 3 kb in adeno-associated virus, and infect all cell types with close to 100% efficiency while other transfection viruses may show cell-type specific infection efficiency34. AAVs, unlike adenoviruses, show low immunogenicity and pathogenicity, and have integration competence into a known site, decreasing the chance for mutagenesis found in retrovirus infections. Counter to lentiviruses, AAVs are not replicated and their genes do not remain within target cells upon cell division, but studies have found MSCs capable of stable gene expression using AAV transfection from as little as 8 days up to 1 month35. Lentivirus infection produces MSCs that continually express a gene of interest that is incorporated within the genome, replicated and passed to all daughter cells. Lentiviruses have a cloning capacity intermediate between adenovirus and adeno-associated virus and only have a transfection efficiency of ~30%. Though lentivirus vectors provide long-term stable expression, they can accidentally insert transgenes into genomic locations that cause destabilization, reversion of the virus to the wild type, or proto-oncogene activation. However, newer generations of lentivectors are comparatively stable and less likely to generate wild type virus or proto-oncogenes. Oncogenic risks depend on several variables including “the vector copy number, the target cell type, the proliferation and/or activation status of the target cells, the nature of the transgene itself, the vector design, the underlying disease and the possible selective advantage of rapidly growing cells, protocol-specific cofactors, and finally the intrinsic genotypic variation of the model animals and the treated patients” 36, as reviewed recently by Mátrai et al.
MSC Delivery Methods in Tumor-Homing Animal Models
Tracking MSCs in animal models is crucial to understanding how normal or transplanted MSCs migrate. Clinically, most intravenously administered MSCs become trapped within capillary beds, often in the lungs, or are cleared from circulation by the liver and spleen. Despite this, MSC transfusions for disease achieve success by using high doses of MSCs, but more efficient MSC delivery remains a challenge37. In animal models, tumor-homing is typically assessed by injecting MSCs into circulation using intravenous ( i.v.), usually tail vein, injections38,18, but can also be assessed with intratracheal15, internal carotid artery8, intraperitoneal13, and subcutaneous injections30. Some glioma studies have been unable to detect MSC tumor-homing or effects from i.v. injections, but extensive MSC migration within glioma tumors upon intratumoral injection has been observed. These observations suggest different MSC homing patterns and hence different treatments for glioma patients23. Our group has produced a tissue model demonstrating MSC breast tumor-homing from a bone-like environment, rather than from circulation, that may capture more steps of inherent bone-marrow derived MSC tumor-homing11.
In Vivo Imaging of MSC Tumor-homing
Personalized treatment using autologous and allogeneic stem cells is a reality and the need for non-invasive tracking methods is escalating. Tracking MSC fate in animal models is performed most often using optical techniques due to the non-invasiveness of light detection, the ability to section explants and retain optical signals, and the ease and simplicity of in vivo imaging. Non-optical methods such as MRI (magnetic resonance imaging), PET (positron emission tomography) and SPECT (single photon emission computed tomography), which are already clinically used for cell tracking, may be developed for clinical stem cell tracking before optical methods40. A summary of these detection methods, which are often used in combination, and their associated benefits and limitations are found in Table 1. The following sections discuss advantages and disadvantages of non-optical and optical methods for tracking MSCs within animal models and clinical settings.
TABLE 1.
Summary Table of Methods for Tracking MSC Tumor Migration
| Method | Catergory | Cellular Modification | Contrast Agents | Detection Method | Strengths | Weaknesses | Assessment of Cell Function | References |
|---|---|---|---|---|---|---|---|---|
| MRI | Real-time Non- Optical | Magnetic nanoparticles added to cells or coupled to ligands, stable transduction for expression of enzymes or proteins that produce unique MRI signatures. | SPIOs, internalized iron, metal chelates, etc. | Magnetic fields align magnetic moments of atoms; radio frequency fields alter moments and cause atom-specific rotating magnetic fields. | Specific labeling possible based on ligand- expression, clinically used, high sensitivity, fullcontrast agents complicate signal interpretation, expensive tissue penetration, full body analysis, easy cell labeling, safe for multiple uses over time, better spatial resolution than SPECT or PET. | Image lost in contrast artifacts or upon cell division, discarded detection technology, cytotoxicity of certain labeling agents. | No | 41, 44, 47 |
| SPECT | Real-time Non- Optical | Uptake of radioisotope labels. | Gamma-emitting radioisotopes (radionuclides). | Tracer emits gamma radiation that is measured directly. | 3-D imaging, less expensive than PET, longer- lived more easily-obtained radioisotopes vs PET (Ex: indium-111, 2.8 day halflife, ), gives anatomical and physiological data, better labeling efficiency than PET, used to trace human cells clinically. | Lower resolution than PET (1 cm), drawbacks with stability and imaging time, suboptimal photon energies (depending on tracer), not good for longitudinal studies or multiple uses over time. | Maybe | 47, 60 |
