Abstract
Background
Hair cortisol levels are used increasingly as a measure for chronic stress in young children. We propose modifications to the current methods used for hair cortisol analysis to more accurately determine reference ranges for hair cortisol across different populations and age groups.
Methods
The authors compared standard (finely cutting hair) vs. milled methods for hair processing (n=16), developed a 4-step extraction process for hair protein and cortisol (n=16), and compared liquid chromatography-mass spectrometry (LCMS) vs. ELISA assays for measuring hair cortisol (n=28). The extraction process included sequential incubations in methanol and acetone, repeated twice. Hair protein was measured via spectrophotometric ratios at 260/280 nm to indicate the hair dissolution state using a BioTek® plate reader and dedicated software. Hair cortisol was measured using an ELISA assay kit. Individual (n=13), pooled hair samples (n=12) with high, intermediate, and low cortisol values and the ELISA assay internal standards (n=3) were also evaluated by LCMS.
Results
Milled and standard methods showed highly correlated hair cortisol (rs=0.951, p<0.0001) and protein values (rs=0.902, p=0.0002), although higher yields of cortisol and protein were obtained from the standard method in 13/16 and 14/16 samples respectively (p<0.05). Four sequential extractions yielded additional amounts of protein (36.5%, 27.5%, 30.5%, 3.1%) and cortisol (45.4%, 31.1%, 15.1%, 0.04%) from hair samples. Cortisol values measured by LCMS and ELISA were correlated (rs=0.737; p<0.0001), although cortisol levels (median [IQR]) detected in the same samples by LCMS (38.7 [14.4, 136] ng/ml) were lower than by ELISA (172.2 [67.9, 1051] ng/ml). LCMS also detected cortisone, which comprised 13.4% (3.7%, 25.9%) of the steroids detected.
Conclusion
Methodological studies suggest that finely cutting hair with sequential incubations in methanol and acetone, repeated twice, extracts greater yields of cortisol than does milled hair. Based on these findings, at least three incubations may be required to extract most of the cortisol in human hair samples. In addition, ELISA-based assays showed greater sensitivity for measuring hair cortisol levels than LCMS-based assays.
Keywords: stress, chronic stress, ELISA, assays, protein extraction
Established “gold standard” methods for measuring acute stress are used widely, although there is a paucity of methods for measuring chronic stress [1]. Measurements of cortisol from different biological sources (blood, saliva, urine) provide a measure of acute cortisol production, and thus may not reliably reflect chronic stress [2, 3]. On the other hand, hair cortisol is a good candidate for measuring chronic stress since the hair shaft grows at rates of 256±44 µm/day in African-Americans and 396±55 µm/day in Caucasians, averaging at rates of around 1 cm/month [4, 5]. Hair cortisol levels were originally measured in the hair of athletes thought to be abusing anabolic steroids and were later studied among humans and primates as a measure for chronic stress [6, 7]. Multiple studies showed positive correlation between subjective stress and hair cortisol levels [3, 8], further corroborated with serum and salivary cortisol in elementary school girls [9]. In earlier studies, liquid chromatography mass spectroscopy (LCMS) analysis was used more commonly to measure hair cortisol levels [6, 10, 11], however, since 2007 enzyme-linked immunosorbent assays (ELISA) [12] have been widely used for measuring hair cortisol [13].
Relatively few studies have examined hair cortisol as a marker for chronic stress in pediatric patients. Yamada et al. (2007) first reported hair cortisol levels in newborns receiving neonatal intensive care [14], showing that those requiring mechanical ventilation had higher hair cortisol levels than non-ventilated term infants. Palmer et al. (2013) found significantly higher hair cortisol levels in African American infants compared to Caucasian infants at 1 year of age [15], correlated with measures of maternal prenatal adversity, maternal postpartum depression, parenting stress and the child’s socioemotional development at age 1 year [15]. Among preschool children, hair cortisol levels were negatively correlated with the parent’s educational level, but not parental income [16]. Longitudinal studies found a natural decrease in hair cortisol levels with increasing age from 1 to 8 years [17]. Groneveld et al. (2013) reported that hair cortisol levels increased in children after starting school, with greater increases among the children who were fearful before starting school [18].
Despite these studies, the reported analytical methods and hair cortisol values vary significantly between laboratories [19] Thus, it is difficult to develop normative values for children across different ages or investigate hypotheses with long-term developmental effects. Factors that can influence hair cortisol levels include preterm birth and nutritional status [20] in addition to the frequency of hair washing, use of emollients and creams (which may contain steroids), race, socioeconomic status, and biological characteristics of the hair collected [3, 15, 21, 22].
We present three methodological variations in the ELISA-based measurement of hair cortisol. Specifically, our aims were to (1) compare hair cortisol and protein levels between finely cutting (standard) and milled methods for hair preparation (n=16), (2) investigate the fractions of hair protein and hair cortisol extracted by alternating incubations in methanol and acetone, and (3) compare hair cortisol levels between ELISA and LCMS testing methods (n=28). We postulated that there would be no differences in the hair cortisol extracted and levels measured by these methodological variations. Hair cortisol data based on a single extraction may measure partial cortisol content. Although each laboratory can establish reference ranges based on populations they serve, however, similar amounts of cortisol may not be extracted from each sample because of differences between individual hair samples (such as hair texture, color, culturally-dependent cleaning practices, or other factors). Extracting all the cortisol content from each hair sample will generate more precise values, quantitative reference ranges, and may reveal the hair-related factors that lead to cortisol differences between hair samples. Previously used methods using a single extraction have greater time economy, but cannot guarantee accuracy. A lack of consistency in hair cortisol data from different laboratories using single extraction methods contributes to greater variability and inconsistency in the reported reference ranges, an inability to perform quantitative meta-analyses, or to examine age-related changes.
Materials and Methods
Testing Strategy
Analyses were conducted to test our hypotheses on two sets of samples. First, 16 hair samples from individual children were used to compare standard vs. milled methods for hair preparation and cortisol/protein extraction. The standard method involves finely cutting the hair to a powder consistency and the milled method includes mechanically grinding the hair to a powder. Protein and cortisol levels were detected in the reconstituted residue from each sample. Second, the ELISA vs. LCMS testing methods were compared for measuring hair cortisol in 28 samples, obtained from individual subjects (n=13), internal controls from the ELISA kit (n=3), and pooled hair samples (n=12) derived from the low, intermediate, or high ranges of cortisol levels (4 pooled samples from each range). Pooled samples, a common approach for assay validation with limited sample volumes [23], contained the hair residue extracts from 20 different subjects (remaining after the ELISA assay) that were combined for specific age groups if their cortisol values were within the low, intermediate, or high ranges defined a priori.
