Abstract
Binge drinking is common during adolescence and can lead to the development of psychiatric disorders, including alcoholism in adulthood. Here, the role and persistent effects of histone modifications during adolescent intermittent ethanol (AIE) exposure in the development of anxiety and alcoholism in adulthood were investigated. Rats received intermittent ethanol exposure during post-natal days 28-41, and anxiety-like behaviors were measured after 1 and 24 hrs of the last AIE. The effects of AIE on anxiety-like and alcohol-drinking behaviors in adulthood were measured with or without treatment with the histone deacetylase (HDAC) inhibitor, trichostatin A (TSA). Amygdaloid brain regions were collected to measure HDAC activity, global and gene-specific histone H3 acetylation, expression of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein and dendritic spine density (DSD). Adolescent rats displayed anxiety-like behaviors after 24 hrs, but not 1 hr, of last AIE with a concomitant increase in nuclear and cytosolic amygdaloid HDAC activity and HDAC2 and HDAC4 levels leading to deficits in histone (H3-K9) acetylation in the central (CeA) and medial (MeA), but not in basolateral nucleus of amygdala (BLA). Interestingly, some of AIE-induced epigenetic changes such as, increased nuclear HDAC activity, HDAC2 expression, decreased global histone acetylation persisted in adulthood. In addition, AIE decreased BDNF exon I, IV and Arc promoter specific histone H3 acetylation that was associated with decreased BDNF, Arc expression and DSD in the CeA and MeA during adulthood. AIE also induced anxiety-like behaviors and enhanced ethanol intake in adulthood, which was attenuated by TSA treatment via normalization of deficits in histone H3 acetylation of BDNF and Arc genes. These novel results indicate that AIE induces long-lasting effects on histone modifications and deficits in synaptic events in the amygdala, which are associated with anxiety-like and alcohol drinking behaviors in adulthood.
Keywords: Amygdala, Anxiety, Adolescent Binge Drinking, Alcohol Intake, Epigenetic, HDAC2, Histone Acetylation
Introduction
Binge drinking is prevalent during adolescence (Donovan, 2004; Matthews, 2010; O'malley et al., 1998; Wechsler et al., 2000). Both clinical and preclinical studies have shown that alcohol use during adolescence leads to a greater risk for developing alcoholism and other psychiatric disorders in adulthood (DeWit et al., 2000; Guerri and Pascual, 2010; Grant and Dawson, 1997). Adolescence is an important developmental period during which the brain undergoes maturation including changes in neurotransmission, gene expression, and synaptic remodeling, specifically, the formation and pruning of axons, dendrites, and synapses in various brain regions (Craiu, 2013; Fumagalli et al., 2007; Tau and Peterson, 2010; Spear, 2013). In general, the central (CeA), medial (MeA), and basolateral (BLA) nuclei of the amygdala have been shown to be involved in the processes that regulate emotion, anxiety, and alcoholism (Koob and Volkow, 2010; LeDoux, 2000; Pandey et al., 2006; Whalen et al., 2001). One major hypothesis of alcohol dependence involves alterations in the allostatic state driven by negative emotional adaptations within the circuitry of the amygdala that is characterized as the “dark side of addiction” (Koob, 2013; 2003; Koob and Volkow, 2010).
Epigenetic processes, such as histone acetylation and DNA methylation mechanisms, have been shown to play a role in neuromaturation by contributing to the stability of gene expression during brain development (Mehler, 2008; MacDonald and Roskams, 2008; Murgatroyd and Spengler, 2011; Szyf, 2013; Witt 2010). Well-studied epigenetic mechanisms include histone modifications via lysine acetylation where histone deacetylases (HDACs) remove acetyl groups from histones, leading to a condensed chromatin state, blocking transcriptional activator access to DNA and thereby decreasing gene transcription (de Ruijter et al., 2003; Feng and Fan, 2009; Gräff and Tsai, 2013; Jenuwein and Allis, 2001; Thiagalingam et al., 2003). Several genes, such as brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein are involved in regulating dendritic spine density (DSD) in the brain and are epigenetically controlled by histone acetylation (Bramham and Messaoudi, 2005; Maddox et al., 2013; Moonat et al., 2013; Tsankova et al., 2004). We recently reported that higher expression of HDAC2, but not HDAC1, 3, 4, or 5 isoforms regulate deficits in histone H3-K9 acetylation, BDNF and Arc expression and DSD in the CeA and MeA of alcohol preferring (P) compared with alcohol non-preferring (NP) adult rats, and HDAC2 in the CeA is mechanistically involved in anxiety-like and alcohol-drinking behaviors of P rats (Moonat et al., 2013). Additionally, withdrawal after chronic ethanol exposure was associated with reduced histone (H3&H4) acetylation, reduced expression of BDNF and Arc and decreased DSD in the CeA and MeA as well as upregulation of HDAC activity in the amygdala of adult Sprague-Dawley (SD) rats (Pandey et al., 2008a; You et al., 2014). Blocking up-regulation of HDAC activity with trichostatin A (TSA) treatment during ethanol withdrawal attenuated anxiety-like behaviors and restored deficits in histone acetylation (Pandey et al., 2008a). These results suggest that amygdaloid HDAC-induced chromatin remodeling may be involved in the process of alcohol preference and dependence in adult rats.
It has been shown that epigenetic modifications in the developing brain, such as histone modifications and DNA methylation due to exposure to environmental stimuli or early life adversity, may lead to both short-and long-term changes in synaptic plasticity that affect behaviors in adulthood (Fagiolini et al., 2009; Morris et al., 2010; Roth et al., 2009; Szyf et al., 2008). Currently, it is unknown how epigenetic factors interact with early life alcohol exposure to shape emotional brain circuitries during development and their role in anxiety and alcoholism in adulthood. We therefore investigated effects of adolescent intermittent ethanol (AIE) treatment on HDAC-mediated histone deacetylation in the amygdala and anxiety-like behaviors in adolescent rats, and if AIE-induced histone modifications can persist in adulthood and coordinate shifts in synaptic plasticity-associated gene expression in the amygdala and regulate anxiety-like and alcohol-drinking behaviors. Furthermore, we investigated the effects of TSA on the reversal of AIE-induced behavioral phenotypes and changes in gene-specific histone H3 acetylation in the amygdala during adulthood.
Materials and Methods
Animals and intermittent ethanol exposure
Pregnant female Sprague Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN, USA) and maintained in 12:12 hr light/dark cycle with ad libitum access to water and food. All animal experimental procedures followed the NIH guidelines for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee. Male pups were weaned at the post-natal day (PND) 21 from dams and were group-housed (2 or 3 rats) in cages at the same animal facility with ad libitum access to water and food. For the adolescent intermittent ethanol (AIE) or normal saline (AIS) exposure, adolescent male rats received eight intraperitoneal (IP) injections of either ethanol (2 g/kg, 20% w/v) or equivalent volume of normal saline during PND 28-41 on a 2-days-on and 2-days-off basis. This paradigm of ethanol exposure has been used by other investigators as well (Alaux-Cantin et al., 2013; Pascual et al., 2009) and we found that 2g/kg ethanol dose is non-sedative and produced anxiolysis in adolescent rats (Sakharkar et al., 2014a).
Adolescent intermittent ethanol and anxiety-like behaviors during adolescence
A cohort of the adolescent male rats treated with AIS or AIE exposure, as described above, were subjected to behavioral measurements during the light phase of light/dark cycle using light/dark box exploration (LDB) and elevated plus-maze (EPM) tests of anxiety measurements as described below at 1 hr (AIE) or 24 hr withdrawal (AIW group) after the last injections along with control rats (AIS group). A separate cohort of control rats was tested with the 1hr and 24hr withdrawal AIE group, however we observed no significant differences in anxiety measures between these two control groups (data not shown) and therefore have merged the data together and presented it as one control group. Under anesthesia (pentobarbital 50 mg/kg), rats were decapitated and brain tissues were dissected and quickly frozen. Some rats were perfused with normal saline followed by 4% paraformaldehyde (PFA) solution prepared in phosphate buffer (pH 7.4); brains were isolated and post-fixed overnight in PFA and soaked in graded sucrose solution (10%, 20% and 30%). All brains were frozen and kept at −80 °C until further use. Blood was also collected for the measurement of ethanol levels using the Analox alcohol analyzer (Analox Instruments, Lunenburg, MA) as reported earlier by our laboratory (Sakharkar et al., 2014a).