| PET | Real-time Non- Optical | Viral modification (ex: Herpes simplex virus type 1 thymidine kinase (HSV1-sr39tk ) (tk ) or other a PET reporter gene) or uptake of radioisotope labels. | Positron-emitting radionuclides. | Pairs of gamma rays detected represent collisions of positrons (emitted by a positron-emitting radionuclides) and electrons within patient at site of tracker. | Clinically used to track human cells, full body analysis, high sensitivity, higher resolution and more current reporter genes than SPECT. | Radiation exposure, costly equipment, genetic modifications of MSCs required, short biological and chemical half-lives of contrast reagents(ex: F-18 FDG, 110 minute halflife), not stable for multiple uses over time, more complex probe construction (tight quality control, advanced chemistry) vs SPECT, can only detect 1 probe (vs SPECT which can detect multiple), needs active uptake (vs SPECT reagents that can diffuse into cells). | Yes | 6, 12, 50 |
| Fluorescent gene- Live imaging | Real-time Optical | GFP/other marker transduction. | Fluorescence from fluorescent proteins. | Excitation with specific wavelengths excite molecules to high energy state, relaxation releases photons. | In animal models: excellent for longitudinal studies and imaging different cell types. | Need immunocompatible//FDA-approved fluorescent protein expression for clinical use, high signal attentuationin vivo , not clinically useful, low sensitivity. | Yes | 9, 23, 30 |
| Fluorescent dye- Live imaging | Real-time Optical | Fluorescent dye labeling of membrane | Fluorescence from fluorescent dyes. | Excitation with specific wavelengths excite molecules to high energy state, relaxation releases photons. | In animal models: excellent for longitudinal studies and imaging different cell types. | Need immunocompatible/FDA-approved dyes for clinical use, high signal attentuation in vivo , not clinically useful, low sensitivity. | Yes | 8, 11 |
| BLI-Live imaging | Real-time Optical | Luciferase transduction. | Bioluminescence from luciferase/luciferin reaction. | Whole animal bioluminescence detection unit quantifies emitted photons from luciferase-labeled cells. | In animal models: excellent for longitudinal studies and imaging different cell types. | Need immunocompatible luciferase expression for clinical use, rapid signal attentuation in vivo , not clinically useful, variable sensitivity. | Yes | 10, 13, 38, 63 |
Abbreviations: MSCs (Mesenchymal Stem Cells), NMRI (Nuclear Magnetic Resonance Imaging), SPIO (Superparamagnetic Iron Oxide), SPECT (Single Photon Emission Computed Tomography) PET (Positron Emission Tomography), 3-D (Three-Dimensional), GFP (Green Fluorescent Protein), BLI (Bioluminescent Imaging)
Non-Optical Methods for Tracking MSCs
MRI in Animal Models
MRI is useful for tracking spatial and temporal homing of cells due to the high spatial resolution and three-dimensional, whole-body imaging41. This non-invasive detection method uses magnetic fields and radio frequency waves to perturb these fields and detect labeled cells. Current model systems use MSCs pre-labeled in vitro, but if a procedure for in vivo labeling was designed, it would probably be widely implemented. In direct labeling MRI model systems, MSCs are labeled with magnetic nanoparticles such as superparamagnetic iron oxides (SPIOs), Mn, Eu or Gd chelates, and perfluorocarbon nanoparticles42,43. Contrast agents can enter cells using polycationic transfection agents, liposomes, “gene-guns”, microinjection, electroporation, or receptor-mediated endocytosis (as reviewed in44).
MSCs may also be indirectly labeled for MRI tracking by stable transduction to express enzymes or proteins such as intracellular metalloproteins (ex: transferrin, ferritin) that will produce unique MRI signatures from internal iron accumulation. Over-expressing ferritin in mouse myoblasts increased iron internalization and made them identifiable via MRI, and this technique could potentially be translated to human MSCs45. Another study found that swine stem cells can be transduced with human ferritin heavy chain (hFTH) and used as a reporter gene in a myocardial infarction model. They were able to identify this MRI signal for 4 weeks in vivo using a 1.5 Tesla MRI scanner and a multiecho T2* gradient echo sequence (clinical standards) and found no effects on cardioreparative or differentiation potential46. The toxicity of high iron concentrations, production of reactive oxygen species and dilution of signal upon cell division may impede the clinical development of ferritin-based MRI imaging and large animal studies are needed before clinical utility of this technique can be determined47
This indirect method may be better than direct labeling because of its dependence on gene expression, which is correlated much more tightly with cell viability and can provide more functional information. Indirect labeling, or using reporter gene expression to produce contrast, can be used in both non-optical and optical labeling and is very versatile; this allows MSCs to be detected at any time, or only upon differentiation, if the enzyme is controlled by a transcription factor or promoter of interest47. The possibilities are nearly endless; reporter proteins could be driven by doxycycline-inducible promoters, controlling temporal expression, or by pathway-specific promoters to examine biological action after MSC tumor-homing.
