Abstract
Background
In laboratory experiments, ω-3 polyunsaturated fatty acids (PUFAs) reduce inflammatory eicosanoids resulting from ω-6 PUFA metabolism via competitive inhibition; and the ω-3 induced cytotoxic environment increases apoptosis and reduces cell growth in breast cancer cells. To the authors' knowledge, epidemiologic investigations regarding whether dietary ω-3 PUFA intake benefits survival following breast cancer, are limited and inconsistent.
Methods
The authors used resources from a population-based follow-up study conducted on Long Island, NY, among 1,463 women newly diagnosed with first-primary breast cancer who were interviewed, on average, about three months after diagnosis to assess risk and prognostic factors, including dietary intake (using a food frequency questionnaire). Vital status was determined through 2011, yielding a median follow-up time of 14.7 years and 485 deaths. Adjusted hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated using Cox-proportional hazards regression.
Results
All-cause mortality was reduced among women with breast cancer reporting the highest quartile of intake (compared to never) for: tuna (HR=0.71, 95% CI=0.55,0.92); other baked/broiled fish (HR=0.75, 95% CI=0.58,0.97); and dietary long-chain ω-3 PUFAs docosahexanoic (DHA, HR=0.71, 95% CI=0.55,0.92) and eicosapentanoic (EPA, HR=0.75, 95% CI=0.58,0.97) acid.
Conclusions
All-cause mortality was reduced by 16–34% among women with breast cancer who reported a high intake of fish and long-chain w-3 PUFAs. Long-chain ω-3 PUFA intake from fish and other dietary sources may provide a potential strategy to improve survival after breast cancer.
Keywords: polyunsaturated fatty acids, breast cancer, survival, all-cause mortality
Introduction
Breast cancer is the second cause of cancer death among women in the U.S. with approximately 40,000 estimated new deaths in 2013 1. Clinical and demographic indicators of breast cancer prognosis include large tumor size, lymph node involvement, hormone receptor-negative subtype, early age at diagnosis, and low socioeconomic status 2. Weight maintenance and physical activity may improve survival following breast cancer 3, 4; but because breast cancer is a multi-factorial disease, it is plausible that additional strategies, including intake of specific nutritional factors, would also improve survival among women diagnosed with breast cancer.
One potential nutritional risk reduction strategy is polyunsaturated fatty acids (PUFAs), of which ω-3 and ω-6 are the two primary classes. Inflammatory eicosanoids of arachidonic acid (AA), an ω-6 PUFA, have been shown in laboratory studies to: increase cell proliferation, metastatic potential, aromatase activity, and angiogenesis; and reduce apoptosis, and cellular differentiation 5. ω-3 fatty acids bind to the same enzymes utilized in AA metabolism, thus, potentially lowering levels of inflammatory eicosanoids generated by ω-6 metabolism 5. Also, the cytotoxic environment induced by ω-3 has been reported to increase apoptosis and reduce cell growth in transformed and malignant breast cancer cells 5, 6. Long-chain ω-3 PUFA have demonstrated ability to chemo-sensitize breast cancer tumors and, thus, potentially improve treatment efficacy 7. Thus, it is plausible that intake of ω-3, for which fish is the major dietary source, could provide an opportunity for improving survival among women with breast cancer.
Despite this laboratory evidence, few epidemiologic studies have examined the association between dietary PUFA intake and survival after breast cancer 8–10. Two 8, 9 utilized cross-sectional study designs, thus, limiting inferences regarding the potential association between PUFA intake and breast cancer survival. Another study examined the relation between adipose tissue biomarkers of PUFA on survival after breast cancer 10 and reported no associations; however, interpretation of results was limited due to the small number of deaths. Thus, the epidemiologic evidence for the potential association between dietary PUFA intake and survival among women with breast cancer is limited.
Previous studies examining fish intake and mortality among breast cancer survivors are inconsistent 11, 12. One Japanese investigation followed cases for 9 to 12 years, and reported increased breast cancer mortality with high fish consumption 12; however, the study population was based on a small number of deaths. The second study utilized participants from the Women’s Healthy Eating and Living (WHEL) study, a low-fat dietary intervention that manipulated total fat intake, and found that higher intake of ω-3 fatty acids (eicosapentanoic acid, EPA; and docosahexanoic acid, DHA) from fish was associated with reduced breast cancer recurrence and all-cause mortality 11. This U.S.-based study, however, did not assess short-chain ω-3 PUFA (i.e., alpha-linolenic acid, ALA), which are readily obtained in the diet of Western populations.
For the study reported here, we examined whether higher intake of fish, as well as any other dietary sources of ω-3 PUFAs, are associated with improved survival among women diagnosed with breast cancer on Long Island, New York. We also considered associations with ω-6 PUFAs and the balance between ω-3 and ω-6 intake, and to our knowledge, the first study to consider multiple PUFA interaction with genes in relation to survival.
