Abstract
Objectives
To determine if the ratio of positive to negative lymph nodes, or lymph node ratio (LNR), is a prognostic variable in patients with node-positive endometrial cancer and the impact of adjuvant therapy on survival.
Methods
After IRB approval, a retrospective review of patients diagnosed with stage IIIC endometrioid or mixed endometrioid endometrial cancer at a single institution from January 2000 through October 2011 was performed. Clinicopathologic and adjuvant treatment data was collected. Univariate and multivariate analysis were used to identify prognostic factors for progression-free (PFS) and overall survival (OS).
Results
One hundred twenty-four patients with stage IIIC1 (n=64) and IIIC2 (n=60) endometrial cancer were included in the analysis. Median age was 60 years (range 25-84) and median follow-up was 49.4 months (range 0.1-301.6). Age >70 years was identified as a prognostic factor for worse PFS (p=0.0002) and OS (p=0.0002) on multivariate analysis. Patients in this cohort receiving any adjuvant radiotherapy showed improved PFS (HR 0.34, 95% CI 0.13-0.90, p=0.03) compared to those receiving any adjuvant chemotherapy (HR 2.33, 95% CI 1.16-4.65, p=0.02). In a subgroup analysis, patients with ≥ 10 nodes removed (n=81) with a LNR >50% had a PFS of 25.2 months compared to 135.6 months with a LNR ≤50% (HR 3.87, 95% CI 1.15-13.04, p=0.03).
Conclusions
LNR may define a subgroup of stage IIIC endometrial cancers at increased risk for recurrence. Adjuvant radiotherapy was associated with decreased recurrence risk.
Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States with an estimated 49,560 new cases diagnosed in 2013 leading to an estimated 8,190 deaths. [1] The majority of patients is diagnosed with early stage (I-II) disease and has an excellent prognosis. However, patients that are diagnosed with advanced stage (III-IV) disease have been shown to have a significantly worse outcome due to variable responses to traditional therapies including chemotherapy and radiation. Involvement of the retroperitoneal lymph nodes is an important prognostic factor for survival, with an estimated 5-year disease-specific survival ranging from 10 to 75%. Wide survival estimates suggests potential heterogeneity in node-positive endometrial cancers and the need for better stratification to determine better treatment strategies. [2]
In 2008, the International Federation of Gynecology and Obstetrics (FIGO) revised staging for endometrial cancer, and stratified stage IIIC tumors based on positivity of either pelvic (IIIC1) or para-aortic (IIIC2) nodes. [3] This stratification has important implications for treatment planning, as multimodality therapy with both radiation and chemotherapy is now frequently used for women with nodal metastases. [4] However, significant toxicity can be associated with this multimodality therapy, and individualizing tailored therapies are preferred in low-risk patients to prevent overtreatment.
The extent of lymph node involvement is an important prognostic factor in most solid tumors including lung [5], breast [6], colorectal [7-8], cervical [9], and vulvar cancers [10]. The ratio of positive nodes to the total number of nodes harvested, the lymph node ratio, has been found to be an independent predictor of survival in pancreatic [11], esophageal [12], gastric [13], colorectal [14-15], and breast cancers [16-17]. There has been recent interest in using a lymph node ratio as a prognostic tool in endometrial cancer. This allows assessment of the comprehensive nature of lymphadenectomy and burden of nodal disease. Previous multi-center retrospective studies in endometrial cancer have found lymph node ratio to be associated with worse progression-free and overall survival. [2,18] These studies also highlighted that lymph node ratios are most meaningful when comprehensive lymphadenectomy is utilized routinely in surgical practice. [18]
The purpose of this study is to examine the relationship between lymph node ratio and progression-free and overall survival in endometrial cancer patients with predominant endometrioid histology from a large academic institution with central pathology review. The relationship between lymph node ratio and other important clinicopathologic factors was assessed. The impact of adjuvant therapy in node-positive endometrial cancers was also evaluated.
