Abstract
Background
Indirect calorimetry is a noninvasive and reliable means of determining resting metabolic rate in humans. Barriers to obtaining an accurate measure of resting metabolic rate in hospitalized patients include the expense and the requirement of technical expertise for maintenance.
Methods
A literature search on hand-held calorimeters was conducted using PubMed and OVID. The search resulted in a total of 54 published articles; 23 of these specifically are about hand-held calorimeter devices.
Results
Results from a hand-held calorimeter were similar to those obtained from metabolic cart studies. The Douglas bag method compared to the MedGem indicated a significant agreement with a p=0.286. The hand-held device compared to metabolic carts in 9 studies with mixed results. The predictive equations (Harris-Benedict, Mifflin St. Joer and FAO/WHO equations) were found to over and underestimate RMR compared to the MedGem. The Harris-Benedict was found to overestimate the RMR by 3-11%, the Mifflin St Joer equation overestimated the RMR by 1% and the FAO/WHO equation overestimated RMR by 12%.
Conclusion
The present study examines the validity and reliability of hand-held calorimeters for measuring resting energy expenditure based on published literature. Hand-held calorimeters are more accurate than predictive equations based on gender, age and ethnicity for determining resting metabolic rate and are therefore a viable alternative for clinical evaluation of the hospitalized patient.
Keywords: energy expenditure, indirect calorimetry, MedGem, resting metabolic rate
Introduction
Total energy expenditure (TEE) consists of three major components: basal metabolic rate (BMR), thermogenic effect of food, and the effect of physical activity or exercise.1 Basal metabolic rate is defined as the measurement of resting energy expenditure under the following conditions: a state of complete rest, ambient temperature is between 68° to 77° Fahrenheit. The subject is tested immediately upon waking after a minimum of a four hour sleep and in a post-absorptive state (12 hours after a meal). Thermogenic effect of food refers to the heat the body generates as food is digested. Physical activity is any activity in which work is performed which results in an increase in metabolic rate. Resting metabolic rate (RMR), which is synonymous with resting energy expenditure (REE), is not measured under basal conditions. It is a measurement taken during a fast (two to four hours after a meal) and allows for some movement with a rest period prior to testing.1,2 The RMR is usually higher than the BMR due to less restrictive conditions for measureing RMR. Therefore, basal metabolic rate accounts for 60- 75% 1 of TEE while RMR accounts for 50-70% of TEE.. Since the difference between BMR and RMR is only about 10%, it is more practical to measure RMR in a clinical setting.2 In clinical settings RMR is used to determine accurate energy expenditure of a patient's caloric needs. Resting metabolic rate can be measured either directly or indirectly.
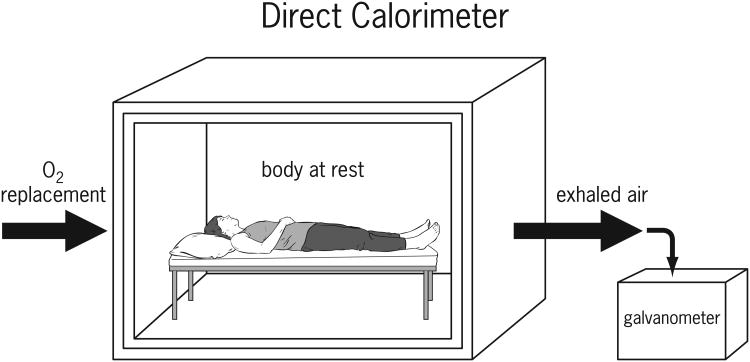
Direct calorimetry is the measurement of whole body heat released from an individual in a specialized chamber (Figure 1). A direct calorimeter is a large insulated, air-tight chamber in which oxygen is introduced while a subject is at rest. Oxygen used is replaced by the addition of a weighed amount of oxygen as required. Expired carbon dioxide and water vapor that is expended by the subject is measured. The temperature within the chamber is maintained with a cooling circuit. Thermal equilibrium within the chamber must be obtained for accurate measurements. The measurements for an individual take approximately an hour due to the large size of the chamber and the time-lag for the chamber to reach thermal equilibrium once a subject is placed in the environment.3
Figure 1.
Heat produced by a subject is measured from ventilating air current and a galvanometer in the thermo-electric junctions within the insulated walls. Therefore, heat given off by the subject can only escape by way of the circulating air and the specially designed walls. Measurements of temperature changes are calculated based on changes in the air or water circulating through the insulated walls. Since the measurements are calibrated for a single person, subjects must remain in the chamber alone with minimal movement for a period of several hours. Direct calorimetry is not practical in most settings. It requires an expensive apparatus and it is logistically impractical to leave a patient unattended for the extended period of time. It is therefore not the optimal method of measurement to be utilized in a large population such as with patients in a hospital.
