Abstract
Conversion from asexual proliferation to sexual differentiation initiates the production of the gametocyte, which is the malaria parasite stage required for human-to-mosquito transmission. This protocol describes an assay designed to probe the effect of drugs or other perturbations on asexual replication, sexual conversion and early gametocyte development in the major human malaria parasite Plasmodium falciparum. Synchronized asexually replicating parasites are induced for gametocyte production by the addition of conditioned medium, and they are then exposed to the treatment of interest during sexual commitment or at any subsequent stage of early gametocyte development. Flow cytometry is used to measure asexual proliferation and gametocyte production via DNA dye staining and the gametocyte-specific expression of a fluorescent protein, respectively. This screening approach may be used to identify and evaluate potential transmission-blocking compounds and to further investigate the mechanism of sexual conversion in malaria parasites. The full protocol can be completed in 11 d.
INTRODUCTION
After injection by a mosquito into a human host and a single round of mitotic expansion within hepatocytes, malaria parasites undergo an indefinite number of asexual replication cycles within red blood cells (RBCs). During each of these intra-erythrocytic developmental cycles (IDCs), a small proportion of parasites stop replicating and initiate development into sexual precursor cells in a process known as sexual conversion. These sexual precursor cells, called gametocytes, are at maturity the only parasite form capable of establishing an infection in the mosquito vector. Sexual conversion and proper gametocyte development are thus essential for transmission of malaria.
Strategies for preventing transmission of parasites from humans to mosquitos are essential for efforts aiming at malaria elimination and eradication1. As gametocytes are required for transmission and are produced in low numbers, they represent an important and potentially vulnerable target for transmission-blocking therapies. However, most antimalarial drugs in current use perform poorly at killing mature gametocytes and are thus unable to fully prevent malaria transmission. Some reports have suggested that treatment with antimalarial compounds, such as pyrimethamine2–4, chloroquine5 and mefloquine6, may even increase the number of gametocytes produced in patients or under in vitro conditions, possibly by increasing sexual conversion rates7. Similarly, enhancement of gametocyte production and transmission has been observed with drug-resistant parasites in rodent infections8. Clearly, there is an important but unmet need for methods for identifying agents that inhibit gametocytes throughout all stages of development. In addition, better methods are required to ensure that future antimalarial drugs do not stimulate gametocyte production.
The assay presented here focuses on P. falciparum, the parasite species responsible for the vast majority of >600,000 malaria deaths each year9. The presented protocol allows experimenters to manipulate the rate at which P. falciparum parasites commit to either the asexual or the sexual developmental pathway in a highly targeted and reproducible manner. It describes an updated and streamlined version of our previously developed drug screening assay10, which was the first assay capable of targeting both sexual conversion and the earliest stages of gametocyte development. The assay involves the simultaneous measurement of total parasite proliferation and gametocyte production during a single blood-stage replication cycle. We have used variations of this assay to complete dose-response and window-of-action analyses of antimalarial compounds under development11,12. The protocol presented here (Fig. 1) enables screening of drugs or other perturbations against highly synchronous early gametocytes. It additionally allows experimenters to interrogate the biology of sexual conversion and to test antimalarial compounds under development for potential inhibitory or stimulatory effects on gametocyte production.
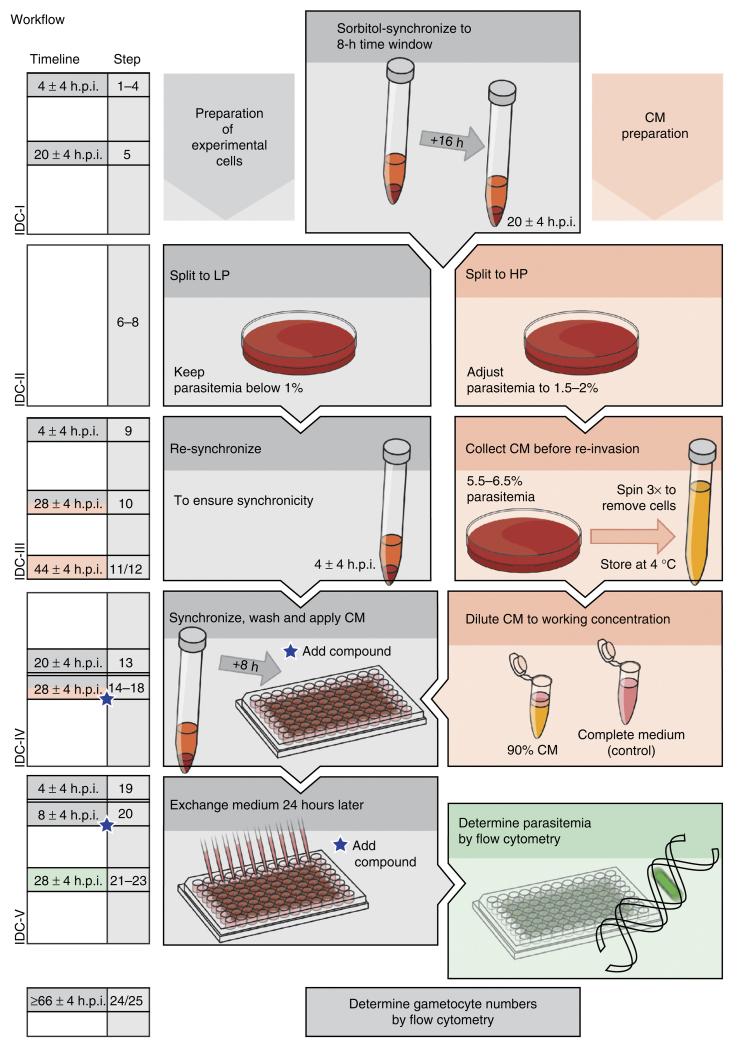
Figure 1.
Flowchart depicting individual steps of the protocol. The timeline represents consecutive parasite developmental cycles (IDCs). IDC-I: parasite cultures are sorbitol-treated twice to synchronize the population to an 8-h age window. IDC-II: the population is split into lower (LP) and higher parasitemia (HP) cultures containing below 1% and 1.5–2% infected red blood cells, respectively. IDC-III: the LP culture is sorbitol-synchronized at 4 ± 4 h.p.i. The HP culture is adjusted to a parasitemia of 5.5–6.5% at 28 ± 4 h.p.i. and incubated for 16 h before collecting the CM by three centrifugation steps. IDC-IV: the LP culture is sorbitol-synchronized at 20 ± 4 h.p.i. and adjusted to a parasitemia of 0.3%. At 28 ± 4 h.p.i., the cells are washed and resuspended in 90% (vol/vol) CM (complemented with complete medium) and seeded into wells of a 96-well plate. These conditions will induce sexual conversion in a high proportion of forming schizonts, and the compound to be screened may be either added at this time point or during the subsequent IDC (indicated by blue stars). Parasites cultured in the absence of the compound and under noninducing conditions, i.e., with complete medium, serve as positive and negative controls, respectively. IDC-V: after the parasites have reinvaded fresh erythrocytes (4 ± 4 h.p.i.), the culture medium is exchanged with complete medium. The medium is henceforth exchanged daily. At 28 ± 4 h.p.i., parasitemia is determined by flow cytometry using an aliquot of SYBR Green–stained cells. At 66 ± 4 h.p.i., young gametocytes have acquired enough tdTom fluorescence for cytometry-based quantification.