Materials and Methods
After Institutional Review Board approval, women with stage IIIC1 and IIIC2 endometrioid or mixed endometrioid endometrial cancer were identified from our institutional tumor registry at M.D. Anderson Cancer Center from January 2000 through October 2011. Patients with mixed histology were included if the non-endometrioid histology was a minor component (<50%) of the histology. All patients underwent hysterectomy, bilateral salpingoophorectomy, and lymphadenectomy. Lymphadenectomy was performed at the discretion of the surgeon at M.D. Anderson based on intraoperative frozen section of the hysterectomy specimen. At our institution, a lymphadenectomy is not routinely performed in patients with intraoperative frozen section assessment suggesting a grade 1 or 2 endometrioid adenocarcinoma, <50% myometrial invasion, and with tumor size <2cm. A pelvic lymphadenectomy was omitted in cases with intraoperative assessment suggesting high-risk factors requiring pelvic radiotherapy. Patients were included in the analysis if they had endometrioid or mixed endometrioid adenocarcinoma histology confirmed by M.D. Anderson pathology, and received adjuvant therapy with radiation and/or chemotherapy. Patients with pure non-endometrioid histologies such as serous or clear cell were excluded due to variations in the disease course and adjuvant therapy with these histologies. Demographic and clinicopathologic data was also abstracted from the patient's medical record.
Descriptive statistics were used to summarize the demographic and clinic characteristics of the patients. The product-limit method of Kaplan and Meier was used to estimate progression-free (PFS) and overall survival (OS). For PFS, an event will be disease progression, recurrence, or death. Cox proportional hazards regression was used to model PFS and OS as functions of potential prognostic factors, including age, stage, grade, chemotherapy, radiation therapy, number of lymph nodes removed, number of positive lymph nodes, and lymph node ratio. From the univariate analysis of OS and RFS we selected all factors with P<0.20 and included them in a saturated model. We then used backward elimination to remove factors from the model until all remaining factors were statistically significant. Patients with missing data for any factor considered in the model were excluded from the model. Age ≤70 and > 70 years was chosen as age-adjusted variables for both univariate and multivariate analysis based on prognostic implications previously reported in the literature. [19] Methods previously described by Williams et al. were used to identify the optimal cutpoint for lymph node ratio for PFS and OS. [20] Based on previously reported lymph node ratio cutoffs [2,18], a subset analysis was performed in patients who had at least 10 lymph nodes removed. Adjuvant radiation was tailored based on stage IIIC1 or stage IIIC2 disease. When pelvic only positive nodes were identified, patients received pelvic external beam radiation in a AP/PA field or 4-field technique. A total of 45 Gy was delivered to those areas at risk for microscopic disease. Up to 57 Gy was delivered to areas of gross disease or extracapsular extension. The pelvic treatment area generally extended superiorly to include L5. When lateral fields were used, the posterior border encompassed S2. When para-aortic nodes were identified, treatment fields were extended to include nodes up to T12. Chemotherapy regimens were not uniform but included standard combination regimens used in endometrial cancer therapy. The standard treatment regimen for node-positive endometrial cancer in our institution is typically radiation with concurrent weekly ciplatin followed by carboplatin and paclitaxel for 4 to 6 cycles. P<0.05 was considered statistically significant. All analyses were performed with SAS 9.1 for Windows (SAS Institute Inc, Cary, NC).