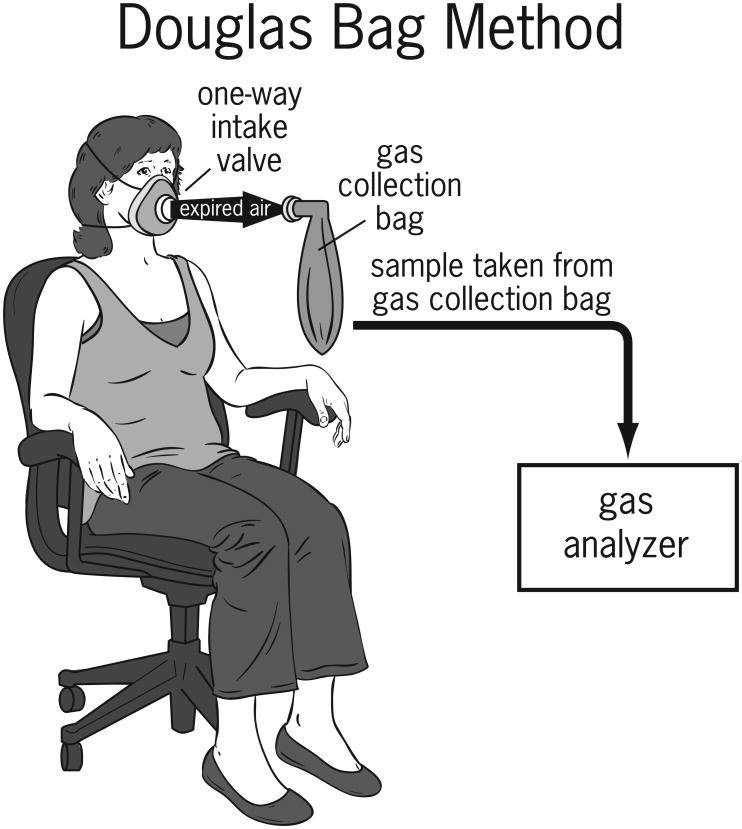
Indirect calorimetry measures the volume of oxygen (VO2) consumed compared to the volume of carbon dioxide (VCO2) expired by a subject. A metabolic cart is a device used to measure the oxygen consumed and the carbon dioxide produced by a subject and then calculates (using the modified Weir equation) the RMR for the patient. Oxidation of substrates to generate energy results in the release of water and carbon dioxide during the chemical reactions in vivo. Water and carbon dioxide are the two waste products resulting from this chemical reaction. The original Weir equation utilized liters of oxygen consumed and carbon dioxide produced from the Douglas Bag method (Figure 2) to determine RMR. The Douglas Bag method involves the collection of expired air over a period of 5-15 minutes4 to measure the consumption of oxygen and production of carbon dioxide. The Douglas Bag method is reliable and simple to use; however, limitations include potential for air leakage, small amount of sample that can be collected in the bag and the high cost of the equipment used for analysis.
Figure 2.
Weir equation
The indirect calorimeter measures VO2 and VCO2 so the RMR can be calculated using the modified Weir equation. The modified Weir equation replaces liters of oxygen consumed and liters of carbon dioxide produced with VO2 and VCO2 from the indirect calorimeter.5, 6
Modified Weir equation
However, since the difference in calculations for energy expenditure do not differ significantly when calculated with or without nitrogen excretion values, the abbreviated Weir equation omits urinary nitrogen:7, 8
Abbreviated Weir Equation
The ratio of carbon dioxide produced to the volume of oxygen inspired (VCO2/VO2) is called the respiratory quotient (RQ). Energy is derived from the release of heat through the oxidation of food substrates. Carbon based nutrients get converted to carbon dioxide, water and heat. The RQ will vary dependent on the fuel being oxidized by a subject. Complete oxidation of carbohydrate results in a RQ of 1.0, fat oxidation results in a RQ of 0.7 and a mixed diet results in a RQ of 0.85.1,6 Respiratory quotient has been used to determine the contribution of different substrates even though some authors have questioned the validity of the utility of RQ. Based upon the RQ it is possible to estimate the relative oxidative state of carbohydrate, fat and protein and therefore useful in clinical practice.8
Limited availability of direct and indirect calorimeters result in frequent utilization of predictive equations as an estimate of RMR in clinical practice. There are more than 200 predictive equations for measuring energy expenditure. 1 Commonly used predictive equations include the Harris-Benedict equation, Mifflin-St. Joer equation, Penn State equation, Ireton-Jones equations and calories/kilogram. Predictive equations are a quick and low cost means of obtaining RMR, but are imprecise especially in the critically ill patient population. 1 Predictive equations are constantly evaluated and developed as a result of advances in technology, availability of equipment, differences in body composition, health status and activity levels, to determine the most accurate means of determining RMR.
In recent years, indirect calorimeter devices have become more portable making it more convenient to perform these evaluations at bedside or outpatient settings. Indirect calorimetry is believed to be a more accurate measurement of RMR than predictive equations that are commonly used.6 These devices produce immediate results at the completion of the study. However, indirect calorimeters are expensive and require technical expertise for maintenance and use.
