Abstract
Intracerebral microdialysis enables continuous measurement of changes in brain biochemistry. In this study intracerebral microdialysis was used to assess changes in cytokine levels after tumor resection and in response to treatment with temsirolimus. Brain tumor patients undergoing craniotomy participated in this non-therapeutic study. A 100 kDa molecular weight cut-off microdialysis catheter was placed in peritumoral tissue at the time of resection. Cohort 1 underwent craniotomy only. Cohort 2 received a 200 mg dose of intravenous temsirolimus 48 h after surgery. Dialysate samples were collected continuously for 96 h and analyzed for the presence of 30 cytokines. Serial blood samples were collected to measure systemic cytokine levels. Dialysate samples were obtained from six patients in cohort 1 and 4 in cohort 2. Seventeen cytokines could be recovered in dialysate samples from at least 8 of 10 patients. Concentrations of interleukins and chemokines were markedly elevated in peritumoral tissue, and most declined over time, with IL-8, IP-10, MCP-1, MIP1β, IL-6, IL-12p40/p70, MIP1α, IFN-α, G-CSF, IL-2R, and vascular endothelial growth factor significantly (p < 0.05) decreasing over 96 h following surgery. No qualitative changes in intracerebral or serum cytokine concentrations were detected after temsirolimus administration. This is the first intracerebral microdialysis study to evaluate the time course of changes in macromolecule levels in the peritumoral microenvironment after a debul-king craniotomy. Initial elevations of peritumoral interleukins and chemokines most likely reflected an inflammatory response to both tumor and surgical trauma. These findings have implications for development of cellular therapies that are administered intracranially at the time of surgery.
Keywords: Intracerebral microdialysis, Craniotomy, Cytokines, Brain tumor
Introduction
Despite advances in molecularly targeted therapies for cancer, effective pharmacologic treatment for the vast majority of malignant brain tumors remains elusive. In addition to targeted agents, engineered cellular therapies and viral vectors injected directly into the brain are being investigated as potential new treatments for brain cancer. Although these local therapies are typically administered at the time of craniotomy for tumor resection, some cellular-based therapies also take advantage of their ability to distribute throughout the brain. The migration of genetically-modified neural stem cells to tumor cells in the brain, for example, is mediated by certain cytokines, such as interleukin-6 (IL-6) [1] and vascular endothelial growth factor (VEGF) [2, 3]. A better understanding of changes that occur in the peritumoral microenvironment during the early post-operative period could influence further development of these therapies.
Microdialysis is a technique for continuously analyzing the concentration of a drug or biomolecule in the extra-cellular fluid of body tissues, without significantly disturbing the function of these tissues [4–6]. It is an accepted research tool for monitoring changes in neurochemistry from acute brain injury [7–13]. More recently, we [14–16] and others [17, 18] have applied microdialysis to the pharmacologic monitoring of drug levels in brain tissue.
There has also been interest in exploring the use of this sampling technique to measure intracerebral levels of macromolecules, such as cytokines. In this study, we examined the feasibility of using a microdialysis catheter whose semi-permeable membrane has a molecular weight (MW) cut-off of 100 kDa, to determine the type and time course of cytokine changes in the brain after a debulking craniotomy. We also wanted to investigate whether this microdialysis catheter could be used to assess the neuropharmacodynamics of a targeted agent by measuring changes in intracerebral cytokine levels after intravenous administration of temsirolimus (Torisel, Pfizer Inc., New York, NY, USA).
Temsirolimus and its metabolite sirolimus (rapamycin) inhibit the mammalian target of rapamycin (mTOR), an important downstream effector in the phosphatidylinositol 3-kinase/Akt signaling pathway, which is often overactivated in cancer cells. One of mTOR's many functions is to activate the hypoxia inducible factor (HIF)-1α pathway. When HIF-1α binds to VEGF, production of VEGF by the tumor cell is increased, leading to angiogenesis. Inhibiting mTOR should result in decreased levels of VEGF in extracellular fluid. There are also preclinical data showing that treatment with rapamycin can decrease levels of IL-1β, TNF-α, [19] and MCP-1 [20].
Materials and methods
Study subjects
Subjects who were at least 18 years old, had either a primary or metastatic brain tumor(s), and were planning to undergo a craniotomy for tumor resection were asked to participate in this non-therapeutic pilot study. Because temsirolimus and its active metabolite, sirolimus, are substrates for cytochrome P450 (CYP), patients receiving temsirolimus (cohort 2) could not be taking anti-seizure medications that induce the hepatic CYP isoenzyme system or any concomitant medications that were strong CYP3A4 inducers or inhibitors, except for dexamethasone.
Other inclusion criteria: (a) a Karnofsky performance status (KPS) of ≥60 %; (b) recovery from toxicity of any prior therapy; (c) adequate bone marrow (absolute neutrophil count ≥1,500 cells/mm3 and platelet count ≥100,000 cells/mm3), hepatic (total bilirubin ≤2.0 mg/dl; aspartate aminotransferase ≤4× the upper limit of normal), and renal function (serum creatinine ≤1.5× the upper limit of normal).
Patients were excluded from the study if they were (a) receiving radiation, chemotherapy, or enrolled in another clinical trial; (b) pregnant or breast feeding; (c) taking anticoagulant medication; (d) allergic to temsirolimus, sirolimus, or dextran; or had (e) uncontrolled diabetes; or (f) a serious medical or psychiatric illness that could potentially interfere with the completion of treatment according to the protocol.
