Abstract
This study explores whether inflammatory biomarkers act as moderators of clinical response to omega-3 (n-3) fatty acids in subjects with Major Depressive Disorder (MDD). 155 subjects with DSM-IV MDD, a baseline 17-item Hamilton Depression Rating Scale (HAM-D-17) score ≥ 15 and baseline biomarker data (IL-1ra, IL-6, hs-CRP, leptin, adiponectin), were randomized between 05/18/06 and 06/30/11, to 8 weeks of double-blind treatment with eicosapentaenoic acid (EPA)-enriched n-3 1060 mg/day, docosahexaenoic acid (DHA)-enriched n-3 900 mg/day, or placebo. Outcomes were determined using mixed model repeated measures (MMRM) analysis for “high” and “low” inflammation groups based on individual and combined biomarkers. Results are presented in terms of standardized treatment effect size (ES) for change in HAM-D-17 from baseline to treatment week 8. While overall treatment group differences were negligible (ES=−0.13 to +0.04), subjects with any “high” inflammation improved more on EPA than placebo (ES=−0.39) or DHA (ES=−0.60) and less on DHA than placebo (ES=+0.21); furthermore, EPA-placebo separation increased with increasing numbers of markers of high inflammation. Subjects randomized to EPA with “high” IL-1ra or hs-CRP or low adiponectin (“high” inflammation) had medium ES decreases in HAM-D-17 scores versus subjects “low” on these biomarkers. Subjects with “high” hs-CRP, IL-6 or leptin were less placebo-responsive than subjects with low levels of these biomarkers (medium to large ES differences). Employing multiple markers of inflammation facilitated identification of a more homogeneous cohort of subjects with MDD responding to EPA versus placebo in our cohort. Studies are needed to replicate and extend this proof of concept work.
Introduction
The heterogeneity of both symptoms and underlying pathophysiology confounds the development of targeted treatments for major depressive disorder (MDD).(1) Therefore, the discovery of biomarkers that characterize more homogeneous subgroups of patients with MDD is critical to our understanding of its pathogenesis and to the development of personalized therapies. (1, 2)
Chronic inflammation is involved in the etiology of heart disease, stroke, cancer, and diabetes (3, 4), and is thought to play a role in the etiology of MDD for some individuals. (5) Preclinical work has established that fatigue, anorexia, sleep disturbance, and anhedonia are part of the behavioral component of a systemic inflammatory response. (5) Inflammation may cause glucocorticoid insensitivity as well as shunting of tryptophan away from monoamine production and toward production of kynurenine and its metabolites (6, 7) thus decreasing the synthesis of monoamine neurotransmitters, while disrupting brain glutamatergic systems. (8–10) Epidemiological studies demonstrate that MDD is associated with a greater prevalence of elevated markers of inflammation. (11) Conversely, interferon-α (IFN-α) therapy-induced MDD can be successfully prevented by prophylactic antidepressant medications or pre-treatment with the omega-3 polyunsaturated fatty acid (n-3 PUFA) eicosapentaenoicacid (EPA).(12, 13) In a clinical trial of subjects with treatment resistant MDD, those with high sensitivity C-reactive protein (hs-CRP) levels > 5 had a positive response to therapy with infliximab, an anti-tumor necrosis factor-α (TNF-α) antibody. (14)
The epidemiological literature suggests that individuals who eat diets rich in n-3 PUFA have less cardiovascular disease and a decreased incidence of mood disorders. (15, 16) This led to the investigation of n-3 PUFA supplementation for a heterogeneous group of medical and psychiatric disorders. (17, 18) Clinical studies investigating the efficacy of EPA, docosahexaenoic acid (DHA) and a combination of EPA + DHA as augmenting agents suggest that EPA-enriched supplementation of antidepressant medications is associated with a greater improvement in depression ratings than placebo augmentation. (19–21) The few trials of EPA or DHA monotherapy for the treatment of MDD have found inconsistent benefits for n-3 therapy. (21–23) We completed the first double-blind randomized monotherapy trial of EPA versus DHA versus placebo treatment of MDD; the effect sizes (ES) were −0.179 for EPA versus placebo and −0.228 versus DHA, and +0.049 for DHA versus placebo (Mischoulon et al., 2014).(24) This finding agrees with the reviews and meta-analyses that suggest EPA or EPA + DHA (but not DHA alone) have a small ES advantage over placebo. (25–27) Additionally, in two independent re-analyses of the Bloch and Hannestad meta-analyses, Martins et al (2012) report an adjusted ES of 0.468 for studies with ≥ 60% EPA, while Lin et al (2012) reported an ES of 0.58 for these studies (28, 29). One postulate that reconciles the disparate data about n-3 therapy for MDD is that only a subset of patients with MDD benefit from n-3 treatment. EPA and its metabolites are important for an array of biological functions, including competing with the n-6 fatty acid metabolite arachidonic acid (AA) to shift synthesis away from inflammatory eicosanoids and toward the production of anti-inflammatory eicosanoids. (30, 31) Based on the evidence that some patients with MDD have increased inflammatory markers, and data suggesting that increasing n-3 intake shifts eicosanoid metabolism toward production of anti-inflammatory substances, we hypothesized that PUFA monotherapy would be more effective than placebo for patients with MDD who manifest elevated markers of inflammation. We further postulated that the response to EPA would be enhanced for a more homogenous subset of patients characterized by elevated inflammatory markers.
Materials and Methods
This collaborative R01 was based at Massachusetts General Hospital (MGH) and Cedars-Sinai Medical Center (CSMC), and approved by their Institutional Review Boards. All subjects reviewed and signed an informed consent form.
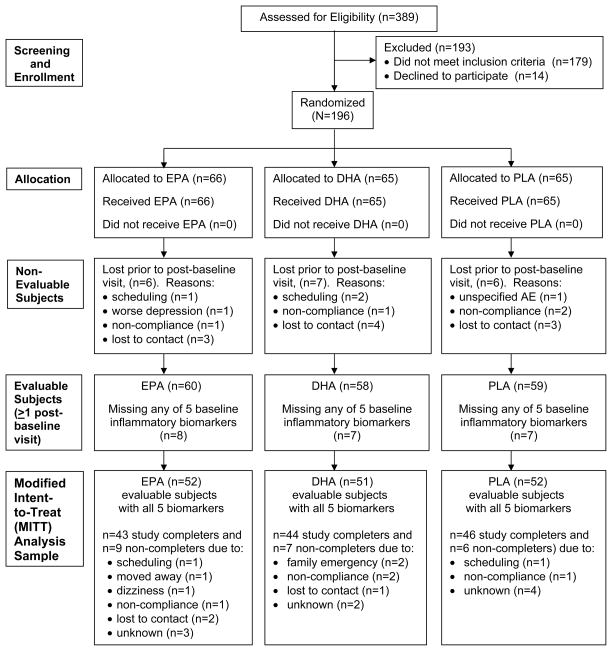
We recruited 389 outpatients with MDD, ages 18–80, from 05/18/06 to 06/30/11 through advertisements and outpatient referrals. Inclusion criteria were a diagnosis of MDD according to the Structured Clinical Interview for DSM-IV – Axis I Disorders – Patient Edition (SCID I/P)(32), a Clinical Global Impressions-Severity score ≥ 3, and a baseline HAM-D-17(33) score ≥ 15 (Figure 1).
