Abstract
Glypican-3 is a promising target for immunotherapy for hepatocellular carcinoma, but limited data exist regarding its immunogenicity in patients with diverse HLA types, immunogenicity for CD4+ T-cells, and the impact of inhibitory co-stimulation on glypican-3-specific T-cells. Using a 15mer overlapping peptide library for glypican-3, PBMC from patients with HCC were assessed ex vivo and after short-term in vitro expansion for tumor antigen-specific T-cell responses with and without blockade of PD-1/PD-L1 and CTLA-4 signaling. Glypican-3-specific T-cells were undetectable ex vivo, but primarily IFNγ+ TNFα+ CD4+ T-cells expanded with short-term in vitro stimulation in 10/19 (52%) patients. Glypican-3-specific CD8+ T-cells predominantly produced TNFα, but did not secrete IFNγ nor degranulate. CTLA-4 and PD-1 blockade minimally impacted the cytokine secretion and proliferation of glypican-3-specific T-cells. These data suggest that CD8+ T-cell-directed tumor vaccines in HCC may have limited potential for efficacy unless optimal co-stimulation conditions can be identified but CD4+-directed vaccines merit consideration.
Keywords: Hepatocellular carcinoma, T-cells, Interferon-gamma, PD-1, CTLA-4
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and third-leading cause of cancer death worldwide [1]. Hepatocellular carcinoma most frequently develops in patients with cirrhosis related to chronic viral hepatitis [2] at an approximate rate of 4% per year, often prior to the development of other complications of cirrhosis or portal hypertension. In these individuals, HCC primarily causes morbidity and mortality due to acceleration of hepatic failure and portal hypertension rather than via metastatic spread. Usually asymptomatic until late stages, HCC most often presents at an advanced stage at which time treatment options are quite limited [3]. For this reason, novel approaches including immunotherapy have been considered as a potential adjunctive therapy in advanced HCC.
There are several theoretical and empiric reasons to expect that hepatocellular carcinoma might be poorly immunogenic and thus a challenging target for tumor vaccines. First, the liver microenvironment exerts a toleragenic effect due to various properties of liver sinusoidal endothelial cells and Kupffer cells [4,5]. Second, HCC arises primarily in a cirrhotic liver, an immunosuppressive environment associated with phagocyte and antigen-presenting cell dysfunction [6,7]. Third, chronic viral infections, such as hepatitis B and C, which underlie the antecedent cirrhosis, induce intrahepatic antigen-specific effector T-cell exhaustion as well as regulatory T-cell generation [8,9]. Furthermore, there is mounting evidence that hepatocellular carcinomas may directly recruit regulatory cell populations, such as M2 macrophages, myeloid-derived suppressor cells, and regulatory T-cells, to suppress tumor-directed T-cell effector responses [10,11]. Not surprisingly, tumor-antigen specific T-cells in patients with HCC, when detectable, have generally exhibited restricted effector functions [12]. Nonetheless, some studies suggest that the presence of T-cell responses against panels of tumor antigens in HCC may be associated with improved prognosis [13]. Furthermore, T-cell activation by cytokines [14] and/or by therapeutic embolization of tumor-lysate-pulsed dendritic cells directly into HCCs has resulted in partial tumor control [15], suggesting that tumor-specific T-cells in HCC could play a role in retarding tumor growth.
Glypican-3 (GPC3), a glycosylphosphatidylinositol-linked heparan-sulfate proteoglycan, has been identified as a highly specific, membrane-associated, tumor antigen found in 66–100% of HCC [16,17] with little or no expression in non-tumorous cirrhotic liver tissue or other normal adult tissues [18,19]. GPC3 fosters HCC growth by altering Wnt signaling [20], modulating growth factors such as IGF-2, BMP-7 and FGF-2 [21], and possibly by playing a role in M2 macrophage recruitment [11]. GPC3 may be cleaved from the surface of expressing hepatocytes, thereby entering the circulation to allow serological detection [22]. While several groups have reported the ability to expand glypican-3-specific T-cells in mice [23–25] and from a small number of human subjects [23,26], this work has focused primarily on expanding CD8+ T-cell using specific HLA-types and highly immunodominant epitopes [23,24,26] limiting the applicability of findings to more heterogeneous populations. Such approaches have also precluded the examination of the potential important role of tumor antigen-reactive CD4+ T-cells [27,28]. Furthermore, cytolytic capacity of expanded GPC3-specific cells remained fairly weak even after long-term in vitro expansion under optimized conditions [23] suggesting that antigen-specific T-cells expanded from HCC patients remain dysfunctional.
In this study, we sought to quantify the in vivo frequency and capacity for expansion of polyfunctional CD8+ and CD4+ antigen-specific T-cell response against glypican-3 in a cohort of patients with heterogeneous HLA types and to define the role of inhibitory co-stimulatory molecule expression on suppressing tumor antigen-specific peripheral T-cells. Our studies indicate that glypican-3-specific T-cells are functionally suppressed ex vivo in patients with HCC. However, IFNγ-producing CD4+ T-cells are expanded with short term in vitro stimulation in approximately half of HCC-bearing individuals. Glypican-3-reactive CD8+ T-cells are also expanded in approximately half of the HCC patients, but these CD8+ T-cells are functionally constrained to produce TNFα alone. CTLA-4 and PD-1 inhibitory co-stimulation pathways, which are quite important in suppressing hepatitis virus-specific T-cells, only modestly impact the cytokine secretion and proliferation of peripheral glypican-3-specific T-cells in HCC patients. Thus, peripheral glypican-3-specific T-cells may be poor targets for effective vaccine-induced augmentation or for expansion in adoptive immunotherapy protocols unless mechanisms to reverse tolerance are identified.
2. Materials and methods
2.1. Patients
Subjects and controls were recruited from the Gastroenterology Clinics at the Philadelphia Veterans Affairs Medical Center following informed consent on an institutional review board-approved protocol. All patients were assessed for baseline demographics, hepatitis viral serologies, alcohol use history, and prior therapy for hepatocellular carcinoma. HCC patients were diagnosed histologically or via standard radiological and serological criteria [29]. Controls included patients with hepatitis C-induced cirrhosis with no evidence of HCC by serial imaging and alpha-fetoprotein screening (cirrhotic group, CIR), hepatitis C patients with F1-2 fibrosis by biopsy within the preceding 5 years (early-stage viral hepatitis, EVH), and healthy donors (HD) with no evidence of chronic liver disease.
2.2. HLA Typing
In all patients, HLA-A2.1 expression was determined using flow cytometry with anti-HLA-A2.1 (clone BB7.2, BD Biosciences, Franklin Lakes NJ). Results were confirmed with Terasaki HLA Class I Typing Tray (One Lambda, Canoga Park CA).
2.3. Peptides and proteins
Libraries of 15mer peptides offset by 6 and overlapping by 9 amino acids for the human (580aa, NP_004475) and survivin (142aa, NP_001159) were commercially synthesized (Proimmune, Oxford UK). Selected individual 9–10mer peptides predicted to bind to human HLA-A2.1 based on online algorithms (BIMAS [30], SYPEITHI [31], and RankPep [32]) were also synthesized. Recombinant human glypican-3 (Gln 25–His 559) and survivin were obtained commercially (R&D Systems, Minneapolis MN and Genemega Inc., San Diego CA respectively). In previous studies using 15mer peptide libraries for the hepatitis C virus, 15mer peptides were shown to be immunogenic for both CD4+ and CD8+ T-cells [33]. A mixture of CMV, EBV, and Influenza (CEF) 9–10mer control peptides (Cellular Technology Ltd., Cleveland, OH) were used as positive controls for CD8+ effector T-cell responses.
2.4. Isolation of PBMC
Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Histopaque (Sigma, St. Louis MO) density centrifugation. Cells were resuspended in RPMI 1640 with L-glutamine (Invitrogen) with 10% human AB serum (Sigma Inc., St. Louis, MO), 1.5% HEPES (Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
2.5. In vitro T-cell expansion
4 × 106 PBMC were stimulated with media control, pooled glypican-3 15mer peptides (95 peptides, 1 µg/ml each), or CEF peptide pool (1 µg/ml) for 8 days at 37 °C 5% CO2 with rhIL-2 100U/ml (Novartis/ Chiron, Emeryville, CA) added on day 2 and day 5. Additional antigen-presenting cells (APC) were not utilized due to the presence of sufficient number of APCs in whole PBMC. For proliferation and blocking experiment, PBMC were labeled with 5 uM CFSE-DA (Molecular Probes, Eugene OR) per manufacturer’s instructions, then incubated with survivin-, glypican-3- or control peptide pools in the presence of NA/LE control Ig (Biolegend, San Diego CA), anti-PD-L1 (clone 29A.2E3, Biolegend) and/or anti-CTLA-4 (clone BNI3, BD Biosciences, San Jose CA) with rhIL-2 added on day 2 and day 5.