Stage conversion and transmission of P. falciparum parasites
Recent discoveries have begun to shed light on the mechanisms governing the production of gametocytes in Plasmodium, which show that environmental, genetic and epigenetic factors can drive sexual conversion. For example, recent studies have identified a transcription factor, AP2-G (refs. 13,14), as well as the epigenetic factors PfHP1 and PfHDA2 (refs. 15,16), as regulators of sexual commitment. In addition, it was shown that parasites use microvesicle-mediated cellular communication that seems to modulate the rate at which sexual commitment is initiated17,18. Finally, various cellular components have been reported to be required for sexual conversion, including environmental sensing and signaling molecules19 (i.e., receptors, kinases and G proteins20–24), cell cycle control proteins, and cellular stress responses25,26. Through an as-yet-unknown pathway, external stimuli are believed to manipulate cell fate decision during the IDC that precedes gametocyte development. Parasites are hence induced by certain microenvironments to commit to either the sexual or the asexual cell fate27, and their progeny will either develop into gametocytes or continue asexual proliferation. Altogether, these data suggest that multiple layers of regulation act in concert in order to induce the production of gametocytes. Interestingly, the protein classes suggested to be involved in P. falciparum sexual conversion include numerous major drug targets in other eukaryotes. Identifying the factors associated with conversion in P. falciparum is therefore likely to uncover promising targets for future transmission-intervention strategies.
Comparison with other published P. falciparum in vitro assay protocols
A number of high-throughput–compatible whole-cell assay methods have been developed and applied to the screening of compound libraries against Plasmodium asexual and sexual blood stages, as well as liver-stage parasites. Existing screens to identify compounds with an effect on sexual development generally focus on gametocytes in the second half of the 8–12-d maturation process28–32. For example, Peatey et al.30 and Sanders et al.29 recently screened compounds against pure populations of mature sexual-stage parasites by using ATP bioluminescence or the gametocytes’ ability to exflagellate, respectively, as a readout. In contrast, D’Alessandro et al.33 assessed the viability of purified mid-stage gametocytes in a lactate dehydrogenase assay, whereas Lucantoni et al.34 screened against early-to-mid-stage gametocytes using a luciferase reporter–based system.
Screens targeting young gametocytes are largely under-represented, which is at least partially attributable to the fact that these stages are difficult to separate from the bulk of asexually replicating parasites. As we demonstrate with the assay presented here, flow cytometry combined with an appropriate reporter system can make such screens possible. Other than the presented screen, Wang et al.35 developed the only cytometry-based approach that enables targeting sexual-stage parasites, including early gametocytes. In that assay, drug susceptibility of developing gametocytes is tested after they have been purified from asexual cells by sequential treatments with sorbitol, Percoll and heparin. Our protocol, by contrast, uses flow cytometric detection of a gametocyte-specific fluorescent reporter to quantify early gametocytes within the asexual population, and thus it avoids the necessity of purifying gametocytes or applying compounds that specifically kill asexual cells. The improved protocol allows experimenters to reproducibly generate either (i) asexual parasite populations containing negligible numbers of gametocytes or (ii) cultures that are greatly enriched in highly synchronous gametocytes—a feature that is unique to this screen. This allows screening for compounds that inhibit sexual conversion and/or development, as well as for conditions that trigger gametocytogenesis. Consequently, diverse questions about drug susceptibility and the biology of sexual differentiation may be addressed using the same basic and simple assay.
Owing to its excellent performance in inducing highly synchronous gametocyte populations, and in contrast with most published screens, the presented assay does not require physical purification of sexual-stage parasites or removal of gametocytes formed during previous IDCs. Further, the effect of the perturbation of interest on asexual parasite proliferation is detected in the same well, making the assay ideal for screens to identify molecules that specifically target gametocytes but not asexual blood stages and vice versa.
Experimental design
In the assay presented here, modified from Buchholz et al.10, stage-synchronized blood-stage parasites are seeded in 96-well plates (220 μl per well, 0.3% parasitemia, 2.5% hematocrit (HC)) at 28 ± 4 h post invasion (h.p.i.; IDC-IV; Fig. 1). In wells to be stimulated for high gametocyte production, sexual conversion is induced at that time point by the addition of parasite-conditioned medium (CM) for 24 h under well-defined conditions. The CM is produced on a separate parasite culture maintained at high parasitemia (HP; IDC-III; Fig. 1).
When screening for inhibitory effects on sexual conversion and/or gametocyte development, the culture is induced by applying CM, and test compounds are simultaneously added to the cells or at any later time point, depending on the experimenter’s requirements. When screening for stimulatory effects on sexual conversion, cells are not induced before treatment with test compounds. In a set of control wells, parasites are cultured without drug addition. In the presence of CM, these will produce high proportions of gametocytes, whereas only few gametocytes are formed under non-inducing control conditions. After the parasites have re-invaded erythrocytes, flow cytometry is first used to determine parasitemia (IDC-V, 28 ± 4 h.p.i.; Fig. 1) and, 38 h later, to evaluate the proportion of early gametocytes (IDC-V, 66 ± 4 h.p.i.; Fig. 1) using DNA dye staining and fluorescent protein expression, respectively. The effect of drug treatment on the proportion of parasite offspring that become gametocytes (referred to as the conversion rate), on the overall asexual parasite proliferation rate, and/or on gametocyte survival is determined by comparison with the high-gametocyte and low-gametocyte control wells (see PROCEDURE).
Gametocyte detection relies on the expression of the red fluorescent reporter tandem Tomato (tdTom), which is driven by the promoter of the gametocyte-specific gene etramp10.3 (PF10_0164/PF3D7_1016900) (refs. 10,36,37). The fluorescence intensity of gametocytes produced in parasite lines carrying this reporter system (termed 164-tdTom) allows for stage-diagnostic signal detection by flow cytometry after 62 h.p.i. At this time point, gametocytes formed during the next IDC are not yet detectable10.
As described above, multiple assay setups are available to address different questions. Drugs or other perturbing conditions can be evaluated for negative effects on sexual conversion or early gametocyte development, as well as for effects that potentially induce or enhance the production of gametocytes. As the assay does not require purification of gametocytes, the effect of treatment on asexual parasites can be measured simultaneously, which allows the investigation of differences in drug susceptibility between asexual- and sexual-stage parasites in the same well.
Optimization
On the basis of our published protocol10, we have streamlined and updated the assay setup. For simplicity and ease of use with large culture volumes, we replaced the combination of sorbitol and Percoll synchronization steps10 with a series of four sorbitol treatments (IDC-I through IDC-IV; Fig. 1), to generate parasites synchronized to an 8-h age window. The assay presented here is now based on the gametocyte-specific expression of a tdTom reporter. Compared with the former protocol10, in which we used GFP fluorescence as a readout, this has significantly improved the signal-to-noise ratio from below 10 to 1,142 ± 186 (values represent the mean ± s.d. of five biological replicates) by avoiding an overlap between the spectrum of our reporter and the autofluorescence of erythrocytes in green wavelengths38. Moreover, we found P. falciparum strain Pf2004 to be favorable for the purposes of our assay. In comparison with reference isolates, Pf2004 is a more recently culture-adapted, gametocyte-producing isolate from Ghana39,40. Compared with other laboratory strains such as 3D7 or HB3, the Pf2004/164-tdTom line is more responsive to environmental stimuli that induce sexual conversion (Fig. 2), whereas the rate at which gametocytes are formed under normal growth conditions (that is, baseline conversion rate) is negligible. The assay thus allows for the controlled production of highly synchronous gametocyte populations that can be perturbed as soon as sexual commitment is initiated.