Figure 1.
CONSORT Statement Flow Diagram.
Exclusion criteria included: pregnancy or women of child bearing potential who were not using contraception; suicidality; homicidality; unstable medical illness; current or past history of organic mental disorders, substance use disorders, psychotic disorders, or bipolar disorder; allergy to the study compounds; concurrent use of psychotropic medications, systematic corticosteroids, steroid antagonists, anticoagulants, or immunosuppressant agents; ECT during the current episode; any trial of ≥ 6 weeks with citalopram 40 mg/day or equivalent antidepressant during the current episode; history of n-3 PUFA supplement use; an average daily intake of ≥ 3.0 g of total n-3 between screening and baseline visit per the Food Processor 7.8 questionnaire (ESHA Research Inc, Salem, OR); psychotherapy; smoking > 10 cigarettes per day; vitamin E supplementation or regular non-steroidal anti-inflammatory use.
Subjects were randomized in a double-blind 1:1:1 manner to 2 capsules of EPA-enriched mix (ProEPAxtra, 530 mg EPA / 130 mg DHA per soft gel) and 2 placebo capsules, 4 capsules of DHA-enriched mix (ProDHA, 225 mg DHA / 45 mg EPA per soft gel), or 4 placebo (1000 mg soybean oil) capsules per day for 8 weeks (Nordic Naturals, Watsonville, CA).
Subjects were evaluated every 2 weeks for 8 weeks. The primary clinical outcome measure was decrease in the HAM-D-17 score.
The Baseline Body Mass Index (BMI) formula employed was: BMI = (pounds * 703.06942) / (inches2). We used the standard conventions for defining BMI categories: < 18.50 = underweight; 18.50 to < 25.00 = normal weight; 25.00 to < 30.00 = overweight; and ≥ 30.00 = obese.
Biological Measures and Assay Methodology
Blood samples for biomarkers were drawn at baseline. Plasma Interleukin (IL)-1ra concentrations were determined using an enzyme-linked immunosorbent assay (ELISA) from R&D Systems (Minneapolis, MN). Intra- and inter-assay coefficients of variation for the IL-1ra ELISAs were 3.9 and 5.1%, respectively. Plasma concentrations of IL-6 were measured using a high sensitivity ELISA from R&D Systems (Minneapolis, MN). Intra- and inter-assay coefficients of variation for the IL-6 ELISAs were 7.0 and 9.9%, respectively. Plasma hs-CRP concentrations were assessed using an immunoturbidimetric assay kit from Sekisui Diagnostics (Framingham, MA). Intra- and inter-assay coefficients of variation for the hs-CRP assays were 4.4 and 5.5%, respectively. Plasma concentrations of leptin and adiponectin were measured using separate ELISAs from R&D Systems (Minneapolis, MN). Intra- and inter-assay coefficients of variation for the leptin ELISAs were 6.6 and 8.9%, respectively, and 5.2 and 8.7%, respectively for the adiponectin ELISAs.
Statistical Analyses
This investigation of high inflammation as a moderator of efficacy of the primary efficacy outcome measure, HAM-D-17 score, was based on a modified intent-to-treat (MITT) sample of 155 evaluable subjects with data on all five inflammatory biomarkers at baseline and at least one post-baseline visit (for HAM-D-17). Baseline comparisons across treatment groups were made by ANOVA for continuous measures and chi-square tests for categorical variables. All 5 inflammatory biomarkers had highly skewed distributions, even after log transformation, so distributions of biomarker values are presented as quartile values and ranges. Spearman Rank correlations were used to describe their interrelationship.
Since conventions are not established for plasma levels of inflammatory biomarkers except hs-CRP, and values of all biomarkers were highly skewed, “high” levels of inflammation were defined based on inflection points in stem-and-leaf plots of baseline values for all subjects entering the study. Using this method, “high” inflammation was defined as >1.92 pg/ml for IL-6 and >500 pg/ml for IL-1ra, along with >3.0mg/l for hs-CRP which agrees with the CDC and American Heart Association convention. (34) “High” inflammation for cut points on leptin and adiponectin were based on separate stem-and-leaf plots for males and females, since leptin and adiponectin levels differ substantially by sex. (35, 36) The definition of “high” inflammation for leptin was ≥250 mg/l for females and ≥70 mg/l for males, while high inflammation on adiponectin (primarily a biomarker of anti-inflammatory activity) was <80 mg/l for females and <60 mg/l for males. In this paper, subjects in the “not high” group will be referred to as being “low”.
Mixed model repeated measures analysis (MMRM) was carried out to examine the effect of treatment group on changes in HAM-D-17 scores from baseline to treatment week 8. Models included subjects as a random effect and treatment group, treatment week, and their interaction as fixed effects. Baseline HAM-D-17 scores were included as a covariate. Since these analyses demonstrated similar results for DHA and placebo, we focused further analysis on inflammatory moderators of EPA versus placebo response. MMRM was used to test EPA versus placebo treatment effect based on each of the 5 biomarkers individually, and to examine whether the EPA versus placebo separation was increased by any combination of 2 markers. MMRM was performed to test “high” versus “low” levels of individual biomarkers, within EPA and placebo groups; to explore whether being “high” on particular biomarkers was responsible for EPA response or placebo non-response. An auto-regressive covariance structure was used for MMRM because it provided the best fit for the data. In light of the small numbers of cases available for comparisons of sub-groups, our outcome of interest was standardized treatment ES for change in HAM-D-17 from baseline to week 8 (defined as the difference in least-square mean change divided by the pooled standard deviation of change), rather than the significance of differences in slopes over the entire treatment period.
Treatment response was defined as an improvement of ≥ 50% in HAM-D-17 score from baseline and remission was defined as a HAM-D-17 score ≤ 7. Comparisons of response and remission rates at subjects’ last study visit were computed across treatment groups using an extension of Fisher’s Exact Test (37) for the MITT sample and for study completers.
All statistical analyses were carried out using SAS 8.2 software (SAS Institute Inc., 2001). A two-tailed alpha level of 0.05 was used to determine statistical significance, uncorrected for multiple comparisons as is appropriate for preliminary analyses (37). Analyses were performed based on blind treatment codes.
Results
Baseline characteristics of the sample are shown in Table 1. The three treatment groups did not differ on demographic variables, clinical characteristics, body mass index (BMI) or prevalence of high levels of inflammation except that there were more “high” IL-1ra subjects in the EPA group (Supplemental Table 1). There were no site differences. One third of each treatment group fell into the obese BMI category and another third into the overweight category, regardless of sex.
Table 1.