Microdialysis catheters
A 100 kDa cut-off catheter (71 High Cut-Off Brain MD Catheter, membrane length 10 mm, shaft length 100 mm, Ref. No. 8010320, M Dialysis, Solna, Sweden) was used. This catheter is CE-marked (approved for human use) in Europe. However, because this catheter does not have FDA (510 k) clearance, and because we used a larger single dose of temsirolimus than is approved for routine weekly use, the study was conducted under an Investigational New Drug Application (IND # 102755). The City of Hope Institutional Review Board approved the study, and all participants gave written informed consent. The study was registered at ClinicalTrials.gov (NCT00784914).
Study design
During craniotomy, the microdialysis catheter was inserted into peritumoral brain interstitium within 5 mm of the resection cavity. Proper placement of the catheter was confirmed by postoperative non-contrast CT scan of the brain, after which the catheter was connected to a portable syringe pump (107 MD Pump, Ref. No. P000127, M Dialysis, Solna, Sweden), which perfused it with 2 % dextran 40 at 0.3 μL/min.
The catheter remained in place for 96 h, and dialysate samples were collected continuously every 6 h. After the first 3 subjects in cohort 1 completed study treatment, we decided to also measure serum levels of cytokines every 6 h for comparison. Cohort 2 patients received a 200 mg dose of temsirolimus intravenously 48 h after placement of the microdialysis catheter. This dose was chosen because it is within range of the temsirolimus doses that were assessed in glioma clinical trials [21, 22], and a previous study [23] documented measurable levels of temsirolimus in the brain with this dose range. During the period of collection, patients were mobile (e.g., able to move from bed to chair or walk around the nurses' station).
Analytical method for determining cytokine concentrations in dialysate and serum
The Human Cytokine Thirty-Plex Antibody Bead Kit (Invitrogen, Camarillo, CA) was used according to manufacturer's instructions. The 30 cytokine panel included: epidermal growth factor (EGF), eotaxin, basic fibroblast growth factor (FGF-basic), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interferon alpha (IFN-α); IFN-gamma (γ), interleukin-1 beta (IL-1β), interleukin-1 receptor antagonist (IL-1RA), IL-2; interleukin-2 receptor (IL-2R), IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40/p70, IL-13, IL-15, IL-17, IFN-γ-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), monokine induced by IFN-γ (MIG), monocyte inflammatory protein-1 alpha (MIP-1α), monocyte inflammatory protein-1 beta (MIP-1β), regulated upon activation, normal T cell expressed and secreted cytokine (RANTES), tumor necrosis factor (TNF)-α, and VEGF. Cytokine concentrations were measured using the Bio-plex HTF Luminex instrument and results calculated using Bio-plex Manager 3.0 Software. The assay was performed using the perfusion fluid containing 2 % dextran 40 as the diluent for the controls. Therefore, the final solutions for both the controls and unknowns were identical. The inter-assay precision for all cytokines is <10 % and the lower limit of quantitation is between 1 and 15 pg/ml, depending on the target. Data are reported as absolute values that have not been corrected for recovery.
Statistical analysis
Each cytokine was evaluated independently. Initially, we sought to observe a change in cytokine levels due to temsirolimus, but with no evidence of a temsirolimus-induced change, we pooled all time points to better describe the cytokine changes over time. To conduct this analysis, the time courses of log10 transformed cytokine levels were fit with a linear model separately for each patient. The slope of each resulting linear model (log linear) was treated as an independent observation (one per patient). A one-sample t test was then carried out on these slope values to assess whether the observations were inconsistent with an average slope of zero (no detectable change in cytokine values over time), using a type I error of 5 %. A 95 % confidence interval was also calculated for the average slope. A similar slope analysis was carried out for the serum cytokine values. When there was a clear two phase process, such as with IL-6 in serum, representing an increase in serum cytokine levels due to surgery followed by a decay in the cytokine, a piecewise linear regression was also applied to investigate possible trend changes during the study period. This was to avoid under-reporting a change in cytokines over time when an up-then-down process was apparent.
Results
Patient characteristics
From October 2008 through February 2010, 12 participants were enrolled. Initially the plan was to obtain dialysate samples from six patients in each cohort. A total of eight patients were accrued to the first cohort; one patient withdrew consent shortly after surgery, and another patient's catheter occluded, which prevented the collection of any dialysate samples. Both were replaced so that we were able to obtain dialysate samples from six patients in cohort 1. Only four patients were accrued to cohort 2 because after data analysis of these first 4 participants indicated that further accrual would not change the conclusions, the study was closed.
Placement of the catheters and collection of dialysate samples were tolerated well by all patients. There were no grade 3 or higher adverse events, as assessed by the NCI Common Terminology Criteria for Adverse Events, Version 3.0. Table 1 summarizes characteristics of the 10 patients from whom dialysate samples were collected. All patients received tapering doses of dexamethasone during the 96 h study period. The median doses of dexamethasone at the beginning and end of the study period were similar between the 2 cohorts.
Table 1.