Adolescent intermittent ethanol and anxiety-like behaviors in adulthood
To examine the effect of AIE exposure in adulthood, groups of AIE and AIS adolescent male rats were allowed to mature until adulthood (PND 92 days). For baseline comparisons between the AIS and AIE group, animals were subjected to either LDB or EPM behavioral tests on PND 92 followed by collection of brain tissue after decapitation under anesthesia for biochemical studies, or perfusion with 4% PFA for the collection of fixed brains and processed for histochemistry, as described above.
Trichostatin A treatment and anxiety-like behaviors and gene-specific histone acetylation in AIS and AIE adult Rats
For examination of the effect of trichostatin A (TSA) on anxiety-like behaviors, AIS or AIE adult (PND 92) rats received either vehicle (AIS+Vehicle and AIE+Vehicle) or TSA (AIS+TSA or AIE+TSA) treatment. TSA was prepared, as our laboratory has previously described (Pandey et al., 2008a; Sakharkar et al., 2012; You et al., 2014). TSA (2 mg/kg) or vehicle (DMSO in PBS, 1:5 dilution; 2 ml/kg) was injected (i.p.) once daily for three consecutive days (each injection 24 hrs apart) and rats were subjected to behavioral measurements two hours after the last TSA or vehicle injection using LDB and EPM exploration tests. Immediately after behavioral tests, amygdaloid brain tissues were collected after decapitation under anesthesia for chromatin immunoprecipitation (ChIP) studies which are described below.
Elevated plus maze exploration test
Anxiety-like behavior was examined using the EPM test, as previously described by our and other laboratories (File, 1993; Pandey et al., 2008a; 2006; Sakharkar et al., 2012). The results were calculated as mean (±SEM) percent open arm entries and mean (±SEM) time spent on open arms. Mean (± SEM) number of closed arm entries are represented as general activity of rats (File, 1993).
Light /dark box exploration test
The LDB exploration test was employed for the measurement of anxiety-like behaviors as our laboratory has previously described (Pandey et al., 2008a; Sakharkar et al., 2014b; 2012). Data is presented in terms of percent time spent (mean± SEM) in light and dark compartment and total ambulation (mean± SEM) as the general activity of the rat.
Measurement of HDAC activity in the amygdala
HDAC activity in amygdaloid tissues was measured, as our laboratory has previously described (Pandey et al., 2008a; Sakharkar et al., 2014b; 2012). The optical density (O.D.) /mg of protein were calculated and the results are presented as the mean (±SEM) percent of controls.
Gold Immunolabeling for HDACs, acetylated histones and BDNF and Arc in the amygdala
Gold immunolabeling procedure was employed for the measurement of HDAC2, HDAC4, acetylated histone H3-K9, Arc and BDNF protein levels in the amygdaloid brain structures as our laboratory has previously described (Pandey et al., 2008a; Moonat et al., 2013; Sakharkar et al., 2014b). Briefly, brains were cut into 20 μm thick coronal sections and immunostained using antibodies against HDAC2 (1:200 dilution) (MBL International, Woburn, MA), HDAC4 (1:200 dilution) (MBL International), acetylated histones H3-K9 (1:500 dilution) (Millipore, Billerica, MA), Arc (1:200 dilution) and BDNF (1:200 dilution) (Santa Cruz Biotechnology Santa Cruz, CA). Immunogold particles were quantified using the computerized Image Analyzer connected to a light microscope. The threshold of the non-immunostained area in the amygdaloid region was set to zero. After the threshold determination, the software counted the number of immunogold particles/100 μm2 area within three object areas from each of three brain sections of each rat at high magnification (100X). The object fields for each brain area were consistent across the groups and sections were bregma matched. The values were averaged for each rat. The results are represented as number of immunogold particles per 100μm2 area. Two individuals independently observed the slides, however the individual who analyzed histochemical images was not blind to the experimental groups.
In situ Reverse transcription (RT)-PCR for mRNA expression in the amygdala
In situ RT-PCR was performed in 40 μm thick coronal brain sections, as our laboratory has previously described (Pandey et al., 2008a; Sakharkar et al., 2012; Moonat et al., 2013; 2011) for the mRNA measurements of Arc, BDNF I, IV exons, and HDAC2 using the primers (Arc: Forward-5’-ACAGAGGATGAGACTGAGGCAC-3’ and Reverse-5’-TATTCAGGCTGGGTCCTGTCAC-3’; BDNF exon I: Forward-5’-AGGACAGCAAAGCCACAATGTTCC-3’ and Reverse-5’-TGGACGTTTGCTTCTTTCATGGGC-3’; and BDNF exon IV: Forward-5’-TCTCACTGAAGGCGTGCGAGTATT-3’ and Reverse-5’-TGGTGGCCGATATGTACTCCTGTT-3’; and HDAC2: Forward, 5’-CGGTGGCTCAGTTGCTGGGG-3’ and Reverse, 5’-GGCCTCTGACTTCTTGGCGTGG-3’) and digoxigenin (DIG)-11-dUTP (Roche Diagnostics, Indianapolis, IN) instead of dTTP. These primers were synthesized by Integrated DNA Technologies (Coralville, IO). Following PCR cycling, sections were immunolabeled with alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics), and stained with nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Roche Diagnostics). The O.D. of the NBT/BCIP-positive cell bodies from three object fields within each brain area from three different coronal sections was measured, and the values were averaged for each rat. The results are represented as mean (±SEM) O.D. /100 pixels of area for mRNA levels.
Chromatin immunoprecipitation (ChIP) assay
In order to examine the histone acetylation levels specifically at the promoter of the Arc and BDNF exons I and IV in the amygdala of AIS and AIE adult rats, we performed ChIP assay using the antibodies against acetylated histone H3-K9&14 (Millipore) as our laboratory has previously described (Moonat et al., 2013; Sakharkar et al., 2014b). Tissues were fixed with 1% formaldehyde (15 min at 37 °C) and homogenized in lysis buffer following sonication that achieves DNA fragment size of 200-500 base pairs. The sheared chromatin was pre-cleared with agarose beads (Santa Cruz Biotechnology) for 2 hrs and further incubated with the antibodies and agarose beads overnight at 4 °C. After immunoprecipitation, chromatin was eluted; DNA fragments were isolated and quantified by quantitative real-time PCR using primers designed within the promoter regions of Arc and BDNF exons I & IV. Input DNA was used for the normalization as internal control. The primer sequences used were as follows: Arc: Forward-5’-CAGGCACTTCTGAGGTTGCA-3’, Reverse-5’-GCTGATGCGCCTATCCTGA-3’; BDNF exon I, Forward-5’-GCGCCCAAAGCCCACCTTCT-3’, Reverse-5’-GCGTCGGCTCCGTGCTTCTT-3’; BDNF exon IV, Forward-5’-GTTCGCTAGGACTGGAAGTGG-3’, Reverse-5’-CCTCTGCCTCGAAATAGACAC-3’. The c(t) value of immunoprecipitated DNA was corrected with the c(t) value of respective input DNA. The levels of acetylated histone H3-K9&14 within the gene promoters in the amygdala of AIS and AIE rats were calculated using the ΔΔc(t) method (Moonat et al., 2013; Schmittgen and Livak 2008).
Golgi-Cox method for measurement of dendritic spines in the amygdala
The Golgi-Cox staining procedure was performed to measure the dendritic spine density (DSD) in the amygdaloid brain structures of AIS and AIE adult rats using the FD Rapid Golgi Stain Kit (FD Neuro Technologies, Baltimore, MD), as described previously by us (Pandey et al., 2008b; Moonat et al., 2011; You et al., 2014). Spines from neurons where dendrites are connected to soma and showing complete impregnation were marked and then counted using sholl analysis of Neurolucida program (MicroBrightField, Williston, VT). Spines from dendrites (a total of 9 dendrites) from three adjacent brain sections were counted and then averaged for each rat. DSD was represented as mean (±SEM) of the number of dendritic spines/10 μm of dendritic length.
Adolescent intermittent ethanol and alcohol-drinking behavior in adulthood
A subset of the AIS and AIE adult rats were also used for examining the effect of AIE on alcohol-drinking behaviors in adulthood using the two-bottle free-choice paradigm as our laboratory has previously described (Pandey et al., 2006; Moonat et al., 2013; Sakharkar et al., 2014b). AIS and AIE rats (PND 88) were housed singly and received water in two bottles until they started drinking equally from both the bottles, which approximately took two weeks of training. After this AIS and AIE adult rats received water in one bottle and increasing concentrations of ethanol (3% of ethanol for 3 days, 7% of ethanol for 3 days and 9% of ethanol for 3 days) in the other bottle. The position of the bottles was exchanged every day to avoid habit formation for the position of the ethanol bottle. All rats received fresh bottles containing either water or ethanol every day in the evening between 5:00 to 6:00 pm, and ethanol and water intake for the previous day (last 24 hrs) was measured (ml/day) at the same time.