Baseline Demographic, Clinical, and Inflammatory Biomarker Characteristics for N=155 Evaluable Subjects with All Five Inflammatory Biomarkers at Baseline
| Demographic Characteristics | |||
| Study Site | Cedars-Sinai Medical Center | N (%) | 94 (60.6) |
| Massachusetts General Hosp. | N (%) | 61 (39.4) | |
| Age in Years (N=148) | Mean (sd) [Range] | 46.1 (12.6) [21 – 73] | |
| Sex | Female | N (%) | 91 (58.7) |
| Male | N (%) | 64 (41.3) | |
| Race | Caucasian | N (%) | 104 (67.1) |
| African American | N (%) | 29 (18.7) | |
| Other | N (%) | 13 (8.4) | |
| Prefer Not to Say | N (%) | 9 (5.8) | |
| Ethnicity (N=149) | Hispanic | N (%) | 23 (15.4) |
| Non-Hispanic | N (%) | 126 (84.6) | |
| Education (N=148) | High School or Less | N (%) | 39 (26.4) |
| Some College or More | N (%) | 109 (73.6) | |
| Marital Status (N=139) | Married or Living Together | N (%) | 27 (19.4) |
| Separated/Widowed/Divorced | N (%) | 47 (33.8) | |
| Never Married | N (%) | 65 (46.8) | |
| Employment Status (N=148) | Employed | N (%) | 72 (48.6) |
| Homemaker | N (%) | 8 (5.4) | |
| Student | N (%) | 9 (6.1) | |
| Other | N (%) | 59 (39.9) | |
|
| |||
| Clinical Characteristics | |||
| Hamilton Depression Rating Scale, 17-Item (HAM-D17) | Mean (sd) [Range] | 19.3 (3.1) [15 – 30] | |
| Comorbid Anxiety Disorder (N=148) | Current | N (%) | 40 (27.0) |
| Lifetime | N (%) | 47 (31.8) | |
| Body Mass Index (BMI)1 (N=144) | Underweight | N (%) | 6 (4.2) |
| Normal Weight | N (%) | 45 (31.2) | |
| Overweight | N (%) | 45 (31.2) | |
| Obese | N (%) | 48 (33.3) | |
| Distribution per Inflammatory Biomarker | Group | Median | Q1 – Q3 | [Range] | High Inflammation Definition |
|---|---|---|---|---|---|
| hs-CRP (mg/l) | Total | 1.3 | 0.4 – 2.9 | [0.1 – 28.2] | >3.02 |
| IL-6 (pg/ml) | Total | 1.3 | 0.8 – 2.0 | [0.4 – 9.7] | >1.923 |
| IL-1ra (pg/ml) | Total | 381.8 | 239.9 – 570.2 | [100.5 – 2599.0] | >5003 |
| Leptin4 (mg/l) | Female | 150.0 | 58.0 – 336.0 | [11.0 – 1187.0] | ≥2503 |
| Male | 37.0 | 20.5 – 72.5 | [2.0 – 229.0] | ≥703 | |
| Adiponectin4,5 (mg/l) | Female | 97.0 | 62.0 – 138.0 | [0.04 – 319.0] | <803 |
| Male | 59.0 | 32.5 – 93.0 | [0.04 – 220.0] | <603 |
| High Inflammation Status per Inflammatory Biomarker | Group | High Inflammation Rates by BMI Category1 | All Subjects6 N / N (%) |
||
|---|---|---|---|---|---|
| Underweight or Normal Weight N / N (%) |
Overweight N / N (%) |
Obese N / N (%) |
|||
| hs-CRP | Total | 6/51 (11.8) | 6/45 (13.3) | 23/48 (47.9) | 37/155 (23.9) |
| Female | 4/39 (10.3) | 3/18 (16.7) | 17/29 (58.6) | 25/91 (27.5) | |
| Male | 2/12 (16.7) | 3/27 (11.1) | 6/19 (31.6) | 12/64 (18.8) | |
| IL-6 | Total | 4/51 (7.8) | 7/45 (15.6) | 26/48 (54.2) | 39/155 (25.2) |
| Female | 3/39 (7.7) | 2/18 (11.1) | 21/29 (72.4) | 27/91 (29.7) | |
| Male | 1/12 (8.3) | 5/27 (18.5) | 5/19 (26.3) | 12/64 (18.8) | |
| IL-1ra | Total | 10/51 (19.6) | 10/45 (22.2) | 23/48 (47.9) | 47/155 (30.3) |
| Female | 4/39 (10.3) | 4/18 (22.2) | 17/29 (58.6) | 27/91 (29.7) | |
| Male | 6/12 (50.0) | 6/27 (22.2) | 6/19 (31.6) | 20/64 (31.2) | |
| Leptin | Total | 1/51 (2.0) | 7/45 (15.6) | 35/48 (72.9) | 46/155 (29.7) |
| Female | 1/39 (2.6) | 3/18 (16.7) | 23/29 (79.3) | 30/91 (33.3) | |
| Male | 0/12 (0.0) | 4/27 (14.8) | 12/19 (63.2) | 16/64 (25.0) | |
| Adiponectin | Total | 11/51 (21.6) | 20/45 (44.4) | 33/48 (68.8) | 67/155 (43.2) |
| Female | 7/39 (18.0) | 7/18 (38.9) | 19/29 (65.5) | 35/91 (38.5) | |
| Male | 4/12 (33.3) | 13/27 (48.2) | 14/19 (73.7) | 32/64 (50.0) | |
| Number of Biomarkers with High Inflammation | Group | High Inflammation Rates by BMI Category1 | All Subjects6 N / N (%) |
||
|---|---|---|---|---|---|
| Underweight or Normal Weight N / N (%) |
Overweight N / N (%) |
Obese N / N (%) |
|||
| 4 or 5 | Total | 0/51 (0.0) | 2/45 (4.4) | 18/48 (37.5) | 21/155 (13.5) |
| Female | 0/39 (0.0) | 0/18 (0.0) | 14/29 (48.3) | 15/91 (16.5) | |
| Male | 0/12 (0.0) | 2/27 (7.4) | 4/19 (21.0) | 6/64 (9.4) | |
| 2 or 3 | Total | 6/51 (11.8) | 9/45 (20.0) | 21/48 (43.8) | 38/155 (24.5) |
| Female | 3/39 (7.7) | 5/18 (27.8) | 11/29 (37.9) | 20/91 (22.0) | |
| Male | 3/12 (25.0) | 4/27 (14.8) | 10/19 (52.6) | 18/64 (28.1) | |
| 1 | Total | 18/51 (35.3) | 22/45 (48.9) | 5/48 (10.4) | 50/155 (32.3) |
| Female | 12/39 (30.8) | 8/18 (44.4) | 2/29 (6.9) | 24/91 (26.4) | |
| Male | 6/12 (50.0) | 14/27 (51.8) | 3/19 (15.8) | 26/64 (40.6) | |
| Any of the Above (1 or More) | Total | 24/51 (47.1) | 33/45 (73.3) | 44/48 (91.7) | 109/155 (70.3) |
| Female | 15/39 (38.5) | 13/18 (72.2) | 27/29 (93.1) | 59/91 (64.8) | |
| Male | 9/12 (75.0) | 20/27 (74.1) | 17/19 (89.5) | 50/64 (78.1) | |
| None | Total | 27/51 (52.9) | 12/45 (26.7) | 4/48 (8.3) | 46/155 (29.7) |
| Female | 24/39 (61.5) | 5/18 (27.8) | 2/29 (6.9) | 32/91 (35.2) | |
| Male | 3/12 (25.0) | 7/27 (25.9) | 2/19 (10.5) | 14/64 (21.9) | |
BMI was calculated as (pounds × 703.06942) / inches2. Resulting BMI <18.50 = underweight; 18.50 to <25.00 = normal weight; 25.00 to <30.00 = overweight; and ≥30 = obese. Due to the small number of underweight subjects (N=6), this group was combined with the normal weight BMI category for analyses. Height and weight data are missing for N=11 subjects (5 females and 6 males).