Characteristics of study patients
| Cohort 1 (surgery alone) | Cohort 2 (surgery & temsirolimus) | |
|---|---|---|
| Number of patients | 6 | 4 |
| Median age (range) | 53 (39–63) | 52 (31–70) |
| Female/Male | 4/2 | 4/0 |
| Median KPS (range) | 85 (60–100) | 90 (90–90) |
| Primary cancer diagnosis (# of patients) | breast cancer (1) | breast cancer (2) |
| chondrosarcoma (1) | glioblastoma (1) | |
| glioblastoma (1) | leiomyosarcoma (1) | |
| low-grade glioma (1) | ||
| lung cancer (2) | ||
| Median dose of dexamethasone | ||
| Start of study period | 16 mg | 16 mg |
| End of study period | 6 mg | 6 mg |
Recovery of cytokines
Prior to enrolling the first patient, in vitro recovery experiments were performed to determine which cytokines, out of a panel of 30, could be recovered by the microdialysis catheter, and which perfusion fluid resulted in the highest fractional recovery for most cytokines. It was determined that a solution of 2 % dextran 40 perfused at a rate of 0.3 μL/min produced the best in vitro recovery for the majority of cytokines. Please see Supplementary Data for details of the experimental set up and in vitro recovery results (Supplementary Table 1).
Dialysate samples from patients in cohort 1 served to assess baseline cytokine concentrations shortly after craniotomy. Levels of most recoverable cytokines were elevated within the first 8 h after surgery and then decreased over the remainder of the study period, with the greatest decline observed within the first 48 h. Median concentrations and ranges of the 17 intracerebral cytokines detectable initially in at least 8 of 10 patients, along with corresponding cytokine levels in blood at the same time points, are listed in Table 2. The number of patient dialysate samples containing a particular cytokine decreased over the study period (Table 2, second column), likely due to concentrations dropping below the level of detection of the assay in many patients by the end of the monitoring period.
Table 2.
Summary of cytokines measurable in initial dialysate samples from at least 8 of 10 study patients
| Cytokine | Pt numberb | Brain interstitial fluid |
Serum |
||||
|---|---|---|---|---|---|---|---|
| Baselinec | 48 hc | 96 hc | Baselinec | 48 hc | 96 hc | ||
| IL-8a | 10,10,7,7,6,6 | 7,949.2 (303.7–25,650) | 399.5 (69.0–2,217.7) | 378.5 (14.8–5,715.3) | 61.8 (24.0–1,077.7) | 39.5 (20.1–892.0) | 37.3 (21.9–138.0) |
| IP-10a | 10,10,7,7,6,6 | 87.8 (9.3–2,359) | 115.6 (4.4–2,720.2) | 56.0 (2.7–131.2) | 23.5 (12.4–173) | 18.6 (10.4–141.5) | 17.0 (10.9–115.0) |
| MCP-1a | 10,10,7,7,6,6 | 13,864.7 (3,453.8–24,360) | 2,426.1 (61.5–7,819.8) | 2,139.1 (38.8–7,771.8) | 297.2 (190.1–435.6) | 229.0 (101.6–318.8) | 414.8 (179.3–464.8) |
| MIP-1βa | 10,10,7,7,6,6 | 577.8 (47.1–2,580.0) | 55.5 (15.6–307.7) | 51.1 (7.4–217.9) | 101.3 (46.0–1,163.1) | 70.5 (40.5–96–0) | 55.8 (30.2–122.7) |
| IL-6a | 10,10,7,7,6,4 | 1,108.5 (248.6–6,900) | 309.0 (39.3–1,160.6) | 76.0 (8.3–836.5) | 12.4 (3.0–22.2) | 9.4 (4.2–15.0) | 8.9 (3.7–19.2) |
| IL12p40p70a | 10,9,6,7,6,6 | 34.8 (8.2–205.2) | 6.8 (3.9–23.0) | 6.9 (3.4–14.6) | 109.0 (53.9–495.0) | 95.3 (57.5–453.5) | 98.2 (50.1–462.3) |
| IL-1RA | 10,9,6,6,5,5 | 627.1 (91.0–2,458.8) | 110.2 (27.8–708.4) | 115.3 (18.6–844.1) | 158.3 (112.0–693.5) | 108.5 (60.1–159.6) | 88.8 (58.8–226.0) |
| MIP-1αa | 10,8,7,7,6,6 | 169.2 (53.1–513.4) | 20.5 (9.3–76.2) | 10.6 (5.4–64.2) | 34.4 (9.1–626.9) | 25.7 (7.1–298.7) | 30.5 (7.1–376.2) |
| RANTES | 10,7,1,3,2,1 | 325.5 (13.1–1,638.3) | 48.8 (20.9–92.6) | 18.8 | 8,380 (7,994.0–11,462.9) | 9,412.7 (8,380–10,445.5) | 8,380 |
| IFN-αa | 9,7,3,7,5,6 | 21.9 (14.8–76.8) | 17.2 (5.3–29.1) | 8.6 (7.1–13.1) | 29.6 (22.7–124.9) | 33.3 (25.9–150.4) | 35.4 (32.6–158.2) |
| G-CSFa | 9,7,1,7,4,4 | 66.0 (16.5–2,226.8) | 24.0 (9.4–277.0) | 4.1 | 65.7 (28.4–198.1) | 89.0 (46.7–163.4) | 89.5 (24.6–168.8) |
| IL-2Ra | 9,5,1,7,6,6 | 82.4 (42.5–317.1) | 49.2 (20.1–63.6) | 32.4 | 256.2 (132.9–710.8) | 249.5 (121.2–698.4) | 220.2 (122.5–704.9) |
| VEGFa | 8,7,2,5,4,4 | 11.4 (4.8–46.2) | 6.2 (2.6–17.5) | 5.4 (2.1–8.7) | 3.2 (2.1–22.4) | 3.6 (3.1–10.2) | 4.1 (3.3–10.1) |
| IL-17 | 8,5,3,4,2,3 | 47.2 (16.9–88.3) | 43.4 (11.1–63.5) | 44.8 (37.7–73.3) | 55.7 (10.2–58.8) | 51.1 (47.6–54.7) | 48.0 (15.3–56.3) |
| IL-10 | 8,6,0,6,5,2 | 9.3 (1.8–19.7) | 3.0 (1.6–7.7) | n/d | 5.0 (2.4–11,1) | 3.0 (1.8–21.0) | 5.7 (2.0–9.4) |
| MIG | 8,4,1,6,5,3 | 17.6 (6.8–84.7) | 14.4 (10.4–18.2) | 21.1 | 52.9 (17.8–391.2) | 31.6 (18.2–151.4) | 63.6 (28.4–104.3) |