After establishing the ethanol intake paradigm, another batch of AIS and AIE adult (PND 88) rats were used to examine the effect of TSA on ethanol intake. After the training of drinking water from two bottles, rats were given various concentration of ethanol as described above. Upon stabilization of three days of 9% (v/v) ethanol drinking, AIE and AIS adult (PND 111) rats continued to receive 9% ethanol and were concomitantly injected (i.p) with either vehicle [DMSO in PBS, 1:5 dilution; (AIS+ Vehicle) and (AIE+ Vehicle)] or TSA [2 mg/kg; (AIS+TSA) and (AIE+TSA)] (once daily for three consecutive days). Rats received the vehicle or TSA injection before the water and ethanol bottles were given in the evening between 5:00 to 6:00 pm. After three days of vehicle and TSA injection and drinking measurements, rats continued to receive 9% ethanol for next three days to examine the drug washout effects on ethanol intake. Alcohol and water intakes were measured daily (ml/day) and ethanol-drinking in terms of g/kg/day was calculated for each rat and presented as mean (±SEM). The body weights of rats were measured. Total fluid intake (ml/day) was calculated.
Statistical analysis
Student's t-test and one or two-way analysis of variance (ANOVA) were employed for testing the significance of differences between the two groups and more than two groups respectively. Significance of the differences in the alcohol intake by AIS and AIE rats was examined using the two-way repeated measures ANOVA. Post hoc comparisons for all ANOVAs were performed using Tukey's test. The p<0.05 value was considered to be significant.
Results
Effect of AIE on anxiety-like behaviors during adolescence
To examine the effects of AIE on the anxiety-like behaviors, rats were subjected to LDB and EPM exploration tests after 1 hr and 24 hrs from the last AIE or AIS (Fig. 1A, B). One-way ANOVA indicated significant group differences for time spent in light and dark compartments of LDB (F2, 32 = 7.1, p<0.01). Although AIE adolescent rats did not display anxiety-like behaviors at 1 hr (ethanol on-board) as compared to AIS rats, anxiety-like behaviors were observed after 24 hrs of the last AIE injection (AIW). In the LDB exploration test, AIW adolescent rats spent significantly (post-hoc comparisons; p<0.01) less time in the light compartment and more time in the dark compartment as compared to AIS adolescent rats (Fig. 1A). In EPM test, one-way ANOVA indicated significant group differences for % open arm entries (F2, 33 = 72.1, p<0.001), % time spent on open arms (F2, 33 = 27.6, p<0.001) and for total number of closed arm entries (F2, 33 = 5.95, p<0.01). Post-hoc analysis revealed that AIW rats (24 hrs after last AIE) spent significantly (p<0.001) less time and had fewer entries to open arms as compared to AIS rats (Fig. 1B). On the other hand, closed arm entries were significantly (p<0.01) increased in AIE rats due to overall increase in total arms entries but not in AIW rats as compared with AIS rats in the EPM test (Fig. 1B). No significant differences were observed between AIS and AIE rats at 1 hr and 24 hrs in terms of total ambulation in the LDB test (Fig. 1A). Blood ethanol levels (Mean ± SEM; n=23) in AIE (1hr) group were 183 ± 4.9 mg%. These results indicate that anxiety-like behaviors during withdrawal (24hrs) after last AIE exposure are not due to changes in general activity.
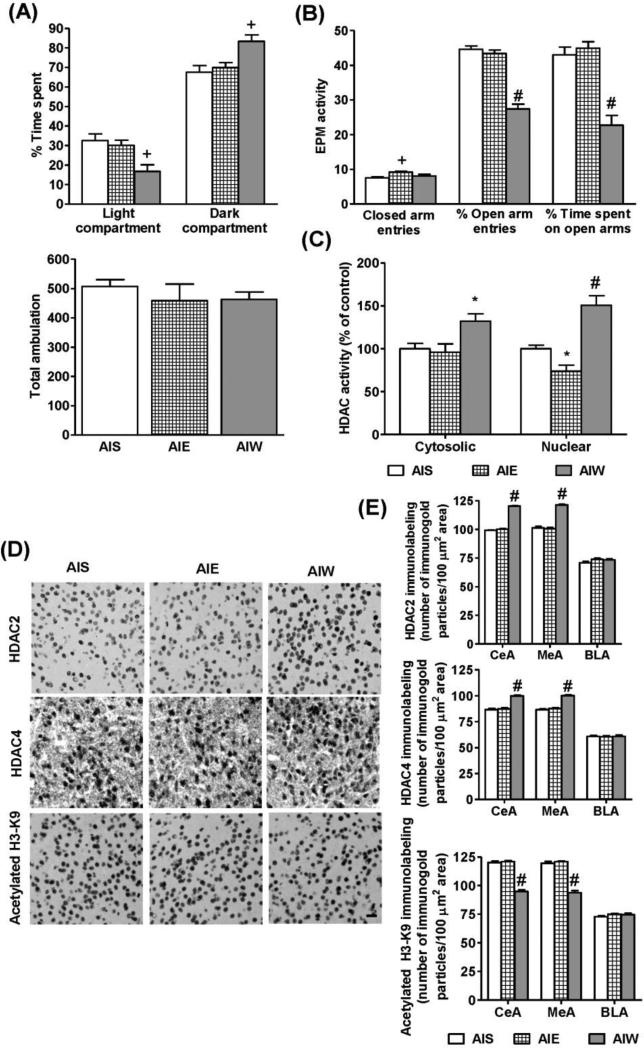
Figure 1.
Effects of adolescent intermittent ethanol (AIE) exposure and its withdrawal on anxiety-like behaviors in adolescent rats. Anxiety-like behaviors were measured by light/dark box (LDB) exploration test (A) and elevated plus maze (EPM) exploration test (B) in adolescent intermittent saline (AIS) treated, AIE (1hr withdrawal), and AIW (24 hr withdrawal) adolescent rats. Values are represented as the mean ± SEM of 11-12 rats per group. Significantly different from AIS control group (+p<0.01, #p<0.001; one way ANOVA followed by post hoc analysis by Tukey's test).
C. HDAC activity in the nuclear and cytosolic fractions of the amygdala obtained from AIS, AIE, and AIW adolescent rats. Values are represented as % of control and mean ± SEM of 6-7rats per group. Significantly different from AIS control group (*p<0.05, #p<0.001; one way ANOVA followed by post hoc analysis by Tukey's test).
D. Representative low-magnification photomicrographs (Scale bar = 50 μm) of gold-immunolabeling of HDAC2, HDAC4, and H3-K9 acetylation in the central nucleus of amygdala (CeA) of AIS, AIE (1hr withdrawal), and AIW (24 hr withdrawal) adolescent rats.
E. Quantification of gold immunolabeling of HDAC2, HDAC4, and acetylated H3-K9 proteins in the amygdaloid structures [CeA; medial nucleus of amygdala (MeA), and basolateral amygdala (BLA)] of AIS, AIE, and AIW rats. Values are represented as the mean ± SEM of the number of immunogold particles per 100 μm2 area of 5 rats per group. Significantly different from AIS control group (#p<0.001; one way ANOVA, followed by post hoc analysis by Tukey's test).