Results
One-hundred twenty-four patients with stage IIIC endometrioid or mixed endometrioid endometrial cancer were identified and included in the analysis. Clinicopathologic data is shown in Table 1. Median age was 60 years (range 25-84), median body mass index (BMI) was 30.1 (range 18.8-62.2), and median follow-up was 49.4 months (range 0.1-301.6 months). Ninety-two patients (74%) underwent staging by open laparotomy compared to 32 patients (26%) by minimally invasive surgical methods. One hundred seventeen patients (94%) underwent pelvic lymphadenectomy and 97 patients (78%) underwent para-aortic lymphadenectomy. Median number of pelvic nodes removed was 9 (range 0-49) and para-aortic nodes was 3 (range 0-24). Sixty-four patients (52%) were diagnosed with stage IIIC1 and 60 patients (48%) with stage IIIC2 disease. Fifty-one patients (41%) received adjuvant radiation alone, 60 patients (48%) combined chemotherapy and radiation, and 6 patients (5%) chemotherapy alone. Details of adjuvant therapy was unknown in 7 patients. In the entire series, 39 patients (31%) recurred.
Table 1. Demographic and clinical characteristics.
| Total | < 10 Lymph Nodes N=43 | ≥ 10 Lymph Nodes N=81 | p-value | |
|---|---|---|---|---|
|
|
|
|
|
|
| Median age, years (range) | 60 (25.4-83.5) | 62.8 (25.4-83.5) | 59.4 (35.9-82.5) | 0.25 |
| Median BMI (range) | 30.1 (18.8-62.2) | 29.9 (20.9-59.5) | 30.3 (18.8-62.2) | 0.40 |
| Stage | 0.35 | |||
| IIIC1 | 64 (52%) | 25 (58%) | 39 (48%) | |
| IIIC2 | 60 (48%) | 18 (42%) | 42 (52%) | |
| Histology | 0.99 | |||
| Endometrioid | 104 (84%) | 36 (83.7%) | 68 (84%) | |
| Mixed | 20 (16%) | 7 (16.3%) | 13 (16%) | |
| Grade | 0.57 | |||
| 1 | 6 (5%) | 3 (7%) | 3 (4%) | |
| 2 | 78 (63%) | 25 (58%) | 53 (65%) | |
| 3 | 38 (31%) | 14 (33%) | 24 (30%) | |
| Unknown | 2 (1%) | 1 (2%) | 1 (1%) | |
| LVSI | 0.99 | |||
| No | 6 (5%) | 2 (5%) | 4 (5%) | |
| Yes | 99 (80%) | 32 (74%) | 67 (83%) | |
| Unknown | 19 (15%) | 9 (21%) | 10 (12%) | |
| Adjuvant therapy | 0.004 | |||
| Chemotherapy alone | 6 (5%) | 4 (9%) | 2 (3%) | |
| Radiation alone | 51 (41%) | 24 (56%) | 27 (33%) | |
| Chemo + radiation | 60 (48%) | 12 (28%) | 48 (59%) | |
| Unknown | 7 (6%) | 3 (7%) | 4 (5%) | |
| Recurrence | 0.22 | |||
| No | 85 (69%) | 26 (60%) | 59 (73%) | |
| Yes | 39 (31%) | 17 (40%) | 22 (27%) | |
| LNR | < 0.0001 | |||
| ≤50% | 106 (85%) | 28 (65%) | 78 (96%) | |
| >50% | 18 (15%) | 15 (35%) | 3 (4%) |
Our statistical methods identified LNR of 50% and 40% as cutpoints for PFS and OS, respectively. Those patients with a LNR >50% did not show any statistical difference in PFS compared to those with a LNR ≤50% (38.4 vs. 160.8 months, HR=1.92, 95% CI 0.99-3.72, p=NS; Table 2). Similarly, on analysis for OS, those patients with a LNR >40% did not show any statistical difference in OS compared to those with a LNR ≤40% (116.4 vs. 199.2 months, HR=1.66, 95% CI 0.67-4.12, p=NS; Table 3). A subset analysis was performed on those patients that had at least ≥ 10 nodes removed and based on LNR cutoffs previously reported in the literature of 0-10%, >10-50%, and >50%. [2,12] In patients with ≥ 10 nodes removed (n=81), a LNR >50% had a PFS of 25.2 months compared to 135.6 months with a LNR ≤50% (HR 3.87, 95% CI 1.15-13.04, p=0.03). These differences were not seen in OS analysis when stratifying by ≥ 10 nodes and previous defined LNR cutoffs (Tables 2 and 3).