Human Subjects
After approval from the University of Tennessee Health Science Center’s Institutional Review Board (IRB), hair samples were obtained from children enrolled in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. This includes children residing in urban and suburban areas of Shelby County, Tennessee born to women between 16–40 years of age enrolled during the second trimester of pregnancy. To obtain a healthy child cohort, maternal exclusion criteria included existing chronic maternal disease of any kind (such as hypertension, diabetes, sickle cell disease etc.) and known pregnancy complications (such as pre-eclempsia, placenta previa, oligohydramnios). More detailed descriptions of the CANDLE cohort [24] and race distribution [15] were published earlier. Informed consent was given by the mothers or by their legally authorized representatives. Characteristics of the study participants used for hair cortisol analyses are listed in Table 1.
Table 1.
Demographic Data related to Individual Samples
| Standard vs. Milled methods |
ELISA vs. LC/MS methods* |
|
|---|---|---|
| Age groups | ||
| 12–17 months | - | 3 (23.1%) |
| 24–27 months | 3 (19%) | 3 (23.1%) |
| 36–40 months | 5 (31%) | 4 (30.7%) |
| 48–64 months | 8 (50%) | 3 (23.1%) |
| Sex | ||
| male | 3 (19%) | 8 (61.5%) |
| females | 13 (81%) | 5 (38.5%) |
| Race | ||
| African-Americans | 9 (56%) | 6 (46.2%) |
| Caucasians | 7 (44%) | 7 (53.8%) |
| Health Insurance | ||
| Medicaid/Tenncare | 7 (44%) | 7 (53.8 %) |
| Other (private, employer, military, none) | 9 (56%) | 6 (46.2%) |
Data for individual hair samples only (N=13)
Collection of Hair
Hair samples were cut as close to the scalp as possible from the posterior vertex of children (1–3 cm length), taped at the cut end, weighed in an analytical balance (Mettler-Toledo scale AL54, Greifensee, Switzerland), sealed in plastic bags and stored at room temperature (RT) until analysis. Only hair samples weighing 100 mg or more were used for the standard vs. milled comparisons. Each of 16 hair samples were divided into 2 equal parts with 50 mg reserved for each method.
Preparation of Hair
(a) Standard method
Pre-weighed hair was finely cut to a powder consistency using scissors (ROBOZ RS-5853; Gaithersburg, MD), then 4 successive extractions were performed on each hair sample (n=16): Hair was extracted alternating 1 mL of methanol incubated at 52°C for 15 hours, rotated at 200 rpm followed by 1 mL of acetone rotated at 200 rpm for 5 minutes at room temperature (RT). These extraction steps were repeated twice and the supernatants for each individual subject were pooled. Pooled supernatants from each sample were kept in an explosion-proof refrigerator (4°C) for air evaporation. The completely dried residue was reconstituted in phosphate-buffered saline (PBS) according the hair sample’s weight (i.e., 350 µl for 50 mg hair).
(b) Milled method
Hair samples from the same subjects (n=16) were precut to approximately 0.5 cm, milled at 20,000 rpm with 0.2 mm zirconium beads for 10 minutes using a Bullet Blender (Next Advance Inc., Averill Park, NY), followed by the same 4-step extraction process as described above. Each of the four extracts was centrifuged at 10000 rpm for 10 minutes and supernatants were not pooled but collected in separate glass vials; hair protein and cortisol were measured separately in each of these four fractions. As with the standard method, supernatants from the milled samples were air evaporated at 4°C and the dried residues were reconstituted in PBS according to the hair weight and fraction (fraction 1 in 150 µl, fractions 2, 3, and 4 in 67 µl each; total 350 µl for 50 mg hair).
Protein and Cortisol Quantification
Measurements of the protein levels extracted from hair indicate the hair dissolution state for the release of cortisol. Therefore, total protein yield (mcg/ml) of supernatants isolated using the standard method was compared to the total protein levels from the milled method fractions. The Epoch BioTek® plate reader with Nanodrop attachment was used to read protein concentrations by calculating the ratio of spectrophotometric absorption at 260 nm and 280 nm. A calibration curve is not required for this method. We used the Take 3, Gen5 2.05 program (BioTeK plate reader software, Winooski, VT) for calculating protein concentrations (mcg/ml).
Total cortisol yield of standard method supernatants was compared to the individual and total cortisol levels from the milled samples. Hair cortisol was quantified with a salivary cortisol ELISA assay kit (ALPCO Diagnostics, Salem, NH), according to the manufacturer’s instructions. The Epoch BioTek® plate reader (BioTeK Instruments, Winooski, VT) was used to quantify samples against a generated standard curve (ng/ml). Our intra- and inter-assay coefficients of variation were 6% and 7% respectively.
Mass Spectroscopy
A representative set of 28 samples was sent to the Wisconsin National Primate Center (University of Wisconsin, Madison) for confirmation of cortisol expression and evaluation of the presence of cortisone by LCMS, as reported previously [25]. The antibody in the ELISA cortisol kit (ALPCO Diagnostics, Inc.) reports a cross-reactivity of 6.2% with cortisone; thus, it was important to determine if the levels of detected cortisol may have been influenced by cortisone cross-reactivity. We evaluated hair cortisone since our goal was to establish accurate reference intervals of hair cortisol content for the age groups and ethnicities of the CANDLE subjects. All samples were first evaluated using the ELISA assay prior to evaluation by LCMS. The sample set included 13 individual hair samples, 3 positive controls from the ELISA kit at high (100 ng/ml, standard), intermediate (47.4 ng/ml quality control-1), and low (11.7 ng/ml, quality control-2) concentrations, and 12 pooled hair samples (each consisting of 20 individual samples with known cortisol ranges per pool; see Table 1 for details). Technicians at the Wisconsin National Primate Center were blinded to the sample type and reported cortisol and cortisone levels in ng/ml of the reconstituted hair extracts.
Statistical Analyses
GraphPad Prism version 6.0d (GraphPad Software, La Jolla, CA) was used to perform descriptive statistics, Bland-Altman plots and Spearman rank correlations (rs). Non-parametric tests were used because the values for hair protein and hair cortisol did not satisfy the conditions of a normal distribution. Measurements of each milled hair fraction (1–4) were calculated for the percentage of protein and cortisol obtained from that fraction, compared to the total amounts obtained from standard assay of the same hair sample. Percent differences between the standard vs. milled method were plotted for paired cortisol and protein levels using the formula: (standard value minus milled value)/standard value) ×100. Similarly, the formula for the percent differences between the ELISA and LCMS measures of cortisol content was (ELISA value minus LSMS value)/ELISA value) × 100. Levels of cortisol and cortisone detected by LCMS in each sample were totaled to calculate the percentage of cortisone measured in each sample and descriptive statistics were obtained from these data.