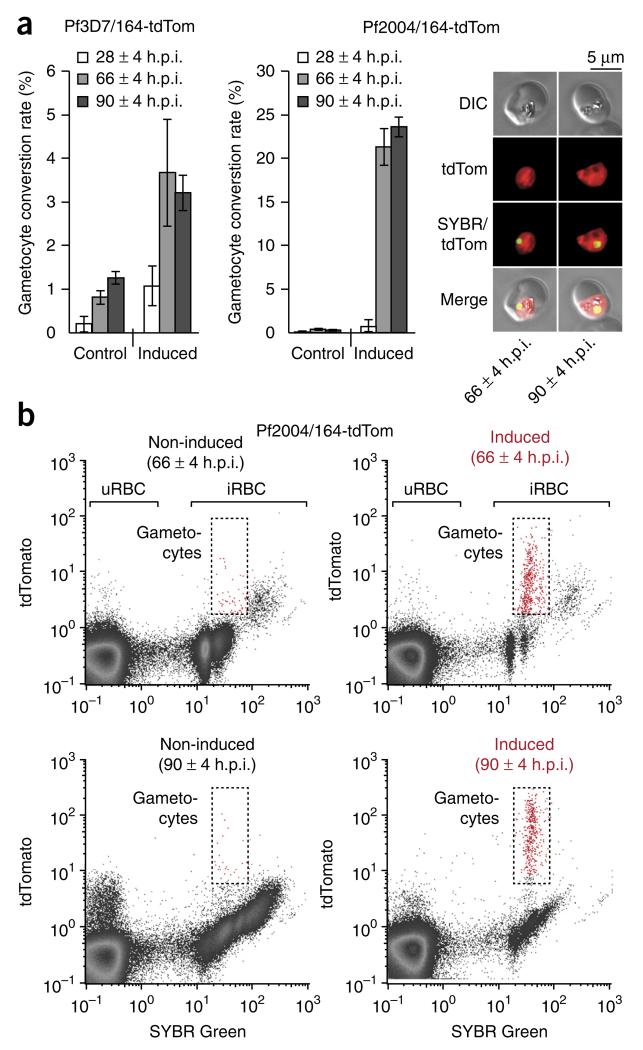
Figure 2.
Cytometric readout of gametocyte production and survival. (a) Gametocyte induction. Bar graphs quantifying the number of sexual cells produced under gametocyte-inducing and control conditions for cell lines 3D7/164-tdTom and Pf2004/164-tdTom (mean ± s.d., n = 3) in example experiments. The time point of flow cytometry–based quantification is indicated in h.p.i. Live cell images are shown for Pf2004/164-tdTom gametocytes and illustrate tdTom reporter expression (red) at 66 ± 4 h.p.i. and 90 ± 4 h.p.i. (b) Cytometry-based gametocyte quantification. Example of cytometric scatterplots quantifying SYBR Green (x axis) and tdTom (y axis) fluorescence for Pf2004/164-tdTom parasites cultured under control (left plots) and gametocyte-inducing (right plots) conditions. Flow cytometry was carried out at 66 ± 4 and 90 ± 4 h.p.i. Gates indicate tdTom-positive gametocytes within the infected RBC (iRBC) population. DIC, differential interference contrast.
Although we provide a protocol for the reproducible induction of gametocytogenesis, sexual-stage conversion remains a multi-layered process that is sensitive to a variety of both environmental and parasite-intrinsic factors. Consequently, gametocyte conversion rates vary between different inductions, depending on the exact composition of the experimental cell population and the batch of CM used (referred to as an ‘induction batch’). However, it is noteworthy that the presented protocol allows for a steady induction of gametocytogenesis. Z′-factors within individual induction batches consistently remain above 0.7 (0.79 ± 0.01, mean ± s.d.; n = 9), indicating that the assay should be adaptable to high-throughput platforms. Z′-factors were calculated according to Zhang et al.41, using induced Pf2004/164-tdTom cultures treated with lethal doses of dihydroartemisinin as negative controls and nontreated cells as positive controls. In contrast to other screens, in which the dynamic range of the signal is defined by technical constraints, variability of CM composition is an inherent component of the presented assay, and therefore Z′-factors calculated from across induction batches fail to describe the screens’ performance. To prevent false positive and/or negative hits, screening results must thus always be interpreted in relation to ‘induced’ (that is, treated with CM) and ‘noninduced’ control conditions from the same induction batch (see PROCEDURE). Compared with the consistently low conversion rates of noninduced populations (0.3 ± 0.06%, mean ± s.d., n = 5), CM-treated Pf2004/164-tdTom cultures produce at least 40 (61.8 ± 17.8, mean ± s.d., n = 5) times more gametocytes, typically converting to the sexual pathway at a rate of 14 to 24% (17.8 ± 5.4%, mean ± s.d., n = 5). This high responsiveness of the Pf2004/164-tdTom line to CM treatment is particularly important when screening compounds or growth conditions that induce gametocytogenesis, and it generally allows one to identify hits with high accuracy.
Although we recommend the use of the Pf2004/164-tdTom line, the assay can also be carried out using transgenic 3D7 parasites, as reported in our original screen10, or by using HB3 parasites carrying the respective reporter plasmid. However, higher baseline conversion rates of both of these strains compared with Pf2004 may limit the ability to detect perturbations that stimulate sexual conversion. Transgenic 3D7, HB3 and Pf2004 strains can be requested from our laboratory, and they are available with GFP or tdTom reporters under control of the gametocyte-specific promoters of PF3D7_1016900, PF3D7_1477300 or PF3D7_1477700. In addition, the assay should be adaptable for screening of nontransgenic parasites using immunolabeling of gametocytes as readout.
Assay variations
The standard assay as provided below is designed to screen compounds for inhibitory effects on gametocyte production and development, as well as on asexual proliferation. The compounds of interest are hence added simultaneously with the gametocyte-inducing CM or at any later time point, depending on the question being asked. Alternatively, using the same assay setup, compounds or changes in culture condition can be probed for a positive effect on the rate at which parasites switch to the sexual pathway. If screening for such gametocyte-enhancing effects is performed, noninduced parasite populations are probed instead, whereas gametocyte-induced cultures serve as a positive control.
The protocol presented here uses CM at a defined working concentration of 90% (vol/vol) to induce gametocytogenesis in P. falciparum. The use of different CM concentrations may increase or decrease gametocyte production while having the inverse effect on asexual replication. Consequently, there is a fine line between optimal induction of gametocytogenesis and provoking parasite death. In our hands, highest sexual conversion rates are obtained with a 90% (vol/vol) CM working solution. Before starting the screen, it may, however, be advisable to test the performance of different CM concentrations. This optional evaluation is performed by following the protocol as described in the PROCEDURE section, and it should include parasites cultured in either malaria culture medium (MCM; negative control) or CM at 90, 92.5 and 95% (vol/vol).
Limitations
We strongly recommend that personnel performing this screen have experience in P. falciparum in vitro culture or are supervised by an expert in the field. As compared with plate reader–based methods, the flow cytometry readout of our assay places a limit on the number of compounds that can be screened per unit of time. Labor is a limiting factor of this assay because of the multiple synchronization and readout steps, as well as the long time span of the full assay (Fig. 1), and it can be beneficial to have two individuals share steps in the protocol. Because multiple cell cycles are required for sexual conversion and gametocytes require time to develop to a distinguishable stage, assays that screen gametocytes, whether mature or immature, necessarily occur over a longer time span than assays targeting asexual blood stages.
When developing the original assay, we observed highly increased gametocyte levels after treatment with sublethal doses of some antimalarials such as atovaquone and methylene blue10. Further investigation of this phenomenon revealed that the cytostatic effect of these drugs results in an immediate but transient ‘faux’ activation of the fluorescent reporter preceding reporter expression during early gametocyte development. Importantly, owing to the temporal differences in reporter activation, the assay presented here is capable of distinguishing these ‘faux-induced’ parasites from sexually committed cells (see Supplementary Fig. 1 and ANTICIPATED RESULTS).
MATERIALS
REAGENTS
-
P. falciparum line Pf2004/164-tdTom, tested for gametocyte production (Marti Lab; the following alternative transgenic parasite strains and fluorescent reporter cassettes are available: strain 3D7 is available as a transgenic line carrying GFP under control of either gametocyte-specific promoters PF3D7_1016900, PF3D7_1477300 or PF3D7_1477700 or TdTomato under PF3D7_1016900 or PF3D7_1477700 promoters, Pf2004 and HB3 are available as transgenic lines carrying TdTom under control of the gametocyte-specific PF3D7_1016900 promoter)
! CAUTION P. falciparum is a human pathogen; consult local regulations for handling and waste disposal requirements. Wear gloves and a lab coat.