Hand-held calorimeters such as the MedGem™ and BodyGem™ (Microlife, Dunedin, FL, USA) have been developed to measure energy expenditure. While traditional indirect calorimeters measure VO2 and VCO2, the hand-held devices measure only VO2 where RQ is assumed to be 0.85.9 Subjects hold the hand-held device and breathe into a mask or mouthpiece. Advantages of hand-held devices include portability, lesser degree of technical expertise required, and greater cost effectiveness than traditional calorimeters. These devices cannot be used on patients requiring mechanical ventilator support, do not measure VCO2, and have not been validated in various inpatient or hospitalized patient populations.
The present systematic review was performed to determine the validity and reliability of hand-held calorimeters for measuring RMR based on published literature.
Methods
A literature search on hand-held calorimeters was conducted using PubMed, EMBASE, Google Scholar and OVID. The key terms searched included “hand-held indirect calorimeter”, “portable indirect calorimeter”, “metabolic calorimeter”, “validation calorimeter”, “respiratory calorimeter”, and “MedGem”. Bibliographies for each study were cross referenced for additional publications to assure a comprehensive review of the literature. For the purpose of this review, a metabolic cart is defined as a device where the mixing chamber, gas analyzer and monitor can be moved via a wheeled cart and the subject is placed under a canopy or utilizes a face mask.
The search resulted in a total of 54 published articles; 23 of those about hand-held calorimeter devices. Of the 23 articles, 10 compared MedGem to an indirect calorimeter, one compared MedGem against Douglas Bag, five compared MedGem to predictive equations, four were evaluations of the BodyGem, four used the MedGem for assessments and two were review articles. The two hand-held calorimeters that are most widely used are the MedGem and BodyGem. Other portable devices include Fitmate™ (Cosmed, Pavona di Albano, Rome, Italy), VO2000 ™ (MedGraphics, St. Paul, Minnesota, USA) and Oxycon Mobile ™ (CareFusion, San Diego, California USA); however, the MedGem was not compared to these devices. Hand-held devices were compared to the following indirect calorimeters: Vmax Encore 29 (VIASYS Healthcare Inc., Yorba Linda, CA), Deltatrac II (VIAYSIS Healthcare Inc., SensorMedics, Yorba Linda, CA), Physiodyne (Physio-Dyne Instrument Corp., Quogue, NY) and the Douglas Bag method. Previous reviews of the literature10,11 focused on the MedGem and BodyGem devices and did not focus on the utility in a clinical setting. McDoniel10 determined that the hand-held device is valid and reliable for assessment of RMR. Van Loan11 determined that there is not enough evidence to support the use of a hand-held device for assessing RMR. This study focuses on usefulness of a hand-held calorimeter in an acute care setting. Our review of the published data showed that there were significant differences in the populations studied. However, since the studies reviewed measured REE using at least two instruments, the same subjects served both as the test and the control. Therefore, despite the heterogeneity of the populations the data remain robust and the interpretations are valid.
Inclusion criteria
All English-language published manuscripts, indexed journals comparing hand-held calorimeters to predictive equations, portable calorimeters and indirect calorimeters on human subjects.
Exclusion criteria
Articles/studies containing products no longer available, studies using the BodyGem (not FDA approved and does not display VO2) that is unlikely to be utilized in clinical practice, and articles that did not compare a hand-held calorimeter to another calorimeter device. Of the 23 published articles produced related to hand-held devices, ten were excluded from review based on the aforementioned criteria.
Results
The manufacturer's protocol prior to use of the MedGem recommends 10-15 minutes of rest, four hours fasting (water allowed, recommend no caffeine), four hours abstinence from exercise, and one hour abstinence from smoking or nicotine.9 There were some deviations from the MedGem manufacturer recommendations in several of the studies reviewed. All reviewed studies satisfied the following criteria: 3-12 hour fasting, 3-24 hour abstinence or light from exercise, rested for 5 - 30 minutes before the procedure.
Hand-held Device vs. Metabolic Cart
The MedGem device was compared directly against a metabolic cart in 9 studies.12-20 There was no published literature comparing the MedGem device with other types of portable indirect calorimeters (Fitmate, VO2000, and Oxycon Mobile). In the Cooper study21 there were 5 indirect calorimeters compared to the Deltatrac (VIASYS Healthcare Inc, SensorMedics, Yorba Linda, CA). The indirect calorimeters studied include the MedGraphics CPX Ultima (Medical Graphics Corp, St. Paul, MN), TrueOne 2400 (Parvo Medics, Sandy, UT), Vmax Encore 29 (VIASYS Healthcare Inc, Yorba Linda, CA), Korr Ree Vue (Korr Medical Technologies, Salt Lake City, UT), and the MedGem. In this multicenter study performed at 3 sites, RMR was measured for the MedGem and the Deltatrac at the University of Minnesota, St Paul. The results from the University of Minnesota for the MedGem were compared to measurements with the other devices at the other two sites: University of Wisconsin-Madison and Loyola University. MedGem was compared with the metabolic carts from the other sites for analysis of RMR.