| IFN-γ | 8,2,0,4,3,3 | 15.1 (2.3–47.9) | 4.3 (2.8–5.8) | n/d | 67.7 (9.1–76.9) | 58.9 (12.1–68.8) | 57.5 (15.8–83.1) |
n/d Not detectable
Cytokines whose intracerebral levels significantly decreased over the 96 h study period
The number of patient samples represented in each column (baseline brain, 48 h brain 96 h brain, baseline serum, 48 h serum 96 h serum) respectively
Median (range) in units of pg/ml
In addition to high levels of some pro-inflammatory interleukins, such as IL-8 and IL-6, release of chemokines (MCP-1, MIP-1β, and RANTES) involved in monocyte chemotaxis and activation was detected in study patients. Particularly, levels of MCP-1, a strong chemoattractant for monocytes, lymphocytes, and mesenchymal stem cells in a variety of tumors [24–26], were elevated initially. Although MCP-1 concentrations decreased significantly throughout the 96 h monitoring period, they still remained relatively high. We also identified rising levels of IP-10, which is a chemoattractant for T helper cells and an inhibitor of angiogenesis [27].
In contrast to detecting cytokine levels in brain with a microdialysis catheter, recovery of cytokines from serum was not an issue; however, not all cytokines were measurable in study patients' serum samples at each time point either (Table 2, second column). A likely explanation is that many of the cytokine concentrations in serum were low, often just at the level of detection of the assay, and due to normal fluctuations, these levels sometimes dropped below our ability to detect them.
Table 3 displays results of the slope analysis of the log-transformed cytokine values that were found in initial dialysate samples from at least 8 of 10 patients. Data from cohorts 1 and 2 were combined in the analysis because of the lack of an effect of temsirolimus described below. Over the entire 96 h post-surgical period, 11 cytokines in brain interstitial fluid showed a statistically significant (p < 0.05) decreasing linear trend: IL-8, IP-10, MCP-1, MIP1β, IL-6, IL-12p40/p70, MIP1α, IFN-α, G-CSF, IL-2R, and VEGF. On this log scale, slopes ranged from −0.025 to −0.004.
Table 3.
Estimates of slope over 96 h with log-transformed cytokines measured in initial dialysate samples from at least 8 of 10 study patients
| Cytokine | Brain interstitial fluid |
Serum |
||||||
|---|---|---|---|---|---|---|---|---|
| Slope | p value | Lowera | Upperb | Slope | p value | Lowera | Upperb | |
| IL-8 | −0.013 | 0.002 | −0.02 | −0.006 | 0.006 | 0.054 | 0 | 0.012 |
| IP-10 | −0.009 | 0.014 | −0.016 | −0.002 | −0.001 | 0.245 | −0.003 | 0.001 |
| MCP-1 | −0.014 | <0.001 | −0.02 | −0.008 | 0 | 0.557 | −0.001 | 0.002 |
| MIP-1β | −0.013 | <0.001 | −0.018 | −0.008 | −0.001 | 0.029 | −0.002 | 0 |
| IL-6c | −0.013 | 0.002 | −0.02 | −0.006 | 0.065, 0.013 | 0.004 | 0.03 | 0.1 |
| 0.01 | −0.02 | −0.005 | ||||||
| IL12p40p70 | −0.015 | 0.026 | −0.027 | −0.002 | 0 | 0.573 | 0 | 0.001 |
| IL-1RA | −0.005 | 0.073 | −0.01 | 0.001 | −0.001 | 0.493 | −0.003 | 0.002 |
| MIP-1α | −0.013 | 0.002 | −0.02 | −0.006 | 0.001 | 0.429 | −0.002 | 0.005 |
| RANTES | −0.015 | 0.101 | −0.034 | 0.004 | −0.005 | 0.400 | −0.02 | 0.01 |
| IFN-α | −0.01 | 0.043 | −0.02 | 0 | 0.001 | 0.109 | 0 | 0.002 |
| G-CSF | −0.021 | 0.049 | −0.042 | 0 | −0.001 | 0.452 | −0.004 | 0.002 |
| IL-2R | −0.025 | 0.044 | −0.05 | −0.001 | 0 | 0.808 | −0.001 | 0.001 |
| VEGF | −0.005 | 0.002 | −0.007 | −0.003 | 0 | 0.761 | −0.004 | 0.005 |
| IL-17 | −0.008 | 0.110 | −0.019 | 0.003 | −0.001 | 0.367 | −0.003 | 0.001 |
| IL-10 | −0.006 | 0.145 | −0.016 | 0.003 | −0.003 | 0.185 | −0.009 | 0.002 |
| MIG | −0.014 | 0.215 | −0.04 | 0.012 | −0.003 | 0.164 | −0.007 | 0.003 |
| IFN-γ | −0.013 | 0.264 | −0.037 | 0.012 | 0.01 | 0.411 | −0.002 | 0.003 |
n/a Insufficient patient data (n<2) for analysis
Lower boundary for the 95 % confidence interval
Upper boundary for the 95 % confidence interval
For IL-6 serum only, the results are presented for the two-phase linear regression. The first row represents 0–12 h, and the second row represents 12–96 h. The p values and 95 % confidence intervals of the slopes of the two lines are presented
In contrast to cytokine levels in brain, only MIP-1β showed a statistically significant decreasing linear trend over time in serum (slope of −0.001, p < 0.03). Applying a piecewise linear regression model to the serum data also identified IL-6 as having a statistically significant increase from 0 to 12 h (p < 0.01), and then a significant decrease over time after 12 h (p < 0.02), likely reflecting an acute reaction of IL-6 in serum to surgery.