High inflammation status for hs-CRP was based on conventionally defined level (32).
High inflammation cut-points for biomarkers other than hs-CRP were based on inflection points in stem-and-leaf plots, such that ‘high’ inflammation included the skewed end of the distribution of values.
Levels of leptin and adiponectin vary greatly by sex, so distribution data are shown separately for females and males, and thresholds for defining high inflammation were determined separately by sex.
Low values on adiponectin indicate high inflammation.
All N=155 subjects with all 5 inflammatory biomarkers present at baseline, including 11 subjects missing BMI.
The prevalence of “high” inflammation in the total sample ranged from 24% for hs-CRP to 43% for adiponectin (Table 1), with rates varying by BMI and sex. In obese subjects, the prevalence of “high” inflammatory markers ranged from 48% for hs-CRP to 73% for leptin. Approximately 90% of obese subjects had at least one biomarker in the high range. Obese women had a greater prevalence of high hs-CRP, IL-6, and IL-1ra biomarkers than obese men, and were more than twice as likely to have 4–5 biomarkers of high inflammation than obese men. Spearman correlation scores among the biomarkers were higher for female than for male subjects (Supplemental Table 2).
Classifying the treatment sample by the number of “high” biomarkers of inflammation led to important observations about treatment response (Table 2). While overall treatment group differences for the entire sample were negligible (ES=−0.13 to +0.04), subjects with one or more “high” biomarker of inflammation improved more on EPA than placebo (ES=−0.39) or DHA (ES=−0.60) and less on DHA than placebo (ES=+0.21). Subjects randomized to EPA treatment with one or more “high” biomarkers consistently had a greater than 11-point decrease in HAM-D-17 scores by treatment week 8, while subjects randomized to placebo treatment were progressively less responsive as the number of high biomarkers of inflammation increased, resulting in an increasing EPA-placebo gradient of separation (from ES= −0.20 associated with 1 marker of “high” inflammation, to ES= −0.59 for 2–3 “high” markers, to ES= −1.10 for the subjects with 4–5 markers of “high” inflammation). Conversely, subjects without any “high” marker of inflammation were less responsive to EPA than to placebo or DHA (ES=+0.91). Most placebo-responding subjects fell into this low inflammation group (Supplemental Table 3). Remission and response rates were consistent with our continuous data: among subjects with 4–5 markers of “high” inflammation, remission rates were 40% for EPA, 14% for DHA, and 25% for placebo, while remission rates for subjects without any markers of “high” inflammation were: 19% for EPA, 43% for DHA, and 44% for placebo. Response rates followed a similar pattern. Inflammatory biomarkers did not consistently differentiate DHA from placebo in any analysis.
Table 2.
Change in HAM-D-17 Total Score from Baseline to Treatment Week 8 by Number of “High” Biomarkers at Baseline1
| Number of Biomarkers Reflecting ‘High” Inflammation | Change from Baseline to Treatment Week 8 | Standardized Treatment Effect Size3 at Treatment Week 8 | Paired Comparison of Groups at Treatment Week 8 | Significance of Treatment- by-Time Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| EPA | DHA | PLA | EPA vs. PLA | DHA vs. PLA | EPA vs. DHA | EPA vs. PLA | DHA vs. PLA | EPA vs. DHA | ||||||
| 4 or 5 Biomarkers (N=21) | LS-Mean (seM) [N] | −11.14 (1.79) [10] | −4.90 (2.17) [7] | −5.022 (2.52) [4] | ES (95% CI) | −1.11 (−2.35 to +0.13) | + 0.02 (−1.21 to +1.25) | −1.10 (−2.14 to −0.05) | t | −2.01 | +0.04 | −2.13 | F | 0.94 |
| df4 | 34.4 | 31.9 | 31.5 | df4 | 2, 79.8 | |||||||||
| P | 0.052 | 0.972 | 0.041 | P | P=0.396 | |||||||||
| 2 or 3 Biomarkers (N=38) | LS-Mean (seM) [N] | −12.38 (1.47) [13] | −11.52 (1.35) [13] | −9.43 (1.35) [12] | ES (95% CI) | −0.59 (−1.39 to +0.21) | − 0.44 (−1.23 to +0.36) | −0.17 (−0.94 to +0.60) | t | −1.48 | −1.09 | −0.42 | F | 0.70 |
| df4 | 82.2 | 82.2 | 82.1 | df | 2, 135 | |||||||||
| P | 0.142 | 0.279 | 0.672 | P | P=0.498 | |||||||||
| 1 Biomarker (N=50) | LS-Mean (seM) [N] | −11.76 (1.28) [13] | −7.31 (1.11) [17] | −10.80 (1.10) [20] | ES (95% CI) | −0.20 (−0.90 to +0.50) | + 0.73 (+0.06 to +1.40) | −0.97 (−1.73 to −0.20) | t | −0.57 | +2.23 | −2.62 | F | 1.20 |
| df | 122 | 123 | 120 | df | 2, 177 | |||||||||
| P | 0.569 | 0.027 | 0.010 | P | P=0.303 | |||||||||
| Any (1 to 5) Biomarkers (N=109) | LS-Mean (seM) [N] | −11.46 (0.82) [36] | −8.59 (0.77) [37] | −9.57 (0.80) [36] | ES (95% CI) | −0.39 (−0.86 to +0.08) | +0.21 (−0.25 to (+0.67) | −0.60 (−1.07 to −0.13) | t | 1.66 | 0.88 | 2.55 | F | 0.86 |
| df | 251 | 249 | 250 | df | 2, 405 | |||||||||
| P | 0.099 | 0.381 | 0.011 | P | P=0.423 | |||||||||
| No Biomarker (N=46) | LS-Mean (seM) [N] | −7.78 (0.85) [16] | −11.65 (0.96) [14] | −10.85 (0.83) [16] | ES (95% CI) | +0.91 (+0.18 to +1.64) | − 0.23 (−0.95 to +0.49) | + 1.11 (+0.33 to +1.88) | t | +2.60 | −0.63 | +3.03 | F | 4.09 |
| df | 215 | 215 | 215 | df | 2, 215 | |||||||||
| P | 0.010 | 0.528 | 0.003 | P | P=0.018 | |||||||||
|
| ||||||||||||||
| All Subjects with 5 Baseline Biomarkers (N=155) | LS-Mean (seM) [N] | −10.14 (0.57) [52] | −9.61 (0.57) [51] | −9.79 (0.55) [52] | ES (95% CI) | −0.09 (−0.47 to +0.30) | +0.04 (−0.34 to +0.43) | −0.13 (−0.52 to +0.26) | t | −0.44 | +0.22 | −0.65 | F | 0.17 |
| df | 716 | 716 | 716 | df | 2, 716 | |||||||||
| P | 0.661 | 0.823 | 0.513 | P | 0.840 | |||||||||
HAM-D-17 was administered at Baseline and at 2-week intervals during the 8-week study. Mixed Model Repeated Measures (MMRM) analyses were performed on change from Baseline to Week 8 for subsets of N=155 evaluable subjects with all 5 biomarkers present at baseline, testing the significance of effects of treatment, time, and treatment-by-time interaction, covarying for the Baseline HAM-D-17 score.
Change at 8 weeks is not significantly different from zero; all other means are significantly different from zero, at P<0.02 to P<0.0001.