Table 2. Univariate and multivariate analyses for PFS.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. patients | Median PFS (months) | HR | 95% CI | p-value | HR | 95% CI | p-value | |
|
|
|
|
|
|
|
|
|
|
| Age | ||||||||
| ≤ 70 | 102 | 160.8 | Ref | --- | --- | Ref | --- | --- |
| >70 | 22 | 43.2 | 3.75 | 2.07-6.77 | 0.0001 | 4.62 | 2.30-9.25 | <.0001 |
| Any Chemo | ||||||||
| No | 51 | 135.6 | Ref | --- | --- | Ref | --- | --- |
| Yes | 66 | 63.6 | 1.52 | 0.81-2.84 | 0.19 | 2.33 | 1.16-4.65 | 0.02 |
| Any RT | ||||||||
| No | 7 | 31.2 | Ref | --- | --- | Ref | --- | --- |
| Yes | 111 | 135.6 | 0.37 | 0.15-3.96 | 0.04 | 0.34 | 0.13-0.90 | 0.03 |
| LNR | ||||||||
| ≤50% | 106 | 160.8 | Ref | --- | --- | |||
| >50% | 18 | 38.4 | 1.92 | 0.99-3.72 | 0.30 | |||
|
| ||||||||
| Patients with ≥10 nodes removed | ||||||||
| LNR | ||||||||
| 0-10% | 36 | NR | Ref | --- | --- | |||
| >10-50% | 42 | 135.6 | 1.21 | 0.52-2.81 | 0.65 | |||
| >50% | 3 | 25.2 | 4.33 | 1.16-16.14 | 0.03 | |||
Chemo=chemotherapy, RT=radiation, LNR=lymph node ratio, NR=not reached, NS=not significant
Table 3.
Univariate and multivariate analyses for OS.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. patients | Median OS (months) | HR | 95% CI | p-value | HR | 95% CI | p-value | |
|
|
|
|
|
|
|
|
|
|
| Age | ||||||||
| ≤ 70 | 102 | NR | Ref | --- | --- | Ref | --- | --- |
| >70 | 22 | 66 | 5.42 | 2.23-13.18 | 0.0002 | 5.42 | 2.23-13.18 | 0.0002 |
| Any Chemo | ||||||||
| No | 51 | 199.2 | Ref | --- | --- | |||
| Yes | 66 | NR | 1.51 | 0.60-3.82 | 0.38 | |||
| Any RT | ||||||||
| No | 7 | NR | Ref | --- | --- | |||
| Yes | 111 | 199.2 | 0.77 | 0.18-3.30 | 0.72 | |||
| LNR | ||||||||
| ≤40% | 102 | 199.2 | Ref | --- | --- | |||
| >40% | 22 | 116.4 | 1.66 | 0.67-4.12 | 0.30 | |||
|
| ||||||||
| Patients with ≥10 nodes removed | ||||||||
| LNR | ||||||||
| 0-10% | 36 | NR | Ref | --- | --- | |||
| >10-50% | 42 | 135.6 | 1.11 | 0.35-3.51 | 0.86 | |||
| >50% | 3 | NR | 2.51 | 0.29-21.77 | 0.40 | |||
Chemo=chemotherapy, RT=radiation, LNR=lymph node ratio, NR=not reached, NS=not significant
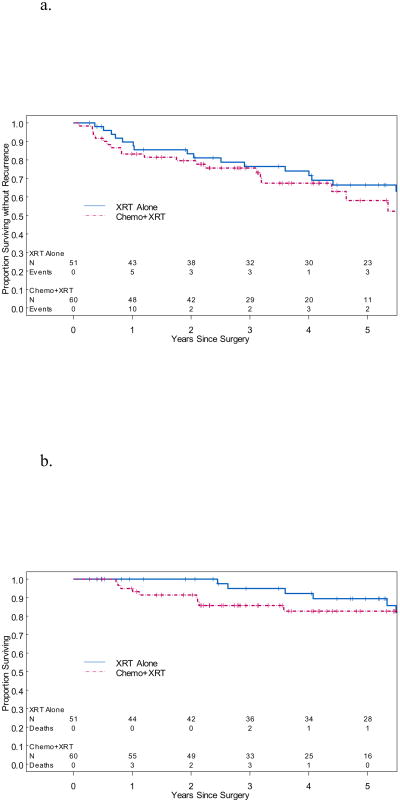
Age >70 years was identified as a prognostic factor for worse PFS (HR=4.62, 95% CI 2.30-9.25, p=0.0001) and OS (HR=5.42, 95% CI 2.23-13.18, p=0.0002) on multivariate analysis. Patients in this series receiving any adjuvant radiation showed improved PFS (HR 0.34, 95% CI 0.13-0.90, p=0.03) compared to those receiving any adjuvant chemotherapy (HR 2.33, 95% CI 1.16-4.65, p=0.02, Table 2) on