Methods
This follow-up study utilizes resources from the Long Island Breast Cancer Study Project (LIBCSP), a population-based study 3, 4, 13. Institutional Review Board approval was obtained from all participating institutions.
Study Population
Women eligible for the LIBCSP follow-up study were English-speaking residents of Long Island, New York (Nassau and Suffolk counties) who were newly diagnosed with a first primary in situ (16%) or invasive breast (84%) cancer between August 1, 1996 and July 31, 1997. After obtaining physician approval, study personnel contacted pathology departments from participating hospitals (2–3 times per week or daily, for the hospitals with large numbers of newly diagnosed cases) to identify potentially eligible subjects. The final LIBCSP follow-up sample consisted of 1,508 women with breast cancer. Within approximately three months of diagnosis, 98% (n=1,479) completed a validated self-administered 101-item modified Block food frequency questionnaire (FFQ) 14–16. Subjects with implausible total energy intake (±3 standard deviations from the mean) were excluded (n=16). Thus, the final analytic cohort for this ancillary study included 1,463 women with newly diagnosed breast cancer.
At diagnosis (baseline) with the first primary breast cancer, participants ranged in age from 20–98 years, 67% were postmenopausal, and 94% reported their race as white, 4% as black, and 2% as other, which reflects the underlying racial distribution of Nassau and Suffolk counties at the time of data collection 3, 4, 13.
Outcome Assessment
Vital status through December 31, 2011 for all LIBCSP participants was determined via linkage with the National Death Index, a standard epidemiologic resource for ascertaining mortality data in the U.S. 17. We identified women who died from all-causes (death from any cause), and those whose deaths were breast cancer-related (breast cancer-specific mortality). Breast cancer-related deaths were determined using the International Classification of Disease (codes 174.9 or C-50.9). Among the 1,463 participants included in this study, the median follow-up time was 14.7 years after breast cancer diagnosis (range=0.2–15.4 years), and we identified 485 total deaths, of which 210 were breast cancer-specific.
Assessment of PUFA Intake and Other Prognostic Factors
LIBCSP participants self-completed the FFQ, and were administered a baseline, structured questionnaire by a trained interviewer, within three months, on average, after diagnosis. The FFQ assessed dietary intake in the year prior to the interview. Other factors assessed included: demographic characteristics; reproductive and menstrual history; exogenous hormone use, family history of breast cancer, and other medical history; body size, physical activity, and alcohol use; active and passive cigarette smoking; occupational history and other environmental exposures 13. Medical records were abstracted to determine tumor characteristics of the first primary breast cancer and the first course of treatment for the first primary breast cancer. Concordance between the medical record and the self-reported treatment data was high (kappa>90%), and thus, the self-reported data are used here.
PUFA intake from any dietary source was estimated by linking participant responses from the FFQ (i.e., grams per day for each line item) with average nutrient values for foods included in each line item available in the U.S. Department of Agriculture databases for ω-3 and ω-6 PUFAs 18. The following PUFA subtypes were estimated: (1) ω-3 PUFA including, ALA, docosapentanoic acid (DPA), DHA, EPA; and (2) ω-6 PUFA including, linoleic acid (LA) and AA. An estimate of total ω-3 and ω-6 PUFA intake (henceforth, total PUFA intake) was calculated by combining all individual PUFA subtypes. Additionally, an estimate of total ω-3 and ω-6 PUFA was obtained by summing each individual fatty acid within category (e.g., total ω-3=ALA+DPA+DHA+EPA), representing total ω-3 and ω-6 intakes that are commonly consumed among U.S. populations.
Fish and/or seafood consumption were also assessed by FFQ as: (1) tuna, tuna salad, tuna casserole; (2) shell fish (shrimp, lobster, crab, oysters, etc.); and (3) other fish (broiled/baked).
Statistical analyses
Kaplan-Meier survival curves were constructed, and Cox-proportional hazards regression 19 models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CI), to assess the associations between intake of fish, as well as PUFAs from any dietary source, and all-cause and breast cancer-specific mortality for the full 15-years of follow-up. As a sensitivity analysis, we also estimated HRs for 5-years of follow-up.
For regression analyses, the proportional hazards assumption was assessed using exposure interactions with time, and also using Martingale residuals 19. No violations were observed. Quartiles (Q) were created for each PUFA exposure (total PUFA, total ω-3, ALA, DPA, DHA, EPA, total ω-6, LA, AA, ratio of ω-3/ω-6) based on the distributions among the 1,463 women with breast cancer included in our study. Other exposure cut-points were considered (e.g., tertiles, quintiles, linear, splines); however, the shape of the dose-response between PUFAs and the log-hazard of mortality was best captured with quartiles, balancing both parsimony and non-linearity of the exposure. Similarly, quartiles were created for exposures related to fish intake (i.e., tuna, shell fish, other fish). We conducted linear trend tests by modeling the exposure continuously 20.