-
Human RBCs, type O+ (Research Blood Components or local vendor)
! CAUTION Blood cells may contain human pathogens; consult local and institutional regulations for handling and waste disposal requirements. Wear gloves and a lab coat. ! CAUTION RBCs must be obtained in accordance with all relevant institutional and governmental regulations, and where applicable, should be obtained with informed consent.
▲ CRITICAL Erythrocytes must always be fresh (that is, <10 d old).
MCM components
dH2O (for example, from Millipore dH2Osystem)
RPMI 1640 with phenol red (Sigma, cat. no. R6504)
HEPES-free acid (Calbiochem/EMD Millipore, cat. no. 391338)
Gentamycin (Sigma, cat. no. G1397)
Human serum (Interstate Blood Bank or local vendor). Store it at −20 °C until immediately before use ! CAUTION Human serum may contain human pathogens; consult relevant local and institutional regulations for handling and waste disposal requirements. Wear gloves and a lab coat. ! CAUTION Human serum must be obtained in accordance with all relevant institutional and governmental regulations, and where applicable, should be obtained with informed consent.
Hypoxanthine (Sigma, cat. no. H9377)
NaHCO3 (Sigma, cat. no. S5761)
Flow cytometry buffers and calibration beads (Miltenyi Biotec, or as appropriate for your instrument)
Running buffer (Miltenyi, cat. no. 130-092-747)
Washing solution (Miltenyi, cat. no. 130-092-749)
Storage solution (Miltenyi, cat. no. 130-092-748)
MACSQuant calibration beads (Miltenyi, cat. no. 130-093-607)
Tissue culture consumables
Glass slides (VWR, cat. no. 16004-368)
Sterile filter, 0.22-μm pore size (Thermo Fisher/Nalgene, cat. no. 569-0020)
Sterile 20-μl, 200-μl and 1-ml tips (Denville, cat. nos. P1121, P1122, P1123)
Sterile 15- and 50-ml Falcon tubes (Corning, cat. nos. 430791, 430829)
Sterile tissue culture–treated 96-well plates, flat bottom (Corning, cat. no. 353072)
Sterile 100 × 15 mm and 150 × 15 mm Petri dishes (Corning, cat. nos. 351029, 351058)
Sterile 5-, 10-, 25- and 50-ml pipettes (VWR, cat. nos. 89130-896, -898, -900, -902)
Sterile 250-ml media bottles (Thermo Fisher/Nalgene, cat. no. 2019-0125)
WR 99210 (Sigma, cat. no. W1770)
Gas mix for malaria culture 5% CO2, 1% O2, balance N2 (Med-Tech Gases or local vendor)
Diff-Quik, Giemsa-based or similar slide staining system
Methanol (EMD, cat. no. MX0475P)
Hemacolor Solution II (EMD, cat. no. 65044B-85)
Hemacolor Solution III (EMD, cat. no. 65044C-85)
SYBR Green (Lifetech, cat. no. S7567) ! CAUTION SYBR Green binds DNA and may be a mutagen.
Sterile PBS (Lonza, cat. no. 17-517Q)
Small-molecule libraries ! CAUTION Some molecules in the library may be toxic; suitable precautions should be taken.
DMSO (Sigma, cat. no. D2650)
Sorbitol (Calbiochem, cat. no. 56755)
EQUIPMENT
Light microscope (e.g., Olympus CX21) for viewing stained slides
Biosafety level 2 rated (Level II A2) tissue culture cabinet (e.g., Nuare Model NU-425-400)
Tissue culture incubator maintained at 37 °C (e.g., Forma Scientific, Model 3110)
Culturing chamber (e.g., Billups-Rothenberg, cat. no. MIC-101) or gassed incubator system
Pipetboy (e.g., Integra, cat. no. 155000)
Tabletop centrifuge with microtest-plate buckets (e.g., Eppendorf 5810R with MTP/Flex bucket)
pH meter (e.g., Mettler Toledo SevenEasy)
Glass flask, >4 liters
Water bath at 37 °C
-
Flow cytometer (e.g., MACSQuant VYB, Miltenyi Biotec)
▲ CRITICAL The flow cytometer used must be able to excite and detect both SYBR Green and TdTomato fluorescence and must be compatible with 96-well plates.
FlowJo (Tree Star) or similar flow cytometry analysis program
REAGENT SETUP
Parasite cell preparation
For thawing, freezing and maintenance of P. falciparum cells, refer to Methods in Malaria Research42. Keep Pf2004/164-tdTom parasite cultures at 37 °C in MCM containing 4 nM WR 99210. Replace the medium daily. Unless otherwise stated, keep the culture hematocrit at 5%. Maintain cultures in sterile 100 × 15 mm Petri dishes (10-ml culture volume per dish) or 150 × 15 mm dishes (30-ml culture volume per dish). We recommend minimizing handling time outside ideal growth conditions (37 °C in gas mixture optimized for malaria culture). After each step involving handling parasites, promptly return the dishes to a culturing chamber and add optimized gas mixture, and then return the chamber to an incubator at 37 °C. Alternatively, return it to a gassed TC incubator system at 37 °C.
Per screening plate, start the experiment with ~10 ml of culture at a ring-stage parasitemia of at least 2%. To evaluate parasitemia (percentage of parasite-infected RBCs), place 2 μl of packed RBCs on a glass slide and use the edge of another glass slide to smear. Stain using Diff-Quik or another staining system. Count infected cells per 2,000 total RBCs using a light microscope. ! CAUTION P. falciparum is a human pathogen; consult local regulations for handling and waste disposal requirements. Wear gloves and a lab coat. ▲ CRITICAL To maintain sterility of cells, all steps involving cells that will be returned to culture, as well as culture reagent preparation, must be carried out under sterile conditions in a biosafety cabinet.
▲ CRITICAL P. falciparum lines can lose the ability to produce gametocytes after extended in vitro culturing43. Keep multiple aliquots of the original parasite stabile in liquid nitrogen, and maintain gametocyte-producing cultures at a low passage number.
Incomplete malaria culture medium
In a clean and sterile flask, prepare 4 liters of medium as follows: dissolve 41.76 g of RPMI powder with phenol red and 23.76 g of HEPES (free acid) in 2.8 liters of ddH2O and stir it for at least 30 min. Separately, add 400 mg of hypoxanthine to 80 ml of boiling water and mix to dissolve. Add 40 ml of the hypoxanthine and 1 liter of ddH2O to the 4-liter flask. Use a pH meter to adjust the pH to exactly 6.75 with 1 M NaOH. Filter-sterilize the solution by passing it through a filter with a 0.22-μm pore size in a tissue culture hood, and then store it at 4 °C for up to 4 weeks.
Complete malaria culture medium
Dissolve NaHCO3 to 3.6% (wt/vol) in water and filter-sterilize it. To 210 ml of incomplete MCM, add 14 ml of NaHCO3 solution and 25 ml of human serum under sterile conditions. Mix the medium and store it at 4 °C for up to 2 weeks.
5% (wt/vol) sorbitol
Prepare 5% sorbitol (wt/vol) in dH2O. Filter-sterilize the solution by passing it through a 0.22-μm filter in a tissue culture hood, and store it at 4 °C for up to 6 months. Sorbitol treatment lyses erythrocytes infected with 24- to 48-h-old P. falciparum cells, and thus it synchronizes the parasites to 24 h.p.i. and younger.
SYBR Green staining solution
Prepare SYBR Green staining solution immediately before use by diluting SYBR Green stock solution to a 1:5,000 ratio in PBS.
WR 99210
Completely dissolve WR 99210 to 2 mM in 10 ml of RPMI-HEPES in a 15-ml Falcon tube by rotating it for 1–3 h. Filter-sterilize and store this stock solution at −20 °C for at least 5 years. To prepare working solution, dilute the stock to a 1:200 (to 10 μM) ratio in RPMI-HEPES and store it at 4 °C for up to 4 weeks. Add 4 μl of working solution per 10 ml of parasite culture.