Spearman rank correlations (rs) with 99% confidence intervals (CI) were used to assess the relationships between hair protein and hair cortisol levels and between the ELISA and LCMS methods for measuring hair cortisol content. Bland-Altman plots with 95% limits of agreement were used to compare the standard (S) vs. milled (M) methods and the ELISA vs. LCMS testing methods for cortisol detection and to determine the mean bias from differences between the values measured by these two methods. Narrow limits (i.e., small biases) would indicate that the two methods used for detection of cortisol levels were equivalent. The level of significance was set at p<0.05.
Results
The percentages of cortisol and protein levels extracted by each step (Fractions 1–4) of the milled extraction in 16 hair samples are listed in Table 2. Highly significant correlations occurred between the standard and milled methods measuring hair cortisol (ng/ml) and protein (mcg/ ml) content (Table 3). Higher cortisol yields occurred from the standard (103±98 ng/ml) vs. milled (68±94 ng/ml, p=0.0453) methods in 13/16 samples and higher protein yields occurred from the standard (5.98±5.54 mg/ml) vs. milled methods (4.36±4.41 mg/ml, p=0.0387) in 14/16 samples (Figure 1). Bland-Altman bias (mean±SD) are reported and plots were used to assess the level of agreement between the standard and milled methods for both cortisol (Figure 2; bias = 78±77) and protein (Figure 3; bias = 35.2±31.8). Between the two methods, the mean differences in cortisol values were 34.4±63.0 ng/ml and in protein values were 1.62±2.86 mcg/ml, with the standard method yielding higher values compared to the milled method.
Table 2.
Percent of Protein and Cortisol content extracted in each fraction
| Protein (n=16) | Cortisol (n=16) | |||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Fraction 1 | 38.7 (11.6) | 36.5 (32.5, 40.0) | 46.1 (24.7) | 45.4 (39.8, 65.1) |
| Fraction 2 | 26.0 (7.6) | 27.5 (21.7, 31.4) | 32.3 (19.3) | 31.1 (21.0, 39.6) |
| Fraction 3 | 32.0 (13.3) | 30.5 (25.5, 36.9) | 21.0 (23.1) | 15.1 (11.3, 28.0) |
| Fraction 4 | 3.3 (1.6) | 3.1 (2.5, 5.3) | 0.6 (0.9) | 0.04 (0.0, 0.86) |
Table 3.
Correlation of Protein and Cortisol values from Standard (S) vs. Milled (M) methods
| M-Protein | M-Cortisol | |||
|---|---|---|---|---|
| rs* | p-value | rs* | p-value | |
| M-Protein | - | - | 0.699 | 0.0142 |
| M-Cortisol | 0.699 | 0.0142 | - | - |
| S-Protein | 0.902 | 0.0002 | 0.552 | 0.0667 |
| S-Cortisol | 0.755 | 0.0062 | 0.951 | 0.0000 |
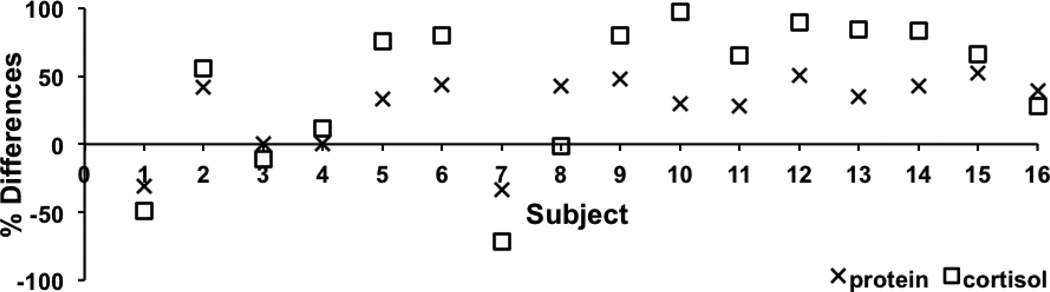
Figure 1.
Percent differences of Protein and Cortisol values per subject between Standard vs. Milled methods (calculated using the formula = (standard value minus milled value)/standard value) × 100) suggest that percent differences of hair protein and cortisol between the standard and milled methods both occurred in the same direction.
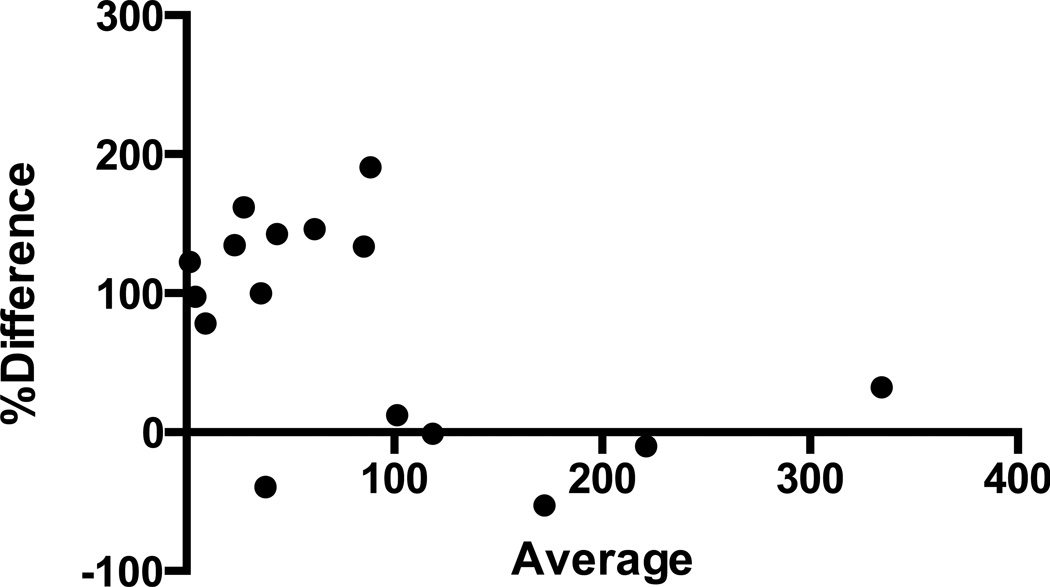
Figure 2.
The Bland-Altman plot for percent difference vs. average values of cortisol measured by standard and milled methods show that hair cortisol extracted by our standard method had greater yields in 13/16 samples (95% agreement limits −72.98, +228.9).