2.6. Flow cytometry
All antibodies were purchased from Becton Dickinson (Becton Dickinson, Franklin Lakes, NJ) except where specified. The cutoff for each marker was based on the isotype antibody. Data were acquired on a modified FACSCanto (BD), and ana\lyzed using Flow Jo (Tree Star Inc., Ashland OR).
2.7. Intracellular cytokine staining
In vitro expanded cells were restimulated for 6 h with survivin- or glypican-3-derived 15mer peptide pools and controls in the presence of anti-CD107a-PE and monensin, fixed and permeabilized using BD Cytoperm/Cytofix (BD), then stained intracellularly for IFNγ PE-Cy7 and TNFα APC (BD).
2.8. IFNγ Elispot
Antigen-specific T cell IFNγ responses were examined ex vivo and after in vitro expansion in cytokine Elispot assay as previously described [34]. In ex vivo assays, PBMC (2 × 105/well) were plated in 96-well Elispot plates pre-coated with anti-IFNγ (5 µg/ml, Pierce, Rockford IL) and stimulated with peptide matrices for glypican-3 (95 peptides, 7×7×2 matrix) and human survivin (22 peptides, 5×5 matrix), pooled glypican-3 peptides (95 peptides), pooled surviving peptides (22 peptides)(1µg/ml), recombinant glypican-3, and recombinant survivin proteins (10 mg/ml) in duplicates. Negative control wells without peptides (6 replicates) and positive control wells with 2 µg/ml phytohemagglutinin, 1 µg/ml CEF and 20 µg/ml C. albicans. After 8 days in vitro expansion, 5×104 cells/ well were stimulated with each peptide pool (1 µg/ml) in duplicates with positive (PHA) and negative controls (media, irrelevant peptide pool). Plates were analyzed for spot forming units (SFU), excluding assays with high background (average > 10 SFU/well in negative control wells) or no response to positive control stimulation on an AID Elispot ELF04 (AID Diagnostika, Strassberg, Germany).
2.9. HLA-A2.1 pentamer staining
In HLA-A2+ patients, HCC-specific T-cells were detected ex vivo and after in vitro expansion using peptide-loaded HLA-A2.1 pentamers (Proimmune, Oxford UK) loaded with following 9–10mer epitopes: Survivin96–104 (LMLGEFLKL)(Sur1M2), Glypican-344–52 (RLQPGLKWV) [23], Glypican-3136–145 (SLTPQAFEFV), Glypican-3144–152 (FVGEFFTDV)[23], Glypican-3169–177 (ELFDSLFPV)[23], Glypican-3563–572 (KLLTSMAISVV), HTLV-I tax11–19 (LLFGYPVYV), HCV NS31073–1081 (CINGVCWTV) and Influenza Matrix58–66 (GILGFTFVL). 1 × 106 PBMC were stained with APC-labeled pentamer and the following antibodies (from BD, except as indicated): a dump channel containing anti-CD4 PerCP (RPA-T4), anti-CD14 PerCP (MDP9), anti-CD19 PerCP (SJ25C1) and Viaprobe; anti-CD8 APC-H7 (SK1); anti-PD-1 FITC (MIH4); and biotinylated anti-LAG-3 (BAF2319, R&D Systems) detected with streptavidin-Alexa700 (Invitrogen). Cells were permeabilized and fixed using BD Cytoperm/Cytofix and stained intracellularly for anti-CD152 PE (CTLA-4, BNI3).
2.10. Statistical analysis
The median values for clinical and immunologic parameters were compared using ANOVA, the nonparametric Kruskal–Wallis ANOVA, Wilcoxon Rank Sum or Mann–Whitney U test. The frequency of positive responses was compared using χ2 or Fisher’s exact test based on sample size. Spearman rank correlation was used for bivariate correlation of variables. Multivariate regression was performed using JMP 7 (SAS Institute Inc, Cary NC). A p-value < 0.05 was considered significant.
3. Results
3.1. Patient characteristics
Thirty-three patients with hepatocellular carcinoma provided samples (Table 1). Median age was 57 years (range 49–67), 21 (63%) patients were black, and 12 (36%) were HLA-A2.1 positive. Except for one patient each with hepatitis B and non-alcoholic steatohepatitis, all patients were infected with hepatitis C virus and 25 had prior histories of alcohol dependence. Solitary tumors were present in 19/33 (58%, range 1–3 tumors) with a median size of the largest tumor being 3.8 cm (range 1.5–11.5 cm). 8/33 cases were confirmed histologically, the remainder confirmed using standard noninvasive criteria [29]. The distribution of BCLC tumor stages were as follows: A 16; B 13; C 3; D 1. Five patients received transarterial chemoembolization prior to recruitment, but none within 3 months of enrollment. Serum AFP levels were >20 mg/dl in 22 patients (67%) and >200 mg/dl in 12/33 (36%). Serum glypican-3 was detectable in 22/33 (66.7%). Controls included 10 patients with HCV cirrhosis with no evidence of HCC, 6 HCV patients with F1–2, and 15 healthy donors with no evidence of chronic liver disease (Table 2). HCC patients were all male and modestly older than healthy donors, a group that included 5 women, but were well matched with respect to age, gender and ethnicity with cirrhotic and non-cirrhotic hepatitis C controls.
Table 1.
Baseline characteristics of hepatocellular carcinoma patients.
| Patient ID | Age | Eth | Gnd | Underlying liver disease |
Biopsy confirmed? |
HLA-A02 | Serum AFP mg/dI |
Serum glypican-3 pg/mi |
BCLC stage |
|---|---|---|---|---|---|---|---|---|---|
| HCCOO1 | 57 | W | M | HCV/EtOH | + | + | 58,700 | 219.2 | B |
| HCCOO2 | 57 | B | M | HCV/EtOH | + | + | 13 | <50 | A |
| HCCOO3 | 54 | B | M | HCV/EtOH | − | − | 1720 | <50 | A |
| HCCOO4 | 60 | W | M | HCV/EtOH | + | − | 23,700 | 1724.4 | B |
| HCCOO5 | 60 | B | M | HCV/EtOH | − | + | 12,600 | 820.7 | B |
| HCCOO6 | 49 | B | M | HCV/EtOH | − | − | 58 | 406.0 | A |
| HCCOO7 | 62 | B | M | HCV/EtOH | − | − | 421 | 1012.8 | B |
| HCCOO8 | 58 | B | M | HCV/EtOH | − | − | 94 | 213.5 | A |
| HCCOO9 | 61 | W | M | HCV/EtOH | − | − | 430 | 442.8 | B |
| HCCO1O | 53 | B | M | HCV/EtOH | − | − | 70 | 327.4 | A |
| HCCO11 | 63 | B | M | HCV/EtOH | + | + | 5 | 169.8 | B |
| HCCO12 | 61 | W | M | HCV | − | − | 3 | <50 | A |
| HCCO13 | 58 | B | M | HCV/EtOH | − | − | 17 | <50 | A |
| HCCO14 | 58 | B | M | HCV | − | − | 7230 | 253.2 | B |
| HCCO15 | 57 | B | M | HCV/EtOH | + | − | 24 | 206.0 | B |
| HCCO16 | 56 | B | M | HCV/EtOH | − | − | 4 | 310.3 | A |
| HCCO17 | 55 | B | M | HCV | − | + | 9 | <50 | A |
| HCCO18 | 52 | B | M | HCV | − | − | 118 | 163.6 | A |
| HCCO19 | 50 | B | M | HCV/EtOH | − | + | 1240 | 431.5 | C |
| HCCO21 | 59 | B | M | HCV/EtOH | + | + | 21,000 | <50 | B |
| HCCO22 | 59 | B | M | HCV | − | − | 50 | <50 | B |
| HCCO23 | 57 | W | M | HCV/EtOH | + | + | 1130 | 152.9 | B |
| HCCO24 | 67 | W | M | HCV | − | + | 12 | <50 | A |
| HCCO25 | 59 | W | M | NAFLD | + | − | 82 | <50 | A |
| HCCO26 | 56 | B | M | HCV/EtOH | − | − | 1150 | <50 | B |
| HCCO27 | 57 | W | M | HCV | − | − | 12 | 556.7 | A |
| HCCO28 | 56 | B | M | HCV/EtOH | − | + | 46 | 115.4 | A |
| HCCO29 | 58 | B | M | HCV/EtOH | − | − | 14 | 76.0 | D |
| HCCO3O | 57 | W | M | HCV/EtOH | − | − | 9 | <50 | A |
| HCCO31 | 62 | B | M | HCV/EtOH | − | − | 2110 | 68.2 | C |
| HCCO32 | 59 | W | M | HCV/EtOH | − | − | 53 | 850.1 | B |
| HCCO33 | 54 | W | M | HCV/EtOH | − | + | 119 | 3040 | C |
| HCCO34 | 56 | W | M | HCV/EtOH | − | + | 18 | 328.0 | A |
Ethnicity: W (White), B (Black).