EQUIPMENT SETUP
Flow cytometer setup
Regularly run calibration and cleaning protocols on your flow cytometer according to the manufacturer’s instructions. On the MACSQuant VYB, tdTomato is detected in the 615/20 channel (Y2) and SYBR Green is detected in the 525/50 channel (B1). For both parasitemia readout (Steps 21–23) and gametocytemia readout (Steps 24 and 25), optimized settings on the MACSQuant VYB are as follows: FSC (forward scatter) channel (trigger on this channel), 270 V; Y2 channel, 375 V; B1 channel, 270 V; use low flow rates.
If an alternative flow cytometer is used, the specific settings may need to be optimized to ensure good separation between the uninfected RBC, asexual parasite and gametocyte populations.
PROCEDURE
IDC-I: first sorbitol synchronization ● TIMING 40 min
▲ CRITICAL The following procedure describes the parallel generation of enough CM and experimental cells for 180 gametocyte inductions in the 96-well format. If the assay is scaled up, it may be advisable to perform the labor-intensive production of CM (Steps 8 and 10–12) in advance.
-
1|
Prewarm MCM and sorbitol solution to 37 °C in a water bath.
-
2|
Evaluate parasitemia of a 30-ml (asynchronous) parasite culture by light microscopy using Diff-Quik or Giemsa-stained blood smears (see Reagent Setup).
▲ CRITICAL STEP Only start the protocol if >2% of the erythrocytes are infected with early ring-stage parasites.
-
3|
Pellet the cells in a 50-ml conical centrifuge tube (800g, 5 min, 20 °C), discard the supernatant and resuspend the cells in eight times the pellet volume of sorbitol solution. Pipette the cells up and down to mix and incubate the mixture at 37 °C for 5–10 min. This initial sorbitol treatment synchronizes the parasites to a 24-h time window (0–24 h.p.i.).
-
4|
Wash the cells once in 10 ml of prewarmed MCM, and then resuspend the culture in 30 ml of prewarmed MCM.
Second sorbitol synchronization ● TIMING 40 min
-
5|
16 h after the first sorbitol synchronization (Step 3), repeat Steps 1–4 to bring parasites to an 8-h time window. After this second synchronization step, parasites have a mean age of 20 h.p.i. and range from 16 to 24 h.p.i. (20 ± 4 h.p.i.).
IDC-II: preparation of experimental cells and cultures producing the CM ● TIMING 30 min
-
6|
Evaluate parasitemia by light microscopy using Diff-Quik– or Giemsa-stained blood smears. Perform this step and Steps 7 and 8 after parasite reinvasion is completed (i.e., 40–48 h after Step 5).
▲ CRITICAL STEP A minimum of 2% parasitemia is required.
? TROUBLESHOOTING
-
7|
Prepare a low-parasitemia (LP) culture by splitting the synchronous parasite population into a new dish at parasitemia of ~0.3%, while maintaining the HC at 5%. In this LP culture, the proportion of infected cells will be kept below 1% to prevent stress responses that may lead to a premature induction of gametocytogenesis.
▲ CRITICAL STEP This culture will provide the experimental cells, used in Steps 15 and 16.
-
8|
Prepare a second culture with a parasitemia of 1.5–2% (HP) and expand the culture volume according to your requirements (220 μl of CM is needed per well of a 96-well plate; save an additional 110 μl per well for the washing procedure given in Steps 15 and 16).
▲ CRITICAL STEP The HP culture is used for the preparation of CM, as described in Steps 10–12.
IDC-III: third sorbitol synchronization of experimental cells ● TIMING 40 min
-
9|
Resynchronize early ring-stage parasites (at 4 ± 4 h.p.i.) of the LP culture by repeating Steps 1–4. This step is only necessary if the LP culture is substantially contaminated with schizont stages (i.e., >2% of parasitized erythrocytes).
Collection of CM ● TIMING 40 min plus culturing time
-
10|
Adjust the HP culture to a parasitemia of 5.5–6.5% when parasites are 28 ± 4 h.p.i. Allow cells to develop for 16 h to 44 ± 4 h.p.i.
▲ CRITICAL STEP Parasites of the HP culture are used to produce the CM, and hence the medium should be exchanged completely with prewarmed MCM at this time point.
-
11|
When parasites are at 44 ± 4 h.p.i., pellet the cells (800g, 5 min, 20 °C) in a conical centrifuge tube and collect the supernatant (this is the CM).
-
12|
Centrifuge the supernatant two more times at 1,600g for 5 min at 20 °C to remove remaining cells, and collect the supernatant (CM). You can discard the culture pellet.
▲ CRITICAL STEP Culturing late-stage asexual parasites of the line Pf2004/164-tdTom at a parasitemia >5% may drastically affect their development. At the time of CM collection, expect the parasites to deviate from the typical morphology of schizonts at 44 ± 4 h.p.i. (Supplementary Fig. 2).
■ PAUSE POINT If you are producing CM in advance, CM can be kept for at least 90 d at 4 °C or frozen at −20 °C.
IDC-IV: fourth sorbitol synchronization of experimental cells ● TIMING 40 min
-
13|
Resynchronize late ring-stage parasites (at 20 ± 4 h.p.i.) of the LP culture (as in Steps 1–4) and accurately determine parasitemia by light microscopy or by flow cytometry. Dilute the culture to a parasitemia of exactly 0.3% and expand the culture to the desired volume (110 μl is needed per well of a 96-well plate). Confirm that parasitemia is within the range of 0.25–0.35%, and readjust if necessary. Write down the exact parasitemia used for seeding (will be required in Step 27).
▲ CRITICAL STEP If the parasitemia or the culture volume is too low for your requirements, allow the parasites to complete another IDC and repeat this step.
? TROUBLESHOOTING
Setting up the library screen ● TIMING 2 h
-
14|
Add nine parts of CM (collected in Step 12) to one part of MCM to obtain a 90% CM working solution. In preparation for Step 15, mix and prewarm this CM working solution to 37 °C in a water bath for 30 min.
▲ CRITICAL STEP Using CM working solutions at different concentrations may drastically affect gametocyte conversion rates of the experimental cells. If you have tested different CM concentrations before starting the assay (see ‘Assay variations’ section in the INTRODUCTION), prepare the CM working solution according to the conditions evaluated in this optional optimization step.
-
15|
At 28 ± 4 h.p.i., prepare cells to be treated with drugs (and untreated positive-control cells) by inducing gametocytogenesis as follows: pellet the required volume of the LP culture (calculate the volume on the basis that 110 μl is needed per well of a 96-well plate; e.g., 6.6 ml for 60 wells). Wash it once in an equal volume (e.g., 6.6 ml for 60 wells) of CM working solution. Resuspend the cells in twice the volume (e.g., 13.2 ml for 60 wells) of CM working solution.
-
16|
Also prepare cells to be used as ‘noninduced’ negative controls by pelleting the required amount (110 μl is needed per well of a 96-well plate) of the LP culture in a conical centrifuge tube and by washing the cells in an equal volume of prewarmed MCM. Resuspend the cells in twice the volume of MCM.
▲ CRITICAL STEP If drugs are screened for effects that induce sexual conversion, prepare nontreated control cells as described in Step 15 rather than as described in this step, as the conditions described in Step 15 will control for maximal induction of sexual conversion. In this situation, prepare the experimental cells to be treated, as described in this step (Step 16).
-
17|
Distribute 220 μl of cell suspension per well of a 96-well plate. Note that the parasite culture now contains 0.3% infected erythrocytes at an HC of 2.5%. Fill unused surrounding wells with incomplete medium to prevent evaporation.
-
18|
Add the compounds of interest now (when parasites are at 28 ± 4 h.p.i.) or during Step 20 (IDC-V, 8 ± 4 h.p.i.) and mix by pipetting up and down. Note that administering compounds during IDC-V will probe for effects on early gametocyte development, whereas compounds added during the present cycle (IDC-IV) will also test for potential effects on sexual conversion. Include untreated control wells in every plate. Do not add drug to these wells, but do include the maximum concentration of the compound vehicle (for example, DMSO) used in the experiment.