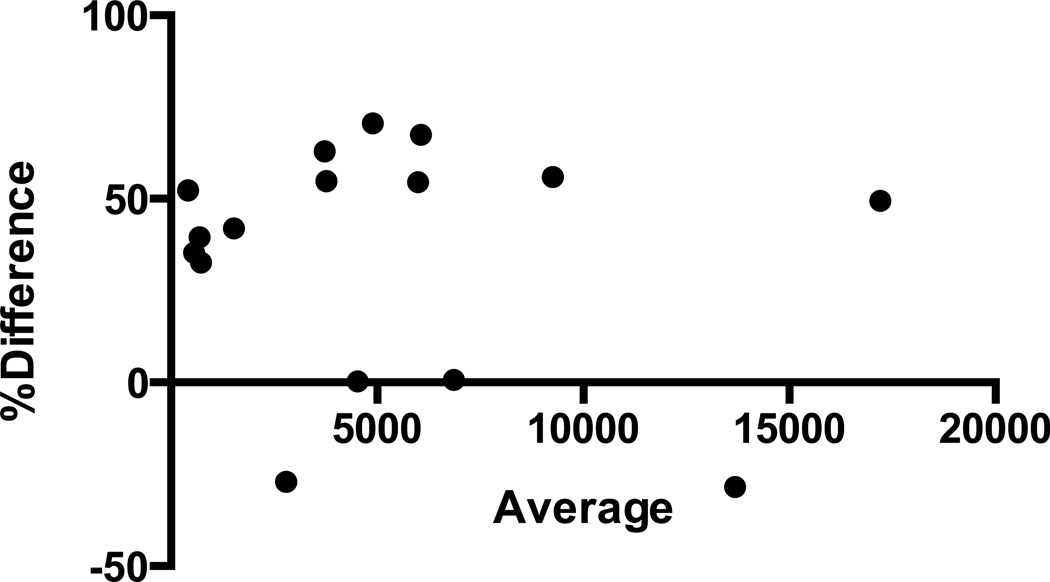
Figure 3.
The Bland-Altman plot for percent difference vs. average values of protein measured by standard and milled methods demonstrates that the hair protein extracted by our standard method had greater yields in 14/16 samples (95% agreement limits −27.07, +97.44).
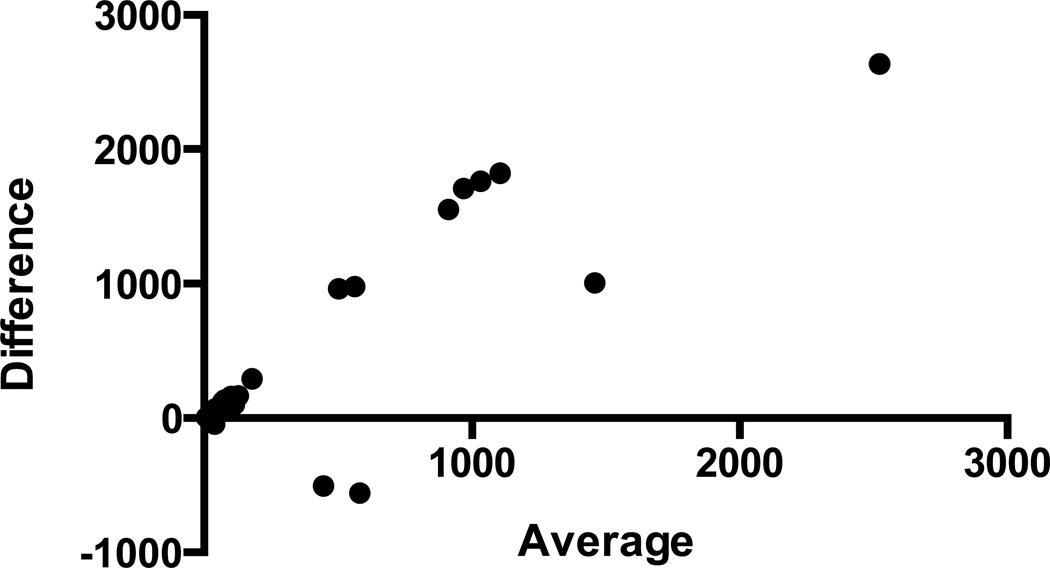
ELISA cortisol values were correlated with LCMS cortisol (rs=0.737; p<0.0001) and cortisone values (rs=0.636; p=0.0003). The LCMS cortisol vs. cortisone values were also correlated (rs=0.735; p<0.0001). Pooled hair samples showed the greatest correlation between the two testing methods (rs=0.972, p<0.0001). In the positive controls provided by the ELISA assay kit, the LCMS method detected lower levels of cortisol (11.7 vs. 6.7, 47.6 vs. 16.7, 100 vs. 48.8 for ELISA vs. LCMS, respectively) and spuriously detected some amounts of cortisone (25%, 11%, 5%) in these samples. On average, lower cortisol levels (median [IQR]) were detected by LCMS (38.7 ng/ml [13.4–130.8]) than by ELISA (172.2 ng/ml [66.8–1034]) and 13.4% [3.7%–25.9%] of the steroids detected by LCMS were measured as cortisone across all the samples (N=28). Bland-Altman plots showed a large bias between the two methods (101.3±96.6 ng/ml, mean±SD) when percent-differences between the two values were plotted against the average of the two values (Figure 4), whereas absolute differences between the cortisol values reported by the two testing methods appeared to be related to the averages of both values (Figure 5).
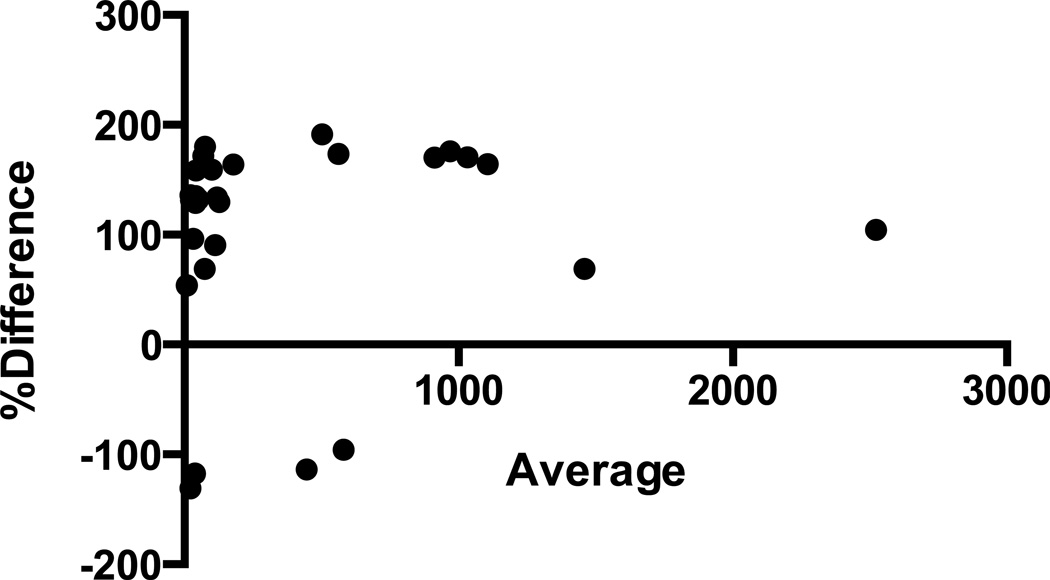
Figure 4.