Though very high concentrations of certain contrast agents, such as ultrasmall SPIOs ((U)SPIOs) can be toxic to cells and released iron can damage metabolic pathways, most reports find that MRI contrast agents do not damage cell differentiation potential, proliferation, or function47, 44. MRI is sensitive enough to detect as few as 1,000 labeled MSCs in co-injection with breast cancer cells in subcutaneous tumors14 and its resolution, 100–200 μm, allows for visualization of small cell clusters. Imaging single cells is difficult due to blurring from intrinsic movements (ex: breathing, muscle twitching) but a solution to this problem, called “white marker tracking”, has been developed48,44. MRI can be combined with other modalities such as PET and SPECT to give greater insight into cell localization and function49,50. Drawbacks of MRI include mislabeling of cells due to labeled-cell uptake by phagocytic cells, decreased signal-to-noise ratios as particles are diluted when cells divide, lack of information regarding cell survival or activity, and short physical and biological half-life of labels (by physical breakdown or natural exit from cells, respectively)47. Consequently, MRI is primarily a short-term monitoring technique. MSCs have been detected using MRI in many rat, mouse, rabbit and swine models of damaged tissue and within glioma models42. MRI has also been used to detect human MSC homing to pulmonary metastases using biocompatible SPIOs in mouse models of human metastatic breast cancer14 (Figure 1E).
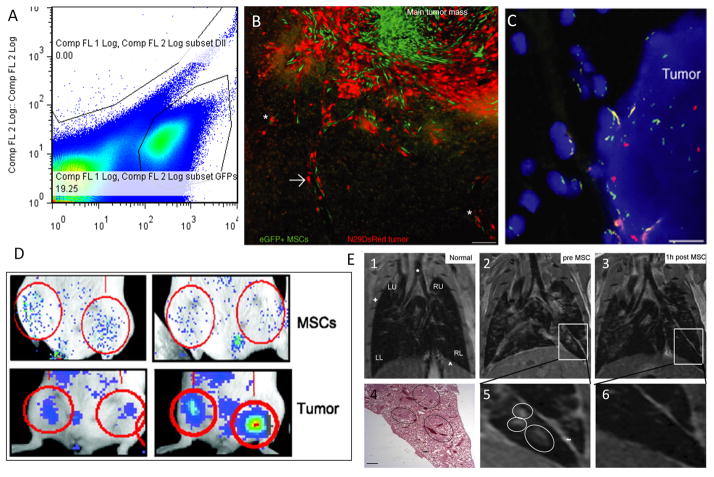
Figure 1.
Imaging modalities for MSC-tumor tropism. A) Flow cytometry plot of cells from a single cell suspension of a digested primary tumor containing three populations of cells (Tumor cells, MSCs, and residual mouse mammary cells.) B) eGFP fluorescent MSCs (green) and glioma tumor cells (red) identified using epifluorescence microscopy of tumor sections show co-localization within tumor. Bar = 100 μm. C) Whole-chromosome FISH painting identifies Y chromosomes (red) from male rat MSCs within female-derived glioma tumors. Nuclei are stained with by DAPI (4′,6-diamidino-2-phenylindole staining) (blue). Scale bar = 15 μm. D) Xenogen images demonstrate the use of BLI to track fLuc-expressing MSC homing from tail-vein specifically to primary orthotopic 4T1 breast tumors (below) compared to non-cancerous mammary fat pads (above). E) Intravenously-delivered SPIO-loaded MSCs localize to lung metastases and can be visualized by MRI. 1, Normal lung. 2,5) Lung with MDA-MB-231 metastases. 3,6) Same lung 1 hr after SPIO-loaded MSC injection shows decreased MRI signal in metastases signal. 4) H&E histologic sections (bar, 100 μm). See references for more details. Figure 1A (unpublished data from our lab). Figures 1B and 1C from Reference 23, Figure 1D and 1E adapted and reprinted by permission from the American Association for Cancer Research from references 18 and 14 respectively.
MRI in Clinical Settings
The sensitivity, specificity, and current clinical use of MRI, along with its full tissue penetration, and high in vivo resolution may make MRI imaging the favorite for clinically tracking MSCs. MRI is currently used clinically to detect malignancies, and many other diseases and injuries51. In terms of tumor-homing, clinical studies have revealed that autologous, immature dendritic cells can be labeled with 111In-oxine and SPIO and imaged using MRI during homing to lymph nodes in stage-III melanoma patients41. The study found MRI cell tracking using iron oxides to be clinically safe and well suited to monitor cellular therapy in humans. SPIOs are FDA-approved, but transfection agents pose a problem in translating many animal model systems into human, until better methods of introducing SPIOs into non-phagocytic cells are developed41. The review by Budde, et al. details MRI methods used to track cells and the potential and challenges for each in clinical translation43. Detection of MSCs using MRI in humans is likely to develop once the health risks of MSCs are fully elucidated and standards for treatment and imaging are developed. To date, only four human clinical trials using SPIOs for cell tracking have been performed, all outside the United States, as reviewed by Bulte et al52. Most FDA approved SPIOs that were previously used in animal studies have now been discontinued from the market, so moving to clinics with SPIO-labeled cells will be difficult in the foreseeable future. Still, once stem cell therapy becomes mainstream and better MRI cellular imaging tools are developed, MRI cell tracking may become a vital tool in cell tracking.