▲ CRITICAL STEP Changes in culture volume may affect gametocyte conversion rate. We thus recommend adding relatively low and equal volumes (2–10 μl) of compound stock solutions to each well. We observed that DMSO concentrations of up to 0.3% do not affect parasite growth or gametocyte production or development; nevertheless, DMSO-alone controls should be included.
▲ CRITICAL STEP Perturbations of interest can be introduced at time points other than those indicated. Any time point between 28 ± 4 h.p.i. of IDC-IV and 36 ± 4 h.p.i. of IDC-V may be chosen to answer more specific questions about sexual commitment or early gametocyte development.
IDC-V: medium exchange ● TIMING 30 min
-
19|
24 h after the addition of CM, spin the 96-well plate at 800g for 5 min at 20 °C to pellet cells. Remove 200 μl of medium from each well and resuspend cells in 200 μl of prewarmed MCM.
▲ CRITICAL STEP The cultures now contain early developing gametocytes that are not yet morphologically distinct from asexual parasites. Depending on the setup of your experiment, you may want to add, re-add or remove the compounds of interest at this time point. In the latter case, remove the medium completely and wash it three times in 200 μl of prewarmed MCM before incubating the cells in 220 μl of MCM.
-
20|
Add compounds to be tested for effects on early gametocyte development when parasites are at 8 ± 4 h.p.i. Mix by pipetting up and down.
IDC-V (and early gametocyte development): measurement of parasitemia by flow cytometry ● TIMING 2.5 h
-
21|
At 28 ± 4 h.p.i., resuspend the cells and transfer 10–20 μl of cell suspension from each well to 100 μl of SYBR Green staining solution (preloaded in a 96-well plate). Mix and incubate the cells at 37 °C for 20 min. Protect the cell mixture from light.
-
22|
Pellet cells by centrifugation (800g, 5 min, 20 °C), discard the supernatant and resuspend the cells in 200 μl of PBS. After repeating this washing procedure once, resuspend the cells in 200 μl of PBS for subsequent cytometry. If protected from light, cells can be kept at room temperature (20 °C) for at least 6 h before starting flow cytometry measurements.
-
23|
Run flow cytometry to determine parasitemia. Measure 100,000 events per well. See Equipment Setup for optimized flow cytometry settings. Parasite multiplication rate of CM-treated cultures typically drops by two- to threefold compared with parasites grown under noninducing conditions. This is indicative for a successful induction of gametocyte production. Owing to the low starting parasitemia, parasite multiplication rates are not restricted by self-induced nutrient depletion, and noninduced parasite cultures are expected to reach a parasitemia of 2.1–2.7% in IDC-V, whereas CM-induced cultures typically grow to a parasitemia of 0.6–0.9%. During subsequent IDCs, however, asexual parasite growth will be hampered owing to high parasite densities. Although this has no effect on gametocyte development, asexual parasite growth rates should thus not be used as a basis for analyses after this time point. Conversion rates will be calculated as a proportion of gametocytes formed from the pool of parasites determined in this step. Therefore, asexual replication can be ignored after IDC-V.
? TROUBLESHOOTING
Measurement of gametocytemia by flow cytometry ● TIMING ~3.5 h
-
24|
At 66 ± 4 h.p.i. (38 h after Step 21), stain the cells with SYBR Green by repeating Steps 21–22. At this time point, young gametocytes have acquired enough tdTom reporter for cytometry-based detection. As the intensity of the fluorescent signal continuously increases during early gametocyte development, detection at a later time point may be favorable.
▲ CRITICAL STEP Measurements should not be carried out after 90 ± 4 h.p.i. to prevent detection of gametocytes formed during subsequent IDCs.
-
25|
Perform flow cytometry to determine gametocytemia. Measure 400,000 events per well. See Equipment Setup for optimized flow cytometry settings.
▲ CRITICAL STEP Although setting up this cytometric readout is not labor-intensive, enough time should be scheduled to allow detection of 400,000 events per well (usually 1–2 min are required per well, depending on the equipment and settings used).
? TROUBLESHOOTING
■ PAUSE POINT Data analysis (next section) can be performed at any time point after data acquisition.
Flow cytometry data analysis and hit selection ● TIMING 1.5 h
▲ CRITICAL Data analysis can be carried out using any cytometry analysis program (e.g., FlowJo).
-
26|
To visualize flow cytometry data, export .fcs data files from the flow cytometer to the cytometry analysis program. Generate dot plots with Y2 intensity (tdTom fluorescence) on the y axis and B1 intensity (SYBR Green fluorescence) on the x axis. Gate double-negative uninfected RBC (uRBC), SYBR Green–positive (asexual parasite) and tdTom/SYBR double-positive (gametocyte) cell populations (Fig. 2b). Use the same gates for all samples from the same flow cytometry run. The gates should be drawn on the basis of scatterplots from induced untreated control samples (Fig. 2b).
▲ CRITICAL STEP Although the cell populations separate clearly, caution is required when gating the tdTom/SYBR double-positive gametocytes. After defining the gate, it should be applied to noninduced control samples to test for an overlap with the asexual (tdTom negative) population. In case such an overlap is observed, the lower gate boundaries have to be shifted toward higher tdTom intensities. While reducing the number of false positive counts, which is essential for the performance of the assay, this procedure may result in the loss of some gametocytes with low tdTom expression. If the asexual and sexual populations are not separating clearly, it is advisable to perform the flow cytometry readout at a later time point (compare Step 24).
? TROUBLESHOOTING
-
27|
Determine parasitized erythrocyte multiplication rate (PEMR) for each well by dividing the proportion of SYBR Green–positive cells (parasitemia) from Step 23 by the starting parasitemia evaluated in Step 13.
E.g.: a starting parasitemia of 0.3% (determined in Step 13) results in a parasitemia of 2.4% during the following IDC (determined in Step 23). PEMR = 2.4%/0.3% = 8.
-
28|
Determine sexual conversion rates or, when testing compounds active against early gametocytes, gametocyte survival rates for each well by dividing the proportion of tdTom/SYBR double-positive cells (gametocytemia) from Step 25 by the proportion of SYBR Green–positive cells (parasitemia) from Step 23.
For example, the induced control population contains 0.12% gametocytes (determined in Step 25), whereas parasitemia (determined in Step 23) is at 0.6%. The conversion rate in this example is 20% (0.6%/0.12% = 0.2).
-
29|
Calculate the mean rate of sexual conversion or gametocyte survival ± s.d. for all test conditions, as well as for the induced, noninduced and vehicle controls.
▲ CRITICAL STEP Confirm the absence of marked effects of the vehicle on parasitemia, gametocytemia and conversion rate by comparing the vehicle-only controls with induced and noninduced control samples.
-
30|
Assess the effect of probed perturbations on sexual conversion and/or gametocyte survival in relation to the induced control wells created in Step 15 (defining maximal sexual conversion and 100% gametocyte survival, respectively) and noninduced control wells created in Step 16 (defining baseline conversion).
E.g: the tested compound is administered under gametocyte-inducing conditions, and it results in a conversion rate of 5% (determined in Step 28), whereas the induced control population produces 20% gametocytes. In this example, the tested compound reduces gametocyte production by 75% down to 25% (5%/20% = 0.25).