Underlying liver diseases: HCV (hepatitis C). EtOH (alcoholic liver disease), NAFLD (non-alcoholic liver disease). HBV (hepatitis B).
Table 2.
Demographics of hepatocellular carcinoma patients and controls.
| HCC | CIR | EVH | HD | p | |
|---|---|---|---|---|---|
| N | 33 | 10 | 6 | 15 | |
| Age (Median [Range]) |
57 [49–67] |
55 [45–69] |
56 [44–64] |
49 [25–60] |
0.00261 |
| Gender (M/F) |
33/0 | 10/0 | 6/0 | 10/5 | 0.00112 |
| Ethnicity (W/B/H) |
12/20/1 | 5/5/0 | 2/4/0 | 10/4/1 | 0.2 |
| Hepatitis C infected (N [%]) |
31 [94%] |
10 [100%] |
6 (100%] |
0 [0%] |
HCC vs. HD p = 0.0001; HCC vs. CIR p = 0.049; and HCC vs. EVH p = 0.11.
Difference entirely related HD group.
3.2. IFNγ+ T-cell responses against glypican-3 cannot be detected ex vivo in HCC patients or controls
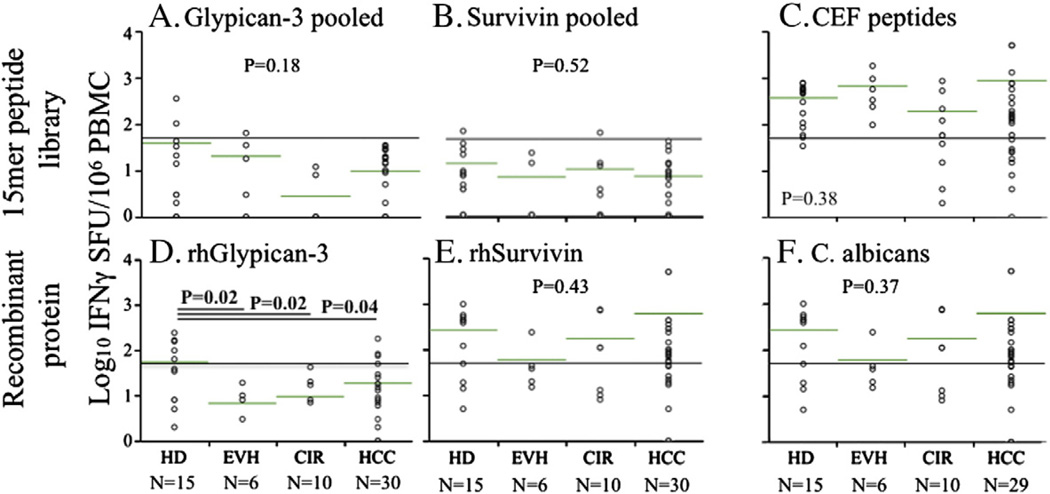
Utilizing 15mer overlapping peptides in IFNγ Elispot, we quantified total T-cell responses against glypican-3 in patients with HCC and relevant controls. Using an arbitrary cutoff of 50 SFU/106 PBMC (10 SFU/well above background) to define positive responses, we found no positive IFNγ responses in HCC patients against pooled glypican-3 15mer peptides (Fig. 1A) nor against pooled survivin 15mer peptides (Fig. 1B). Positive responses occurred in fewer than 10% of non-HCC controls. Consistent with results for pooled peptides, peptide matrices revealed no convincing detectable T-cell reactivity to any individual glypican-3 15mer peptide in any patient (data not shown). The lack of detection of T-cell IFNγ+ response to the peptide libraries did not reflect global T-cell suppression as CD8+ T-cell reactivity to CEF viral peptides and CD4+ T-cell reactivity against C. albicans lysate remained detectable in the majority of cirrhotic patients (Figs. 1C and F). A significant minority of healthy donor patients (5/ 15) but no EVH, CIR and only a small minority of HCC (4/30, only 1 of whom had detectable serum glypican-3 levels) had detectable CD4+ IFNγ+ responses against recombinant human glypican-3 (Fig. 1D), suggesting that endogenously expanded Th1 effector T-cells against glypican-3 are relatively frequently detectable in health but become suppressed during the course of HCV disease. By contrast, CD4+ T-cell responses against human survivin were readily detected in most HCC patients as well as controls (Fig. 1E) indicating both the absence of a global CD4+ T-cell defect as well as differential regulation of Th1 responses against various tumor antigens. Overall however, these data indicate that CD4+ and CD8+ T-cells reactive to glypican-3 either circulate at extremely low frequencies or are highly suppressed in vivo in HCC patients.
Figure 1.
T-cell reactivity to glypican-3 and control antigens ex vivo in HCC patients and controls. IFNγ SFU/106 PBMC in response to glypican-3 15mer peptides (A), survivin 15mer peptides (B), CEF 15mer peptides (C), recombinant human glypican-3 (D), recombinant human survivin (E), and Candida albicans (F) are shown for healthy donors, non-cirrhotic viral hepatitis, non-tumor-bearing cirrhotics, and HCC patients. Reference line indicates 50 SFU/106 PBMC and green bars show log-normalized mean values. P-values shown from ANOVA on log-normalized data.