Effect of AIE on HDAC activity, HDAC2 and HDAC4 expression and H3 acetylation in the amygdala during adolescence
Nuclear and cytosolic HDAC activities were measured in the amygdaloid tissues of AIS and AIE adolescent rats. One-way ANOVA indicated significant group differences for HDAC activities (F2, 17 = 5.98, p<0.01 for cytosolic HDAC activity; F2, 17 = 23.3, p<0.001 for nuclear HDAC activity). Post-hoc analysis showed that while nuclear HDAC activity was found to be significantly (p<0.05) inhibited, cytosolic HDAC activity remained unaffected in the amygdala of AIE rats at 1 hr after last injection (Fig. 1C) as compared to AIS rats. However, both cytosolic (p<0.05) and nuclear (p<0.001) HDAC activities were significantly increased in the amygdala of the AIE rats after 24 hrs withdrawal (AIW) as compared to AIS rats (Fig. 1C). Similarly, HDAC2 and HDAC4 protein levels were significantly (p<0.001) increased in the CeA (F2, 12 = 491.5, p<0.001 for HDAC2; F2, 12 = 55.7, p<0.001 for HDAC4) and MeA (F2, 12 = 125.3, p<0.001 for HDAC2; F2, 12 = 79.4, p<0.001 for HDAC4) but not in the BLA of AIW rats as compared to AIS rats (Fig. 1D, E). Additionally, it was found that global histone H3-K9 acetylation was significantly (p<0.001) decreased in the CeA (F2, 12 = 132.4, p<0.001) and MeA (F2, 12 = 120.4, p<0.001), but not BLA, of the AIW rats as compared to AIS rats (Fig. 1D, E). These results indicate that increased HDAC2 and HDAC4 expression might be responsible for the increased nuclear and cytosolic HDAC activities, respectively, leading to deficits in histone H3-K9 acetylation in CeA and MeA during withdrawal after last AIE exposure during adolescence.
Effect of AIE on anxiety-like and alcohol drinking behaviors in adulthood
AIS and AIE adult rats were tested for anxiety-like behaviors using LDB (Fig. 2A) and EPM (Fig. 2B) exploration tests. AIE adult rats displayed anxiety-like behaviors, spending significantly (t= 7.32; p<0.001) less time in the light compartment and more time in dark compartment of the LDB exploration test as compared to AIS adult rats (Fig. 2A). Moreover, AIE adult rats had significantly lower percent open arm entries (t=6.49; p<0.001) and spent less time in open arms (t= 4.24; p<0.001) in the EPM test as compared to AIS adult rats (Fig. 2B). Two-way repeated measures of ANOVA revealed a significant difference in alcohol intake by groups and ethanol concentrations (Group: F1, 30 = 23.5, p<0.001; Concentration: F2, 30 =8.1, p<0.01), but there was no significant interaction between group × ethanol concentrations. Post-hoc comparisons indicated that AIE adult rats drank significantly (p<0.01) higher amounts of 7% and 9% ethanol (measured by the two-bottle free choice paradigm) as compared with AIS adult rats (Fig. 2C). These results suggest that AIE produced anxiety-like behaviors during adolescence that persisted into adulthood. In addition, AIE induced alcohol-drinking behaviors in adulthood.
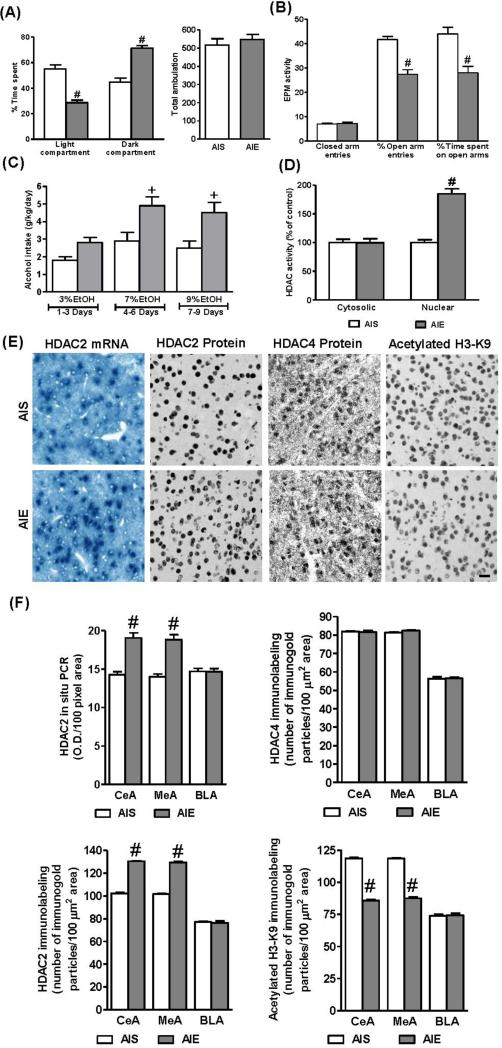
Figure 2.
Effects of adolescent intermittent ethanol (AIE) exposure on anxiety-like behaviors in adulthood. Anxiety-like behaviors were measured by light/dark box (LDB) exploration test (A) and elevated plus maze (EPM) exploration test (B) in adolescent intermittent saline (AIS) and AIE treated adult rats. Values are represented as the mean ± SEM of 12-13 rats per group for LDB and 11 rats per group for EPM. Significantly different from AIS adult group (#p<0.001, student's t test).
C. The effect of AIE on alcohol intake in adulthood. AIS and AIE adult rats were subjected to a two-bottle choice alcohol-drinking paradigm with increasing concentration of ethanol (3% to 9%) and their water and alcohol intakes were measured daily. The data is represented as mean alcohol intake (g/kg/day) for three days for each concentration of the ethanol. Values are represented as the mean ± SEM of 6 rats per group. Significantly different from AIS adult group (+p<0.01; Repeated measures of ANOVA, followed by post hoc analysis by Tukey's test).
D. The HDAC activity in the nuclear and cytosolic fractions of the amygdala obtained from AIS and AIE adult rats. Values are represented as the % of control and mean ± SEM of 7-8 rats per group. Significantly different from AIS adult group (#p<0.001, student's t test).
E. Representative low-magnification photomicrographs (Scale bar = 50 μm) of gold-immunolabeling of HDAC2, HDAC4, and H3-K9 acetylation and HDAC2 mRNA (in situ RT-PCR) in the central nucleus of amygdala (CeA) of AIS and AIE adult rats.
F. Quantification of gold immunolabeling of HDAC2, HDAC4, and acetylated H3-K9 proteins and HDAC2 mRNA levels in the amygdaloid structures [CeA; medial nucleus of amygdala (MeA), and basolateral amygdala (BLA)] of AIS and AIE adult rats. Values are represented as the mean ± SEM of the number of immunogold particles/100 μm2 area for gold immunolabeling derived from 6 rats per group and optical density (O.D.)/100 pixel area for HDAC2 mRNA levels (in situ RT-PCR) derived from 5 rats per group. Significantly different from AIS adult group (#p<0.001, Student's t test).
Effect of AIE on HDAC activity, HDAC2 and HDAC4 expression and histone H3-K9 acetylation in the amygdala during adulthood
The long-lasting influence of AIE on epigenetic changes in the amygdala was also investigated. Nuclear and cytosolic HDAC activities were analyzed in the amygdala of AIS and AIE adult rats (Fig. 2D). Whereas nuclear HDAC activity was significantly (t=8.05; p<0.001) higher in the amygdala of AIE adult rats as compared to AIS adult rats, cytosolic HDAC activity did not differ between these two groups (Fig. 2D). Similarly, HDAC2 mRNA and protein levels were significantly increased in the CeA (t=6.21, p<0.001 for mRNA; t=25.64, p<0.001 for protein) and MeA (t=6.67, p<0.001 for mRNA; t=28.56, p<0.001 for protein) but not in the BLA of AIE adult rats, as compared to AIS adult rats (Fig. 2E, F). However, HDAC4 protein levels were not altered in AIE adult rats as compared to AIS adult rats (Fig. 2E, F). Moreover, histone H3-K9 acetylation levels were significantly decreased in the CeA (t=26.51, p<0.001) and MeA (t=24.62, p<0.001) but not in the BLA of AIE adult rats, as compared to AIS adult rats (Fig. 2E, F). These results indicate that the AIE induced changes in HDAC2-mediated chromatin remodeling that occurred during adolescence persisted in adulthood, showing long-lasting effects of AIE on histone acetylation mechanisms in the amygdala.