By Cohen’s d effect size = (difference between LS-Mean change) / pooled sd for each pair of treatments (sd per group computed from se of LS-Mean from MMRM). A negative effect size indicates that the 1st group (in the comparison pair) improved more than the 2nd one (had a larger negative LS-mean change).
Degrees of freedom were determined using the Satterthwaite approximation method.
Table 3 indicates a benefit to employing a combination of biomarkers to define the “high” inflammation group. EPA versus placebo ESs for subjects with high inflammation on individual biomarkers ranged from −0.368 to −0.775. By contrast, EPA versus placebo ESs for subjects with “high” inflammatory status on 5 of the 10 possible pairs of biomarkers were −0.924 or higher. Three pairs (hs-CRP plus IL-6, hs-CRP plus adiponectin, and IL-6 plus leptin) had effect sizes between −1.297 and −1.718
Table 3.
Change in HAM-D-17 Total Score from Baseline to Treatment Week 8 for Subjects Treated with EPA vs. Placebo (PLA) with High Inflammation on Individual Biomarkers and Pairs of Biomarkers at Baseline1
| High Inflammatory Status on | Change from Baseline to Treatment Week 8 | Standardized Treatment Effect Size at Treatment Week 8 | EPA vs. PLA at Treatment Week 8 | Significance of Treatment-by-Time Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPA | PLA | |||||||||||||
| Individual Biomarkers: | LS-Mean | (seM) | [N] | LS-Mean | (seM) | [N] | ES2 | (95% CI) | t | df3 | P | F | df | P |
| hs-CRP | −12.44 | (1.54) | [15] | −7.99 | (1.86) | [8] | −0.78 | (−1.67 to +0.11) | −1.84 | 42.8 | 0.073 | 1.70 | 1, 90.6 | 0.195 |
| IL-6 | −9.78 | (1.44) | [15] | −7.84 | (1.40) | [12] | −0.37 | (−1.13 to +0.40) | −0.96 | 54.0 | 0.341 | 0.42 | 1, 104 | 0.518 |
| IL-1ra | −12.14 | (1.16) | [23] | −9.63 | (1.61) | [11] | −0.46 | (−1.18 to +0.27) | −1.26 | 72.1 | 0.213 | 0.31 | 1, 127 | 0.580 |
| Leptin | −8.99 | (1.29) | [19] | −5.61 | (1.46) | [13] | −0.62 | (−1.34 to +0.11) | −1.72 | 63.3 | 0.090 | 1.01 | 1, 101 | 0.318 |
| Adiponectin | −11.69 | (1.10) | [21] | −8.81 | (1.06) | [21] | −0.58 | (−1.20 to +0.04) | −1.88 | 94.9 | 0.063 | 1.69 | 1, 159 | 0.195 |
|
| ||||||||||||||
| 10 Pairs of Biomarkers: | ||||||||||||||
| hs-CRP + IL-6 | −12.00 | (1.68) | [10] | −4.44 | (2.30) | [4] | −1.47 | (−2.77 to −0.17) | −2.65 | 22.2 | 0.015 | 4.13 | 1, 51.9 | 0.047 |
| hs-CRP + IL-1ra | −12.74 | (1.60) | [13] | −7.05 | (3.66) | [2] | −0.99 | (−2.53 to +0.54) | −1.42 | 25.1 | 0.167 | 1.18 | 1, 54.2 | 0.281 |
| hs-CRP + Leptin | −10.56 | (1.91) | [12] | −6.30 | (2.52) | [5] | −0.67 | (−1.74 to +0.40) | −1.34 | 28.3 | 0.192 | 0.87 | 1, 64.6 | 0.354 |
| hs-CRP + Adip. | −12.12 | (1.70) | [9] | −3.56 | (2.33) | [4] | −1.72 | (−3.10 to −0.34) | −2.92 | 18.9 | 0.009 | 3.66 | 1, 47.5 | 0.062 |
| IL-6 + IL-1ra | −11.53 | (1.98) | [9] | −7.41 | (2.21) | [6] | −0.72 | (−1.79 to +0.35) | −1.35 | 23.7 | 0.189 | 0.68 | 1, 56.1 | 0.415 |
| IL-6 + Leptin | −10.15 | (1.63) | [12] | −3.15 | (1.96) | [6] | −1.30 | (−2.38 to −0.22) | −2.74 | 32.1 | 0.010 | 3.94 | 1, 65.3 | 0.051 |
| IL-6 + Adip. | −10.27 | (1.68) | [11] | −7.46 | (2.03) | [6] | −0.52 | (−1.53 to +0.49) | −1.06 | 27.7 | 0.300 | 0.28 | 1, 65.7 | 0.597 |
| IL-1ra + Leptin | −10.22 | (1.66) | [14] | −5.80 | (2.54) | [5] | −0.73 | (−1.78 to +0.32) | −1.43 | 32.1 | 0.162 | 0.60 | 1, 71.4 | 0.441 |
| IL-1ra + Adip. | −12.99 | (1.44) | [12] | −10.44 | (2.33) | [4] | −0.52 | (−1.67 to +0.63) | −0.92 | 28.1 | 0.367 | 0.00 | 1, 52.9 | 0.954 |
| Leptin + Adip. | −9.88 | (1.27) | [15] | −5.42 | (1.86) | [6] | −0.92 | (−1.92 to +0.07) | −1.99 | 38.2 | 0.054 | 1.22 | 1, 72.1 | 0.274 |
HAM-D-17 was administered at Baseline and at 2-week intervals during the 8-week study. Mixed Model Repeated Measures (MMRM) analyses were performed on change from Baseline to Week 8 for subsets of N=155 evaluable subjects with all 5 biomarkers present at baseline, testing the significance of effects of treatment, time, and treatment-by-time interaction, covarying for the Baseline HAM-D-17 score.
By Cohen’s d effect size = (difference between LS-Mean change) / pooled sd for each pair of treatments (sd per group computed from se of LS-Mean from MMRM). A negative effect size indicates that the EPA group improved more than the PLA group (had a larger negative LS-mean change).
Degrees of freedom were determined using the Satterthwaite approximation method.
Table 4 describes the impact of “high” versus “low” levels of individual inflammatory biomarkers on treatment response for the EPA and placebo groups. Subjects treated with EPA who were categorized as “high” on hs-CRP, IL-1ra or “low” on adiponectin demonstrated a moderate ES for differences in HAM-D-17 score improvement compared to subjects who were “low” on these biomarkers. For placebo-treated subjects (bottom of Table 4), being classified as “high” for hs-CRP or IL-6 (moderate ES difference) or leptin (large ES difference) was associated with a decreased response to placebo when compared to subjects who were classified as being low on hs-CRP, IL-6 or leptin.
Table 4.