Bland-Altman plot showing average values of cortisol derived from the ELISA and LCMS methods presented on the x-axis and percent differences between the two methods presented on the y-axis. The scatterplot shows no relationship between the percent differences and average cortisol values (95% agreement limits −88, +291).
Figure 5.
Bland-Altman plot showing average values of cortisol derived from the ELISA and LCMS methods presented on the x-axis and absolute differences between the two methods presented on the y-axis. Scatterplot suggests a positive relationship between the absolute differences and average cortisol values (95% agreement limits −1075, +1991).
Discussion
Identifying hair cortisol as a putative marker for chronic stress has led to several different laboratories measuring and reporting cortisol values from hair samples in children. A single-phase extraction procedure is commonly performed to capture hair cortisol content for quantification (Table 4). Our data suggest that repeated extractions with methanol and acetone are required to maximize the extraction of cortisol and protein from human hair. Caucasian and Asian hair mostly contains protein, whereas African hair contains significant amounts of lipid moieties as well [26]. Methanol extraction denatures the protein by breaking non-covalent bonds, thus allowing release of hair cortisol. When heated to 52°C in methanol, Africoid hair forms clumps, thus not allowing cortisol to be released into the supernatant. Acetone solubilizes the lipids in these hair clumps, by breaking annular and non-annular lipid-protein bonds. Hair dissolution is simply a marker for the amounts of cortisol released from the hair sample. A 5-minute acetone wash is the minimum time required to dissociate the hair, prevent further clumping, while also ensuring that acetone does not over-dry the precipitated protein. Alternating between methanol and acetone exposures improves the effectiveness of these chemical processes. Acetone also dissolves the lipid/protein film on the inner surface of the glass vial, speeds the evaporation time of supernatant and increases the yield of residue to be solubilized in PBS.
Table 4.
Analytical Methods used for the Measurement of Hair Cortisol: Summary of the Published Literature
| Author Name(s) |
Year | Journal Name |
Extraction method | Purification method | Detection method | Comments |
|---|---|---|---|---|---|---|
| Russell, Kirschbaum, Laundenslager [19] | 2014 | Ther Drug Monit |
Washing:
isopropanol 2–3 times, 3 min-Dresden, Colorado, & Western NW- Erasmus Cutting: CH-Western & Erasmus MH-Dresden & Colorado Extraction: All groups-MeOH |
n/a |
ELISA:
Western- (Alpco Salem, NH), Colorado (Salimetric, Pennsylvania), Erasmus- (DRG Instruments GmbH, Marburg, Germany). Luminescence immunoassay: Dresden- (IBL International, Hamburg, Germany) LCMS: Dresden & Erasmus |
Method comparison: 4 labs Unit: ng/g Subjects: human hair- Dresden, Western, Erasmus vervet monkey- Colorado Age: not reported |
| Noppe, Van Rossum, Koper [27] | 2014 | Horm Res Paediatr | NW; CH, 25–40 mg; MeOH 16 hr, 52°C; evap, 37°C, N2; 250 µl PBS | n/a | ELISA range, 2–80 ng/ml (DRG Instruments GmbH, Marburg, Germany) |
Unit: pg/mg Subjects: children Age: 4–14 yr Sex: mixed Race: Caucasian |
| Simmons, Whittle, Patton [34] | 2014 | BMC Peds | Isopropanol wash, 3min, 2–3 times; MH, 50 mg; MeOH, 24 hr, 25°C; evap, 37°C N2; 4 ml PBS | n/a | ELISA range, 0.012–3.000 µg/dL (Salimetric,Pennsylvania) |
Unit: not reported Subjects: children Age: m=9.51yr Sex: mixed Race: not reported |
| Steude, Kirschbaum, Gao [35] | 2013 | Biol Psychiatry | Isopropanol wash; MH, 10 mg; MeOH 18 hr, 45°C; evap conditions not reported; 250 µl ddH20 | n/a | LCMS |
Unit: pg/mg Subjects: adults Age: m=36.41 Sex: mixed Race: Caucasian |
| Palmer, Anand, Graff [15] | 2013 | J Peds | NW; CH, 5–50 mg; MeOH, 52°C, overnight (2–3 times); air evap 4°C; PBS (150 µl/10mg) | n/a | ELISA range, 0–100 ng/ml, (Alpco Diagnostics, Salem, NH) |
Unit: ng/mg Subjects: Children Age: 1 yr Sex: mixed Race: African & Caucasian |
| Grunau, Cepada, Chau [36] | 2013 | PLOS One | Isopropanol wash (twice); CH, 10–15 mg; MeOH, 16 hr, 50°C; evap, hot plate N2; 250 µl PBS | n/a | ELISA range, 0–100 ng/ml, (Alpco Salem, NH) |
Unit: pg/mg Subjects: Children Age: mean= 7.8 yrs Sex: mixed Race: Caucasian & Asian |
| Hinkelmann Muhtz, Dettenborn [37] | 2013 | Biol Psychiatry | Isopropanol wash; 10 mg hair; MeOH, 18 hr, 45°C; 250 µl ddH2O | n/a | LCMS |
Unit: pg/mg Subjects: Humans Age: not reported Sex: not reported Race: not reported |
| Karlen, Frostell, Theodorsson [17] | 2013 | Peds | NW; MH, at least >5mg hair; N2 frozen; MeOH overnight, 25°C (once); evap, vac; 150 µl 0.1 mol/L PBS, 0.02% BS | n/a | RIA |
Unit: pg/mg Subjects: Children Age: 1,3,5,8 yr Sex: mixed Race: Caucasian |
| Vaghri, Guhn, Weinburg [16] | 2013 | Psychoneuroendocrino | Isopropanol wash (twice); MH, 30 mg hair; MeOH 24 hr, 25°C (twice); evap, air dried; ELISA kit dil | n/a | ELISA range, 0.012–3.000 µg/dL (Salimetric, Pennsylvania) |
Unit: ng/mg Subjects: children Age: 4–6 yr Sex: mixed Race: mixed |
| Groeneveld, Vermeer, Linting [18] | 2013 | Stress | NW; CH, 20–30 mg; MeOH, 16 hr, 52°C; evap N2; 250 µl PBS |
n/a | ELISA range, 2–80 ng/ml (DRG Instruments GmbH, Marburg, Germany) |
Unit: pg/mg Subjects: Children Age: 4–5 yr Sex: mixed Race: Caucasian |
| Manenschijn, Koper, van den Akker [38] | 2012 | J Clin Endocrinol Metab |
NW; CH, 10 mg; MeOH, 16 hr, 52°C; evap; PBS dil (volume not reported) |
n/a | ELISA range, 2–80 ng/ml (DRG GmbH, Marburg, Germany) |
Unit: pg/mg Subjects: children & adults Age: 10–76 yr Sex: mixed Race: Caucasian |