multivariate analysis, however the difference did not persist in OS analysis (Table 3). Only 6 patients received chemotherapy alone and thus did not serve as a direct comparison group in the adjuvant therapy analysis. Patients who received chemotherapy and radiation (n=60) did not have significantly different OS or PFS than patients who received radiation alone (n=51). Median OS for patients who received radiation alone was 199.2 months, and median PFS for patients who received radiation alone was 135.6 months (Figures 1a and 1b). Median OS and PFS for patients who received chemotherapy and radiation were not reached. However, median follow-up for the 51 patients remaining alive who received chemotherapy and radiation was only 44.4 months (range 6 to 110.4 months). Stage, grade, and lymphovascular space invasion were not significant prognostic factors for PFS or OS in our analysis.
Figure 1.
a. Kaplan-Meier curves for PFS. Median PFS in patients receiving radiation alone was 135.6 months, however the median PFS was not reached in patients receiving chemotherapy and radiation. This difference was not statistically significant.
XRT=radiation, chemo=chemotherapy
b. Kaplan-Meier curves for OS. Median OS in patients receiving radiation alone was 199.2 months, however the median OS was not reached in patients receiving chemotherapy and radiation. This difference was not statistically significant.
XRT=radiation, chemo=chemotherapy
Patients receiving chemotherapy alone (n=6) recurred more frequently (83%) compared to those receiving radiation alone (29%) or combined chemotherapy and radiation (30%, p=0.03, Table 4). In the small subgroup receiving chemotherapy alone, these patients were more likely to recur in either the pelvis (40%) or abdomen (40%). Patients receiving radiation alone were more likely to recur in either the abdomen (33.3%) or distant (33.3%). Those that received combination chemotherapy and radiation were most likely to recur either distant (55.6%) or in the abdomen (27.8%, Table 5.) There were no significant differences in type of adjuvant therapy received as stratified by LNR or in those patients with ≥ 10 nodes removed.
Table 4. Recurrence by adjuvant treatment.
| Adjuvant Treatment | No recurrence (n=79) | Recurrence (n=38) |
|---|---|---|
|
|
|
|
| Chemotherapy alone | 1 (1.3%) | 5 (13.2%) |
| Radiation alone | 36 (45.6%) | 15 (39.5%) |
| Chemo + Radiation | 42 (53.2%) | 18 (47.4%) |
Fisher's exact test p-value=0.03
Table 5. Recurrence location by adjuvant treatment.