Caution should be exercised when comparing cytokine levels in serum to those in brain, since the uncorrected cytokine concentrations measured in dialysate (Table 2) are likely underestimations of the true intracerebral levels, given the relatively low in vitro fractional recoveries of many of the cytokines (see Supplementary Table 1). Keeping this in mind, the following conclusions can be made: (1) for many of the cytokines, intracerebral concentrations were several fold higher than in serum, and (2) with the exception of IL-6, no other corresponding peaks in systemic cytokine production were observed. These data reflect the highly localized nature of the inflammatory response that occurs in the brain due to the presence of tumor and in response to craniotomy.
There were no detectable temsirolimus-specific effects on the time course of intracerebral or serum cytokine levels, as measured by a difference in the estimated slopes of the log-transformed cytokine levels. Figure 1 shows representative temporal profiles of 3 cytokines (IL-6, IP-10, and MCP-1) which were consistently recovered from brain interstitial fluid and serum from the most study patients. The bold lines overlaid on the raw data represent the best-fit linear regression line. Only raw data from dialysate samples were plotted. Since the slope is scale invariant, it was not necessary to use recovery-adjusted values. For IL-6 in serum, we present the piecewise linear regression fit, as noted above, showing that serum levels of IL-6 increased initially and then fell over time.
Fig. 1.
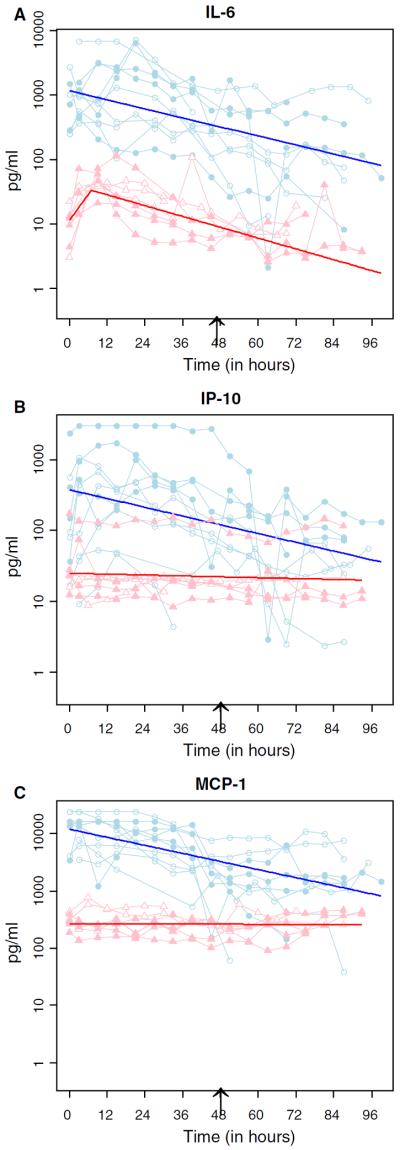
Temporal profiles of representative cytokines in brain interstitial fluid and serum. Raw data for IL-6 (a), IP-10 (b), and MCP-1 (c) in brain interstitial fluid (light blue circles) and serum (pink triangles) from each patient in cohort 1 (open circles/triangles) and cohort 2 (filled circles/triangles) were connected by straight line segments. The fitted bold lines (dark blue for brain interstitial fluid and red for serum) were overlaid on the lined scatter plot. The black arrow indicates the time at which cohort two patients were given a single 200 mg dose of temsirolimus. Note that for IL-6 serum data, a piecewise linear regression analysis (joint point at 12 h) was applied to reflect the trend changes (from increasing to decreasing) during the study period
Discussion
To our knowledge, this is the first microdialysis study to document the time course of cytokine changes that occur in the brain after craniotomy for tumor resection. Despite large inter-patient variability, we observed consistent temporal changes of many macromolecules in the peritumoral tissue. Although it is not clear if the cytokine levels measured in this study reflect baseline concentrations in the tumor microenvironment in addition to acute changes in response to surgery itself, our findings have implications for future development of local therapies for malignant brain tumors.