Four studies12, 15, 20, 21 found the results obtained from the MedGem overestimated RMR compared to the metabolic cart. Alam et al12 did not find the MedGem to be valid and overestimated RMR compared to Deltatrac by 8-11%. However, the MedGem results were reproducible and the measured mean RQ per the Deltatrac was similar to the assumed RQ of the MedGem (Table 1). A paired t-test for the MedGem versus the Deltatrac in the Cooper study21 did not show a significant difference for RMR with a p-value of 0.32 for RMR in 16 subjects. However, a Bland-Altman analysis of the same data indicated a significant bias (p=0.02) with increasing RMR for the MedGem. Fares et al20 assessed the MedGem in older adults greater than 65 years of age and found that it overestimated RMR 30% higher on average than the Europa Gas Exchange Monitor (GEM; NutrEn Technology Ltd, Cheshire, UK). Hlynsky et al15 did not find the MedGem to be reliable when compared to Deltatrac in healthy or anorexia nervosa population. The correlation of RMR between the MedGem and Deltatrac for anorexia nervosa subjects was r= 0.6 and p = 0.04; control subjects r = 0.04 and p = 0.89.
Table 1. Resting Metabolic Rate and Respiratory Quotient.
| Author/year | Population/N | MG1 RQ2 | Measured mean RQ | RMR3 (REE) mean kcal/day | Primary outcome |
|---|---|---|---|---|---|
| Alam et al12 2005 | Healthy women n=37 | 0.85 | 0.83 | MG session 1=1390 MG session 2=1390 DTC4 session 1=1278 DTC session 2=1235 |
Reproducibility and validity for RMR of the MG using the DTC as a reference. |
| Compher et al13 2005 | Home nutrition support patients. n=24 | 0.85 | 0.77 | MG session 1=1302 MG session 2=1296 DTC (only 1 session)= 1446 |
Reproducibility of the MG in a clinical population compared with the DTC. |
| Cooper et at21 | Healthy Adults n=38 | 0.85 | N/A | N/A | MedGem was not valid compared to the DTC. |
| Fares et al20 2008 | Healthy adults. n=48 | 0.85 | N/A | MG =1489 GEM=1149 ** |
Validity and acceptability of MG for measurement of REE in older adults. |
| Fields et al14 2006 | Healthy children. n=100 | 0.85 | N/A | MG =1452 ** DTC =1349 |
Assess the validity of the MG in children. |
| Hlynsky et al15 2005 | Anorexia nervosa patients vs healthy adults n=27 | 0.85 | Anorexia=0.9 Healthy=0.78 |
MG anorexia =1243 MG healthy = 1398 DTC anorexia = 1369 DTC healthy = 1519 |
Compare the MG with the DTC to determine if the tests agree |
| Reeves, et al16 2004 | Cancer Patients vs healthy adults n=35 | 0.85 | Cancer=0.71 Healthy=0.72 |
MG cancer=1351 * MG healthy=1258 VMax6 cancer=1526 VMax healthy=1371 |
Compare measurements of REE using the MG device and VMax in pts with cancer vs. healthy subjects. |
| Rubenbauer et al17 2006 | Healthy adults. n=19 | 0.85 | REE=0.83 FEE7=0.87 AEE8=0.88 |
MG REE=1551 Physiodyne REE=1552 MG FEE=1825 Physiodyne FEE=1875 MG AEE=3489 Physiodyne AEE=3333 |
Determine how closely the MG compared with the Physiodyne in 3 different physiologic conditions. |
| Stewart et al18 2005 | Healthy adults n=50 | 0.85 | N/A | Mean difference in RMR=4.66±113.4 kcal/day | Compare simultaneous measurements of RMR with MG and DTC |
| St-Onge, et al19 2004 | Healthy adults n=15 | 0.85 | N/A Food quotient provided was similar to RQ of MG |
MG REE=1543 DTC REE=1546 MG MEE9=1619 DTC MEE=1678 MG PE EE10=1630 DTC PE EE=1697 |
Determine if the MG can measure post-prandial energy expenditure compared with DTC |
MG = MedGem
RQ = Respiratory quotient
RMR (REE) = Resting metabolic rate (Resting energy expenditure)
DTC = Deltatrac
GEM = Europa Gas Exchange monitor
VMax = Vmax Encore 29
FEE = Fed energy expenditure
AEE = Activity energy expenditure
MEE = Measured energy expenditure
PP EE = Post-prandial energy expenditure
p<0.05 only for MG vs Vmax in patients with cancer
p<0.001
Three studies17, 18, 19 indicated a significant agreement between the results from the MedGem compared that obtained using a metabolic cart. Rubenbauer et al17 compared the MedGem to the Physiodyne (Physio-Dyne Instrument Corp., Quogue, NY) in three physiologic states. They found strong agreement between the MedGem and Physiodyne for RMR with r = 0.80, p > 0.0001, fed energy expenditure (FEE) with r = 0.89 and p < 0.0001, and activity energy expenditure (AEE) with r = 0.75 and p < 0.0002. The RQ was similar for all three states compared to the assumed RQ of the MedGem. Stewart et al18 performed a simultaneous comparison of the MedGem under the hood of the Deltatrac. They found no significant difference between the MedGem and Deltatrac when subjects were studied under the same conditions for both devices with a high correlation between the results (r = 0.941, p < 0.01) for RMR. They found that the MedGem and Deltatrac differed by only 86.58±72.32 kcal/day. The RQ was not reported in this study. St. Onge et al19 found the MedGem is accurate and valid for assessing thermogenic effect of food (p<0.001) and no significant difference in RMR (p > 0.005) and post-prandial energy expenditure (PP EE) between the Deltatrac and MedGem (p = 0.07). St. Onge et al19 found the MedGem underestimated group means differences for RMR by 3.5% for total measured energy expenditure and 3.9% for PP EE.