PET in Animal Models
PET is arguably more sensitive than MRI in animal models, but has a lower resolution (on the order of mm53, compared to μm resolution in MRI54). Direct labeling of rat adipose-derived stem cells (ASCs)50 and leukocytes55 with copper and cobalt isotopes can provide longer term imaging capabilities than found with MRI. For indirect labeling, MSCs can be transduced with a gene for mutant herpes simplex virus type 1 thymidine kinase (HSV1-tk), which increase their uptake of an injected radioactive substrate (18F-labeled 9-(4-fluoro-3-hydroxymethylbutyl)-guanine ([18F]-FHBG)) and causes increased PET signal6. However, the short half-life of 18F (110 min) considerably limits its use in clinical and model systems. Still, 18F- labeled MSC migration to subcutaneously-implanted colon adenocarcinoma and self-renewal abilities were assessed over one month using PET in a mouse model12. MSCs can also be labeled with mutant dopamine receptors or transmembrane proteins such as the sodium iodide symporter (NIS), which can be used for PET or SPECT imaging when using tracers 124I (for PET) or 123I or 99Tc-pertechnetate 47.
PET in Clinical Settings
Novel dual-modality (PET/MRI) contrast agent nanoparticles are currently being developed to label cells without transfection reagents; these may prove to be paramount in animal models and in the clinic based on their high cell-labeling efficiency and low cytotoxicity50. PET and PET/CT (computed tomography) scans are commonly used clinically to detect human malignancies and have been used to detect cytolytic T cells (CTLs), or other therapeutic cells, labeled with HSV1-tk or mutant HSV1-sr39tk reporter genes. Reporter gene expression, detected by 18F–FHBG injection, and can be used to image cell migration towards glioblastomas or other tumors51,6. The clinical utility of PET scans makes them easily translatable to short-term or long-term MSC tracking applications in patients, depending on the contrast reagent used.
SPECT in Animal Models and Clinical Settings
SPECT utilizes the radioactive decay of radionuclides and gamma rays to provide 3-D information on cell location using tomographic reconstruction. Most usable and FDA-approved SPECT isotopes are short-lived (e.x.: Tc-99m (360 minutes), Ga-67 (4320 minutes), In-111 (4020 minutes) and I-123 (780 minutes))56. SPECT can also be combined with PET and CT imaging and has been successful at imaging labeled leukocytes, human MSCs (hMSCs), and progenitor cells in rat, mice and pig models, though the effects of SPECT contrast reagents on hMSC function remain debated55,57,58,59,49. Although SPECT has not been used to track MSCs or other therapeutic cells during tumor-homing in patients, it is clinically used for tracking leukocyte migration and could easily be expanded to track MSCs in tumor-homing applications. Table 1 summarizes the strengths and limitations of SPECT, specifically in comparison to PET. Notably, PET depends on active uptake by glucose transporters for cell labeling while SPECT contrast reagents such as [In-111]oxine can passively diffusive into cells due to their lipophilic nature60.
Optical Methods for Tracking MSCs
End-point tracking of MSC engraftment is done in models using histology, immunohistochemistry, immunofluorescence (IF), fluorescent in situ hybridization (FISH) and even flow cytometry, but these techniques are not translatable to the clinic because of the lack of inherent MSC specific markers and FDA approval for genetically-modified MSCs or agents for optical labeling. Real-time tracking of MSCs using optical techniques is also unrealistic in clinical settings due low light penetration through the body. Non-invasive fluorescence-based approaches, which may prove to be relatively inexpensive, are being developed for clinical applications by redesigning FDA-approved fluorescent dyes and adapting quantum dots, antibody-conjugated labels, activatable fluorescent imaging probes, surface-enhanced Raman scattering (SERS) nanoparticles, and target peptides as reviewed elsewhere61.
In model systems, end-point techniques are essential for validation of MSC engraftment and evaluation of spatial orientation, morphology, differentiation, and function within tumors. Histology can detect MSCs using anti-GFP9 (green fluorescent protein), anti-firefly-luciferase18, or anti-human antibodies29 and can identify MSC differentiation into pericytes12, endothelial cells, adipocytes, and osteoblasts38,12,8 (Figure 1B). FISH has been used to identify male MSCs within gliomas of female mice utilizing the y-chromosome (Figure 1C)23. Flow cytometry allows for quantification of MSCs within a tumor after digesting the tumor into a single cell suspension (Figure 1A).