Change in HAM-D-17 Score from Baseline to Treatment Week 8 by High vs. Low Inflammation Status on Each of Five Biomarkers at Baseline for Subjects Treated with EPA or Placebo, Based on Mixed Model Repeated Measures Analysis1
| Treatment Group and Inflammatory Marker | Change from Baseline to Treatment Week 8
|
High vs. Low Inflammation Effect Size2 at Treatment Week 8 (95% CI) | High vs. Low Inflammation at Treatment Week 8 | Significance of High/Low- by-Time Interaction | ||
|---|---|---|---|---|---|---|
| High Inflammation | Low Inflammation | |||||
| EPA: | ||||||
| hs-CRP | LS-Mean (SEM) [N] | −11.94 (1.37) [15] | −9.50 (0.78) [37] | −0.497 (−1.105 to +0.111) | t= −1.55 | F=2.04 |
| df=125 | df=1, 188 | |||||
| P=0.124 | P=0.155 | |||||
| IL-6 | LS-Mean (SEM) [N] | −9.90 (1.42) [15] | −10.17 (0.78) [37] | +0.04 (−0.556 to +0.644) | t= +0.17 | F=0.01 |
| df=127 | df=1, 189 | |||||
| P=0.869 | P=0.913 | |||||
| IL-1ra | LS-Mean (SEM) [N] | −11.96 (1.03) [23] | −8.72 (0.89) [29] | −0.667 (−1.230 to −0.104) | t= −2.39 | F=3.10 |
| df=123 | df=1, 188 | |||||
| P=0.018 | P=0.080 | |||||
| Leptin | LS-Mean (SEM) [N] | −9.28 (1.21) [19] | −10.50 (0.83) [33] | +0.246 (−0.320 to +0.813) | t= +0.82 | F=0.23 |
| df=121 | df=1, 187 | |||||
| P=0.412 | P=0.633 | |||||
| Adiponectin | LS-Mean (SEM) [N] | −11.93 (1.06) [21] | −8.86 (0.87) [31] | −0.633 (−1.201 to −0.065) | t= −2.24 | F=2.71 |
| df=125 | df=1, 189 | |||||
| P=0.027 | P=0.102 | |||||
|
| ||||||
| Placebo: | ||||||
| hs-CRP | LS-Mean (SEM) [N] | −8.04 (1.57) [8] | −10.18 (0.71) [44] | +0.458 (−0.301 to +1.217) | t= +1.25 | F=0.24 |
| df=122 | df=1, 185 | |||||
| P=0.215 | P=0.626 | |||||
| IL-6 | LS-Mean (SEM) [N] | −8.09 (1.32) [12] | −10.37 (0.74) [40] | +0.490 (−0.163 to +1.142) | t= +1.50 | F=0.91 |
| df=126 | df=1, 188 | |||||
| P=0.136 | P=0.342 | |||||
| IL-1ra | LS-Mean (SEM) [N] | −9.81 (1.41) [11] | −9.82 (0.74) [41] | +0.002 (−0.663 to +0.667) | t= +0.01 | F=0.03 |
| df=122 | df=1, 186 | |||||
| P=0.996 | P=0.865 | |||||
| Leptin | LS-Mean (SEM) [N] | −6.18 (1.30) [13] | −10.91 (0.72) [39] | +1.042 (+0.382 to +1.702) | t= +3.20 | F=4.71 |
| df=129 | df=1, 185 | |||||
| P=0.002 | P=0.031 | |||||
| Adiponectin | LS-Mean (SEM) [N] | −8.77 (1.01) [21] | −10.55 (0.85) [31] | +0.379 (−0.179 to +0.938) | t= +1.35 | F=1.13 |
| df=124 | df=1, 187 | |||||
| P=0.181 | P=0.289 | |||||
Measures (MMRM) analyses were performed on change from Baseline to Week 8 for subsets of N=155 evaluable subjects with all 5 biomarkers present at baseline, testing the significance of effects of high/low inflammatory status, time, and high/low-by-time interaction, covarying for the Baseline HAM-D-17 score.
By Cohen’s d effect size = (difference between LS-Mean change) / pooled sd for each pair of treatments (sd per group computed from se of LS-Mean from MMRM). A negative effect size indicates that the group with high inflammation improved more than the low inflammation group (had a larger negative LS-mean change).
Discussion
In this proof of concept study we identified an EPA-responsive subgroup of subjects with MDD, based on biomarkers of inflammation. Subjects identified as being “high” on any of the 5 biomarkers that we measured were more likely to respond to EPA than placebo (Table 3). Individuals categorized as high on two or more biomarkers of inflammation demonstrated even greater EPA-placebo separation of HAM-D-17 scores (Tables 2 and 3). To explore the reasons for this EPA-placebo separation we asked two questions: (1) Are elevations in specific biomarkers associated with a greater likelihood of response to EPA? (2) Are elevations in specific biomarkers associated with less response to placebo? We demonstrate that subjects categorized as “high” on IL-1ra or hs-CRP or “low” on adiponectin (which reflects high inflammation) have a greater decrease in HAM-D-17 scores in response to EPA than subjects categorized as having “low” inflammation on these biomarkers (ES: −0.667, −0.497, −0.633 respectively) (Table 4). Conversely, subjects categorized as “high” on hs-CRP, IL-6 or leptin were less responsive to placebo than subjects categorized as “low” on these biomarkers (ES: +0.458, +0.490, +1.042 respectively). The latter observation extends a secondary analysis by Raison. (14) Our preliminary findings suggest that employing an inflammatory biomarker panel in future studies might identify a more homogenous group of subjects responsive to EPA and less responsive to placebo.
Our findings could explain contradictory data about the efficacy of n-3 therapy for subjects with MDD. (25–27) If EPA supplementation only benefits MDD subjects with inflammation as part of their syndrome (38), then there can be only a small effect size improvement in depression ratings associated with EPA therapy for a heterogeneous cohort of subjects with MDD, since it is ineffective for the majority of subjects randomized to EPA treatment. Furthermore, if the antidepressant mechanism of action for EPA is complementary to traditional antidepressant therapy, this would explain why there is increased therapeutic response associated with the combination therapy of traditional antidepressants with EPA.
A possible explanation for the greater effectiveness of EPA over DHA for individuals with high inflammation may be that these 2 n-3’s differ in their influence on the inflammatory cascade. EPA, but not DHA, can decrease the production of arachidonic acid (AA) by inhibiting delta-5-desaturase activity, compete with AA as a substrate for phospholipase A2, and can be converted into anti-inflammatory prostaglandins and leukotrienes (39), thus explaining its potentially unique antidepressant properties when contrasted with DHA.
An intriguing finding was that EPA was less effective than placebo or DHA for subjects “low” on all five biomarkers (Table 2). This agrees with Raison who reported that subjects with “low” hs-CRP levels did worse with infliximab treatment than placebo. (14) We believe that these results support the proposition that anti-inflammatory therapy is only beneficial as a treatment for inflammation-driven MDD and is ineffective and potentially harmful for individuals whose MDD is due to a different physiological disturbance. Such a postulate explains the Warner-Schmidt report (40) that anti-inflammatory agents attenuated the biochemical and behavioral response to SSRIs in a mouse model of MDD and the behavioral response of SSRIs in humans. If one extends this postulate to antidepressant therapies in general, it explains why Gueorguieva et al (2011) observed that 24% of duloxetine-treated subjects became worse over the course of the duloxetine trials. (41) These data highlight the need for targeted therapies to avoid ineffective treatment and unnecessary exposure to side effects.