Three studies reported mixed results.13, 14, 16 Compher et al13 found the RMR for the MedGem to be within an acceptable degree of reproducibility with a mean difference of -6.8 kcal and limits of agreement within the 250 kcal margin of error. They found that when the MedGem was compared to the Deltatrac the MedGem consistently had lower measures of RMR with a mean difference of -162 kcal/day with wide limits of agreement of 577 to -253 kcal/day. The measured RQ was lower than the assumed RQ for the MedGem (Table 1). Fields et al14 studied 100 children and determined that the MedGem is valid for children, but it statically overestimated the RMR compared to the Deltatrac (p<0.001) with a mean difference of 103 kcal/day. The authors stated that although the difference was significant, the results were of limited clinical utility. Reeves et al16 determined that there was poor clinical accuracy for the MedGem compared to the VMax in healthy subjects and patients with different forms of malignancy (p<0.05), neither individual nor overall group differences were observed. In addition, the RQ for VMax was considerably lower than the MedGem (Table 1). The MedGem was therefore found to generate data similar to those obtained from metabolic carts in healthy subjects.
Subject position may account for differences in the measured RMR.14, 18, 20 Fares et al20 and Fields et al14 allowed the subjects to remain in a seated position for the MedGem and supine for the metabolic cart. Fields et al14 did additional testing to determine if subject position had an impact on the study. They found the RMR for the seated position significantly higher (p<0.05). After accounting for the difference in position, they found the RMR to remain significantly higher than the Deltatrac (p<0.01).
Hand-held Device vs. Douglas Bag Method
Only one study22 was found that compared the MedGem to the Douglas Bag. Nieman et al22 found that the results obtained using the Douglas bag were similar to those obtained using the MedGem in children ages between 7 and 13 years old (r = 0.909, p=0.286).
Hand-held Device and Predictive Equations
Even though predictive equations and their limitations are well known to investigators and practioners of nutritional interventions, comparing these with the results obtained from hand-held calorimeters provides an objective rationale to determine the optimum method to quantify RMR.
The MedGem was compared to predictive equations as seen in Table 2. Three studies compared the MedGem with predictive equations and a traditional metabolic cart.16, 17, 19 Reeves et al16 found that the MedGem had poor correlation with the Harris-Benedict equation in patients with cancer (limits of agreement of -30 to 20%) as well as healthy subjects (limits of agreement -27 to 34%). Rubenbauer et al17 found the mean RMR calculated by Harris-Benedict equation was ∼3% greater than the measured RMR for the Physiodyne and MedGem. The calculated RMR for the Mifflin St. Joer equation was ∼1% less than the measured value by the devices (Table 2). The St Onge et al19 study indicated a good agreement between the metabolic cart and the predictive equations and found that the Harris-Benedict equation overestimated RMR by 11% (p=0.01) and FAO/WHO (Food and Agriculture Organization/World Health Organization) equations overestimated by 12% (p<0.01) compared to the MedGem.
Table 2.