-
31|
Identify ‘hits’ according to self-determined thresholds.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 6 | Parasitemia is too low to generate the LP and HP cultures |
Parasitemia of the starting culture was too low, parasites proliferated poorly or many cells were lost during synchronization |
Allow parasites to complete another IDC before returning to this step |
| 13 | Not enough cells are obtained after synchronization to seed the required number of 96-well plates |
Parasitemia of the starting culture was too low, parasites proliferated poorly or many cells were lost during synchronization |
Allow parasites to complete another IDC before returning to this step. Use fresh RBCs. Meanwhile, the CM can be stored at 4 °C for at least 90 d |
| 23 | Low parasite multiplication rate under noninduced conditions |
Poor asexual proliferation. The RBCs may be too old or, for other reasons, do not support optimal parasite growth |
Repeat the assay with a fresh batch of RBCs. It may be beneficial to combine RBCs from multiple donors for culturing |
| Low parasite multiplication rate under CM-induced conditions |
Note that parasite multiplication rate of CM-treated cultures typically drops by two- to threefold compared with parasites grown under noninducing conditions. This is indicative of a good induction of gametocyte production, rather than indicating a problem |
If the parasite multiplication rate drops below 2, it may be necessary to further optimize the assay by using different CM conditions (e.g., lower the concentration of the CM working solution from 90% to 87.5%) |
|
| Drug treatment induces a premature induction of the tdTom reporter at 28 ± 4 h.p.i. |
Parasites show a ‘faux’ induction response to drug treatment (see ANTICIPATED RESULTS) |
Perform gametocyte quantification at 90 ± 4 h.p.i. The transient signal from ‘faux’-induced parasites is not detectable anymore at this time point (see ANTICIPATED RESULTS) |
|
| 25 | CM treatment fails to induce gametocytogenesis |
Small changes in assay conditions may drastically alter the rate at which gametocytes are produced. Whereas CM collected from cells at a parasitemia below 5.5% may lead to poor inductions, the application of other CM conditions may result in the complete killing of the experimental parasite population. Note that different stains may respond differently to CM treatment |
We recommend performing an initial testing of the assay using CM working solutions at 90, 92.5 and 95% (vol/vol). This should allow achieving similar results to those shown in Figure 2. In the case of low gametocyte production, increase the concentra- tion of the CM working solution (e.g., from 90% to 95%). Alternatively, increase the parasitemia of the culture used to produce the CM (see Step 10). If CM treatment causes complete killing of the experi- mental cell population, lower the concentration of the CM working solution. Alternatively, lower the parasitemia of the culture used to produce the CM |
| Many gametocytes are produced under noninducing conditions |
Parasite culture conditions may have caused a self-induction of gametocyte production. Cells of the parasite strain used may convert to the sexual pathway at a high intrinsic rate (high baseline conversion) |
Repeat the assay by strictly maintaining parasitemia of the experimental culture below 1% before seeding. Exchange the medium of this LP culture daily. Perform the assay with a cell line showing lower baseline sexual conversion |
|
| 26 | The tdTom-positive gametocyte population cannot be clearly distinguished from asexual (tdTom negative) parasites |
Some gametocytes are too young and have not yet acquired enough tdTom reporter protein for detection |
Perform flow cytometry to determine gametocytemia (Steps 24 and 25) at a later time point (e.g., 90 ± 4 h.p.i.) |
● TIMING
Steps 1–4, day 1, first sorbitol synchronization: 40 min
Step 5, day 2, second sorbitol synchronization: 40 min
Steps 6–8, day 3, preparation of HP and LP cultures: 30 min
Step 9, day 5, third sorbitol synchronization of LP culture: 40 min
Step 10, dilution and medium exchange on HP culture: 20 min
Steps 11 and 12, day 6, collection of CM from HP culture: 20 min
Step 13, day 8, fourth sorbitol synchronization of LP culture: 40 min
Steps 14–18, setting up the library screen: 2 h
Step 19, day 9, medium exchange: 30 min
Steps 21–23, day 10, flow cytometry measurement of parasitemia: ~1 h of preparation; cytometry requires ~1.5 h per plate (may vary depending on flow cytometer and settings used)
Steps 24 and 25, day 11, flow cytometry measurement of gametocytemia: ~1 h of preparation; cytometry requires ~2.5 h per plate (may vary depending on the flow cytometer and the settings used)
Steps 26–31, day of choice, data analysis and hit selection: ~1.5 h
ANTICIPATED RESULTS
The protocol presented here is designed to probe the effect of drugs and other changes in culture conditions on asexual parasite replication and early sexual development. Gametocytes are detected using the stage-specific expression of a fluorescent reporter. This renders the assay independent from gametocyte purification steps, and it enables screening the effects of perturbations on the earliest phases of gametocyte development including sexual commitment.
Tightly synchronized parasites of the Pf2004/164-tdTom cell line (others are available, see MATERIALS) are induced for gametocytogenesis by applying a standardized CM working solution. In contrast to other protocols, the induced culture is kept at a low starting parasitemia of 0.3% in this assay. Therefore, culture conditions are only marginally influenced by the parasite population itself, which allows for a standardized and highly reproducible induction process. Whereas parasites cultured under noninducing conditions typically multiply by a factor of 7–9, the addition of CM is expected to reduce PEMR to 2–3, resulting in a parasitemia of 2.1–2.7% and 0.6–0.9%, respectively, during the following cycle. As early as 62 h.p.i., gametocytes expressing the red fluorescent reporter tdTom can be quantified by flow cytometry. Compared with noninduced cultures, CM-treated parasite populations are expected to increase sexual conversion rates by 40- to 80-fold from ~0.3% to 14–24% (Fig. 2). Consequently, 350–900 tdTom-positive cells can be expected under induced and nontreated conditions when counting 400,000 erythrocytes. In contrast, baseline conversion of the noninduced cells will give rise to only 10–30 gametocytes per 400,000 RBCs.
Gametocyte development may be perturbed by introducing the compound of interest at any time during early maturation or even before sexual commitment. For example, dose response to drugs can be measured after administering the compounds at 28 ± 4 h.p.i. (Fig. 3). At this time point, notably, sexually committed cells cannot yet be physically separated from asexually replicating parasites. In addition, because the assay does not require gametocytes to be purified, the effect of drug treatment on asexual parasites can be monitored simultaneously in the same well. Further, the low rate at which gametocytes are produced in Pf2004/164-tdTom cells under noninducing conditions (Fig. 2a) allows the identification of drugs and other perturbations that induce sexual conversion.
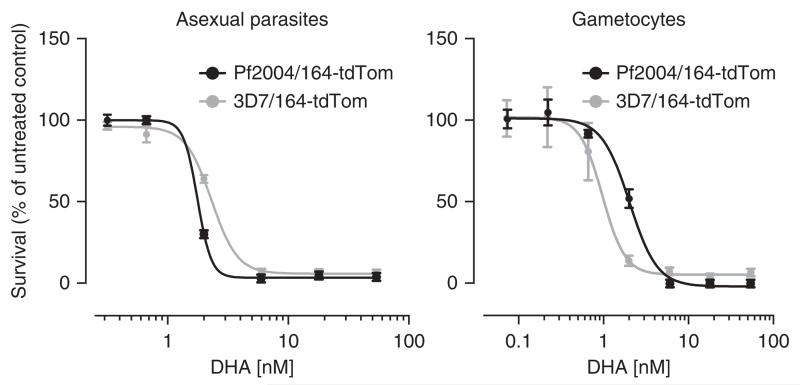
Figure 3.
Drug screening: DHA treatment affects asexually replicating parasites and early gametocytes. Determination of half-maximal inhibitory concentration (IC50). Shown is the effect of DHA on asexual parasite multiplication (left) and early gametocyte development (right). Mitotic proliferation and gametocyte survival was monitored 48 h after administering DHA at 28 ± 4 h.p.i. Note that both cell lines reveal similar dose–response curves. Curves were generated using a four-parameter nonlinear regression fit. The presented data exemplifies the outcome of a single screening run (mean ± s.e.m., n = 3), as described. Specific dose-response information gained by using the presented assay is available from Buchholz et al.10.