| Recurrence location | Chemotherapy (n=5) | Radiation (n=15) | Chemo + Radiation (n=18) |
|---|---|---|---|
|
|
|
|
|
| Pelvic | 2 (40%) | 2 (13.3%) | 3 (16.7%) |
| Pelvic + Abdomen | 1 (20%) | ||
| Pelvic + Distant | 1 (6.7%) | ||
| Abdomen | 2 (40%) | 5 (33.3%) | 5 (27.8%) |
| Distant | 5 (33.3%) | 10 (55.6%) | |
| Abdomen + Distant | 2 (13.3%) |
Discussion
Our study found that in patients with stage IIIC endometrioid or mixed endometrioid endometrial cancers that underwent surgical staging with ≥ 10 nodes removed, a LNR >50% showed a significantly worse PFS compared to those with LNR ≤10% or 10-50%. Our study also highlights that outcomes in node-positive endometrioid or mixed endometrial cancers are closely tied to adjuvant therapy, particularly, with the use of adjuvant radiotherapy.
The use of lymphadenectomy in apparent stage I disease has been a controversial topic of discussion over the last several years. In 2008, Benedetti Panici et al. reported results from a randomized clinical trial in preoperative stage I endometrial cancer patients with or without pelvic lymphadenectomy. The authors concluded that pelvic lymphadenectomy significantly improved detection of lymph node metastases (13% vs. 3%), however, had no impact on disease-free or overall survival. [21] Similarly, in 2009, the authors of the ASTEC trial reported their randomized trial results also concluding no survival benefit for pelvic lymphadenectomy in women with preoperative, clinical stage I endometrial cancer. [22] In contrast, many gynecologic oncologists still see the benefit in lymphadenectomy for staging intermediate-risk and high-risk endometrial cancers due to its added prognostic information and guidance on adjuvant therapy. Data obtained from lymphadenectomy may allow patients with intermediate-risk disease to be managed without adjuvant radiotherapy and/or chemotherapy.
A large registry study from the National Cancer Institute revealed that removing an increasing number of lymph nodes was associated with a higher likelihood of identifying patients with lymph node metastases. [23] Also, several retrospective studies have demonstrated potential therapeutic benefits to comprehensive lymphadenectomy in endometrial cancer. These studies showed that more extensive lymph node dissection led to improved survival outcomes in these patients. [24-25] Although lymphadenectomy has been shown to be important for staging of endometrial cancer, it is also associated with potential surgical and postoperative morbidity including longer operative times, longer hospital stays, higher estimated blood loss, postoperative lymphocysts, lymphedema, ileus, and small bowel obstruction. [24]
There has been recent interest in using a lymph node ratio as a prognostic tool in endometrial cancer. This allows assessment of the comprehensive nature of lymphadenectomy and burden of nodal disease. Previous multi-center retrospective studies have found lymph node ratio to be associated with worse progression-free and overall survival. [2,18] Chan et al. reported from a National Cancer Institute Registry study that an increasing lymph node ratio (≤10, 10-50, and >50%) in node positive endometriod endometrial cancers was associated with a decrease in survival from 77% to 61% to 41%, respectively. [2] Similarly, Polterauer et al. reported a decrease in overall survival of 79%, 61%, and 36% with increasing lymph node ratios of ≤10%, 10-50%, and >50% in stage IIIC endometrial cancers. [18] Although similar outcomes were reported, adjuvant treatment received varied between the two studies. Because data was obtained through a registry, the manuscript by Chan et al. did not provide details on adjuvant chemotherapy patients received, and they reported that only 63% of patients received adjuvant radiotherapy. [2] Type of adjuvant therapy received could have influenced prognosis in a large subset of these patients. The data reported by Polterauer et al. from their multi-institutional retrospective manuscript did include data on adjuvant therapy. In their cohort, 23% patients received chemotherapy alone, 28% radiotherapy alone, and 37% combination chemotherapy and radiotherapy. This variance in adjuvant treatment received could play a role in outcomes reported. Also, the data by Polterauer et al. included all endometrial cancer histologies, of which 30% were non-endometrioid. [18] Previously published literature suggests that histologies such as serous or clear cell have a more aggressive clinical behavior leading to worse overall outcomes. [26] This may have had led to a variance in LNR outcomes reported in this study.