Predictive equations for quantifying resting energy expenditure in the studies evaluated.
| Predictive Equation | Calculation |
|---|---|
| Harris-Benedict* using actual weight 16,17,19 | Harris Benedict* × actual weight |
| Harris-Benedict* using adjusted body weight 16 | {[(actual body weight-ideal body weight**).50]+ideal body weight**}+ Harris-Benedict* |
| Mifflin-St. Jeor equation 17 | Men: 5+10 × actual body weight (kg)+6.25 × height (cm)-5 × age Women: -161+10 × actual body weight (kg)+6.25 × height (cm)-5 × age |
| Food and Agriculture Organization 19 (FAO)/World Health Organization (WHO) equation for women | Age 18-30: 13.3 × weight (kg)+334 × height (m)+35 Age 31-60: 8.7 × weight (kg)-25 × height (m)+865 Age >60: 9.2 × weight (kg)+637 × height (m)+302 |
Harris-Benedict equation: Men: kcal/day=66+13.75 × body weight (kg)+5 × height (cm)-6.76 × age
Women: kcal/day=655+9.56 × body weight (kg) +1.85 × height (cm)-4.68 × age
Hamwi's calculation: Men: 106+ [height (inches)-60) × 6], Women: 100+[height (inches)-60)×5]
Discussion
All studies reviewed evaluated healthy free living subjects (n=307)12,14,17-21 except the studies that included patients with anorexia nervosa compared with healthy controls (n=27)15, cancer patients compared with healthy controls (n=35)16 and subjects receiving home nutrition support (n=24).13 Our review of the literature showed that studies using the MedGem in healthy subjects reported that physiologic responses such as anxiety, body position, physical activity or eating within 4 hours of testing and tolerance to the MedGem device may have impacted their observations. However, the potential impact of these on measured RMR was not part of the initial study design and therefore can not be obtained from these studies. Factors that may increase the measured RMR include studies performed in the seated position, anxiety, and physical activity or eating within 4 hours of testing. 23,24 Factors that may decrease the measurement of RMR include measurements in the supine position, undetected air leaks and the assumed RQ of the MedGem.23,24 There are currently no published data on the impact of physiologic changes on measured RMR using a hand-held calorimeter. Few studies have evaluated the MedGem device in diseased populations.
Subject anxiety has been suggested as a potential cause for differences in RMR23,24. Alam et al12 suggested that increase anxiety may contribute to the differences in RMR between the MedGem and the metabolic cart. Anxiety was decreased in subjects tested with the metabolic cart after the initial session and was attributed to familiarization with the procedure. The decrease in anxiety was not reported for measurements taken with the MedGem.12 Compher et al13 also noted a potential for anxiety to result in differences with the MedGem versus the Deltatrac, however; only one subject reported anxiety and was not considered to significantly impact the results of the study. The impact of tolerance to MedGem could also affect the results because previous studies have shown that the RMR variabilities influenced by mouthpiece and noseclip practice procedures.24
The potential for measuring the thermogenic effect of food may result in an elevated RMR if a minimum of 4 hours fasting was not followed. However, Neiman et al22 limited the subjects to only 3 hours of fasting and found no significant difference in RMR measured by the MedGem compared with the Douglas Bag method.
The assumed RQ for the MedGem of 0.85 was not consistent with the measured RQ reported in several studies (Table 1). The lower measured RQ by a traditional metabolic cart may account for the higher RMR compared to the MedGem results. In the studies where the measured RQ from the metabolic cart was <0.80, the MedGem significantly overestimates measured RMR when compared to the metabolic cart.13, 15, 16 Rubenbauer et al17 reported no significant difference in the measured RQ from the metabolic cart and the assumed RQ of the MedGem and no significant difference in the measured RMR (Table 1). Alam et al12 found that the MedGem overestimated the RMR compared to the Deltatrac with no significant difference in the mean RQ between the two methods.
The potential for an undetected air leak around the mouthpiece and nose clip causing the results of the RMR in the MedGem to be lower is difficult to assess. However, the amount of air leak necessary to trigger an error message is not known. The MedGem device will indicate an error message if an air leak is detected. There are no systematic published studies on the assessment of air leaks that go undetected which will result in a low RMR. In three studies12, 15, 20 the subjects reported discomfort in the use of the MedGem. Discomfort was indicated with the nose clips, dry throat, breathing difficulty and excess saliva. Reeves et al16 reported that the RMR measured by the VMax may be higher than the MedGem due to the mouthpiece used by the VMax had a greater diameter and may possibly cause an increase in minute ventilation.
Generally it takes less time to obtain a steady state with the MedGem as compared to a metabolic cart. The MedGem obtains a measurement of RMR within 10 minutes of initiation of the study. The traditional metabolic cart may take as long as 20 minutes to obtain a steady state. Although it took up to 20 minutes to obtain a steady state with the metabolic cart, Rubenbauer et al17 still found agreement between MedGem and the metabolic cart. However, other studies indicate that the time to obtain a steady state may have resulted in differences between the devices studies. 15, 16, 20
In the study by Stewart et al18, many of the aforementioned limitations were addressed. The MedGem was studied simultaneously with the metabolic cart by having the subject breathe through the MedGem device while under the hood of the metabolic cart. Since the two systems were tested at the same time with each subject, body position, manufacturer protocols and other variables that may impact the differences in RMR were eliminated. They found no significant difference between the MedGem and the Deltatrac for measured RMR.