Effect of AIE on BDNF, Arc expression, dendritic spines, and histone acetylation levels of genes in adulthood
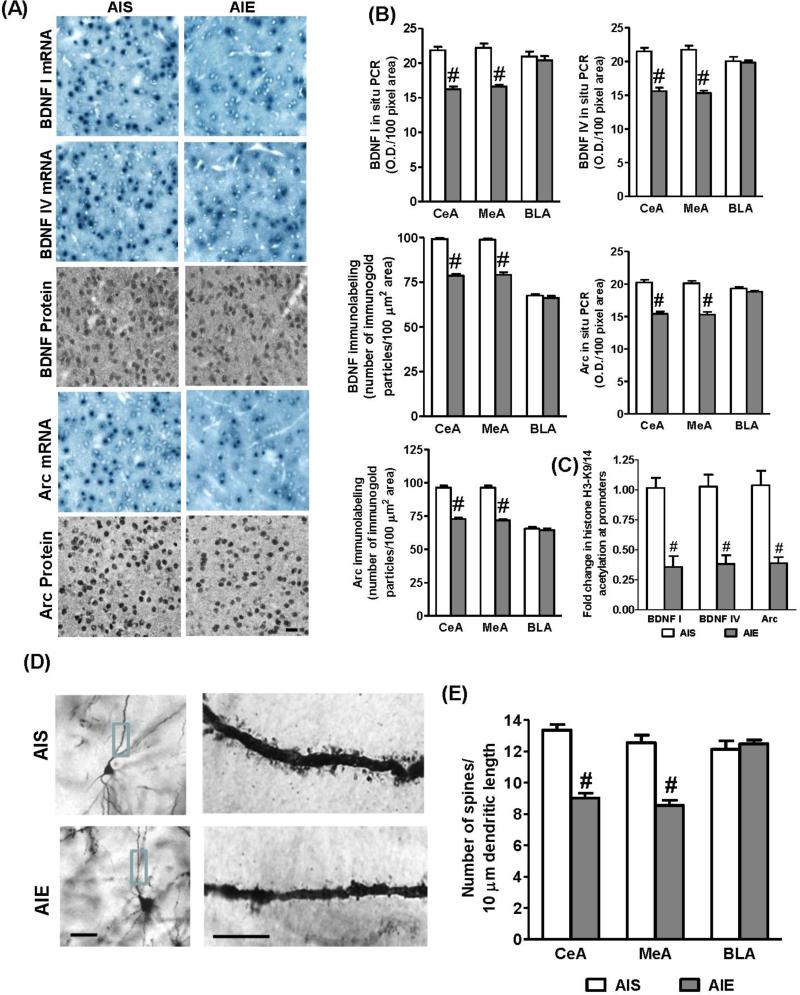
We next investigated AIE effects on synaptic remodeling mechanisms in the amygdala in adulthood. It was observed that BDNF exon I, IV and Arc mRNA levels and BDNF and Arc protein levels were significantly decreased in the CeA (t=9.41, p<0.001 for BDNF exon I mRNA; t=8.05, p<0.001 for BDNF exon IV mRNA; t=8.45, p<0.001 for Arc mRNA; t=18.29, p<0.001 for BDNF protein; t=13.24, p<0.001 for Arc protein) and MeA (t=8.36, p<0.001 for BDNF exon I mRNA; t=8.91, p<0.001 for BDNF exon IV mRNA; t=8.86, p<0.001 for Arc mRNA; t=12.09,p<0.001 for BDNF protein; t=12.86,p<0.001 for Arc protein) but not in the BLA, of AIE adult rats, as compared to AIS adult rats (Figs. 3A, B). We also examined if this decrease in the BDNF and Arc expression is related to lower H3 acetylation of the BDNF (exon I, IV) and Arc genes using a ChIP assay. Similar to global histone H3-K9 acetylation (Figs. 2E, F), AIE produced significant deficits in histone (H3-K9&14) acetylation levels of BDNF exon I (t=5.26, p<0.001), BDNF exon IV (t= 5.34, p<0.001) and Arc (t=5.57, p<0.001) gene promoters in the amygdala (Fig. 3C) in adulthood, as compared to AIS adult rats. We have also employed the Golgi-Cox staining method for analyzing dendritic spine density (DSD) in the amygdala of AIS and AIE adult rats. It was found that the number of dendritic spines were significantly reduced in the CeA (t=9.01, p<0.001) and MeA (t=6.78, p<0.001), but not in the BLA of AIE adult rats, as compared to AIS adult rats (Fig. 3D, E). These results indicate that AIE caused deficits in histone H3 acetylation in the promoters of BDNF and Arc genes and thereby decreased expression of these genes and decreased DSD in the CeA and MeA in adulthood showing that AIE has the ability to induce chromatin and synaptic remodeling in the amygdala.
Figure 3.
A.Representative low-magnification photomicrographs (Scale bar = 50 μm) of gold-immunolabeling of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton associated (Arc) proteins, and BDNF exon I, IV, and Arc mRNA (in situ RT-PCR) in the central nucleus of amygdala (CeA) of AIS and AIE adult rats.
B. Quantification of gold immunolabeling of BDNF and Arc proteins, and BDNF I, IV, and Arc mRNA levels in the amygdaloid structures [CeA; medial nucleus of amygdala (MeA), and basolateral amygdala (BLA)] of AIS and AIE adult rats. Values are represented as the mean ± SEM of the number of immunogold particles/100 μm2 area for gold immunolabeling and optical density (O.D.)/100 pixel area for mRNA levels (in situ RT- PCR) and derived from 6 rats per group. Significantly different from AIS adult group (#p<0.001, Student's t test).
C. Effects of AIE on acetylated histone H3-associated gene promoter levels of BDNF exon I, IV, and Arc in the amygdala of adult rats as determined by chromatin immunoprecipitation (ChIP) assay. Values are represented as the mean ± SEM of the fold change of acetylated H3-K9&14 levels of BDNF and Arc genes normalized to AIS adult group and derived from 6-7 rats per group. Significantly different from AIS adult group (#p<0.001, Student's t test).
D. Effects of AIE on dendritic spine density (DSD) in the amygdaloid structures in adulthood. Representative low-magnification photomicrographs (Scale bar = 50 μm) showing Golgi-impregnated neurons in the CeA of AIS and AIE adult rats. The boxed areas of the dendrites in CeA of low magnification images are shown at high magnification (Scale bar = 10 μm) in the adjacent image showing dendritic spines in the CeA of AIS and AIE adult rat.
E. The quantification of DSD in the amygdaloid (CeA, MeA, and BLA) structures of AIS and AIE adult rats. Values (number of dendritic spines per 10 μm of dendritic length) are represented as the mean ± SEM and derived from 6 rats per group. Significantly different from control AIS group (#p<0.001; Student's t-test).
Effect of trichostatin A (TSA) on gene-specific histone acetylation, AIE-induced anxiety-like and alcohol-drinking behaviors in adulthood
We treated AIS and AIE adult rats with TSA (2 mg/kg, once daily for 3 days) to examine the effect of HDAC inhibition on anxiety-like and alcohol-drinking behaviors, as demonstrated by baseline differences (Fig. 2A-C). Two-way ANOVA revealed a significant difference in LDB measures by groups, treatment and group × treatment (Group: F1, 33 = 7.7, p<0.01; Treatment: F1, 33 =25.2, p<0.001; Group × Treatment: F1, 33 =22.2, p<0.001). Post-hoc comparisons revealed anxiety-like behavior in AIE adult rats, as evidenced by their significant (p<0.001) increased percent time spent in the dark compartment and decreased time in the light compartment of the LDB exploration test, as compared to AIS adult rats (Fig. 4A). Treatment with TSA attenuated the anxiety-like behaviors in the AIE adult rats. TSA treatment did not affect the anxiety measures of the AIS adult rats (Fig. 4A). No significant differences were observed in total ambulation (Fig. 4A) among different groups indicating that neither AIE nor TSA treatment affects the general activity of the rats.
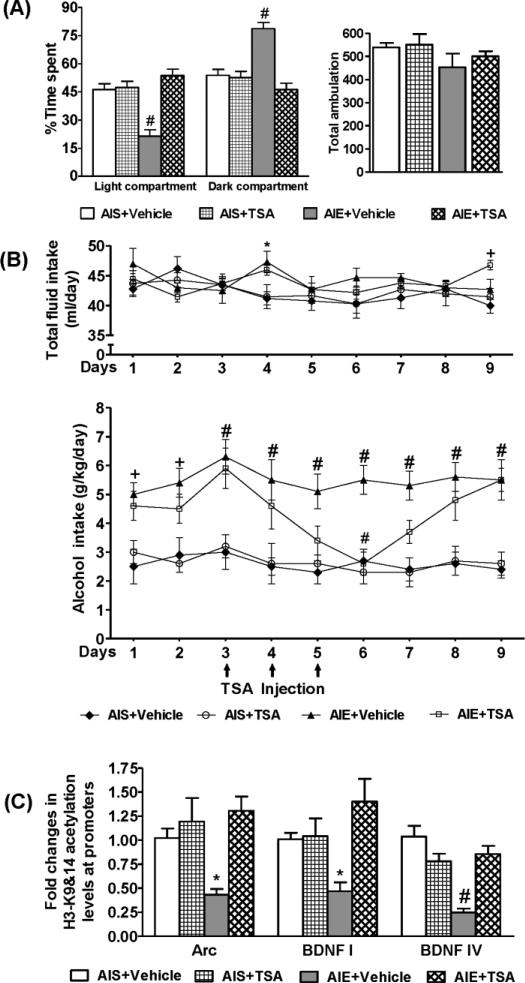
Figure 4.