Assessment of changes in cytokine levels after craniotomy
Dexamethasone inhibits the production and release of proinflammatory cytokines, such as IL-8 [28], IL-6 [29], and IL-1β [30]. Despite standard peri-operative dexamethasone use in study patients, a robust, localized inflammatory response was observed in peritumoral tissue; however, the temporal changes in cytokine concentrations do not clarify whether these factors were secreted by the tumor itself or released by the inflammatory response to surgery. Whereas intracerebral levels of most cytokines peak 12–24 h after traumatic brain injury [31], the initial high levels of macromolecules detected in our patients shortly after tumor resection support their presence in the peritumoral tissue prior to surgery. Furthermore, most of the pro-inflammatory cytokines that were detected early have also been implicated in cancer progression. For example, IL-6 is a key activator of the JAK/Stat pathway in tumors, and IL-8 plays a role in the regulation of angiogenic activity in gliomas [32–34]. While differences may exist regarding cytokine levels in the peritumoral microenvironment of primary brain tumors versus brain metastases, the small number of patients in this pilot feasibility study precluded our ability to assess possible differences.
Multiple research groups [35–46] have used intracerebral microdialysis to assess cytokine concentrations, mainly in patients with traumatic brain injury or subarachnoid hemorrhage; however, Marcus et al. [39] used this technique to measure changes in cytokine levels in brain tumor patients after craniotomy. They reported the average concentrations of 8 cytokines (IL-1α, IL-1β, IL-1 receptor antagonist, VEGF, IL-6, IL-8, transforming growth factor alpha, and EGF) during the first 48 h post-surgery, but fluctuations in cytokine levels were not assessed during this period. To our knowledge, our study is not only the first to fully describe the temporal changes in cytokine levels that occur after craniotomy, it also provides the most extensive data set thus far of cytokine concentrations in the peritumoral microenvironment after craniotomy for tumor debulking.
This information may beneficially contribute to the design of therapies that are intracranially administered shortly after tumor resection. For example, local delivery of engineered cellular therapies and viral vectors are being investigated as treatments for malignant brain tumors. Survival of tumor-implanted cells or vectors depends on a supportive tumor milieu, and high levels of inflammatory cytokines can rapidly abolish these therapies before their effects are accomplished. On the other hand, high levels of chemokines such as MCP-1 can enhance the migration and trafficking of activated T cells or genetically-engineered stem cells to tumor foci [24, 25], thus improving their therapeutic efficacy.
Effect of temsirolimus on intracerebral cytokine levels
One of the objectives of this study was to use microdialysis to assess the effect of temsirolimus on cytokine concentrations in the brain. No temsirolimus-associated effects were observed. One possible explanation is that temsirolimus may not cross the blood–brain barrier sufficiently to achieve therapeutic levels in brain tumor tissue. While Kuhn et al. [23] did report that measurable drug concentrations were present in glioma samples taken from patients receiving 170–250 mg of temsirolimus, it was unclear whether these concentrations were high enough or present for a sufficient amount of time to produce the pharmacologic effect of mTOR inhibition. Moreover, all study subjects received peri-operative dexamethasone, which can increase metabolic elimination of temsirolimus through induction of CYP3A4, and so it is possible that the inability to see an effect of temsirolimus on cytokine levels in the brain or serum was partly due to lower than anticipated systemic exposure of the drug.
We believe that a more plausible explanation for the lack of an observable neuropharmacodynamic effect in our study patients is that release of cytokines from other cells in the tumor microenvironment in response to tissue damage from craniotomy prevented detection of any possible temsirolimus effect. In addition to glioma [32, 47–51] and immune cells (such as microglia and T-cells), other types of cells in the brain can produce cytokines, including capillary endothelial cells, and injured neurons and astrocytes. Thus, the brain's localized inflammatory response to craniotomy may have obscured any cytokine changes due to temsirolimus.
Methodological considerations
Although in vitro fractional recoveries are sometimes used as correction factors for in vivo data (i.e., to estimate true interstitial concentrations), it is problematic to do so because in vitro recovery is rarely an accurate approximation of in vivo recovery, often resulting in underestimating the actual concentration of a biomolecule in brain interstitial fluid. Therefore, we have presented our dialysate data in their uncorrected form only and focused our statistical analysis on the trend in changes of cytokine levels over time, rather than attempting to make conclusions about absolute concentrations of cytokines present in the brain.
As documented by other research groups, we found considerable interpatient variability in intracerebral cytokine levels (Fig. 1), which can make it difficult to interpret microdialysis data. Nonetheless, we detected significant decreases in levels of many cytokines over the 96 h monitoring period. It makes sense that after acute injury to brain tissue from craniotomy, an inflammatory cytokine response would immediately occur and then gradually decrease, particularly in the presence of dexamethasone. It is also possible that the observed decreasing cytokine levels might be due to deterioration in catheter performance over time. Recovery of macromolecules could be affected by membrane pores becoming clogged with cellular debris, as documented on scanning electron microscopy of 100 kDa MW cut-off catheters [52]. However, there is evidence in the literature [44, 46] and within our own data set in support of the ability of these catheters to maintain their dialysis efficiency over many days. Although concentrations of multiple cytokines significantly decreased over 96 h, levels of others (IL-8, MCP-1, MIP-1b, Il-12p40p70, and IL-RA) remained relatively constant from 48–96 h post-surgery. If catheter performance had deteriorated over time, we would have expected to see a uniform decrease in all cytokine levels at 96 h; nonetheless, it is important to note that differential deterioration in probe function for individual cytokines can occur.