3.3. Glypican-3-specific IFNγ+ cells can be expanded in vitro from nearly half of HCC patients
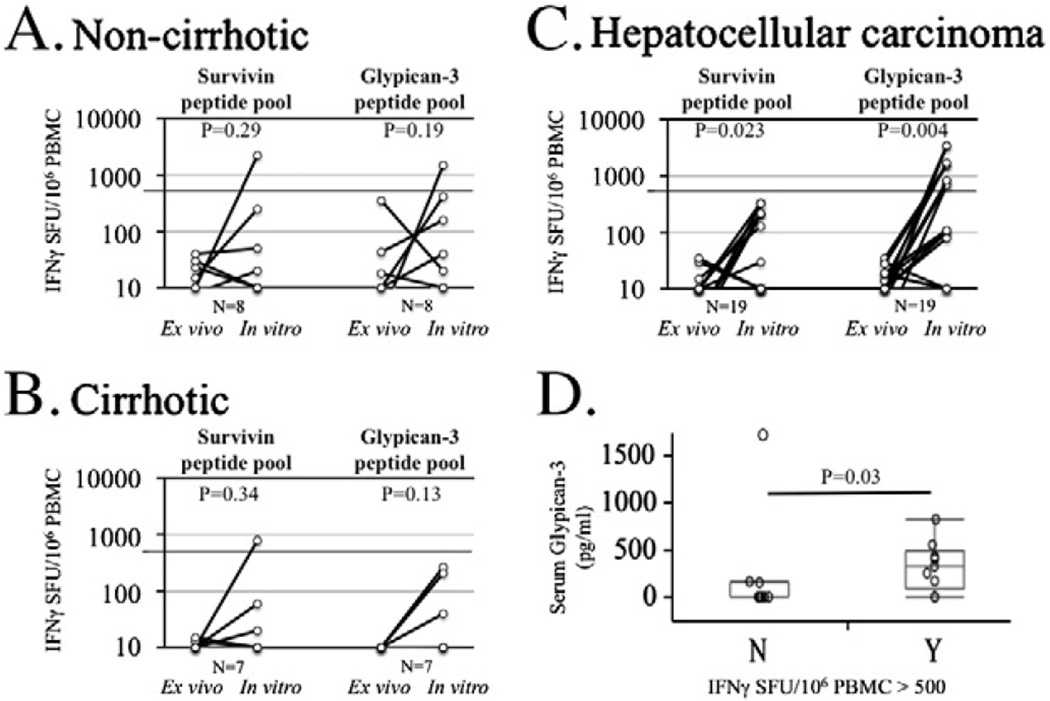
Where adequate lymphocytes were available we performed short-term in vitro expansion of freshly isolated PBMC with pooled 15mer peptides for glypican-3 in the presence of rhIL-2 and then re-examined peptide-specific cytokine secretion by IFNγ Elispot and intracellular cytokine staining. Unstimulated DMSO, survivin and CEF controls were used in all experiments. In all but 1 HCC patient, CEF control peptide stimulation resulted in expansion of antigen-specific IFNγ-secreting T-cells to greater than 1000 SFU/106 PBMC (data not shown) reflecting the absence of a global CD8+ T-cell defect. Glypican-3-specific IFNγ-secreting T-cells could not be detected from PBMC expanded by rhIL-2 alone without peptides in any case (data not shown). As shown in Figs. 2A and B, detection of either glypican-3-or survivin-specific IFNγ+ T-cells in non-HCC patients to levels greater than 500 SFU/106 PBMC (0.005%) with short-term in vitro expansion was a rare event, occurring in only one healthy control subject. By contrast, expansion of glypican-3-specific T-cells to greater than 500 SFU/106 PBMC (0.005%) occurred in 10/19 (52%) of HCC patients (Fig. 2C). Pre- and post-expansion glypican-3-specific IFNγ SFU were markedly increased in HCC patients (mean 9 vs. 815 SFU/106 PBMC, p = 0.0038) but were not statistically increased for either the non-cirrhotic or cirrhotic groups or combination thereof (p = 0.11). Interestingly, expansion of glypi-can-3-specific T-cells to greater than 500 SFU/106 PBMC within the HCC group was associated with greater antigen burden, using serum glypican-3 levels as a surrogate marker of antigen expression (Fig. 2D, p = 0.03), similar to recent findings correlating AFP-specific CD4+ T-cell IFNγ responses and serum AFP levels [27,28]. Thus, among HCC patients, presence of tumor antigen was associated with in vivo expansion of antigen-specific T-cells at a precursor frequency significantly greater than present in healthy donors, non-cirrhotic liver disease and cirrhotic controls.
Figure 2.
Expansion of IFNγ+ T-cells with short-term in vitro expansion by Elispot. PBMC were stimulated with 1 mg/ml survivin or glypican-3 5mer peptide pools for 8 days with rhIL-2 100U/ml added on days 2 and 5. T-cell lines were re-stimulated with peptide pools in Elispot assays. Background was subtracted and results normalized to SFU per 106 PBMC. Comparison of ex vivo and in vitro 15mer peptide responses for non-cirrhotic patients (A), cirrhotic patients (B), and hepatocellular carcinoma patients (C). P-values were obtained by matched pair analyses. (D). Comparison of serum glypican-3 levels in patients with and without IFNγ+ glypican-3-specific T-cells greater than 500 SFU/106 PBMC after expansion.
3.4. Despite expansion of peripheral glypican-3-specific Th1 cells, glypican-3-specific CD8+ T-cells exhibit restricted functional responses
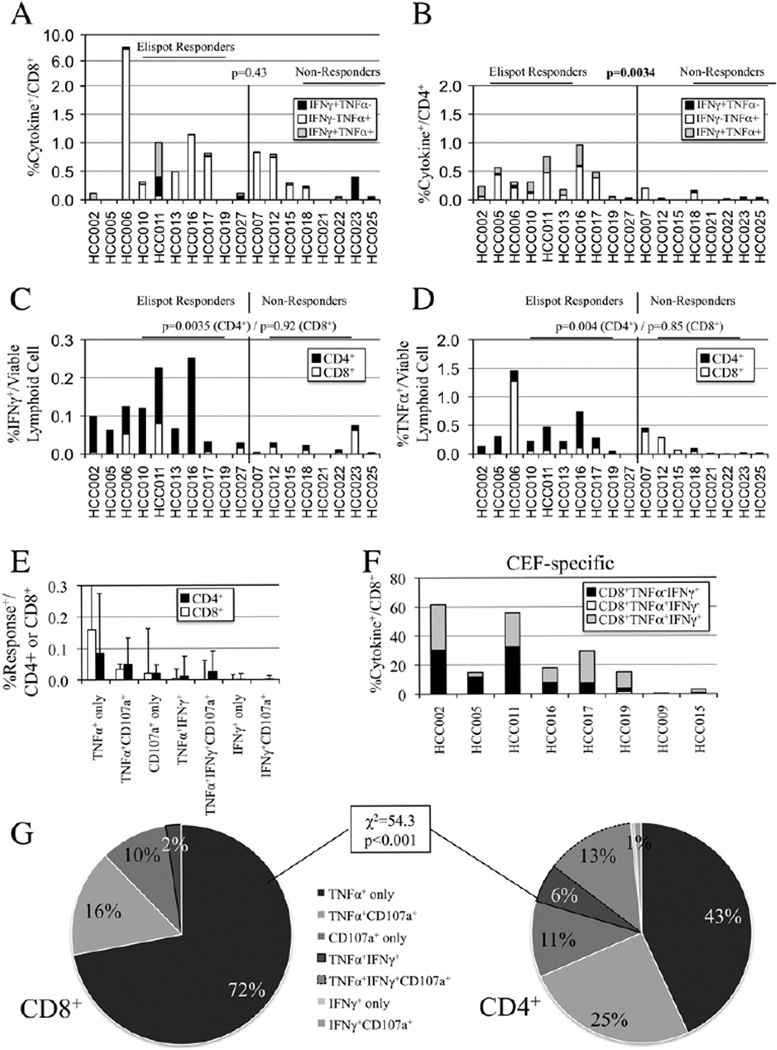
Peptide-expanded T-cells were restimulated on day 8 to measure CD107a uptake, a measure of degranulation, and production of IFNγ and TNFα by intracellular cytokine staining. On a per subset level, the median cytokine response (IFNγ, TNFα, and/or CD107a) was 0.29% of CD4+ and 0.30% of CD8+ T-cells among HCC patients. Expansion of glypican-3-specific IFNγ-secreting CD8+ T-cells to greater than 0.25%/ CD8+ T-cell was only seen in one normal donor (data not shown) and three of 18 HCC patients (median IFNγ+/CD8+ 0.03%, range 0–0.94%, Fig. 3A black plus grey bars). The predominant cytokine profile among CD8+ T-cells was a TNFα+IFNγ− pattern (median of 0.13%/CD8+ T-cells) with 11/ 18 subjects having greater than 0.25% of CD8+ T-cell responding only by TNFα secretion (Fig. 3A). CD8+ T-cell cytokine secretion did not correlate with IFNγ Elispot results. While on a per subtype analysis, median IFNγ+ secretion per CD4+ T-cell was similar to CD8+ T-cells (median IFNγ+/ CD4+ 0.03%, range 0–0.38%), there was a strong correlation between CD4+IFNγ+ frequency and IFNγ Elispot response (Fig. 3B). Reanalyzing these responses relative to all lymphoid cells (compensating for CD4:CD8 ratios), as shown in Fig. 3C, Elispot “responders” had significantly higher frequency of CD4+IFNγ+ (black bars, 0.069 vs. 0.003%, p = 0.0035) but not CD8+IFNγ (white bars, 0.004 vs. 0.004%, p = 0.92). CD4+TNFα+ responses were similarly more frequent in Elispot responders than nonresponders (median 0.167% vs. 0.046%, p = 0.004) while no significant difference was seen for CD8+TNFα+ response (Fig. 3D). Glypican-3-specific CD8+ T-cells exhibited a strong polarization to TNFα secretion as a solitary response (72%) with modest fractions secreting TNFα with degranulation (16%) or degranulation alone (10%) (Figs. 3E white bars and G) with nearly no IFNγ secretion. By contrast, glypican-3-specific CD4+ T-cells a significantly broader array of response patterns with 45% manifesting more than 1 cytokine and/or degranulation function % (Figs. 3E black bars and G). The polarization of CD8+ T-cell responses was antigen-specific and not global as CEF-expanded CD8+ T-cells exhibited a predominant IFNγ response (Fig. 3F) that was most often multicytokine and associated with degranulation (CD107a data not shown).