Our manuscript contributes to the currently body of literature in evaluating the role of using lymph node ratio as a prognostic tool in endometrial cancer with consistent use of adjuvant radiotherapy for stage IIIC disease. Our statistical methods identified LNR of >50% and >40% as cutpoints for PFS and OS, however, were not statistically significant as prognostic variables in our cohort. This may be due to small numbers of patients in this single institution study and low median numbers of lymph nodes retrieved in our cohort. In a subset analysis based on those LNR cutoff previously reported in the literature [2,18], in those patients with ≥ 10 nodes removed, a LNR >50% showed a significantly worse PFS compared to those with LNR ≤10% or 10-50%. This suggests that the benefit of using LNR as a prognostic factor may be limited to those patients that have a set minimum number of nodes removed. This number however, has not been a set standard in clinical practice. Altogether, the number of patients in our study with a LNR>50% is too small to draw any definitive conclusions.
Our manuscript also highlights the importance of adjuvant therapy for those patients diagnosed with stage IIIC endometrial cancers. Our data supports the use of adjuvant radiotherapy in this node-positive group. Patients receiving radiotherapy with or without chemotherapy in our cohort had a decreased recurrence risk and improved PFS, compared to those who received chemotherapy alone. This data is limited due to the small numbers in the cohort of patients that received chemotherapy alone (n=6) in the current study and conclusions should be taken with caution. Also, discrepant results between the univariate and multivariate analysis in the “any chemo” group may also be due to limited power to show such consistent results. However, the results are similar to those previously published from our institution, showing an improved 5-year pelvic relapse-free survival (98% vs. 61%, p=0.001), disease-specific survival (78% vs. 39%, p=0.01), and overall survival (73% vs. 40%, p=0.03) in those patients with stage IIIC endometrial cancer treated with definitive pelvic or extended-field radiotherapy with or without systemic therapy compared to those that received systemic therapy alone. [27]
Although our data and that previously published in the literature supports the potential use of lymph node ratio as a prognostic tool in those patients with node-positive endometrial cancers, it inherently must be tied to adjuvant therapy in this patient population. With large, randomized trials suggesting no survival benefit in performing routine comprehensive lymphadenectomy, we should consider “smarter” lymph node assessments in endometrial cancer, however, closely tie this with adjuvant therapy. The recent interest in sentinel lymph node mapping for endometrial cancer [22-31] may provide the ability for accurate assessment of lymph node metastases to tailor adjuvant therapy, without the associated morbidity of comprehensive lymphadenectomy. We are currently conducting a prospective evaluation at our institution of the use of sentinel lymph node mapping in a high and low-risk endometrial cancer population to assess the accuracy of identification of lymph node metastases and to follow clinical outcomes. We hope that these prospective studies will give us information on the sensitivity and accuracy of sentinel lymph node mapping to detect lymph node metastases and additional prognostic information to help guide therapy in node positive endometrial cancer patients, while limiting morbidity. However, we must also continue to explore if there are certain high-risk patients with positive sentinel lymph nodes that may benefit from a systematic lymphadenectomy such as seen in other disease sites, such as breast cancer.
Acknowledgments
Funding statement: This research is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672 (MFM) and the National Institutes of Health K12 Calabresi Scholar Award (K12 CA088084) (SNW).