Real-time MSC tracking is often done in models using fluorescent dyes and proteins as cell labels. Reporter genes, such as the GFP9,23 are considered indirect labels and produce a signal undiminished by proliferation, which can be detected using in vivo optical imaging30, though the signal will diminish as it travels through tissue. Transgene expression may cause faster MSC clearance and immune response in immunocompetent animals30, but recent data suggests that reporter genes do not significantly alter the biological properties and differentiation capacity of stem cells47. Still, untagged-MSCs are more likely to be accepted clinically, necessitating other methods for MSC tracking in patients.
Similarly, fluorescent dyes, such as Cell TrackerTM dyes, span a range of spectral properties and can directly label cells for weeks or longer for live in vivo imaging8,11. However, dyes can be transferred to surrounding cells and are not preserved with formalin fixation. In vivo confocal microscopy (intravital microscopy) and two-photon video imaging have also been used to image individual progenitor cells using lipophilic dyes, but have not been applied to MSC homing to tumors likely because these are still relatively new techniques and are only capable of examining a small, superficial area within an animal62.
BLI (bioluminescent imaging) is non-invasive, non-destructive, quantitative and commonly used in models of cell migration (Figure 1D)18,32. Different cell types may be distinguished by indirect labeling with different reporter gene luciferase enzymes that utilize unique substrates and whole animals. Single luciferase-transduced cells can be imaged using BLI technology, demonstrating its high sensitivity63, and organ removal improves detection results and reduces noise in endpoint analysis. BLI is ideal for longitudinal studies and only improving in terms of resolution, sensitivity and 3-D imaging capacity10,38
Optical and Non-Optical Labeling Challenges
Recent findings have demonstrated that many stem cells labeled with intracellular labels such as dextran coated SPIOs, bromodeoxyuridine (BrdU) or GFP, can be taken up by resident tissue macrophages, complicating the interpretation of intracellular labels especially during direct implantation of cells, which can result in more than 70% cell death64. The study suggests that histology should be used in combination with MRI, fluorescence microscopy or flow cytometry, since up to 15% of macrophages may be positive for the marker due to phagocytosis of labeled stem cells.
Conclusions and Future Directions
Evaluation of cellular therapy and the design of patient-specific care rely on real-time and endpoint assessment of cellular migration, proliferation, and overall function. Sophisticated animal models give researchers the ability to determine how therapeutic or diagnostic cells, such as MSCs, migrate to and engraft and differentiate within tumors. Regardless of model systems and components used, it is clear that the best clinical and basic research results derive from multi-modal imaging systems which provide functional and anatomical data. This article outlined the characteristics of optical and non-optical imaging using direct and indirect (gene expression) labeling techniques. The article discussed benefits and drawbacks of each in model and clinical settings. Though optical methods will likely remain at the forefront of MSC tracking in animal models, MRI, PET, and SPECT may become more prevalent as the technologies become less expensive and more widespread, due to their clinical utility. Still, these techniques will have to compete with novel fluorophores, bioluminescent enzymes, photon detection devices and cell-labeling technologies that will continue to develop for optical model systems.
Patients and clinicians are demanding better MSC tracking technologies clinically, and the potential use of MSC in many diseases supports the need for better MSC tracking technologies within animal models. The future of therapeutic MSCs will be the expression and delivery of novel proteins, normally expressed proteins, and small hairpin RNAs to help regenerate tissues and kill tumors. As we remain ignorant regarding many of the possible off-target effects inflicted by genetic or non-genetic modifications, we may want to incorporate suicide genes into MSCs driven by inducible promoters as a safety precaution against teratoma formation or other deleterious, unpredicted effects. We will continue to rely on model systems to elucidate the role of MSCs within diseased and healthy tissues and experimental designs that optimally combine real-time, endpoint, and multimodal tracking technologies to gain the greatest insight into cell homing, engraftment, and tumor-host interactions.