Figure 3.
Cytokine and degranulation profile of 15mer peptide short-term in vitro-expanded T-cells. A. Stacked column chart comparing median increase in glypican-3-specific TNFα- and/or IFNγ-secreting CD8+ T-cells in restimulated (subtracting derived from unrestimulated control wells) for patients with (left) and without (right) detectable IFNγ Elispot response. B. Stacked column chart comparing median increase in glypican-3-specific TNFα- and/or IFNγ-secreting CD4+ T-cells in restimulated for patients with (left) and without (right) detectable IFNγ Elispot response. C. Per lymphoid analysis demonstrating relative contribution of CD4+ (black bars) and CD8+ (white bars) T-cells to IFNγ response. D. Per lymphoid analysis demonstrating relative contribution of CD4+ (black bars) and CD8+ (white bars) T-cells to TNFα response. E. Frequency per subset of CD4+ and CD8+ T-cell TNFα, IFNγ, and CD107a responses against glypican-3. F. Stacked column chart comparing median increase in CEF-specific TNFα- and/or IFNγ-secreting CD8+ T-cells under identical expansion conditions. G. Distribution of CD8+ (left) and CD4+ (right) T-cell TNFα, IFNγ, and CD107a responses against glypican-3.
3.5. Proliferation of glypican-3-specific CD8+ T-cells in vitro was not associated with restoration of cytokine responses in HCC patients
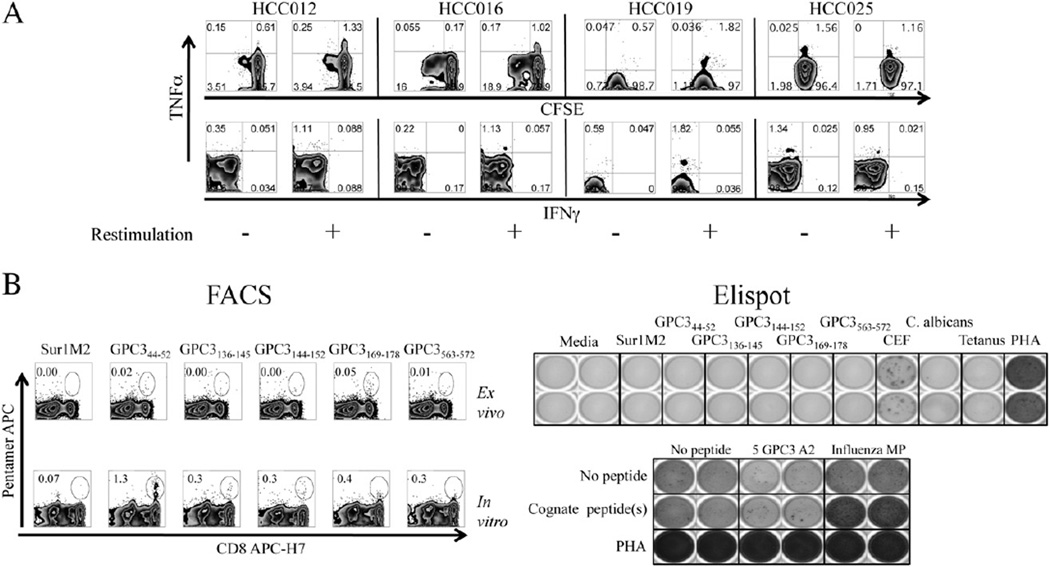
In a subset of patients in whom CFSE-labeling was utilized, as shown with 4 representative patients in Fig. 4A, glypican-3 peptide expansion in the presence of IL-2 led to modest T-cell proliferation (dilution of CFSE), but proliferating CD8+ T-cells did not develop the capacity to secrete cytokine or degranulate; by contrast, peptide-specific responses were found exclusively in mono-functional TNFα-producing non-proliferating cells. While these 15mer peptides efficiently bind to class I MHC molecules (Supplemental Fig. 1), to exclude the possibility that CD8+ T-cell polarization was solely attributable to the use of 15mer rather than 9–10mer optimal peptides, we utilized 9–10mer optimal peptides to expand glypican-3-specific CD8+ T-cells in HLA-A2+ patients and tracked responder cell function by pentamer staining. As shown in Fig. 4B for one representative patient, despite readily detectable expansion of GPC344–52 CD8+ T-cells after 10 days of in vitro expansion, IFNγ secretion by expanded cells restimulated with cognate antigens was minimal-to-absent, particularly when compared to influenza-specific controls. Furthermore, these glypican-3-specific CD8+ T-cells did not produce detectable amounts of IFNγ or TNFα by intracellular staining (data not shown). In total, while modest expansion of glypican-3-specific CD8+ T-cells may occur with stimulation by 15mer peptides in approximately half of HCC patients, despite the activation of CD4+ T-cell type 1 response, expanded CD8+ T-cells do not gain multifunctional effector capacity. CD8+ T-cell cytokine responses that are detected are produced by non-dividing CD8+ cells constrained to produce TNFα.
Figure 4.
Cytokine and degranulation profile of 15mer peptide short-term in vitro-expanded T-cells. A. Representative intracellular cytokine FACS plots showing CFSE dilution versus TNFα (upper) and TNFα vs IFNγ (lower) for four HCC patients with glypican-3-specific TNFα responses after 1 week of in vitro expansion using 15mer pooled peptides. B. Example in which PBMC from HLA-A2+ HCC patient were expanded with 9–10mer optimal peptides. Pentamer frequency ex vivo (top left) and after expansion in vitro (bottom left) for Sur1M2 and 5 GPC3 pentamers are shown. Ex vivo IFNγ Elispot (top right) shows no IFNγ+ responses against any glypican-3 peptide with lack of increase in IFNγ production by Elispot after restimulation with cognate antigen (bottom right).
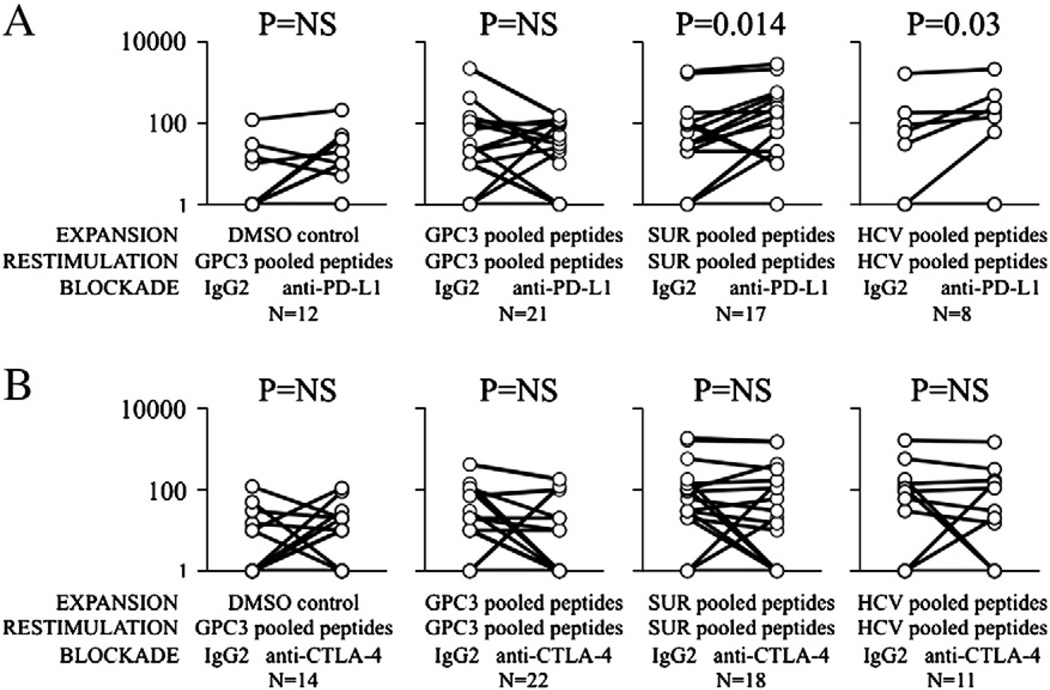
3.6. PD-L1 and CTLA-4 blockade do not significantly enhance the expansion or cytokine production of peripheral glypican-3-specific T-cells
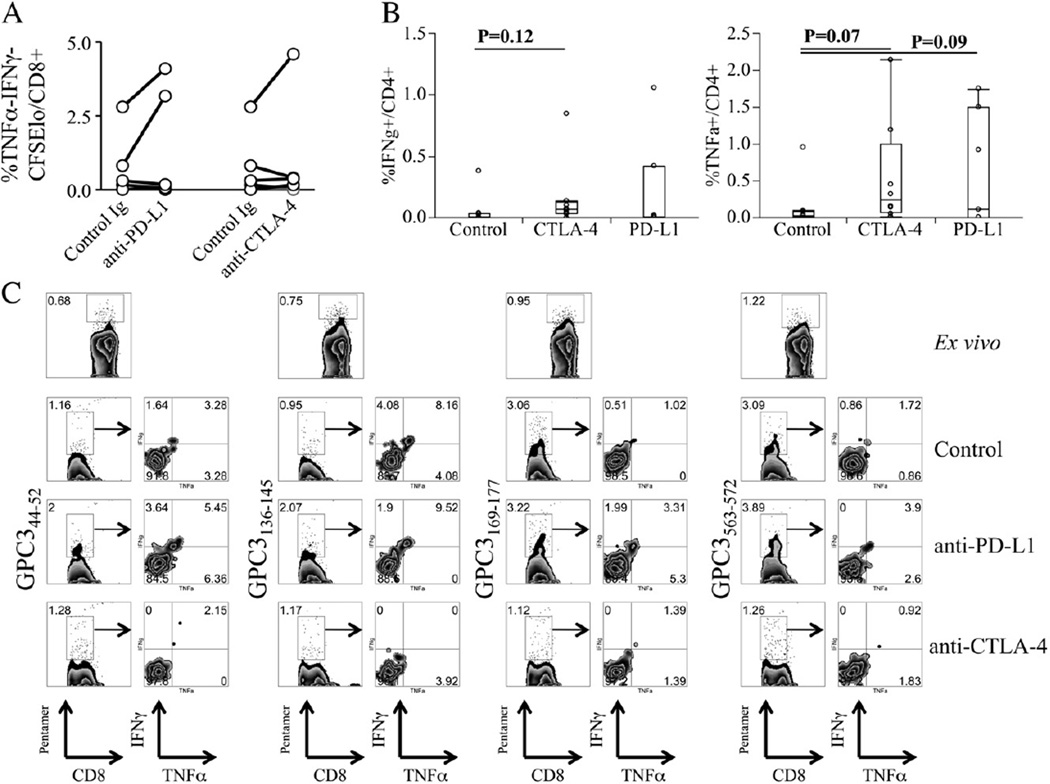