| Li, Xie, Gao [39] | 2012 | Clinica Chimica Acta | MeOH wash, then either: a) UV irradiation @ 254 nm; b) in hot water (40, 65, 80°C), or c) immersed in 10% shampoo solution, 4 hr; 20 mg hair; MeOH, 24 hr, 40°C; N2 dried; 50µl MeOH & 1ml H20 | MeOH elution | HPLC/MS |
Unit: pg/mg Subjects: Adolescents Age: 14–18 yr Sex: mixed Race: Asian |
| D’Anna – Hernandezez, Ross, Natvig [40] | 2011 | Physiol Behav | Isopropanol wash; MH, 50 mg; MeOH 24 hr, 25°C; N2 dried; 400 µl assay buffer | n/a | ELISA range 0.003–3.0 µg/dl (Salimetric, Pennsylvania) |
Unit: pg/mg Subjects: Pregnant females Age: 18–45 yr Sex: female Race: not reported |
| Karlen, Ludvigsson, Frostell [41] | 2011 | BMC Clin Path | NW, 5–17 mg; N2 frozen, MH; MeOH, 10 hr, 25°C (once); evap, N2; PBS, 0.02% BSA (150 µl) | n/a | RIA |
Unit: pg/mg Subjects: Humans Age: not reported Sex: mixed Race: not reported |
| Gao, Xie, Jin [42] | 2010 | Clin Biochem | MeOH wash, 2 min; MH, 50 mg; Soerensen buffer or 0.1 M NaCl or 0.1M NaOH); 16 hr, 40°C; N2 evap; 50 µl MeOH | MeOH | HPLC |
Unit: pg/mg Subjects: adults Age: not reported Sex: mixed Race: Asian |
| Raul, Cirmele, Ludes [43] | 2004 | Clin Biochem | Dichloromethane wash, 2 min (twice); MH, 30–100 mg; Sorensen buffer, 16 hr, 40°C; 5 ml NaOH 0.2M | column eluted | HPLC/MS |
Unit: pg/mg Subjects: Humans Age: not reported Sex: mixed Race: not reported |
Abbreviations: Not applicable (n/a) Milled Hair (MH); Cut Hair (CH); No Wash (NW); Methanol (MeOH); Methylene Chloride (CH2Cl2); hours (hr); minutes (min); Room Temperature (RT); Liquid (LQ), Evaporation (evap); Diluent (dil); Phosphate Buffer Saline (PBS); High Performance Liquid Chromatography (HPLC); Liquid Chromatography (LC) Mass Spectrometer (MS); Radioimmunoassay (RIA); Nitrogen (N2); Bovine Serum (BS); Bovine Serum Albumin (BSA); double-distilled (dd) water (H20); Mean (m); Molar (M); Vacuum (vac)
Further, we found that milling or grinding the hair does not extract more cortisol than by finely cutting the hair and that ELISA-based assays yielded higher cortisol values compared to LCMS, despite greater analytical specificity of the latter method. Before hair cortisol levels can be used as a biomarker for HPA axis development or chronic stress in early childhood, methodological considerations must be applied to ensure the accuracy and reproducibility of the reported data.
Despite meticulous transfers of supernatant, the milling process may lead to sample escaping from the sealed tubes, or the pitting, scratching, and plastic folding or rippling in the tube caused by zirconium beads may prevent exposure of hair particles to the methanol/acetone. Chemical degradation of hair protein and/or steroids may also occur during the milling process. These results imply that the standard method of finely cutting the hair in glass vials is more effective for extraction of protein and cortisol than the milled method. In previous pilot studies (unpublished) conducted within our laboratory, we continued hair sample extractions and analyzed each fraction until a zero level of detection for cortisol was reached, which typically occurred in fraction 3 or 4. Pilot studies showed that fractions 5 or 6 (using additional acetone incubations for 5 minutes at RT) yielded <5 mcg/ml of protein in Fraction 5 and none in Fraction 6 and yielded no detectable levels of cortisol.
Hair dissolution in other laboratories occurs by incubating hair in methanol at varying temperatures (RT or 52°C) [16–18], [19, 27]), although multiple variations in the methods for extraction and detection have been reported (see Table 4). Our data suggest that a single methanol extraction may yield on average 46.1% of cortisol or 38.7% of protein content. Many laboratories fail to adjust for inherent differences of protein/lipid bonding between different ethnicities that may contribute to varying ratios of cortisol extraction in single-phase extractions. A 4-step process to extract protein and cortisol from hair, modeled after standard methods for tissue protein/RNA extraction [28], results in higher cumulative extraction of protein and cortisol (98–100%), arguably leading to more accurate readings for total hair cortisol content. Increasing the accuracy of extraction and analysis methods for cortisol levels in hair is most important before we can establish reference ranges for hair cortisol in children.
Other researchers have also compared ELISA and LCMS-based methods for measuring hair cortisol levels from humans and primates. In a round robin analysis, four different laboratories used specific ELISA-based assays and found high correlations in the measured cortisol values (R2 = 0.91–0.98; all p<0.0001) [19]. The cortisol values measured by LCMS in two laboratories also showed high correlation (R2 = 0.9829, p<0.0001), whereas the ELISA and LCMS values showed lower correlations (R2 = 0.89–0.98; p<0.0001) [19]. Similar to our findings, the round robin reported greater sensitivity in ELISA-based assays than LCMS methods [19]. Pooled hair samples from our study showed greater correlation between the two testing methods (r=0.972, p<0.0001), whereas individual hair samples showed moderate correlation across the two methods (r=0.665, p=0.016). We hypothesize that variable rates of degradation may be associated with the protein content in the reconstituted hair extract. Indeed other labs have found that adding protein in their reconstitution cocktail favors cortisol detection (see Table 4, protein added as bovine serum albumin (BSA)). Thus, hair samples with higher protein content, irrelevant of the cortisol level, may have a slower decay rate for loss of the cortisol signal. If this is true, then the law of averages would tend to protect the cortisol content of pooled samples more than that of individual samples, thus explaining the greater correlation between pooled vs. individual samples across the two testing methods in our study.