In hospitalized patients RMR is frequently evaluated using predictive equations. In three16, 17, 19 studies the MedGem was compared to a metabolic cart and predictive equations (Table 2). All of the studies used the Harris-Benedict equation. Reeves et al16 concluded that REE measured by MedGem was similar than that calculated by Harris-Benedict.. St Onge et al19 showed the Harris-Benedict overestimated the RMR compared to the MedGem by 11%. Rubenbauer et al17 found that the Harris-Benedict equation overestimated the RMR by 3% while the Mifflin St. Joer equation overestimated the RMR by 1% compared to the MedGem. St Onge et al19 also compared the MedGem to the FAO/WHO equation and found it overestimated the RMR by 12% compared to the MedGem. No other study compared FAO/WHO to the MedGem and a metabolic cart.
MedGem was evaluated in a nutritionally compromised population in 3 studies.13,15,16 The RMR obtained by the MedGem was lower in two studies13,16 and higher in one study15 compared with that obtained from the metabolic cart. The Compher study13 included 18 free living subjects age 18-76 years on home parenteral nutrition with malabsorption diagnoses including short bowel syndrome, dysmotility, malabsorption and intestinal transplant. The subjects had RMR measurements with the Deltatrac for one reading and the MedGem for two readings. They found that the MedGem had clinically acceptable results, but values were routinely lower than those obtained with the metabolic cart (Table 1). Hlynsky et al15 studied the MedGem on 15 healthy subjects and 12 inpatients with anorexia nervosa; MedGem did not provide a valid measure of RMR when compared to the Deltatrac. Reeves et al16 utilized the device to measure RMR in 18 patients with cancer at a radiation oncology center and 17 healthy subjects. The end-point outcome in the Reeves study16 indicated RMR was lower for both healthy subjects and patients with cancer compared to the metabolic cart. RQ was only statistically significant in the patients with cancer when compared to the healthy subjects.
Conclusion
Our systematic review of published literature on the hand-held calorimeter device showed that it is a practical, and accurate device to quantify RMR in healthy subjects. However, there is limited data on the validity and utility for its use in the hospitalized patient. Due to cost and logistics, there is an absence of a readily available means to accurately measure RMR in the hospitalized patients. This has resulted in the development of over 200 predictive equations which may have lead to the lack of accuracy for determining the RMR using these equations.
Although some of the studies showed a statistically significant difference in RMR for the MedGem compared to the traditional metabolic cart, these may have limited biological and clinical significance. The mean caloric difference was less than 200 kcal/day in all but one study (Table 1).
The majority of publications that studied the use of a hand-held calorimeter compared to a traditional metabolic cart did not evaluate its use in a nutritionally compromised population. Only three out of 11 studies dealt with subjects that were nutritionally compromised.
A hand-held calorimeter is a viable, reproducible measure of RMR compared to predictive equations and metabolic carts. Recognized limitations include evaluation only in select populations and the assumption of a fixed RQ. Studies are needed to further assess the reliability and reproducibility of hand-held indirect calorimetry compared to metabolic carts across different populations.
Contributor Information
Peggy Hipskind, Email: hipskip@ccf.org, Digestive Disease Institute, Center for Human Nutrition, Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, Phone: 216-445-1144.
Cathy Glass, Email: glassc@ccf.org, Digestive Disease Institute, Center for Human Nutrition, Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, Phone: 216-445-1144.
Denise Charlton, Email: charltd@ccf.org, Digestive Disease Institute, Center for Human Nutrition, Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, Phone: 216-445-1144.
Diane Nowak, Email: nowakd@ccf.org, Digestive Disease Institute, Center for Human Nutrition, Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, Phone: 216-445-1144.
Srinivasan Dasarathy, Email: dasaras@ccf.org, Digestive Disease Institute, Gastroenterology and Hepatology, Cleveland Clinic, 9500 Euclid Ave., Cleveland, OH 44195, Phone: 216-444-2980.