Inhibitory co-stimulation via the PD-1 and CTLA-4 receptors have been previously been shown to be important in suppressing hepat it is virus-specific T-cell effector functions, and PD-1 expression by tumor antigen-specific T-cells may be important in limiting T-cell reactivity to HCC in situ [35,36]. Utilizing glypican-3-specific HLA-A2-restricted pentamers, we first confirmed the expression of PD-1 on peripheral glypican-3-specific CD8+ T-cells, which was detectable ex vivo in 7/9 HCC patients tested ranging in frequency from 0.03 to 1.6% of CD8+ T-cells (Table 3). As shown in Supplemental Fig. 2, PD-1 expression was significantly greater in pentamer-positive versus pentamer-negative CD8+ T-cells for the GPC344–52 (p = 0.013) and GPC3563–572 (strong trend p<0.07) epitopes. By contrast, CTLA-4 expression for GPC3-specific or control CD8+ T-cells was not strongly upregulated (Supplemental Fig. 3). Based on these data, we expanded CFSE-labeled T-cells from HCC patients for 7 days in vitro in the presence glypican-3 peptides, rhIL-2, with either control Ig, anti-PD-L1 mAb, anti-CTLA-4 mAb, or combined PD-L1/CTLA-4 blockade to determine the effect of inhibitory co-stimulation blockade on glypican-3-specific T-cell responses. As shown in Figs. 5A–B, PD-L1 and CTLA-4 had no effect on glypican-3-specific IFNγ+ T-cell responses by Elispot. By contrast, surviving-specific and HCV NS3 responses were augmented by PD-L1. Dual blockade in 12 patients showed no suggestion of effect (data not shown). With PD-L1 and CTLA-4 blockade, 2/7 and 1/7 patients respectively showed significant increases in CD8+ T-cell proliferation (Fig. 6A). However, when assessing mean peptide-induced proliferative and cytokine responses there was no significant difference in CD8+ T-cell proliferation, TNFα production, or IFNγ production under blockade conditions. By contrast, CTLA-4 blockade produced a trend towards a small median increase in CD4+ T-cell IFNγ and TNFα production while PD-L1 blockade also led to trend in CD4+ TNFα production (Fig. 6B). In one patient in which inhibitory receptor blockade could be combined with pentamer analysis, PD-L1 blockade doubled the frequency of GPC344–52- and GPC3136–145-specific CD8+ T-cells after 7 days of expansion; however cytokine production appeared only minimally affected similar to findings with other HCC antigens [12]. In aggregate, these data suggest that PD-1 and CTLA-4 blockade might modestly increase the proliferation of a subset of tumor antigen-specific T-cells in a minority of HCC patients but that co-stimulation blockade does not effectively restore effector function to peripheral tumor-specific CD8+ T-cells.
Table 3.
Frequency of CD8+ pentamer T-cells in nine HLA-Ar hepatocellular carcinoma patients.
| Pentamer | No. of patients |
No. with pentamer+ cluster (%) |
Median pentamer+/ CD8+ cells (range) |
|---|---|---|---|
| 1 SurlM2 | 9 | 1 (11) | 0.20 (0.20–0.20) |
| 2 GPC3 44–52 | 9 | 3 (33) | 0.20 (0.10–0.30) |
| 3 GPC3 136–145 | 8 | 4 (50) | 0.70 (0.05–1.30) |
| 4 GPC3 144–152 | 9 | 2 (22) | 0.47 (0.03–0.90) |
| 5 GPC3 169–178 | 8 | 5 (63) | 1.00 (0.20–1.60) |
| 6 GPC3 563–572 | 9 | 4 (44) | 0.45 (0.05–1.60) |
| 7 HTLV-I | 7 | 1 (14) | 1.00 (0.10–1.00) |
| 8 Flu matrix | 9 | 5 (56) | 0.40 (0.30–1.30) |
| 9 NS3 1073 | 7 | 2 (29) | 0.07 (0.03–0.10) |
Figure 5.
Impact of PD-L1 and CTLA-4 blockade on total T-cell IFNγ production by Elispot. A. IFNγ response (SFU/106 PBMC) after 1 week of in vitro expansion with either DMSO control, glypican-3 15mer peptides, survivin 15mer peptides, or HCV NS3 15mer peptides in the presence of either controlIgor anti-PD-L1 mAb. P-value shown for matched pair analysis onlog-normalized data. B. IFNγ response after in vitro expansion with either DMSO control, glypican-3 15mer pooled peptides, survivin 15mer peptides, or HCV NS3 15mer pooled peptides in the presence of either control Ig or anti-CTLA-4 mAb.
Figure 6.
Impact of PD-L1 and CTLA-4 blockade on CD8 T-cell proliferation and cytokine production by intracellular staining. A. The frequency of proliferated (CFSElo) TNFα−IFNγ− per CD8+ T-cell in presence of control Ig versus anti-PD-L1 and anti-CTLA-4 mAbs for 7 individual patients. B. Percentage of IFNγ+ (left) and TNFα+ (right) T-cells per CD4+ cells in presence of control Ig versus anti-PD-L1 and anti-CTLA-4 mAbs (p by matched pair analysis). C. Example of effect on expansion and cytokine production of pentamer-specific CD8+ T-cells in presence of control Ig, anti-PD-L1 and anti-CTLA-4 mAbs for four glypican-3 pentamers.