Acknowledgments
Grants/Funding Sources: NIH Tissue Engineering Resource Center (P41 EB002520), Susan Komen Breast Cancer Metastasis Grant (BCTR0706887), and Department of Defense CDMRP Breast Cancer Research Program grant, award number W81XWH-10-1-0086. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
The authors have nothing to disclaim.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author Contribution:
Michaela R Reagan: Conception and design, Collection and/or assembly of data, Manuscript writing, Data analysis and interpretation
David L Kaplan: Financial support, Review and final approval of manuscript
N/A: Provision of study material or patients
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. [Accessed July 18, 2010];CA: a cancer journal for clinicians. 2010 60(5):277–300. doi: 10.3322/caac.20073. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20610543. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Hirai M, Cantero S, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells fro. [Accessed February 16, 2011];Journal of cellular biochemistry. 2011 112(4):1206–18. doi: 10.1002/jcb.23042. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21312238. [DOI] [PubMed] [Google Scholar]
- 3.Liang L, Dong C, Chen X, et al. Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Mice TNBS-Induced Colitis. [Accessed March 18, 2011];Cell transplantation. 2011 doi: 10.3727/096368910X557245. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21396175. [DOI] [PubMed]
- 4.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16923606. [DOI] [PubMed] [Google Scholar]
- 5.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. [Accessed July 13, 2010];Stem cells (Dayton, Ohio) 2007 25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17901396. [DOI] [PubMed] [Google Scholar]
- 6.Yaghoubi SS, Gambhir SS. PET imaging of herpes simplex virus type 1 thymidine kinase (HSV1-tk) or mutant HSV1-sr39tk reporter gene expression in mice and humans using [18F]FHBG. [Accessed February 11, 2011];Nature protocols. 2006 1(6):3069–3075. doi: 10.1038/nprot.2006.459. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17406570. [DOI] [PubMed] [Google Scholar]
- 7.Chiu RCJ. "Stealth immune tolerance" in stem cell transplantation: potential for "universal donors" in myocardial regenerative therapy. [Accessed November 12, 2010];The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005 24(5):511–516. doi: 10.1016/j.healun.2004.11.010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15896746. [DOI] [PubMed] [Google Scholar]
- 8.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer research. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15833864. [DOI] [PubMed] [Google Scholar]
- 9.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17914389. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Ginestier C, Ou SJ, et al. Breast Cancer Stem Cells Are Regulated by Mesenchymal Stem Cells through Cytokine Networks. [Accessed January 14, 2011];Cancer research. 2011 71(2):614–624. doi: 10.1158/0008-5472.CAN-10-0538. Available at: http://cancerres.aacrjournals.org/cgi/content/abstract/71/2/614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. [Accessed January 2, 2011];Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. 2010 :10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21159629. [DOI] [PMC free article] [PubMed]
- 12.Hung S-C, Deng W-P, Yang WK, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. [Accessed August 19, 2010];Clinical cancer research : an official journal of the American Association for Cancer Research. 2005 11(21):7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. Available at: http://clincancerres.aacrjournals.org/cgi/content/abstract/11/21/7749. [DOI] [PubMed] [Google Scholar]
- 13.Komarova S, Roth J, Alvarez R, Curiel DT, Pereboeva L. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. [Accessed November 12, 2010];Journal of ovarian research. 2010 3(1):12. doi: 10.1186/1757-2215-3-12. Available at: http://www.ovarianresearch.com/content/3/1/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loebinger MR, Kyrtatos PG, Turmaine M, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. [Accessed November 9, 2010];Cancer research. 2009 69(23):8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. Available at: /pmc/articles/PMC2833408/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin H, Sun R, Kanehira M, et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Molecular medicine (Cambridge, Mass) 15(9–10):321–327. doi: 10.2119/molmed.2009.00059. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem cells (Dayton, Ohio) 2009;27(4):857–865. doi: 10.1002/stem.23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19350687. [DOI] [PubMed] [Google Scholar]
- 17.Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. [Accessed June 24, 2010];Stem cells (Dayton, Ohio) 2007 25(2):520–528. doi: 10.1634/stemcells.2006-0257. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17053212. [DOI] [PubMed] [Google Scholar]
- 18.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Research. 2007;67(24):11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18089798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Stem Cells. 2003;102(10):3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 20.Ramasamy R, Lam EW-F, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. [Accessed June 24, 2010];Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007 21(2):304–310. doi: 10.1038/sj.leu.2404489. Available at: http://dx.doi.org/10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 21.Martin FT, Dwyer RM, Kelly J, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT) Breast cancer research and treatment. 2010 doi: 10.1007/s10549-010-0734-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20087650. [DOI] [PubMed]