In conclusion, results from this microdialysis study elucidate the time course of cytokine changes that occur in the brain after craniotomy for tumor resection and represent the most extensive data set of baseline post-operative peritumoral cytokine concentrations, documenting the presence of an acute inflammatory response involving the release of chemokines that stimulate monocyte chemotaxis and activation. A more detailed understanding of the inflammatory reaction in the peritumoral microenvironment after surgery could impact further development of locally-delivered therapies for brain tumors.
Supplementary Material
Acknowledgments
We thank Brenda Williams, RN, for her management of the microdialysis catheters and Keely Walker, PhD, for her valuable editing of the manuscript. Sources of funding: National Institutes of Health (K12CA01727 to J.P.); Phase One Foundation; Pfizer, Inc.
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
Electronic supplementary material The online version of this article (doi:10.1007/s11060-014-1415-4) contains supplementary material, which is available to authorized users.
References
- 1.Zhao D, Najbauer J, Annala AJ, Garcia E, Metz MZ, Gutova M, et al. Human neural stem cell tropism to metastatic breast cancer. Stem Cells. 2012;2:314–325. 2. doi: 10.1002/stem.784. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt NO, Wojciech P, Yang W, Ziu M, Teng Y, Kim SU, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;6:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, et al. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;12:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 4.Blakeley JO, Portnow J. Microdialysis for assessing intratumoral drug disposition in brain cancers: a tool for rational drug development. Expert Opin Drug Metab Toxicol. 2010;6:1477–1491. doi: 10.1517/17425255.2010.523420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lange EC, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Rev. 1997;25:27–49. doi: 10.1016/s0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Gallo JM. In vivo microdialysis for PK and PD studies of anti-cancer drugs. AAPS J. 2005;7:E659–E667. doi: 10.1208/aapsj070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan TV, Ng SC, Lam JM, Poon WS, Gin T. Monitoring of autoregulation using intracerebral microdialysis in patients with severe head injury. Acta Neurochir Suppl. 2005;95:113–116. doi: 10.1007/3-211-32318-x_24. [DOI] [PubMed] [Google Scholar]
- 8.Kett-White R, Hutchinson PJ, Al-Rawi PG, Gupta AK, Pickard JD, Kirkpatrick PJ. Adverse cerebral events detected after subarachnoid hemorrhage using brain oxygen and microdialysis probes. Neurosurgery. 2003;50:1213–1221. doi: 10.1097/00006123-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Miller CM, Vespa PM, McArthur DL, Hirt D, Etchepare M. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocrit Care. 2007;6:22–29. doi: 10.1385/NCC:6:1:22. [DOI] [PubMed] [Google Scholar]
- 10.Salci K, Nilsson P, Howells T, Ronne-Engstrom E, Piper I, Contant CF, Jr, et al. Intracerebral microdialysis and intracranial compliance monitoring of patients with traumatic brain injury. J Clin Monit Comput. 2006;20:25–31. doi: 10.1007/s10877-006-2864-x. [DOI] [PubMed] [Google Scholar]
- 11.Staub F, Graf R, Gabel P, Kochling M, Klug N, Heiss WD. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery. 2000;47:1106–1115. doi: 10.1097/00006123-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 13.Wang E, Ho CL, Lee KK, Ng I, Ang BT. Changes in brain biochemistry and oxygenation in the zone surrounding primary intracerebral hemorrhage. Acta Neurochir Suppl. 2008;102:293–297. doi: 10.1007/978-3-211-85578-2_55. [DOI] [PubMed] [Google Scholar]
- 14.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portnow J, Badie B, Markel S, Liu A, D'Apuzzo M, Frankel P, Jandial R, Synold TW. A Neuropharmacokinetic Assessment of Bafetinib, a Second Generation Dual BCR-Abl/Lyn Tyrosine Kinase Inhibitor, in Patients with Recurrent High-Grade Gliomas. Eur J Cancer. 2013;49(7):1634–1640. doi: 10.1016/j.ejca.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portnow J, Badie B, Synold T, Lacey S, D'Apuzzo M, Frankel P, Chen M, Aboody K. The Meeting for Society for Neuro-Oncology. 2012. Neural stem cell (NSC)-mediated conversion of 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) in recurrent high-grade glioma patients: a proof of concept. NO-77. [Google Scholar]
- 17.Bergenheim AT, Capala J, Roslin M, Henriksson R. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71:287–293. doi: 10.1007/s11060-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 18.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51–58. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SA, Kim SM, Son YH, Lee CW, Chung SW, Eo SK, et al. Peptidoglycan enhances secretion of monocyte chemoattractants via multiple signaling pathways. Biochem Biophys Res Commun. 2011;408:132–138. doi: 10.1016/j.bbrc.2011.03.136. [DOI] [PubMed] [Google Scholar]
- 20.Sola-Villa D, Camacho M, Sola R, Soler M, Diaz JM, Vila L. IL-1beta induces VEGF, independently of PGE2 induction, mainly through the PI3-k/mTOR pathway in renal mesangial cells. Kidney Int. 2006;70:1935–1941. doi: 10.1038/sj.ki.5001948. [DOI] [PubMed] [Google Scholar]