References
- 1.Wooley JA, Frankenfield . Chapter 2: Energy (pp. 20-26) In: Gottschlich MM, editor. The ASPEN Nutrition Support Core Curriculum: A Case Based Approach-The Adult Patient. 1st. American Society for Parenteral and Enteral Nutrition; 2007. pp. 20–26. pp. 20-26. [Google Scholar]
- 2.Groff JL, Gropper SS, Hunt SM. Energy Balance and Weight Control. In: Purrington L, editor. Advanced Nutrition and Human Metabolism. 2nd. Minneapolis/St. Paul: West Publishing Company; 1995. pp. 466–483. [Google Scholar]
- 3.FAO Corporate Document Repository. Durnin JVGA: Basal Metabolic Rate in Man. Rome: Oct 5-17th, 1981. FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements. Joint. http://www.fao.org/docrep/meeting/004/m2845e/m2845e00.htm. --online source. [Google Scholar]
- 4.Consolazio CF, Johnson RE, Pecora LJ. Physiological Measurements of Metabolic Functions in Man. New York: McGraw-Hill Co; 1963. pp. 313–339. [Google Scholar]
- 5.De Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen HA, Chan LN, Li FL. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22:377–388. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham JJ. Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr. 1991;54:963–969. doi: 10.1093/ajcn/54.6.963. [DOI] [PubMed] [Google Scholar]
- 8.Bursztein S, Saphar P, Singer P, Elwyn DH. A mathematical analysis of indirect calorimetry measurements in acutely ill patients. Am J Clin Nutr. 1989;50:227–230. doi: 10.1093/ajcn/50.2.227. [DOI] [PubMed] [Google Scholar]
- 9.Microlife. MedGem device Step-by-Step Guide. Golden, CO: Microlife Medical Home Solutions, Inc; 2009. [Google Scholar]
- 10.McDoniel SO. A systematic review on use of a hand-held indirect calorimeter to assess energy needs in adults and children. Int J Sport Nutr Exerc Metab. 2007;17:491–500. doi: 10.1123/ijsnem.17.5.491. [DOI] [PubMed] [Google Scholar]
- 11.Van Loan MD. Do hand-held calorimeters provide reliable and accurate estimates of resting metabolic rate? J Am Coll Nutr. 2007;26:625–629. doi: 10.1080/07315724.2007.10719639. [DOI] [PubMed] [Google Scholar]
- 12.Alam DS, Hulshof PJM, Roordink D, et al. Validity and reproducibility of resting metabolic rate measurements in rural Bangladeshi women: comparison of measurements obtained by MedGem and by Deltatrac device. Eur J Clin Nutr. 2005;59:651–657. doi: 10.1038/sj.ejcn.1602122. [DOI] [PubMed] [Google Scholar]
- 13.Compher C, Hise M, Sternberg A, Kinosian BP. Comparison between MedGem and Deltatrac resting metabolic rate measurements. Eur J Clin Nutr. 2005;59:1136–1141. doi: 10.1038/sj.ejcn.1602223. [DOI] [PubMed] [Google Scholar]
- 14.Fields DA, Kearney JT, Copeland KC. MedGem hand-held indirect calorimeter is valid for resting energy expenditure measurements in healthy children. Obesity. 2006;14:1755–1761. doi: 10.1038/oby.2006.202. [DOI] [PubMed] [Google Scholar]
- 15.Hlynsky J, Birmingham CL, Johnston M, Gritzner S. The agreement between the MedGem indirect calorimeter and a standard indirect calorimeter in anorexia nervosa. Eat Weight Disord. 2005;10:e83–e87. doi: 10.1007/BF03327496. [DOI] [PubMed] [Google Scholar]
- 16.Reeves MM, Capra S, Bauer J, Davies PSW, Battistutta D. Clinical accuracy of the MedGem indirect calorimeter for measuring resting energy expenditure in cancer patients. Eur J Clin Nutr. 2005;59:603–610. doi: 10.1038/sj.ejcn.1602114. [DOI] [PubMed] [Google Scholar]
- 17.Rubenbauer JR, Johannsen DL, Baier SM, Litchfield R, Flakoll PJ. The use of hand-held calorimetry unit to estimate energy expenditure during different physiological conditions. JPEN J Parenter Enteral Nutr. 2006;30:246–250. doi: 10.1177/0148607106030003246. [DOI] [PubMed] [Google Scholar]
- 18.Stewart CL, Goody CM, Branson R. Comparison of two systems of measuring energy expenditure. JPEN J Parenter Enteral Nutr. 2005;29:212–217. doi: 10.1177/0148607105029003212. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge MP, Rubiano F, Jones A, Heymsfield SB. A new hand-held indirect calorimeter to measure postprandial energy expenditure. Obes Res. 2004;12:704–709. doi: 10.1038/oby.2004.82. [DOI] [PubMed] [Google Scholar]
- 20.Fares S, Miller MD, Masters S, Crotty M. Measuring energy expenditure in community-dwelling older adults: are portable methods valid and acceptable? J Am Diet Assoc. 2008;108:544–548. doi: 10.1016/j.jada.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JA, Watras AC, O'Brien MJ, et al. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109:128–132. doi: 10.1016/j.jada.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieman DC, Austin MD, Chilcote SM, Benezra L. Validation of a new hand-held device for measuring resting metabolic rate and oxygen consumption in children. Int J Sport Nutr Exerc Metab. 2005;15:186–194. doi: 10.1123/ijsnem.15.2.186. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt WD, O'Connor PJ, Cochrane JB, Cantwell M. Resting metabolic rate is influenced by anxiety in college men. J Appl Physiol. 1996;80:638–642. doi: 10.1152/jappl.1996.80.2.638. [DOI] [PubMed] [Google Scholar]
- 24.Scott CB. Resting metabolic rate variability as influenced by mouthpiece and noseclip practice procedures. J Burn Care Rehabil. 1993;14:573–577. [PubMed] [Google Scholar]