4. Discussion
Among tumor antigens that have been considered as immunotherapeutic targets for hepatocellular carcinoma, glypican-3 has several attractive properties including its high tumor-to-background expression, relatively low extra-hepatic expression, and potential for serological detection. This study was performed to determine the frequency and functional capacity of circulating glypican-3-specific CD4+ and CD8+ T-cells in an HLA-diverse HCC population with underlying cirrhosis, the population most likely to be considered for a therapeutic vaccine. Not unexpectedly, we were unable to detect IFNγ+ T-cells specific for glypican-3 (as well as those specific for survivin, another tumor antigen relevant to HCC) ex vivo using 15mer overlapping peptide libraries and peptide matrices. Interestingly, a small minority of HCC patients did have T-cell responses against recombinant glypican-3, likely derived from CD4+ T-cells. Consistent with previous studies that utilized optimal HCC-related HLA-restricted epitopes in vitro [23,37–39], we were able to expand glypican-3-specific T-cells from approximately half of HCC patients, particularly in patients with evidence of higher serum antigen levels suggesting significant tumor-induced priming. Expansion using the 15mer peptide library approach in an HLA-independent manner generated both CD4+ and CD8+ glypican-3-specific T-cells. Glypican-3-specific CD4+ T-cells retained or gained multiple effector functions during expansion. By contrast, expanded glypican-3-specific CD8+ T-cells failed to degranulate or to produce IFNγ when restimulated and were functionally limited to the production of TNFα. This CD8+ T-cell dysfunction could not be overcome by inhibition of PD-1 and/or CTLA-4 signaling. Thus, despite the presence of Th1-like CD4+ T-cell response, tumor-specific CD8+ T-cells in HCC patients manifest a deeply exhausted or anergized phenotype that could not be reversed simply with inhibitory co-stimulation blockade.
To date, clinical studies using class I peptide-based tumor vaccination for HCC have yet to show convincing, sustained clinical benefit [37,40] while non-specific cytokine-activated lymphocytes [14,41] and/or stimulation with cell lysate-loaded APCs [15] after resection or ablation of tumors has demonstrated some evidence of clinical efficacy. While the lack of benefit of peptide-based approaches could be due to selection of advanced patients unlikely to benefit from inclusion, our data, similar to findings identified utilizing NY-ESO-1b157–165-specific CD8+ T-cells [42] and panels of HLA-A2-restricted tumor antigens [12], suggest that peptide-expanded CD8+ T-cells often remain functionally impaired. Such dysfunction could be due to aberrant liver-specific priming of CD8+ T-cells [43] or tumor-induced upregulation of multiple inhibitory receptors on these T-cells to inhibit TCR-induced activation (reviewed in [44]).
Blockade of inhibitory co-stimulation pathways is an emerging approach to overcome tumor-specific CD8+ T-cell dysfunction for the treatment of solid tumors [45,46]. Previous work from our and other institutions strongly implicates the role of inhibitory co-stimulation by PD-1 and CTLA-4 in the suppression of CD8+ T-cells in chronic hepatitis C [8], the underlying etiology for HCC in 32/33 patients we studied. The expression of PD-L1, an inducible ligand for PD-1, has been to shown to impart a poor prognosis in HCC [35]. PD-L1 is selectively upregulated on malignant hepatocytes and/or tumor-associated Kupffer cells as a result of T-cell-derived IFNγ or tumor-related IL-10, and reversibly inhibits effector PD-1+ CD8+ T-cell proliferation in some studies [36,47]. By contrast, we detected only modest expression of PD-1 on tumor antigen-specific peripheral blood CD8+ T-cells in HCC patients possibly reflecting compartmentalization of PD-1hi activated T-cells to the target environment, a finding suggested by other investigators using different HCC-associated tumor antigen-derived epitopes [12]. While peripheral CD8+ T-cells specific for a subset of glypican-3 epitopes do express elevated levels of PD-1 (but not CTLA-4 and not specifically LAG-3), functional rescue in vitro by PD-L1 blockade did not markedly improve T-cell proliferation, cytokine production or degranulation both in assays using specific HLA-A2 epitopes and the 15mer peptide library. These data contrast with HCV-specific responses which in this and previous work [8] were augmented by PD-L1 blockade. While survivin-specific T-cell IFNγ responses were modestly increased by PD-L1 blockade, overall these data suggest that inhibitory co-stimulation blockade is unlikely to potently augment peripheral T-cell vaccination responses in HCC. However, studies on PBMC cannot be assumed to wholly reflect the impact of interventions within a tumor. Indeed, recent data suggest that PD-L1 blockade in vivo may stimulate effector T-cells in an indirect manner via reduction of the generation of intratumoral Tregs [48]. Thus, our findings would support the investigation of both direct and indirect effects of PD-1 blockade when such therapies are tested in patients.
The expansion and characterization of glypican-3-specific CD4+ T-cells are novel findings that, along with data relating to other tumor antigen-specific CD4+ T-cells in HCC, further highlights a population of cells that might be an important target for immune augmentation. The potential role for CD4+ T-cells to exert a cytolytic effect on HCC has been suggested in both human and murine studies [49,50]. The ability to detect CD4+ T-cells specific for NY-ESO-1 ex vivo has been described in a small number of HCC patients in one study [51]. The detection of AFP-specific CD4+ T-cells has generally required short-term in vitro expansion [27,28,52], resulting in the expansion of either effector [27,28,52] or TGFβ+ regulatory T-cells [53]. We were able to detect responses to recombinant glypican-3 by Elispot ex vivo in a small number of HCC patients, but no early fibrosis or cirrhotic patients, suggesting tumor-induced priming. Half of normal donors in our study had detectable ex vivo CD4+ T-cell responses yet these did not translate into in vitro expansion of glypican-3-specific CD4+ T-cells, also supporting the concept that tumor-priming is critical for establishing an expandable population of CD4+ T-cells [27]. Further support for this concept comes from our finding that the presence of serum antigen correlated strongly with the expansion of glypican-3-specific CD4+ T-cells, as has been suggested by some [28] but not all [52] studies with AFP. The recent study from Witkowski et al. [28] demonstrates that the infrequent detection of HCC-reactive CD4+ T-cells ex vivo is not likely the result of compartmentalization of these cells into the tumors, nor was it likely due to circulation of dysfunctional CD4+ T-cells, but rather due to low circulating precursor frequencies of potential effector cells. While we did not specifically assess TGFβ secretion [53] or a regulatory phenotype in expanded glypican-3-specific CD4+ T-cells, it is unlikely that the peripheral CD4+ T-cells were regulatory given the Th1-like functional profiles. In unpublished observations, we have found that roughly one-third of intratumoral CD4+ T-cells expressed foxp3 and CD25, and that TIL can suppress of glypican-3-specific IFNγ production among TIL and LIL possibly mediated by antigen-specific IL-10 suggesting possible antigen-specific Treg accumulation within the tumors. While the expansion of tumor-reactive peripheral CD4+ T-cells in tumor-bearing patients might support the use of whole-antigen CD4+ T-cell-directed components for therapeutic vaccination, the lack of generation of multifunctional CD8+ T-cells despite the presence of significant CD4+ T-cell responses in our system argues that CD4-directed approaches to increase “help” may not sufficiently help highly dysfunction CD8+ T-cells to regain multi-effector function.
5. Conclusion
We have established that 15mer peptide libraries can expand CD4+ and CD8+ T-cells specific for glypican-3 and survivin in patients with hepatocellular carcinoma in an HLA-nonspecific manner. The CD4+ T-cells retained a broad array of cytokine-secreting capacity, but glypican-3-reactive CD8+ T-cells were functionally constrained suggesting a differentiated or exhausted state. However, this exhausted state could not be reversed by inhibitory co-stimulation blockade in vitro unlike the exhausted state of virus-specific T-cells in chronic viral hepatitis. Whole protein or peptide library-based stimulation of tumor antigen-specific T-cells may be able to generate effector CD4+ T-cell responses in a large proportion of hepatocellular carcinoma patients, but further studies will be necessary to define optimal conditions, including the need for concomitant ablation of the tumor microenvironment, for the concomitant expansion of multifunctional effector CD8+ T-cells to increase the likelihood of clinical efficacy.
Abbreviations
- CIR
cirrhotic group
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- EVH
early viral hepatitis
- GPC3
glypican-3
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HD
healthy donor
- IFN
interferon
- LAG-3
lymphocyte-activation gene 3
- PBMC
peripheral blood mononuclear cells
- PD-1
programmed death-1
- PD-L1
programmed death ligand-1
- PHA
phytohemagglutinin
- SD
standard deviation
- SFU
spot-forming unit
- Th1
type 1 helper T-cell response
- Tc1
type 1 effector T-cell response
Footnotes
Research support: This work was supported by the Research Career Development Award from the Veterans Health Administration (DEK) and academic development funds from the University of Pennsylvania. The content of this article does not reflect the views of the VA or of the US Government.