- 22.Corcoran KE, Trzaska Ka, Fernandes H, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PloS one. 2008;3(6):e2563. doi: 10.1371/journal.pone.0002563. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18575622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bexell D, Gunnarsson S, Tormin A, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. [Accessed July 6, 2010];Molecular therapy : the journal of the American Society of Gene Therapy. 2009 17(1):183–190. doi: 10.1038/mt.2008.229. Available at: http://dx.doi.org/10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi_ s sarcoma. [Accessed June 24, 2010];The Journal of experimental medicine. 2006 203(5):1235–1247. doi: 10.1084/jem.20051921. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2121206&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun B, Roh K-H, Park J-R, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. [Accessed June 24, 2010];Cytotherapy. 2009 11(3):289–298. 1–298. doi: 10.1080/14653240902807026. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19308770. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson L. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. [Accessed June 24, 2010];Experimental and Molecular Pathology. 2003 75(3):248–255. doi: 10.1016/j.yexmp.2003.06.001. Available at: http://dx.doi.org/10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Qiao L, Xu Z-L, Zhao T-J, Ye L-H, Zhang X-D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. [Accessed June 24, 2010];Cancer letters. 2008 269(1):67–77. doi: 10.1016/j.canlet.2008.04.032. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18571836. [DOI] [PubMed] [Google Scholar]
- 28.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer research. 2009;69(10):4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19435900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studeny M, Marini FC, Champlin RE, et al. Advances in Brief Bone Marrow-derived Mesenchymal Stem Cells as Vehicles for Interferon- Gamma Delivery into Tumors 1. Cancer Treatment and Research. 2002;(713):3603–3608. [PubMed] [Google Scholar]
- 30.Wang N, Fallavollita L, Nguyen L, et al. Autologous bone marrow stromal cells genetically engineered to secrete an igf-I receptor decoy prevent the growth of liver metastases. [Accessed November 17, 2010];Molecular therapy : the journal of the American Society of Gene Therapy. 2009 17(7):1241–1249. doi: 10.1038/mt.2009.82. Available at: http://dx.doi.org/10.1038/mt.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. [Accessed September 17, 2010];Advanced drug delivery reviews. 2010 62(12):1156–1166. doi: 10.1016/j.addr.2010.08.010. Available at: http://dx.doi.org/10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svetlana Komarova JR. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. [Accessed November 9, 2010];Journal of Ovarian Research. 2010 3(12) doi: 10.1186/1757-2215-3-12. Available at: /pmc/articles/PMC2883983/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker KL. Principles and practice of endocrinology and metabolism, Page 957. Lippincott Williams & Wilkins; 2001. [Accessed March 7, 2011]. p. 2477. Available at: http://books.google.com/books?id=FVfzRvaucq8C&pgis=1. [Google Scholar]
- 34.Anonymous. [Accessed January 19, 2011];Viral Vector generation, Molecular Biology Services, cDNA libraries, cloning shRNA , Adenovirus Amplification, Adenovirus purification, Transgenic Animal Generation, Recombinant lentiviral vector construction. Available at: http://www.nitanbiotech.com/viralvectors.php.
- 35.Shakhbazau AV, Sevyaryn IN, Goncharova NV, et al. Viral Vectors for Stable Transduction of Human Mesenchymal Stem Cells: Systems Based on Adeno-Associated Viruses and Lentiviruses. [Accessed January 19, 2011];Bulletin of Experimental Biology and Medicine. 2009 146(4):531–533. doi: 10.1007/s10517-009-0320-x. Available at: http://www.springerlink.com/content/h967gk8008r02547/ [DOI] [PubMed] [Google Scholar]
- 36.Mátrai J, Chuah MKL, VandenDriessche T. Recent advances in lentiviral vector development and applications. [Accessed March 18, 2011];Molecular therapy : the journal of the American Society of Gene Therapy. 2010 18(3):477–90. doi: 10.1038/mt.2009.319. Available at: http://dx.doi.org/10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. [Accessed November 9, 2010];Trends in molecular medicine. 2010 16(5):203–209. doi: 10.1016/j.molmed.2010.02.005. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2881950&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Cao F, De A, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem cells (Dayton, Ohio) 2009;27(7):1548–1558. doi: 10.1002/stem.81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19544460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. [Accessed August 26, 2010];Cancer research. 2007 67(13):6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17616689. [DOI] [PubMed] [Google Scholar]
- 40.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. [Accessed November 9, 2010];Circulation. 2005 112(10):1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1456731&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vries IJM, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. [Accessed February 11, 2011];Nature biotechnology. 2005 23(11):1407–1413. doi: 10.1038/nbt1154. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16258544. [DOI] [PubMed] [Google Scholar]
- 42.Anderson SA, Glod J, Arbab AS, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. [Accessed January 25, 2011];Blood. 2005 105(1):420–425. doi: 10.1182/blood-2004-06-2222. Available at: http://bloodjournal.hematologylibrary.org/cgi/content/abstract/105/1/420. [DOI] [PubMed] [Google Scholar]
- 43.Budde MD, Frank JA. Magnetic tagging of therapeutic cells for MRI. [Accessed August 20, 2010];J Nucl Med. 2009 50(2):171–174. doi: 10.2967/jnumed.108.053546. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2633027&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb GA, Hoehn M, Himmelreich U. In: Modern Magnetic Resonance. Webb GA, editor. Dordrecht: Springer Netherlands; 2006. [Accessed January 20, 2011]. pp. 1087–1098-1098. Available at: http://www.springerlink.com/content/p170216l89rn7t79/ [Google Scholar]