Our studies on hair cortisol methods have both advantages and limitations. One advantage is that single extractions using methanol for an overnight incubation may be insufficient to capture all the available cortisol in hair samples, with different assays detecting between 40% and 65% cortisol under these conditions. A second advantage is that pre-washing the hair sample with alcohol has little to marginal effect on hair cortisol content, thus eliminating an unnecessary extraction step. Other investigators reported that cortisol arising from sweat or cortisol solutions is rapidly absorbed into the hair shaft [1]. Therefore, hair samples should not be collected from children just after strenuous activity, when the release of exercise-induced cortisol and sweat may affect hair cortisol values. Finally, another advantage of this study is that the comparison of standard vs. milled methods included 9 African-Americans and 7 Caucasians, whereas the comparison of ELISA vs. LCMS methods included 6 African-Americans and 7 Caucasians, thus accounting for racially dependent variations in hair type in our methods [15].
One limitation in the ELISA-based assay has a known cross-reactivity of the antibody with progesterone (7.2%) and cortisone (6.2%) [29]. However, our hair samples came from children at 1–4 years of age, who were unlikely to have significant progesterone levels and thus would have minimal cross-reactivity at this age [30]. Another limitation is that some of the hair cortisol can be converted to cortisone. The enzyme 11-β-hydroxysteroid dehydrogenase (11β-HSD) metabolizes cortisol to cortisone and vice-versa [31]. The 11β-HSD1 isoform converts cortisone to cortisol, while 11β-HSD2 isoform converts cortisol to cortisone [31]. The 11β-HSD1 isoform is expressed in keratinocytes, dermal mesenchymal cells, and outer root sheath follicles while expression of the 11β-HSD2 gene remains at the background level according to one report [32], while it is detectable at the protein level according to another [33]. Thus inter-conversion of cortisol to cortisone possibly occurs in the skin. It is unlikely, however, that this conversion occurs in the cortisol bound to hair, since the hair shaft is a non-viable structure produced by the hair follicle. Furthermore, the LCMS analyses of our individual and pooled hair samples showed relatively low concentrations of cortisone (on average cortisone comprised 12% (median 13.4%) of the total steroids detected). If 6.2% of this cortisone cross-reacts with the cortisol antibody in the ELISA assay, then the measured cortisol levels would be increased by <1% because of cortisone cross-reactivity in the cortisol ELISA assay, which can be considered minimal.
Conclusion
Further refinements in the methods used for hair cortisol analysis may be required before the data reported in the clinical literature can be considered precise enough for clinical decision-making, or for establishing reference ranges for different age groups. These analyses will be useful for examining early HPA axis development or function, or for determining the long-term effects of chronic stress during early childhood in life-course studies. We propose methods that include finely cutting the hair for processing samples, a four-step extraction procedure to maximize the amount of cortisol extracted, as well as using ELISA-based assays developed specifically for human hair. These and other methodological improvements would allow hair cortisol levels to be a reliable and reproducible measure of chronic stress in childhood.
Acknowledgements
We gratefully acknowledge the commitment of the study participants and staff of the CANDLE Study, funding from The Urban Child Institute, Memphis TN to support the collection of hair samples analyzed in this study; the Principal Investigator for the CANDLE Study (Francis Tylavsky, PhD) and investigators at the University of Tennessee Health Science Center associated with designing and implementing the CANDLE study (Fred Palmer, MD, Laura D. Murphy, EdD, Carolyn Graff, PhD, Marion Hare, MD, Pamela Connor, PhD, Grant Somes, PhD, Owen Phillips, MD). All the laboratory work reported in this study was supported in part by unrestricted grants (to KJSA) from the Oxnard Foundation and Le Bonheur Children’s Hospital Foundation. The LCMS analyses reported were supported in part by the National Institutes of Health (Award Numbers P51OD011106, RR15459-01, RR020141-01) to the Wisconsin National Primate Center, University of Wisconsin-Madison.
Abbreviations
- LCMS
liquid chromatography mass spectroscopy
- ELISA
enzyme-linked immunosorbent assay
- RT
room temperature
- PBS
phosphate-buffered saline
- CANDLE
Conditions Affecting Neurocognitive Development and Learning in Early childhood).
References
- 1.Russell E, Koren G, Rieder M, Van Uum SH. The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Therapeutic drug monitoring. 2014;36:30–34. doi: 10.1097/FTD.0b013e31829daa0a. [DOI] [PubMed] [Google Scholar]
- 2.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Wosu AC, Valdimarsdottir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Annals of epidemiology. 2013;23:797–811. e792. doi: 10.1016/j.annepidem.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic science international. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 5.Loussouarn G. African hair growth parameters. The British journal of dermatology. 2001;145:294–297. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
- 6.Bevalot F, Gaillard Y, Lhermitte MA, Pepin G. Analysis of corticosteroids in hair by liquid chromatography-electrospray ionization mass spectrometry. Journal of chromatography B, Biomedical sciences and applications. 2000;740:227–236. doi: 10.1016/s0378-4347(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 7.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and comparative endocrinology. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien KM, Tronick EZ, Moore CL. Relationship between Hair Cortisol and Perceived Chronic Stress in a Diverse Sample. Stress and health : journal of the International Society for the Investigation of Stress. 2012 doi: 10.1002/smi.2475. [DOI] [PubMed] [Google Scholar]
- 9.Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, Sioen I, Iacoviello L, Gunther K, Molnar D, Lissner L, Rivet N, Raul JS, de Henauw S. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49:1072–1081. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 10.Cirimele V, Kintz P, Dumestre V, Goulle JP, Ludes B. Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. Forensic science international. 2000;107:381–388. doi: 10.1016/s0379-0738(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard Y, Vayssette F, Pepin G. Compared interest between hair analysis and urinalysis in doping controls. Results for amphetamines, corticosteroids and anabolic steroids in racing cyclists. Forensic science international. 2000;107:361–379. doi: 10.1016/s0379-0738(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 12.Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and investigative medicine Medecine clinique et experimentale. 2007;30:E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 13.Albar WF, Russell EW, Koren G, Rieder MJ, van Umm SH. Human hair cortisol analysis: comparison of the internationally-reported ELISA methods. Clinical and investigative medicine Medecine clinique et experimentale. 2013;36:E312–E316. doi: 10.25011/cim.v36i6.20629. [DOI] [PubMed] [Google Scholar]