Subjects’ sex and weight strongly influenced biomarkers in this study. Since women have higher circulating levels of leptin and adiponectin than men, we analyzed our data separately for these biomarkers. (35, 36) Ninety-three percent of the obese women and 89.5% of the obese men had at least one high marker of inflammation, while 86% of the obese women and 74% of the obese men had 2 or more high markers of inflammation. (The adipokines were the most consistently positive markers of inflammation in this cohort.) Obese women were more likely to have 4–5 elevated markers of inflammation than obese men (Table 1). Less than a third of the obese men had elevations in hs-CRP, IL-6, or IL-1ra while approximately 60% or more of the obese women had elevations in these markers. Additionally, the five biomarkers were more highly intercorrelated for female subjects than for male subjects (Supplemental Table 2). However, men and women did not differ in treatment response patterns based on inflammatory biomarkers. Our findings are consistent with the literature suggesting that obesity is associated with chronic inflammation, and that a significant number of MDD patients with elevated biomarkers of inflammation are obese. (42–45) Future studies should explore the relationship between weight, sex, biomarkers of inflammation, and other variables of interest such as the HPA axis and gonadal hormones.
Our choice of IL-6, IL-1ra, and hs-CRP as biomarkers of inflammation was based on review of the psychiatric and autoimmune literature investigating inflammatory biomarkers, the stability of the biomarkers in plasma, and the availability of reliable assay systems. We measured adipokines because: (1) there is a relationship between inflammation, MDD and obesity, (2) leptin stimulates the production of CRP and CRP binds and modifies the actions of leptin (46), (3) leptin and adiponectin are complementary biomarkers of adipocyte function, and (4) at least with respect to metabolic function, adiponectin is a marker of anti-inflammatory activity. (35, 36) We thought it prudent to include at least one anti-inflammatory marker in our panel. Each of the inflammatory biomarkers in this study played a role in enhancing response to EPA and/or decreasing response to placebo. Further investigation is needed to elucidate the complementary effects of these and other inflammatory biomarkers.
As with any proof of concept study, there are limitations. Despite a sample size of 155 subjects, the number of subjects with high biomarkers of inflammation is relatively small for a 3-arm study. Our analysis of the relationship between the number and combinations of “high” inflammatory markers and treatment response is exploratory and needs replication. However, our results strongly suggest that it is important for psychiatry to go beyond measuring a single marker of inflammation and assuming it is sufficient for determining the presence of an inflammatory process. Another unique characteristic of this study is that we employed stem and leaf plots to determine cutoff points for categorizing subjects as “high” on an inflammatory marker. We did so because our biomarker data were not normally distributed and could not be transformed to meet assumptions for parametric analyses. Although one might criticize this approach as “arbitrary”, our cutoff point of >3 for hs-CRP determined by this method matches the established cutoff point for an elevated CRP by the CDC and American Heart Association. (34) Further investigations employing the cut points we have identified and similar assay methodology is warranted.
In conclusion, this proof of concept study employed biomarkers of inflammation to identify a subset of patients who were responsive to EPA monotherapy. We found that subjects with specific combinations of inflammatory markers were more likely to respond to EPA treatment and less likely to respond to placebo treatment. Our preliminary data agree with others who have suggested that obese subjects with MDD are more likely to have “high” inflammatory biomarkers. In future studies, we hope to replicate our preliminary findings and extend them by investigating the influence of other important biological measures (e.g., n-3/n-6 ratios, HPA and estrogen, and other inflammatory markers) and clinical characteristics (e.g., early life trauma and current life stress levels). Biologically classifying patients may pave the way for individualized depression treatment and may inform future study designs.
Supplementary Material
Acknowledgments
Study grant number grant 5R01MH74085.
Support and Acknowledgements
The study was funded by grant 5R01MH74085 from the National Institutes of Health to Drs. Mischoulon and Rapaport. EPA-enriched and DHA-enriched preparations and matching placebos were provided by Nordic Naturals (Watsonville, CA).
Footnotes
ClinicalTrials.gov Identifier is NCT00517036 (available at www.clinicaltrials.gov). Registered on Aug 4, 2006.
The NIMH and Nordic Naturals had no further role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication. This manuscript reflects the views of the authors.
The remaining authors report no conflicts of interest.
Supplementary information is available at Molecular Psychiatry’s website.
Conflict of Interest
Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Nordic Naturals, Methylation Sciences, Inc. (MSI), and PharmoRx. He has received honoraria for speaking from Pamlab, and the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for the book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
Dr. Nierenberg has served as a consultant to: Appliance Computing Inc. (Mindsite), Brain Cells, Inc., Brandeis University, Bristol Myers Squibb, Clintara, Dianippon Sumitomo (Now Sunovion), Eli Lilly and Company, EpiQ, Forest, Novartis, PamLabs, PGx Health, Shire, Schering-Plough, Sunovion, Takeda Pharmaceuticals, Teva, and Targacept. He has consulted through the MGH Clinical Trials Network and Institute (CTNI): Astra Zeneca, Brain Cells, Inc, Dianippon Sumitomo/Sepracor, Johnson and Johnson, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals. Dr. Nierenberg received honoraria or travel expenses including CME activities from: APSARD, Belvoir Publishing, Boston Center for the Arts, University of Texas Southwestern Dallas, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Bayamon Region Psychiatric Society, San Juan, PR, Baystate Medical Center, Canadian Psychiatric Association, Columbia University, Douglas Hospital/McGill University, IMEDEX, International Society for Bipolar Disorders, Israel Society for Biological Psychiatry, John Hopkins University, MJ Consulting, New York State, Massachusetts Association of College Counselors, Medscape, MBL Publishing, Physicians Postgraduate Press, Ryan Licht Sang Foundation, Slack Publishing, SUNY Buffalo, University of Florida, University of Miami, University of Wisconsin, University of Pisa, and SciMed. Dr. Nierenberg is a presenter for the Massachusetts General Hospital Psychiatry Academy (MGHPA). The education programs conducted by the MGHPA were supported through Independent Medical Education (IME) grants from the following pharmaceutical companies in 2008: Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals; in 2009 Astra Zeneca, Eli Lilly, and Bristol-Myers Squibb. No speaker bureaus or boards since 2003. Dr. Nierenberg owns stock options in Appliance Computing, Inc. and Brain Cells, Inc. Additional income is possible from Infomedic.com depending on overall revenues of the company but no revenue has been received to date. Through MGH, Dr. Nierenberg is named for copyrights to: the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery-Asberg Depression Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). Dr. Nierenberg has received grant/research support through MGH from AHRQ, Cephalon, Forest, Mylan, NIMH, PamLabs, Pfizer Pharmaceuticals, Takeda, Elan, and Shire.
Dr. Schettler works part-time both as Senior Research Associate in the Department of Psychiatry and Behavioral Sciences at the Emory University School of Medicine, Atlanta, Georgia; as well as Principal Statistician in the Department of Psychiatry of the School of Medicine at the University of California, San Diego. She has no other direct or indirect affiliations or financial interests in connection with the contents of this paper.
Dr. Rapaport has provided consulting services to PAX, Inc (unpaid) and has been funded by the NIH.