Effects of trichostatin A (TSA) treatment on anxiety-like and alcohol drinking behaviors as well as histone H3 acetylation of BDNF and Arc genes in the amygdala of AIS and AIE adult rats.
A. The light/dark box (LDB) exploration test showed that TSA treatment (2mg/kg once daily for three days and 2 hr after last injections) attenuated AIE-induced anxiety-like behaviors in adulthood, but produced no effects in AIS adult rats. The general activity as measured by total ambulation in LDB test was not different among the treatment groups. Values are represented as the mean ± SEM of the percentage of time spent in each compartment of 9-10 rats per group. Significantly different from AIS control group (#p<0.001, two-way ANOVA followed by Tukey's test).
B. The pattern of ethanol consumption in AIE and AIS rats as measured by the two-bottle free choice paradigm indicated that the AIE+ Vehicle group consumed significantly higher amounts of alcohol (g/kg/day) as compared with the AIS + Vehicle group (+p<0.01, #p<0.001; two-way repeated measures ANOVA followed by post hoc analysis of the group within day comparison using Tukey's test). Line diagrams show daily 9% alcohol intake and its modulation by TSA treatment in AIS and AIE adult rats. Beginning of the third day of 9% ethanol intake, AIS/AIE adult rats treated with either TSA (2mg/kg) or vehicle (once daily for 3 days as shown by arrow) and consumption of ethanol and water was recorded and continued for additional several days after stopping the TSA and vehicle injections. Values are represented as the mean ± SEM (6 rats per group) of either alcohol (g/kg/day) or total fluid intake (ml/day). TSA treatment significantly decreased alcohol intake in AIE adult rats (AIE+TSA) on the 6th day of 9% alcohol drinking as compared with AIE+ vehicle group (#p<0.001, two-way repeated measures ANOVA followed by post hoc analysis within day comparison using Tukey's test). For total fluid intake, AIE+ vehicle group on day 4 and AIE+TSA group on day 9 were significantly different from AIS+ Vehicle group (*p<0.05, +p<0.01; repeated measures ANOVA followed by post hoc analysis of the group within day comparison using Tukey's test).
C. Bar diagram showing the fold changes in the histone acetylation (H3-K9/14) at the promoters of the BDNF exon I, IV, and Arc gene (measured by chromatin immunoprecipitation followed by quantitative real-time PCR) in the amygdala of AIS and AIE adult rats treated with or without TSA. Values are the mean ± SEM of 6-7 rats per group. Significantly different (*p<0.05, #p<0.001; two-way ANOVA followed by Tukey's test) from the AIS+ Vehicle control group.
As described above (Fig. 2C), we observed a baseline increase in alcohol intake by AIE adult rats as compared to the AIS adult rats (Fig. 4B). AIE adult rats drank higher amounts of ethanol (3% for 3 days, 7% for 3 days; data not shown) as compared to AIS adult rats. Two-way repeated measures of ANOVA revealed a significant difference in alcohol intake (9%) between various groups overall and daily (Group: F3, 180 = 68.6, p<0.001; Day: F8, 180 =2.6, p<0.01). Post-hoc comparisons indicated that AIE adult rats drank significantly (p<0.01-0.001) higher amounts of 9% ethanol and after three days of 9% alcohol drinking, AIE and AIS adult rats were treated with TSA or vehicle for three consecutive days and their drinking behaviors were measured (Fig. 4B). It was found that TSA treatment gradually decreased alcohol intake by AIE adult rats and achieved a significant (p<0.001) reduction in alcohol intake following the third day of TSA treatment (Fig. 4B). After TSA or vehicle injections were discontinued, rats continued to receive 9% ethanol in two-bottle free choice paradigm. It was observed that the alcohol intake by the AIE+TSA rats was returned to baseline levels of drinking. However, TSA treatment did not affect the alcohol drinking levels of AIS adult rats (Fig. 4B). Two-way repeated measures ANOVA indicated a significant difference in total fluid intake by groups but not by days (Group: F3, 180 = 4.1, p<0.01). There were no significant differences in total fluid intake (ml/day) between the various groups except AIE+ Vehicle (on day 4; p<0.05) and AIE+TSA (on day 9; p<0.01) groups were significantly higher than AIS+ Vehicle group. There were no significant differences in mean (±SEM) body weight (gm) of rats among various groups (AIS+ Vehicle= 392± 12.6, AIS+ TSA= 370± 6.2, AIE+ Vehicle= 377± 10, AIE+ TSA= 381± 3.1).
We also examined the effects of TSA treatment on the histone (H3-K9&14) acetylation levels in the promoters of BDNF and Arc genes in the amygdala of AIS and AIE adult rats. Two-way ANOVA revealed a significant difference in histone H3 acetylation by treatment and group × treatment for BDNF exon I (Treatment: F1, 21 =8.5, p<0.01; Group × Treatment: F1, 21 =7.6, p<0.05), by groups, treatment, and group × treatment for BDNF exon IV (Group: F1, 21 = 17.9, p<0.01; Treatment: F1, 21 =4.4, p<0.05; Group × Treatment: F1,21 =26.6, p<0.001) and by treatment and group × treatment for Arc (Treatment: F1, 21 =10.2, p<0.01; Group × Treatment: F1,21 =4.6, p<0.05). Post-hoc comparisons found that AIE produced significant reductions in the histone H3 acetylation of BDNF [exon I (p<0.05), exon IV (p<0.001)] and Arc (p<0.05) genes in the amygdala that were normalized by TSA treatment (Fig. 4C). These results indicate that AIE increased alcohol intake and induced anxiety-like behaviors in adulthood and both phenotypes are attenuated by treatment with TSA most likely by normalizing the deficits in histone H3 acetylation of synaptic plasticity associated genes (BDNF &Arc) in the amygdala.
Discussion
The findings of the present study indicate that AIE produced anxiety-like behaviors during withdrawal that persist and are associated with alcohol-drinking behaviors in adulthood. We provided novel evidence that AIE produced HDAC-induced histone H3 deacetylation due to higher HDAC activity and increased protein levels of HDAC2 and 4 in the amygdala during adolescence. Some of these changes, such as increased nuclear HDAC activity and HDAC2 levels and decreased histone H3-K9 acetylation persisted in adulthood and led to subsequent decreases in expression of BDNF exon I and IV, as well as Arc, in the CeA and MeA. These changes were also associated with AIE-induced reductions in dendritic spines in the CeA and MeA in adulthood. Interestingly, when AIE-induced increases in HDAC activity and HDAC2 protein levels were blocked by treatment with TSA in adulthood, it resulted in attenuation of anxiety-like and alcohol-drinking behaviors and normalization of deficits in histone H3 acetylation of BDNF and Arc genes in the amygdala. Early-life adversity has been shown to produce persistent changes in DNA methylation and is responsible for brain disorders later in life (Szyf et al., 2008; Roth et al., 2009). For example, it has been shown that early life stress can induce DNA hypermethylation of BDNF genes in the hippocampus that persist in adulthood and are associated with depression-like symptoms (Roth et al., 2009). Similarly, offspring from mothers who exhibited poor nurturing behaviors in animals were associated with hypermethylation at transcriptional control regions of glucocorticoid receptors in the hippocampus and anxiety-like behaviors in adulthood, which were attenuated by treatment with TSA (Szyf et al., 2008; Szyf, 2013). To our knowledge, this is the first study that demonstrates persistent changes in HDAC-induced histone H3 deacetylation during AIE that is associated with deficits in synaptic plasticity-associated gene expression (BDNF & Arc) in the amygdala resulting in behavioral phenotypes such as anxiety-like and alcohol drinking behaviors in adulthood (Fig. 5).