Supplementary materials related to this article can be found online at doi:10.1016/j.clim.2011.02.014.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Leykum LK, El-Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin. Gastroenterol. Hepatol. 2007;5:508–512. doi: 10.1016/j.cgh.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Schurich A, Berg M, Stabenow D, Bottcher J, Kern M, Schild HJ, Kurts C, Schuette V, Burgdorf S, Diehl L, Limmer A, Knolle PA. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J. Immunol. 2010;184:4107–4114. doi: 10.4049/jimmunol.0902580. [DOI] [PubMed] [Google Scholar]
- 5.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajkovic IA, Williams R, Abnormalities of neutrophil phagocytosis. intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 7.Kakazu E, Ueno Y, Kondo Y, Fukushima K, Shiina M, Inoue J, Tamai K, Ninomiya M, Shimosegawa T. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology. 2009;50:1936–1945. doi: 10.1002/hep.23248. [DOI] [PubMed] [Google Scholar]
- 8.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 1937;134:1927–1937. e1–e2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, Tobias J, Kwok WW, Chang KM. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J. Virol. 2008;82:5043–5053. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Takai H, Ashihara M, Ishiguro T, Terashima H, Watanabe T, Kato A, Suzuki M. Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol. Ther. 2009;8:2329–2338. doi: 10.4161/cbt.8.24.9985. [DOI] [PubMed] [Google Scholar]
- 12.Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, Lim SG, Bertoletti A. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137:682–690. doi: 10.1053/j.gastro.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 13.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 14.Lygidakis NJ, Kosmidis P, Ziras N, Parissis J, Kyparidou E. Combined transarterial targeting locoregional immunotherapy-chemotherapy for patients with unresectable hepatocellular carcinoma: A new alternative for an old problem. J. Interferon Cytokine Res. 1995;15:467–472. doi: 10.1089/jir.1995.15.467. [DOI] [PubMed] [Google Scholar]
- 15.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 16.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 17.Libbrecht L, Severi T, Cassiman D, Borght SV, Pirenne J, Nevens F, Verslype C, Pelt JV, Roskams T. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis dysplastic nodules, and focal nodular hyperplasia-like nodules. Am. J. Surg. Pathol. 2006;30:1405–1411. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Al HM, Makhlouf HR, Wang G, Goodman ZD. Glypican-3 expression in benign liver tissue with active hepatitis C: Implications for the diagnosis of hepatocellular carcinoma. Hum. Pathol. 2008;39:209–212. doi: 10.1016/j.humpath.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: A tissue microarray analysis of 4,387 tissue samples. Am. J. Clin. Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 20.Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J. Biol. Chem. 2005;280:2116–2125. doi: 10.1074/jbc.M410090200. [DOI] [PubMed] [Google Scholar]
- 21.Filmus J, Song H, Shi W, Duenas Gonzalez A, Kaya M, Cano-Gauci D. Glypican-3 is a novel inhibitor of insulin-like growth factor signaling. Medicina (B Aires) 1999;59:546. [PubMed] [Google Scholar]
- 22.Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K, Nezu J, Tsunoda H, Yoshino T, Ohizumi I, Tsuchiya M, Ohnishi S, Makuuchi M, Hamakubo T, Kodama T, Aburatani H. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418–2423. doi: 10.1158/0008-5472.can-03-2191. [DOI] [PubMed] [Google Scholar]
- 23.Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T, Matsui M, Torigoe T, Sato N, Baba H, Nishimura Y. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 24.Motomura Y, Ikuta Y, Kuronuma T, Komori H, Ito M, Tsuchihara M, Tsunoda Y, Shirakawa H, Baba H, Nishimura Y, Kinoshita T, Nakatsura T. HLA-A2 and -A24-restricted glypi-can-3-derived peptide vaccine induces specific CTLs: Preclinical study using mice. Int. J. Oncol. 2008;32:985–990. [PubMed] [Google Scholar]
- 25.Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Furukawa Y, Ogawa M, Nakamura Y, Nishimura Y. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin. Cancer Res. 2004;10:8630–8640. doi: 10.1158/1078-0432.CCR-04-1177. [DOI] [PubMed] [Google Scholar]
- 26.O’Beirne J, Farzaneh F, Harrison PM. Generation of functional CD8+ T cells by human dendritic cells expressing glypican-3 epitopes. J. Exp. Clin. Cancer Res. 2010;29:48. doi: 10.1186/1756-9966-29-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alisa A, Ives A, Pathan AA, Navarrete CV, Williams R, Bertoletti A, Behboudi S. Analysis of CD4+ T-Cell responses to a novel alpha-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin. Cancer Res. 2005;11:6686–6694. doi: 10.1158/1078-0432.CCR-05-0382. [DOI] [PubMed] [Google Scholar]
- 28.Witkowski M, Spangenberg HC, Neumann-Haefelin C, Buttner N, Breous E, Kersting N, Drognitz O, Hopt UT, Blum HE, Semmo N, Thimme R. Lack of ex vivo peripheral and intrahepatic alpha-fetoprotein-specific CD4+ responses in hepatocellular carcinoma. Int. J. Cancer. 2010:8. doi: 10.1002/ijc.25866. Epub ahead of print, http://onlinelibrary.wiley.com/doi/10.1002/ijc.25866/suppinfo. [DOI] [PubMed]
- 29.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 30.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 31.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 32.Reche PA, Glutting JP, Reinherz EL. Prediction of MHC class I binding peptides using profile motifs. Hum. Immunol. 2002;63:701–709. doi: 10.1016/s0198-8859(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J. Hepatol. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 36.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, Economou JS. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepato-cellular cancer. Clin. Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 38.Mizukoshi E, Nakamoto Y, Marukawa Y, Arai K, Yamashita T, Tsuji H, Kuzushima K, Takiguchi M, Kaneko S. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology. 2006;43:1284–1294. doi: 10.1002/hep.21203. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HG, Chen HS, Peng JR, Shang XY, Zhang J, Xing Q, Pang XW, Qin LL, Fei R, Mei MH, Leng XS, Chen WF. Specific CD8(+ )T cell responses to HLA-A2 restricted MAGE-A3 p271–279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol. Immunother. 2007;56:1945–1954. doi: 10.1007/s00262-007-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, McBride WH, Finn R, Glaspy JA, Economou JS. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 41.Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J. Immunother. 2008;31:63–71. doi: 10.1097/CJI.0b013e31815a121b. [DOI] [PubMed] [Google Scholar]
- 42.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin. Cancer Res. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 43.Su YC, Lee CC, Kung JT. Effector function-deficient memory CD8+ T cells clonally expand in the liver and give rise to peripheral memory CD8+ T cells. J. Immunol. 2010;185:7498–7506. doi: 10.4049/jimmunol.1002606. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J. Immunol. 2010;185:7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarci-noma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q, Xiao H, Liu Y, Peng Y, Hong Y, Yagita H, Chandler P, Munn DH, Mellor A, Fu N, He Y. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J. Immunol. 2010;185:5082–5092. doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakao M, Sata M, Saitsu H, Yutani S, Kawamoto M, Kojiro M, Itoh K. CD4+ hepatic cancer-specific cytotoxic T lymphocytes in patients with hepatocellular carcinoma. Cell. Immunol. 1997;177:176–181. doi: 10.1006/cimm.1997.1108. [DOI] [PubMed] [Google Scholar]
- 50.Homma S, Komita H, Sagawa Y, Ohno T, Toda G. Antitumour activity mediated by CD4+ cytotoxic T lymphocytes against MHC class II-negative mouse hepatocellular carcinoma induced by dendritic cell vaccine and interleukin-12. Immunology. 2005;115:451–461. doi: 10.1111/j.1365-2567.2005.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin. Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 52.Behboudi S, Alisa A, Boswell S, Anastassiou J, Pathan AA, Williams R. Expansion of anti-AFP Th1 and Tc1 responses in hepatocellular carcinoma occur in different stages of disease. Br. J. Cancer. 2010;102:748–753. doi: 10.1038/sj.bjc.6605526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alisa A, Boswell S, Pathan AA, Ayaru L, Williams R, Behboudi S. Human CD4(+) T cells recognize an epitope within alpha-fetoprotein sequence and develop into TGF-beta-producing CD4(+) T cells. J. Immunol. 2008;180:5109–5117. doi: 10.4049/jimmunol.180.7.5109. [DOI] [PubMed] [Google Scholar]