References
- 1.American Cancer Society. Cancer Facts and Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Chan JK, Kapp DS, Cheung MK, et al. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer. 2007;97(5):605–11. doi: 10.1038/sj.bjc.6603898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecorelli S. Revised FIGO staging for carcinoma of vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):105–6. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Secord AA, Havrilesky LJ, O'Malley DM, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009;114(3):100–5. doi: 10.1016/j.ygyno.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small cell lung cancer. J Clin Oncol. 2003;21(#):1029–34. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Weir L, Speers C, D'Yachkova Y, Olivotto IA. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20(#):1793–99. doi: 10.1200/JCO.2002.07.112. [DOI] [PubMed] [Google Scholar]
- 7.Tepper JE, O'Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19(#):157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21(#):2912–19. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 9.Moore DH, Stehman FB. What is the appropriate management of early stage cervical cancer (International Federation of Gynecology and Obstetrics stages I and IIA), surgical assessment of lymph nodes, and role of therapeutic resection of lymph nodes involved with cancer? J Natl Cancer Inst Monogr. 1996:43–46. [PubMed] [Google Scholar]
- 10.Van der Velden J, van Lindert AC, Lammes FB, et al. Extracapsular growth of lymph node metastases in squamous cell carcinoma of the vulva. The impact on recurrence and survival. Cancer. 1995;75(#):2885–90. doi: 10.1002/1097-0142(19950615)75:12<2885::aid-cncr2820751215>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13(7):1337–44. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu YP, Ma L, Wang SJ, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol. 2009;36(2):155–59. doi: 10.1016/j.ejso.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, et al. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17(5):1267–77. doi: 10.1245/s10434-010-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KD, Lee SI, Moon HY. Lymph node ratio as determined by the 7th edition of the AJCC staging system predicts survival in stage III colon cancer. J Surg Oncol. 2011;103(5):406–10. doi: 10.1002/jso.21830. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in staging of node-positive colon cancer. Ann SurgOncol. 2008;15(6):1600–8. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman SC, McMasters KM, Scoggins CR, Martin RC, Chagpar AB. Lymph node ratio: a proposed refinement of current axillary staging in breast cancer patients. J Am Coll Surg. 2011;213(1):45–52. doi: 10.1016/j.jamcollsurg.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratio in node-positive breast cancer. J ClinOncol. 2006;24(18):2910–16. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 18.Polterauer S, Khalil S, Zivanovic O, et al. Prognostic value of lymph node ratio and clinicopatholgic parameters in patients diagnosed with stage IIIC endometrial cancer. Obstet Gynecol. 2012;119(6):1210–8. doi: 10.1097/AOG.0b013e318255060c. [DOI] [PubMed] [Google Scholar]
- 19.Keys HM, Robers JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Williams BA, Mandrekar JN, Mandrekar SJ, Cha S, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes Technical Report Series #79. Department of Health Scienes Research, Mayo Clinic; Rochester, MN: Jun, 2006. [Google Scholar]
- 21.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 22.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Lancet. 2009;373(9658):125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JK, Urban R, Cheung MK, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109(12):2454–60. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 24.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23(16):3668–75. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 25.Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107(8):1823–30. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- 26.Greggi S, Mangili G, Scaffa C, et al. Uterine papillary serous, clear cell, and poorly differentiated endometrioid carcinomas: a comparative study. Int J Gynecol Cancer. 2011;21(4):661–7. doi: 10.1097/IGC.0b013e3182150c89. [DOI] [PubMed] [Google Scholar]
- 27.Klopp AH, Jhingran A, Ramondeta L, et al. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115(1):6–11. doi: 10.1016/j.ygyno.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Leitao MM, Jr, Khoury-Collado F, Gardner G, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129(1):38–41. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ballester M, Naoura I, Chereau E, et al. Sentinel node biopsy upstages patients with presumed low- and intermediate-risk endometrial cancer: results of a multicenter study. Ann Surg Oncol. 2013;20(2):407–12. doi: 10.1245/s10434-012-2683-x. [DOI] [PubMed] [Google Scholar]
- 30.How J, Lau S, Press J, et al. Accuracy of sentinel lymph node detection following intra-operative cervical injection for endometrial cancer: a prospective study. Gynecol Oncol. 2012;127(2):332–7. doi: 10.1016/j.ygyno.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125(3):531–5. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]