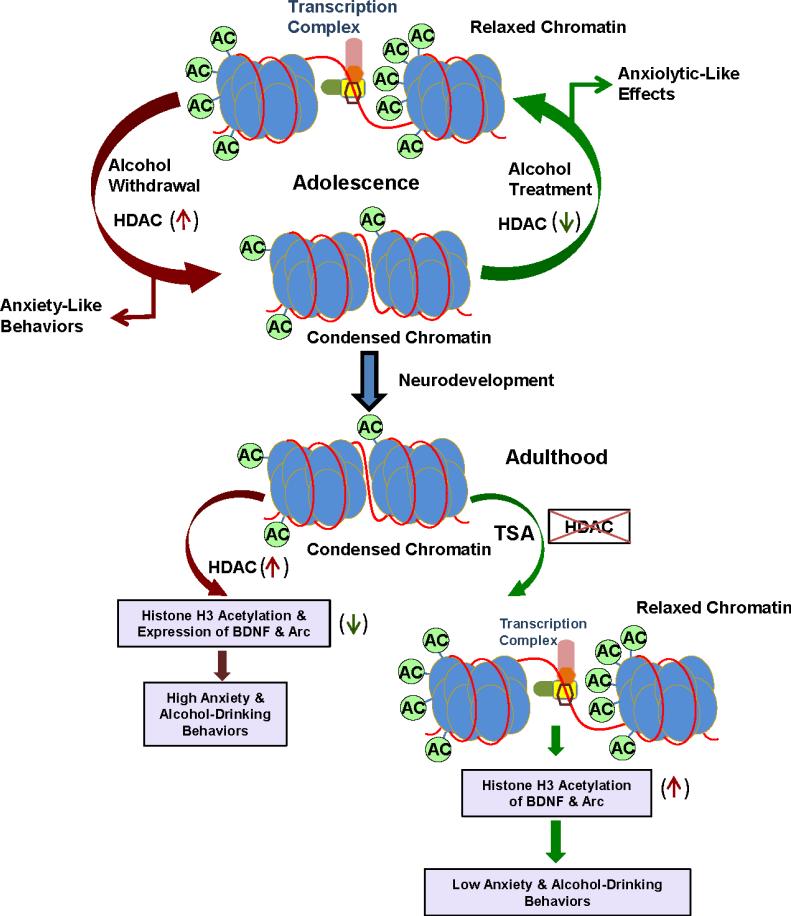
Figure 5.
Hypothetical model of adolescent intermittent ethanol (AIE) exposed chromatin architecture showing opening and closing of chromatin structure due changes in histone H3 acetylation in the amygdala during adolescence and its persistence into adulthood. Acute exposure with higher dose of ethanol inhibits HDAC activity in the amygdala and produced anxiolytic effects in adolescent rats (Sakharkar et al., 2014b). During adolescence, withdrawal after last AIE produced higher HDAC2 and 4 levels and HDAC activity that are associated with reduced histone H3-K9 acetylation in the amygdala and development of anxiety-like behaviors. Some epigenetic modifications such as higher HDAC2 expression and higher HDAC activity and associated deficits in histone H3 acetylation (condensed chromatin) in the amygdala persist in adulthood. AIE also produced lower histone H3 acetylation of BDNF and Arc genes and associated decreased expression of these genes in the amygdala in adulthood. Treatment with the HDAC inhibitor, trichostatin A (TSA) in adulthood was able to normalize the deficits in histone H3 acetylation (relaxing the chromatin structure) in the promoters of BDNF and Arc genes in the amygdala of AIE adult rats and also attenuates AIE-induced anxiety-like and alcohol drinking behaviors.
Epigenetic mechanisms have been shown to play a crucial role in brain development and in shaping behavioral phenotypes later in life (Mehler, 2008; Murgatroyd and Spengler, 2011; Szyf et al., 2008; Roth et al., 2009). Here, we showed that intermittent ethanol exposure during a critical period of brain development (Spear, 2000) in adolescence (PND 28-41) produced increases in expression of HDAC2 and deficits in histone H3-K9 acetylation in the CeA and MeA, which is associated with development of anxiety-like behaviors. Both the epigenetic changes in the amygdala and behavioral phenotypes persisted in adulthood and, additionally, AIE induced an increase in alcohol intake. Interestingly, some of AIE induced changes such as increases in cytosolic HDAC activity and HDAC4 protein levels in the CeA and MeA during withdrawal in adolescence were normalized in adulthood. Thus, AIE induced long-lasting effects (51 days after last AIE) on HDAC2 expression and histone H3-K9 acetylation in the amygdala and this may be involved in the behavioral phenotypes of anxiety and alcoholism in adulthood. Previous studies showed that AIE exposure produced increases in alcohol intake and conditioned place preference in rats during adulthood, and histone modifications within the prefrontal cortex have been implicated in these phenotypes (Alaux-Cantin et al., 2013; Pascual et al., 2009; 2012). We extended these findings and found that HDAC2-mediated chromatin remodeling in the emotional circuitry was not only involved in regulating AIE-induced alcohol intake in adulthood, but also regulated AIE-induced anxiety-like behaviors during both adolescence and adulthood. These results suggest that changes in adolescent rats are long lasting and persist even after several days following the last AIE exposure in adulthood. On the other hand, anxiety-like behaviors that develop in adult rats after chronic ethanol exposure are not long lasting (Aujla et al., 2013; Pandey et al., 1999; Zhang et al., 2007). Furthermore, acute ethanol significantly inhibited HDAC activity in the amygdala and produced anxiolytic-like effects in adolescent rats (Sakharkar et al., 2014a). However, adolescent rats required a higher dose of alcohol to produce anxiolytic-like effects and to inhibit HDAC activity in the amygdala as compared to adult rats (Sakharkar et al., 2012; 2014a; Varlinskaya and Spear, 2002). These results suggest that adolescent and adult rats showed differential initial sensitivity to chromatin remodeling and anxiolytic-like effects of ethanol and possibly enduring AIE effects on histone modifications in the amygdala. The resultant behavioral phenotypes observed in adulthood could be related to the developmental perturbation induced by ethanol exposure.
There are different HDAC isoforms and recently the HDAC2 isoform has been implicated directly in the regulation of synaptic plasticity related to learning and memory and neurodegenerative diseases, as well as in the regulation of anxiety-like and alcohol drinking behaviors (de Ruijter et al., 2003; Gräff et al., 2012; Gräff and Tsai, 2013; Mielcarek et al., 2011; Moonat et al., 2013; Thaiagalingam et al., 2003). Here, we demonstrated that AIE produced increased levels of HDAC2 in the developing amygdala, and this epigenetic regulator remained increased during adulthood. In addition, increases in HDAC2 expression produced deficits in histone H3 acetylation at the promoters of BDNF and Arc genes and was responsible for decreased expression of these genes in the CeA and MeA during adulthood. AIE also produced reductions in dendritic spines in these amygdaloid circuitries. We have shown earlier that decreased BDNF signaling in the CeA and MeA may be involved in regulating anxiety-like and alcohol drinking behaviors in adult rats. Deficits in BDNF cause reductions in Arc expression that directly regulate dendritic spines in CeA and regulate the phenotypes of anxiety and alcoholism (Pandey et al., 2008b; 2006; Moonat et al., 2011). Treatment with TSA was able to normalize deficits in the BDNF and Arc expression as well as DSD in the CeA and MeA of adult Sprague-Dawley (SD) rats undergoing withdrawal after chronic ethanol exposure (You et al., 2014). Combining these previous studies with the observed long-lasting effects of AIE on BDNF and Arc expression as well as on dendritic spines clearly support the involvement of a deficient BDNF system in the regulation of anxiety and alcoholism during adulthood. Recent studies from our laboratory showed that innately higher expression of HDAC2 is responsible for deficits in BDNF and Arc expression and dendritic spines in the CeA and MeA of P rats as compared to NP rats and knock-down of HDAC2 in the CeA by siRNA corrected these deficits and attenuated anxiety-like behaviors and alcohol intake (Moonat et al., 2013). Interestingly, SD adolescent rats treated with intermittent ethanol underwent chromatin remodeling resulting in synaptic plasticity changes in the amygdala that predisposed them to anxiety-like and alcohol-drinking behaviors in adulthood, thus resembling epigenetic and behavioral correlates of P rats (Moonat et al.,2013; Sakharkar et al., 2014b). Together, these studies indicate that HDAC2 in the CeA may play an important role not only in the genetic predisposition to anxiety and alcoholism, but also in AIE-induced these phenotypes in adulthood. Other studies have indicated that adolescent brain is more susceptible to alcohol-induced damage, including inhibition of hippocampal neurogenesis (Crews et al., 2000; 2006; Guerri and Pascual, 2010). In addition, AIE reduces choline acetyltransferase (ChAT) immune-positive cells in the forebrain in adulthood, which is correlated with several behavioral measures of affective states and arousal (Ehlers et al., 2011; Vetreno et al., 2014). Future studies are needed to explore the epigenetic basis of AIE-induced changes in neurogenesis and loss of ChAT positive cells and associated behaviors in adulthood.