- 14.Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 15.Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Volgyi E, Rovnaghi CR, Moore A, Tran QT, Tylavsky FA. Early adversity, socioemotional development, and stress in urban 1-year-old children. The Journal of pediatrics. 2013;163:1733–1739. e1731. doi: 10.1016/j.jpeds.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, Hertzman C. Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology. 2013;38:331–340. doi: 10.1016/j.psyneuen.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlen J, Frostell A, Theodorsson E, Faresjo T, Ludvigsson J. Maternal influence on child HPA axis: a prospective study of cortisol levels in hair. Pediatrics. 2013;132:e1333–e1340. doi: 10.1542/peds.2013-1178. [DOI] [PubMed] [Google Scholar]
- 18.Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EF, van IMH. Children's hair cortisol as a biomarker of stress at school entry. Stress. 2013;16:711–715. doi: 10.3109/10253890.2013.817553. [DOI] [PubMed] [Google Scholar]
- 19.Russell E, Kirschbaum C, Laudenslager ML, Stalder T, de Rijke Y, van Rossum EF, Van Uum S, Koren G. Toward Standardization of Hair Cortisol Measurement; Results of the First International Inter-laboratory Round Robin. Therapeutic drug monitoring. 2014 doi: 10.1097/FTD.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 20.Zain BK, Haquani AH, Qureshi N, el Nisa I. Studies on the significance of hair root protein and DNA in protein-calorie malnutrition. The American journal of clinical nutrition. 1977;30:1094–1097. doi: 10.1093/ajcn/30.7.1094. [DOI] [PubMed] [Google Scholar]
- 21.Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. Effects of shampoo and water washing on hair cortisol concentrations. Clinica chimica acta; international journal of clinical chemistry. 2011;412:382–385. doi: 10.1016/j.cca.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman MC, Karban LV, Benitez P, Goodteacher A, Laudenslager ML. Chemical processing and shampooing impact cortisol measured in human hair. Clinical and investigative medicine Medecine clinique et experimentale. 2014;37:E252. doi: 10.25011/cim.v37i4.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt DW, Lee T, Jones K, Johnston A. Validation of an assay for routine monitoring of sirolimus using HPLC with mass spectrometric detection. Clinical chemistry. 2000;46:1179–1183. [PubMed] [Google Scholar]
- 24.Volgyi E, Carroll KN, Hare ME, Ringwald-Smith K, Piyathilake C, Yoo W, Tylavsky FA. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients. 2013;5:1511–1530. doi: 10.3390/nu5051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor A, Lubach G, Hedman C, Ziegler TE, Coe CL. Hormones in infant rhesus monkeys' (Macaca mulatta) hair at birth provide a window into the fetal environment. Pediatric research. 2014;75:476–481. doi: 10.1038/pr.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz CF, Fernandes MM, Gomes AC, Coderch L, Marti M, Mendez S, Gales L, Azoia NG, Shimanovich U, Cavaco-Paulo A. Keratins and lipids in ethnic hair. International journal of cosmetic science. 2013;35:244–249. doi: 10.1111/ics.12035. [DOI] [PubMed] [Google Scholar]
- 27.Noppe G, Van Rossum EF, Koper JW, Manenschijn L, Bruining GJ, de Rijke YB, van den Akker EL. Validation and reference ranges of hair cortisol measurement in healthy children. Hormone research in paediatrics. 2014;82:97–102. doi: 10.1159/000362519. [DOI] [PubMed] [Google Scholar]
- 28.Evans MA. Role of protein binding in cocaine-induced hepatic necrosis. The Journal of pharmacology and experimental therapeutics. 1983;224:73–79. [PubMed] [Google Scholar]
- 29.ALPCO Cortisol Saliva ELISA for the Quantitative Determination of Cortisol in Human Saliva [package insert] Salem, NF 03079: 26-G Keewaydin Drive; 2013. ts@alpco.com. [Google Scholar]
- 30.Gray SH, Ebe LK, Feldman HA, Emans SJ, Osganian SK, Gordon CM, Laufer MR. Salivary progesterone levels before menarche: a prospective study of adolescent girls. The Journal of clinical endocrinology and metabolism. 2010;95:3507–3511. doi: 10.1210/jc.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase. Best practice & research Clinical endocrinology & metabolism. 2001;15:61–78. doi: 10.1053/beem.2000.0119. [DOI] [PubMed] [Google Scholar]
- 32.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. The Journal of investigative dermatology. 2011;131:30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 33.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. The British journal of dermatology. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons JG, Whittle SL, Patton GC, Dudgeon P, Olsson C, Byrne ML, Mundy LK, Seal ML, Allen NB. Study protocol: Imaging brain development in the Childhood to Adolescence Transition Study (iCATS) BMC pediatrics. 2014;14:115. doi: 10.1186/1471-2431-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, Stalder T. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biological psychiatry. 2013;74:639–646. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Grunau RE, Cepeda IL, Chau CM, Brummelte S, Weinberg J, Lavoie PM, Ladd M, Hirschfeld AF, Russell E, Koren G, Van Uum S, Brant R, Turvey SE. Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PloS one. 2013;8:e73926. doi: 10.1371/journal.pone.0073926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Wingenfeld K, Spitzer C, Gao W, Kirschbaum C, Wiedemann K, Otte C. Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biological psychiatry. 2013;74:e15–e17. doi: 10.1016/j.biopsych.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Manenschijn L, Koper JW, van den Akker EL, de Heide LJ, Geerdink EA, de Jong FH, Feelders RA, van Rossum EF. A novel tool in the diagnosis and follow-up of (cyclic) Cushing's syndrome: measurement of long-term cortisol in scalp hair. The Journal of clinical endocrinology and metabolism. 2012;97:E1836–E1843. doi: 10.1210/jc.2012-1852. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xie Q, Gao W, Xu Y, Wang S, Deng H, Lu Z. Time course of cortisol loss in hair segments under immersion in hot water. Clinica chimica acta; international journal of clinical chemistry. 2012;413:434–440. doi: 10.1016/j.cca.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 40.D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & behavior. 2011;104:348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlen J, Ludvigsson J, Frostell A, Theodorsson E, Faresjo T. Cortisol in hair measured in young adults - a biomarker of major life stressors? BMC clinical pathology. 2011;11:12. doi: 10.1186/1472-6890-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao W, Xie Q, Jin J, Qiao T, Wang H, Chen L, Deng H, Lu Z. HPLC-FLU detection of cortisol distribution in human hair. Clinical biochemistry. 2010;43:677–682. doi: 10.1016/j.clinbiochem.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical biochemistry. 2004;37:1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]