- 45.Naumova AV, Reinecke H, Yarnykh V, et al. Ferritin overexpression for noninvasive magnetic resonance imaging-based tracking of stem cells transplanted into the heart. [Accessed January 21, 2011];Molecular imaging : official journal of the Society for Molecular Imaging. 2010 9(4):201–210. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20643023. [PMC free article] [PubMed] [Google Scholar]
- 46.Campan M, Lionetti V, Aquaro GD, et al. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5T cardiac MRI in a rat model of myocardial infarction. [Accessed February 24, 2011];American journal of physiology. Heart and circulatory physiology. 2011 doi: 10.1152/ajpheart.00935.2010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21335465. [DOI] [PubMed]
- 47.Rodriguez-Porcel M. In vivo imaging and monitoring of transplanted stem cells: clinical applications. [Accessed February 23, 2011];Current cardiology reports. 2010 12(1):51–8. doi: 10.1007/s11886-009-0073-1. Available at: http://www.springerlink.com/content/v0v0341786104g01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seppenwoolde J-H, Viergever MA, Bakker CJG. Passive tracking exploiting local signal conservation: the white marker phenomenon. [Accessed August 27, 2010];Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003 50(4):784–790. doi: 10.1002/mrm.10574. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14523965. [DOI] [PubMed] [Google Scholar]
- 49.Rad AM, Iskander ASM, Janic B, et al. AC133+ progenitor cells as gene delivery vehicle and cellular probe in subcutaneous tumor models: a preliminary study. [Accessed January 25, 2011];BMC biotechnology. 2009 9:28. doi: 10.1186/1472-6750-9-28. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2669076&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel D, Kell A, Simard B, et al. The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents. [Accessed January 4, 2011];Biomaterials. 2011 32(4):1167–1176. doi: 10.1016/j.biomaterials.2010.10.013. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21035183. [DOI] [PubMed] [Google Scholar]
- 51.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. [Accessed February 11, 2011];Nature clinical practice. Oncology. 2009 6(1):53–58. doi: 10.1038/ncponc1278. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19015650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bulte JWM. In vivo MRI cell tracking: clinical studies. [Accessed November 22, 2010];AJR. American journal of roentgenology. 2009 193(2):314–25. doi: 10.2214/AJR.09.3107. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2857985&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wernick MN, Aarsvold JN. Emission tomography: the fundamentals of PET and SPECT. Academic Press; 2004. [Accessed February 4, 2011]. p. 576. Available at: http://books.google.com/books?id=R5slur_hdfEC&pgis=1. [Google Scholar]
- 54.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. [Accessed January 21, 2011];Blood. 2003 102(3):867–872. doi: 10.1182/blood-2002-12-3669. Available at: http://bloodjournal.hematologylibrary.org/cgi/content/abstract/102/3/867. [DOI] [PubMed] [Google Scholar]
- 55.Korf J, Veenma-van der Duin L, Brinkman-Medema R, Niemarkt A, de Leij LF. Divalent cobalt as a label to study lymphocyte distribution using PET and SPECT. [Accessed February 9, 2011];Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998 39(5):836–841. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9591586. [PubMed] [Google Scholar]
- 56.Hans-Jurgen B, Freeman LM. Clinical Nuclear Medicine. Springer; 2007. [Accessed March 19, 2011]. p. 548. Available at: http://books.google.com/books?id=dZIBbLhTOoQC&pgis=1. [Google Scholar]
- 57.Gildehaus FJ, Haasters F, Drosse I, et al. Impact of Indium-111 Oxine Labelling on Viability of Human Mesenchymal Stem Cells In Vitro, and 3D Cell-Tracking Using SPECT/CT In Vivo. [Accessed January 24, 2011];Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2010 doi: 10.1007/s11307-010-0439-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21080231. [DOI] [PubMed]
- 58.Bindslev L, Haack-Sørensen M, Bisgaard K, et al. Labelling of human mesenchymal stem cells with indium-111 for SPECT imaging: effect on cell proliferation and differentiation. [Accessed October 22, 2010];European journal of nuclear medicine and molecular imaging. 2006 33(10):1171–1177. doi: 10.1007/s00259-006-0093-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16763813. [DOI] [PubMed] [Google Scholar]
- 59.Chin BB, Nakamoto Y, Bulte JWM, et al. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. [Accessed October 22, 2010];Nuclear medicine communications. 2003 24(11):1149–1154. doi: 10.1097/00006231-200311000-00005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14569169. [DOI] [PubMed] [Google Scholar]
- 60.Palestro CJ, Love C, Bhargava KK. Labeled leukocyte imaging: current status and future directions. [Accessed February 20, 2011];The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of Radiopharmaceutica. 2009 53(1):105–123. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19182734. [PubMed] [Google Scholar]
- 61.Singhal S, Nie S, Wang MD. Nanotechnology applications in surgical oncology. [Accessed August 19, 2010];Annual review of medicine. 2010 61:359–373. doi: 10.1146/annurev.med.60.052907.094936. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2913871&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. [Accessed November 12, 2010];Nature. 2009 457(7225):92–96. doi: 10.1038/nature07434. Available at: http://dx.doi.org/10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J-B, Urban K, Cochran E, et al. Non-invasive detection of a small number of bioluminescent cancer cells in vivo. [Accessed February 22, 2011];PloS one. 2010 5(2):e9364. doi: 10.1371/journal.pone.0009364. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2826408&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pawelczyk E, Jordan EK, Balakumaran A, et al. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. [Accessed August 29, 2010];PloS one. 2009 4(8):e6712. doi: 10.1371/journal.pone.0006712. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2726631&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]