- 21.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 22.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn JG, Chang SM, Wen PY, Cloughesy TF, Greenberg H, Schiff D, et al. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res. 2007;13:7401–7406. doi: 10.1158/1078-0432.CCR-07-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–3341. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 26.Magge SN, Malik SZ, Royo NC, Chen HI, Yu L, Snyder EY, et al. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J Neurosci Res. 2009;87:1547–1555. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- 27.Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Bourke E, Moynagh PN. Antiinflammatory effects of glucocorticoids in brain cells, independent of NF-kB. J Immunol. 1999;163:2113–2119. [PubMed] [Google Scholar]
- 29.Bessler H, Mendel C, Straussberg R, Gurary N, Aloni D, Sirota L. Effects of dexamethasone on IL-1beta, IL-6, and TNF-alpha production by mononuclear cells in newborns and adults. Biol Neonate. 1999;75:225–2233. doi: 10.1159/000014099. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa MM, Ramilo O, Saez-Llorens X, Olsen KD, Magness RR, McCracken GH., Jr Cerebrospinal Fluid prostaglandins, interleukin 1 beta, and tumor necrosis factor in bacterial meningitis. Clinical and laboratory correlations in placebo-treated and dexamethasone-treated patients. Am J Dis Child. 1990;144:883–887. doi: 10.1001/archpedi.1990.02150320047024. [DOI] [PubMed] [Google Scholar]
- 31.Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 34.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 35.Helmy A, Carpenter KLH, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillman J, Aneman O, Anderson C, Sjogren F, Saberg C, Mellergard P. A microdialysis technique for routine measurement of macromolecules in the injured human brain. Neurosurgery. 2005;56:1264–1268. doi: 10.1227/01.neu.0000159711.93592.8d. [DOI] [PubMed] [Google Scholar]
- 37.Hillman J, Aneman O, Persson M, Andersson C, Dabrosin C, Mellergard P. Variations in the response of interleukins in neurosurgical intensive patients monitored using intracerebral microdialysis. J Neurosurg. 2007;106:820–825. doi: 10.3171/jns.2007.106.5.820. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1α, IL-1β, and their endogenous inhibitor IL-ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 39.Marcus HJ, Carpenter KLH, Price SJ, Hutchinson PJ. In vivo assessment of high- grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors and cytokines. J Neurooncol. 2010;97:11–23. doi: 10.1007/s11060-009-9990-5. [DOI] [PubMed] [Google Scholar]
- 40.Mellergard P, Aneman O, Sjogren F, Pettersson P, Hillman J. Changes in extracellular concentrations of some cytokines, chemokines, and neurotrophic factors after insertion of intracerebral microdialysis catheters in neurosurgical patients. Neurosurgery. 2008;62:151–158. doi: 10.1227/01.NEU.0000311072.33615.3A. [DOI] [PubMed] [Google Scholar]
- 41.Mellergard P, Aneman O, Sjogren F, Saberg C, Hillman J. Differences in cerebral extracellular response of interleukin-1β, interleukin-6, and interleukin-10 after subarachnoid hemorrhage or severe head trauma in humans. Neurosurgery. 2011;68:12–19. doi: 10.1227/NEU.0b013e3181ef2a40. [DOI] [PubMed] [Google Scholar]
- 42.Mellergard P, Sjogren F, Hillman J. Release of VEGF and FGF in the extracellular space following severe subarachnoid haemorrhage or traumatic head injury in humans. Br J Neurosurg. 2010;24:261–267. doi: 10.3109/02688690903521605. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Barcena J, Ibanez J, Brell M, Crespi C, Frontera G, Llompart-Pou JA, et al. Lack of correlation among intra-cerebral cytokines, intracranial pressure, and brain tissue oxygenation in patients with traumatic brain injury and diffuse lesions. Crit Care Med. 2011;39:533–540. doi: 10.1097/CCM.0b013e318205c7a4. [DOI] [PubMed] [Google Scholar]
- 44.Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2010;13:339–346. doi: 10.1007/s12028-010-9432-4. [DOI] [PubMed] [Google Scholar]
- 45.Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1β, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods. 2002;119:45–50. doi: 10.1016/s0165-0270(02)00153-x. [DOI] [PubMed] [Google Scholar]
- 46.Winter CD, Pringle AK, Clough GF, Church MK. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127:315–320. doi: 10.1093/brain/awh039. [DOI] [PubMed] [Google Scholar]
- 47.Ilyin SE, Gonzalez-Gomez I, Romanovicht A, Gayle D, Gilles FH, Plata-Salaman CR. Autoregulation of the interleukin-1 system and cytokine–cytokine interactions in primary human astrocytoma cells. Brain Res Bull. 2000;51:29–34. doi: 10.1016/s0361-9230(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 48.Pietsch T, Valter MM, Wolf HK, von Deimling A, Huang HJ, Cavenee WK, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol (Berl) 1997;93:109–117. doi: 10.1007/s004010050591. [DOI] [PubMed] [Google Scholar]
- 49.Piperi C, Zisakis A, Lea RW, Kalofoutis A. Role of cytokines in the regulation of glioma tumour growth and angiogenesis. Am J Immunol. 2005;1:106–113. [Google Scholar]
- 50.Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 51.Van Meir E, Sawamura Y, Diserens AC, Hamou MF, De Tribolet N. Human glioblastoma cells release interleukin 6 in vitro and in vivo. Cancer Res. 1990;50:6683–6688. [PubMed] [Google Scholar]
- 52.Helmy A, Carpenter KLH, Skepper JN, Kirkpatrick PJ, Pickard JD, Hutchinson PJ. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