As discussed above, perturbation of epigenetic process by AIE resulted in a condensed chromatin structure due to a decrease in histone H3 acetylation in the amygdala during adulthood. We took a pharmacological approach to relax chromatin structure by treating AIE adult rats with TSA and found that the AIE-induced anxiety-like and alcohol-drinking behaviors were attenuated by TSA treatment (Fig. 5). Other studies conducted in adult rats have suggested that TSA is effective in preventing ethanol withdrawal-related anxiety-like behaviors and the development of alcohol tolerance (Pandey et al., 2008a; Sakharkar et al., 2012). Furthermore, it has been shown that TSA treatment significantly decreased alcohol intake in various animal models (Sakharkar et al., 2014b; Warnault et al., 2013). It is important to point out that the dose of TSA (2mg/kg) used here has no effects on anxiety measures and alcohol intake in AIS adult rats. These are similar to our previous findings in control adult SD and NP rats (Pandey et al., 2008a; Sakharkar et al., 2014b; You et al., 2014). Interestingly, there is an increase in HDAC activity and HDAC2 protein levels in the CeA and MeA of AIE adult rats and P rats as compared with AIS adult rats and NP rats, respectively. This may be responsible for the pharmacological effects of TSA in AIE adult rats and P rats, as treatment also inhibited nuclear HDAC but not cytosolic HDAC activity and decreased HDAC2 protein levels in the CeA and MeA of P rats and corrected the deficits in histone H3 acetylation in the promoter of neuropeptide Y gene (Sakharkar et al., 2014b). It is possible that TSA may be able to inhibit nuclear, but not cytosolic activity, as treatment significantly corrected the deficits in histone H3 acetylation in the promoters of BDNF and Arc genes in the amygdala of AIE adult rats. In previous studies, TSA pre-treatment prior to ethanol exposure in mice was shown to increase alcohol intake during the post-ethanol and drug treatment period (Wolstenholme et al., 2011; Qiang et al., 2014). However, TSA treatment after establishment of alcohol drinking in mice (Warnault et al., 2013), P rats (Sakharkar et al., 2014a) and/or in AIE adult rats was able to attenuate alcohol drinking. Although experimenter administered ethanol is a limitation of this study, it nonetheless provides fascinating findings on persistent AIE effects on histone modifications in the emotional circuitry and on behavioral phenotypes in adulthood. The behavioral data collected here confirms the previous findings (Alaux-Cantin et al., 2013; Pascual et al., 2009; 2012) on alcohol intake and suggests that AIE not only increased alcohol intake, but also produced anxiety-like behaviors in adulthood. However some studies reported that AIE produced attenuation of anxiety-like behaviors in rats (Ehlers et al., 2011; Gass et al., 2014; Gilpin et al., 2012). The reason for discrepancies between our studies with some of the previous studies on anxiety-like behaviors is not clear, but may be related to differences in the strains of the rats and the duration and methodology of ethanol exposure (vapor vs i.p. injections). Interestingly, similar to our study, another study using voluntary binge drinking model in rats reported that AIE produced anxiety-like and depression-like symptoms during adolescence (Briones and Woods, 2013). Clinical studies support our preclinical findings and indicate that binge alcohol drinking during adolescence makes individuals more vulnerable to negative affect and alcohol use disorders (DeWit et al., 2000; Grant and Dawson, 1997; Winward et al., 2014). It has been shown that stronger amygdaloid connectivity in adults compared to adolescents may reflect the process of emotional maturation (Guyer et al., 2008). It is possible that AIE may lead to weaker amygdaloid connectivity due to condensed chromatin architecture resulting from deficits in histone H3 acetylation during development. Combining the histone modification data with AIE-induced anxiety and alcoholism phenotypes indicates that targeting the epigenome by HDAC inhibitors can provide a better strategy to reverse AIE-induced behavioral consequences in adulthood.
Conclusions
The data presented here indicate that AIE produced deficits in histone H3-K9 acetylation due to increased expression of HDAC2 and 4 isoforms and increased HDAC activity in the amygdala, which is correlated with anxiety-like behaviors during adolescence. Interestingly, anxiety-like behaviors induced by AIE persist in adulthood and are associated with an increase in alcohol intake. These AIE-induced behavioral phenotypes are correlated with deficits in global and gene specific (BDNF and Arc) histone H3 acetylation due to higher HDAC activity and HDAC2 expression leading to lower BDNF and Arc expression, and fewer dendritic spines in the amygdala in adulthood. The data further suggest the possibility that HDAC linked deficits in histone H3 acetylation and associated synaptic remodeling in the amygdala may be involved in regulating AIE-induced anxiety and alcoholism phenotypes in adults (Fig. 5), as HDAC inhibitor treatment attenuated these behaviors and normalized the deficits in histone H3 acetylation of BDNF and Arc genes.
Research Highlights.
Adolescent intermittent ethanol (AIE) decreased amygdaloid histone H3 acetylation.
AIE produced long-lasting upregulation of HDAC2 levels and HDAC activity.
AIE lowered BDNF and Arc gene expression in adulthood due to histone modifications.
HDAC inhibitors lowered AIE-induced anxiety and alcohol intake in adulthood.
AIE-induced chromatin remodeling may lead to anxiety and alcoholism later in life.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grants AA-019971 (NADIA project), AA-010005, and AA-013341, and by the Department of Veterans Affairs [Merit Review Grant (I01BX000143); Research Career Scientist award] to S.C.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
SCP reports that a US patent application entitled “Histone acetyl transferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th, 2006) is currently pending. Other authors reported no biomedical financial interests or potential conflicts of interest.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romaualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ(N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–334. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiu D. What is special about the adolescent (JME) brain ?. Epilepsy Behav. 2013;28(Suppl 1):S45–S51. doi: 10.1016/j.yebeh.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. J Adolesc Health. 2004;35:529, e7–18. doi: 10.1016/j.jadohealth.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition and increases resistance to extinction of ethanol self administration in adulthood. Neuropsychopharmacology. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety and amygdalar corticotropin releasing factor cells in adulthood in male rats. PloS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013;53:311–330. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE, Ressler KJ. Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Front Psychiatry. 2013;4:62. doi: 10.3389/fpsyt.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB. Adolescence and alcohol: recent advances in understanding the impact of alcohol use during a critical developmental window. Alcohol. 2010;44:1–2. doi: 10.1016/j.alcohol.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol. 2008;86:305–341. doi: 10.1016/j.pneurobio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Benn CL, Franklin SA, Smith DL, Woodman B, Marks PA, et al. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington's disease. PLoS One. 2011;6:e27746. doi: 10.1371/journal.pone.0027746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Karra AS, Monteggia LM. Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain. Behav Pharmacol. 2010;21:409–419. doi: 10.1097/FBP.0b013e32833c20c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley PM, Johnston LD, Bachman JG. Alcohol use among adolescents. Alcohol Health Res World. 1998;22:85–93. [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–878. [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008a;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene Arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008b;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Qiang M, Li JG, Denny AD, Yao JM, Lieu M, Zhang K, et al. Epigenetic mechanisms are involved in the regulation of ethanol consumption in mice. Int J Neuropsychopharmacology. 2014 Oct 31;18(2) doi: 10.1093/ijnp/pyu072. [Epub ahead print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014a;17:2057–2067. doi: 10.1017/S1461145714001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014b;17:1207–1220. doi: 10.1017/S1461145714000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52(Suppl 2):S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Szyf M. DNA methylation, behavior and early life adversity. J Genet Genomics. 2013;40:331–338. doi: 10.1016/j.jgg.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLOS one. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling – a novel strategy to control excessive ethanol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 college alcohol study. J Am Coll Health. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Melnerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res. 2014;38:1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Warner JA, Capparuccini MI, Archer KJ, Shelton KL, Miles MF. Genomic analysis of individual differences in ethanol drinking: evidence for non-genetic factors in C57BL/6 mice. PLoS One. 2011;6:e21100. doi: 10.1371/journal.pone.0021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF, and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, Schulteis G. Dose-and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]