References
- 1.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011 Nov;36(12):2375–94. doi: 10.1038/npp.2011.151. Epub 2011/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother. 2012 Sep;12(9):1143–61. doi: 10.1586/ern.12.98. Epub 2012/10/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub RH. Evolutionary medicine and chronic inflammatory state--known and new concepts in pathophysiology. J Mol Med (Berl) 2012 May;90(5):523–34. doi: 10.1007/s00109-012-0861-8. Epub 2012/01/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutrition reviews. 2007 Dec;65(12 Pt 2):S253–9. doi: 10.1111/j.1753-4887.2007.tb00372.x. Epub 2008/02/05. eng. [DOI] [PubMed] [Google Scholar]
- 5.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009 May 1;65(9):732–41. doi: 10.1016/j.biopsych.2008.11.029. Epub 2009/01/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes M, Ruckoanich P, Chang YS, Mahanonda N, Berk M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Apr 29;35(3):769–83. doi: 10.1016/j.pnpbp.2010.06.008. Epub 2010/06/22. eng. [DOI] [PubMed] [Google Scholar]
- 7.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology & therapeutics. 2011 May;130(2):226–38. doi: 10.1016/j.pharmthera.2011.01.014. Epub 2011/02/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AH. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology. 2013 Aug;38(9):1607–8. doi: 10.1038/npp.2013.140. Epub 2013/07/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Molecular psychiatry. 2007 Nov;12(11):988–1000. doi: 10.1038/sj.mp.4002006. Epub 2007/04/26. eng. [DOI] [PubMed] [Google Scholar]
- 10.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. Epub 2011/08/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper WC, et al. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosomatic medicine. 2011 Jul-Aug;73(6):462–8. doi: 10.1097/PSY.0b013e318222379c. Epub 2011/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, et al. Omega-3 Fatty Acids in the Prevention of Interferon-Alpha-Induced Depression: Results from a Randomized, Controlled Trial. Biol Psychiatry. 2014 Oct 1;76(7):559–66. doi: 10.1016/j.biopsych.2014.01.008. Epub 2014/03/08. Eng. [DOI] [PubMed] [Google Scholar]
- 13.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. The New England journal of medicine. 2001 Mar 29;344(13):961–6. doi: 10.1056/NEJM200103293441303. Epub 2001/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 14.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013 Jan;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. Epub 2012/09/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971 Jun 5;1(7710):1143–5. doi: 10.1016/s0140-6736(71)91658-8. Epub 1971/06/05. eng. [DOI] [PubMed] [Google Scholar]
- 16.Hibbeln JR. Fish consumption and major depression. Lancet. 1998 Apr 18;351(9110):1213. doi: 10.1016/S0140-6736(05)79168-6. Epub 1998/06/27. eng. [DOI] [PubMed] [Google Scholar]
- 17.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012 Sep 12;308(10):1024–33. doi: 10.1001/2012.jama.11374. Epub 2012/09/13. eng. [DOI] [PubMed] [Google Scholar]
- 18.Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacology & therapeutics. 2014 Mar;141(3):272–82. doi: 10.1016/j.pharmthera.2013.10.010. Epub 2013/11/10. eng. [DOI] [PubMed] [Google Scholar]
- 19.Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012 Feb;32(1):61–4. doi: 10.1097/JCP.0b013e31823f3b5f. Epub 2011/12/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. The Australian and New Zealand journal of psychiatry. 2008 Mar;42(3):192–8. doi: 10.1080/00048670701827275. Epub 2008/02/06. eng. [DOI] [PubMed] [Google Scholar]
- 21.Lesperance F, Frasure-Smith N, St-Andre E, Turecki G, Lesperance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011 Aug;72(8):1054–62. doi: 10.4088/JCP.10m05966blu. Epub 2010/06/30. eng. [DOI] [PubMed] [Google Scholar]
- 22.Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu SL, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008 Sep;18(9):639–45. doi: 10.1016/j.euroneuro.2008.04.011. Epub 2008/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 23.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003 May;160(5):996–8. doi: 10.1176/appi.ajp.160.5.996. Epub 2003/05/03. eng. [DOI] [PubMed] [Google Scholar]
- 24.Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry. 2014 Sep 16; doi: 10.4088/JCP.14m08986. Epub 2014/10/02. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Molecular psychiatry. 2012 Dec;17(12):1272–82. doi: 10.1038/mp.2011.100. Epub 2011/09/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007 Jul;68(7):1056–61. doi: 10.4088/jcp.v68n0712. Epub 2007/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 27.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011 Dec;72(12):1577–84. doi: 10.4088/JCP.10m06634. Epub 2011/09/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Molecular psychiatry. 2012 Dec;17(12):1161–3. doi: 10.1038/mp.2012.111. author reply 3–7. Epub 2012/07/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Molecular psychiatry. 2012 Dec;17(12):1144–9. doi: 10.1038/mp.2012.25. discussion 63–7. Epub 2012/04/11. eng. [DOI] [PubMed] [Google Scholar]
- 30.Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins, leukotrienes, and essential fatty acids. 2013 Nov-Dec;89(6):379–90. doi: 10.1016/j.plefa.2013.09.011. Epub 2013/11/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins, leukotrienes, and essential fatty acids. 2014 Jan;90(1):1–4. doi: 10.1016/j.plefa.2013.11.002. Epub 2013/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition (SCID-I/P, Version 2.0) Vol. 1995 Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 33.Hamilton M. Development of a rating scale for primary depressive illness. The British journal of social and clinical psychology. 1967 Dec;6(4):278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. Epub 1967/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 34.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. Epub 2003/01/29. eng. [DOI] [PubMed] [Google Scholar]
- 35.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013 Oct;64(1):1–10. doi: 10.1016/j.cyto.2013.06.317. Epub 2013/07/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marques-Vidal P, Schmid R, Bochud M, Bastardot F, von Kanel R, Paccaud F, et al. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PloS one. 2012;7(12):e51768. doi: 10.1371/journal.pone.0051768. Epub 2012/12/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951 Jun;38(1–2):141–9. Epub 1951/06/01. eng. [PubMed] [Google Scholar]
- 38.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA psychiatry. 2014 Oct 15; doi: 10.1001/jamapsychiatry.2014.1611. Epub 2014/10/17. Eng. [DOI] [PubMed] [Google Scholar]
- 39.Rakel DP. The anti-inflammatory diet. In: DR, editor. Integrative Medicine. 1. Philadelphia: Saunders; 2003. [Google Scholar]
- 40.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011 May 31;108(22):9262–7. doi: 10.1073/pnas.1104836108. Epub 2011/04/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gueorguieva R, Mallinckrodt C, Krystal JH. Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Arch Gen Psychiatry. 2011 Dec;68(12):1227–37. doi: 10.1001/archgenpsychiatry.2011.132. Epub 2011/12/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008 Nov 15;64(10):896–900. doi: 10.1016/j.biopsych.2008.05.019. Epub 2008/07/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine. 2009 Feb;71(2):171–86. doi: 10.1097/PSY.0b013e3181907c1b. Epub 2009/02/04. eng. [DOI] [PubMed] [Google Scholar]
- 44.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Aug 1;45:92–9. doi: 10.1016/j.pnpbp.2013.05.005. Epub 2013/05/21. eng. [DOI] [PubMed] [Google Scholar]
- 45.Zeugmann S, Quante A, Heuser I, Schwarzer R, Anghelescu I. Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. J Clin Psychiatry. 2010 Aug;71(8):1007–16. doi: 10.4088/JCP.08m04767blu. Epub 2010/02/17. eng. [DOI] [PubMed] [Google Scholar]
- 46.Hribal ML, Fiorentino TV, Sesti G. Role of C Reactive Protein (CRP) in Leptin Resistance. Current pharmaceutical design. 2014;20(4):609–15. doi: 10.2174/13816128113199990016. Epub 2013/05/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.