It is worth mentioning that some cytostatic drugs (including methylene blue and atovaquone) seem to deregulate stage-specific expression of gametocyte genes44 or to induce reporter expression in cells that are not committed to the sexual pathway10. This ‘faux’ induction is transient, initiated at an earlier-than-expected time point and only occurs at drug concentrations causing a near-complete block in asexual parasite replication (Supplementary Fig. 1). If such ‘faux’ induction is encountered (see Step 23 of the PROCEDURE), we recommend deferring gametocyte quantification to 90 ± 4 h.p.i. (see Step 24). At this time point, ‘faux’-induced cells have lost tdTom expression.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Ravel for critical reading of the manuscript. This work has been supported through a career development grant from the Burroughs Wellcome Fund and by US National Institutes of Health (NIH) grants R21 AI105328 and RC1AI086222 (M.M.). N.M.B.B. is supported by a postdoctoral fellowship from the Swiss National Science Foundation and K.B. was supported through a Feodor Lynen postdoctoral fellowship from the Alexander von Humboldt Foundation.
Footnotes
Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS I.G., N.M.B.B. and M.M. wrote the manuscript, with input from all coauthors. K.B. and M.M. conceived and developed the originally published protocol. I.G. conceived and performed preliminary experiments and N.M.B.B. conceived and performed later experiments leading to improvements in the protocol. N.M.B.B. and K.W. generated the data shown in the figures. All authors revised the manuscript and contributed to interpretation of the results.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Alonso PL, et al. A research agenda to underpin malaria eradication. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowunmi A, Adedeji AA, Gbotosho GO, Fateye BA, Happi TC. Effects of pyrimethamine-sulphadoxine, chloroquine plus chlorpheniramine, and amodiaquine plus pyrimethamine-sulphadoxine on gametocytes during and after treatment of acute, uncomplicated malaria in children. Mem. Inst. Oswaldo Cruz. 2006;101:887–893. doi: 10.1590/s0074-02762006000800011. [DOI] [PubMed] [Google Scholar]
- 3.Sowunmi A, Fateye BA. Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health. 2003;8:783–792. doi: 10.1046/j.1365-3156.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 4.Fehintola FA, Balogun ST, Adeoye SB. Intermittent preventive treatment during pregnancy with sulphadoxine-pyrimethamine may promote Plasmodium falciparum gametocytogenesis. Med. Princ. Pract. 2012;21:63–67. doi: 10.1159/000332405. [DOI] [PubMed] [Google Scholar]
- 5.Buckling A, Ranford-Cartwright LC, Miles A, Read AF. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118(Part 4):339–346. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 6.Price R, et al. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- 7.Dechy-Cabaret O, Benoit-Vical F. Effects of antimalarial molecules on the gametocyte stage of Plasmodium falciparum: the debate. J. Med. Chem. 2012;55:10328–10344. doi: 10.1021/jm3005898. [DOI] [PubMed] [Google Scholar]
- 8.Bell AS, et al. Enhanced transmission of drug-resistant parasites to mosquitoes following drug treatment in rodent malaria. PLoS ONE. 2012;7:e37172. doi: 10.1371/journal.pone.0037172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization WHO Malaria Report 2014. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/en/
- 10.Buchholz K, et al. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J. Infect. Dis. 2011;203:1445–1453. doi: 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson KK, et al. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc. Natl. Acad. Sci. USA. 2013;110:E2838–E2847. doi: 10.1073/pnas.1306097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Cruz FP, et al. Drug screen targeted at Plasmodium liver stages identifies a potent multistage antimalarial drug. J. Infect. Dis. 2012;205:1278–1286. doi: 10.1093/infdis/jis184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kafsack BF, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha A, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman BI, et al. A Plasmodium falciparum histone deacetylase regulates antigenic variation and gametocyte conversion. Cell Host Microbe. 2014;16:177–186. doi: 10.1016/j.chom.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brancucci NM, Witmer K, Schmid C, Voss TS. A var gene upstream element controls protein synthesis at the level of translation initiation in Plasmodium falciparum. PLoS ONE. 2014;9:e100183. doi: 10.1371/journal.pone.0100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell. Microbiol. 2014;16:344–354. doi: 10.1111/cmi.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regev-Rudzki N, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull. World Health Organ. 1979;57(suppl. 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer M, Day K. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol. Biochem. Parasitol. 2000;108:67–78. doi: 10.1016/s0166-6851(00)00205-x. [DOI] [PubMed] [Google Scholar]
- 21.Peatey CL, Dixon MW, Gardiner DL, Trenholme KR. Temporal evaluation of commitment to sexual development in Plasmodium falciparum. Malar. J. 2013;12:134. doi: 10.1186/1475-2875-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trager W, Gill GS. Plasmodium falciparum gametocyte formation in vitro: its stimulation by phorbol diesters and by 8-bromo cyclic adenosine monophosphate. J. Protozool. 1989;36:451–454. doi: 10.1111/j.1550-7408.1989.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 23.Inselburg J. Stage-specific inhibitory effect of cyclic AMP on asexual maturation and gametocyte formation of Plasmodium falciparum. J. Parasitol. 1983;69:592–597. [PubMed] [Google Scholar]
- 24.Read LK, Mikkelsen RB. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum. J. Parasitol. 1991;77:346–352. [PubMed] [Google Scholar]
- 25.Chaubey S, Grover M, Tatu U. Endoplasmic reticulum stress triggers gametocytogenesis in the malaria parasite. J. Biol. Chem. 2014;289:16662–16674. doi: 10.1074/jbc.M114.551549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter LM, et al. Stress and sex in malaria parasites: why does commitment vary? Evol. Med. Public Health. 2013;2013:135–147. doi: 10.1093/emph/eot011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ankarklev J, Brancucci NM, Goldowitz I, Mantel PY, Marti M. Sex: how malaria parasites get turned on. Curr. Biol. 2014;24:R368–R370. doi: 10.1016/j.cub.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Chevalley S, Coste A, Lopez A, Pipy B, Valentin A. Flow cytometry for the evaluation of anti-plasmodial activity of drugs on Plasmodium falciparum gametocytes. Malar. J. 2010;9:49. doi: 10.1186/1475-2875-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS ONE. 2014;9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peatey CL, Spicer TP, Hodder PS, Trenholme KR, Gardiner DL. A high-throughput assay for the identification of drugs against late-stage Plasmodium falciparum gametocytes. Mol. Biochem. Parasitol. 2011;180:127–131. doi: 10.1016/j.molbiopara.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Lelievre J, et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence ‘transmission blocking’ assay. PLoS ONE. 2012;7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka TQ, et al. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol. Biochem. Parasitol. 2013;188:20–25. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Alessandro S, et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J. Antimicrob. Chemother. 2013;68:2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 34.Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob. Agents Chemother. 2013;57:6050–6062. doi: 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, et al. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS ONE. 2014;9:e93825. doi: 10.1371/journal.pone.0093825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestrini F, et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 2005;143:100–110. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Aingaran M, et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum. Cell. Microbiol. 2012;14:983–993. doi: 10.1111/j.1462-5822.2012.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khandelwal S, Saxena RK. Age-dependent increase in green autofluorescence of blood erythrocytes. J. Biosci. 2007;32:1139–1145. doi: 10.1007/s12038-007-0115-z. [DOI] [PubMed] [Google Scholar]
- 39.Elliott SR, et al. Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am. J. Trop. Med. Hyg. 2007;76:860–864. [PubMed] [Google Scholar]
- 40.Hommel M, et al. Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infect. Immun. 2010;78:1963–1978. doi: 10.1128/IAI.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 42.Moll K, Ljungstroem I, Perlmann H, Scherf A, Wahlgren M. Methods in Malaria Research. (6th edn.) http://www.mr4.org/Publications/MethodsinMalariaResearch.aspx (MR4/ATCC, 2013)
- 43.Alano P, et al. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp. Parasitol. 1995;81:227–235. doi: 10.1006/expr.1995.1112. [DOI] [PubMed] [Google Scholar]
- 44.Chaal BK, Gupta AP, Wastuwidyaningtyas BD, Luah YH, Bozdech Z. Histone deacetylases play a major role in the transcriptional regulation of the Plasmodium falciparum life cycle. PLoS Pathog. 2010;6:e1000737. doi: 10.1371/journal.ppat.1000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.