In the Cox proportional hazards models, we also considered interactions between total ω-3 and total ω-6, and between the ω-3/ω-6 ratio in association with mortality. For these interaction analyses, PUFAs were dichotomized at the median. Interaction was evaluated on the additive scale and measured using relative excess risk due to interaction (RERI), with corresponding 95% CI calculated using the Hosmer and Lemeshow method 21. Multiplicative interactions were also assessed using the Likelihood Ratio Test; however, the conclusions did not change (data not shown).
Effect modification of the PUFA-mortality associations by menopausal status (post- vs. pre-menopausal); hormone receptor status (hormone receptor-positive breast cancer vs. negative), dietary supplement use (yes/no), treatment (chemotherapy, radiation, hormone therapy), and body mass index (BMI, kg/m2; <25, ≥25) were also examined in the PUFA regression models. After stratification, however, little or no heterogeneity was observed for all but BMI (Supplement Tables S3 and S4). We additionally estimated the direct association of PUFA intake and survival while accounting for the potential influence of pre-diagnostic PUFA intake on tumor size using inverse probability weighting 22 (Supplement Table S5).
Potential confounders for the PUFA-mortality regression models were identified using a directed acyclic graph (DAG) 20, and included age (5-year age group), total energy intake (kcal/day), non-steroidal anti-inflammatory drugs (NSAID), family history of breast cancer, income, body mass index, alcohol use, fruit and vegetable intake, physical activity, and race. No adjustment was made for potential intermediates of the hypothesized PUFA-mortality association. However, only age and total energy intake changed the HR estimates by more than 10%, and thus all PUFA-mortality regression models were adjusted for these two confounders.
We conducted sensitivity analyses to determine whether results varied when restricting the cohort to: (a) women with invasive tumors; and (b) whites. The restricted sensitivity results, however, did not vary substantially from those based on all women, thus only the latter are shown.
We are the first to explore interactions between PUFA intake and putatively functional genetic polymorphisms involved in multiple biologically relevant pathways. Laboratory and statistical methods for these exploratory analyses are described in the Supplement.
All statistical analyses were conducted using PROC PHREG (Cox proportional hazards regression models) and PROC LIFETEST (Kaplan-Meier survival curves) in SAS version 9.2 (SAS Institute, Cary, NC).
Results
PUFA and Fish Intake
As shown in Table 1, among our population-based sample of women with breast cancer (n=1,463) baseline intake of total ω-3 fatty acids from any dietary source (average total ω-3 intake 0.99 grams per day, SD=0.69) was lower relative to ω-6 intake (average total ω-6 intake of 7.51 grams per day, SD=5.26). ALA intake was the highest contributor to total ω-3 intake with an average intake of 0.85 grams per day (SD=0.67), whereas LA was the highest contributor to total ω-6 intake with an average intake of 7.44 grams per day (SD=5.24). Tuna intake was higher (8.13 grams per day, SD=10.61) compared to shell fish intake (3.30 grams per day, SD=6.01). As also shown in Table 1, fish was a primary contributor to high intake of long-chain ω-3 PUFAs, including DPA, DHA, and EPA. In contrast, foods that contributed to high ALA intake were biscuits/muffins and other fried foods. Additionally, high AA intake appeared to be driven by eggs and meats, including fish, chicken, and ham.
Table 1.
Distributions of intakes of polyunsaturated fatty acid (PUFA) and fish at baseline among a population-based sample of women newly diagnosed with breast cancer (N=1463), LIBCSP, 1996–1997
| Nutrient/Food | Mean | SD | 25th Pct |
50th Pct |
75th Pct |
Major PUFA-rich foods contributing to high nutrient intake in the LIBCSP |
|---|---|---|---|---|---|---|
| Nutrient (g/day) | ||||||
| Total PUFAa | 8.50 | 5.83 | 4.38 | 7.24 | 10.92 | Butter, mayonnaise/salad dressings, safflower/corn oil, margarine, peanuts/peanut butter |
| Total ω-3b | 0.99 | 0.69 | 0.52 | 0.82 | 1.26 | Biscuits/muffins, butter, fish, mayonnaise/salad dressings, safflower/corn oil |
| ALA | 0.85 | 0.67 | 0.38 | 0.68 | 1.10 | Biscuits/muffins, French fries/fried potatoes, butter, cookies, mayonnaise/salad dressings, safflower/corn oil |
| DPA | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | Fish, tuna, chicken, shellfish, beef |
| DHA | 0.09 | 0.10 | 0.03 | 0.06 | 0.12 | Fish, tuna, eggs, chicken, shellfish |
| EPA | 0.05 | 0.05 | 0.01 | 0.03 | 0.06 | Fish, tuna, shellfish, chicken |
| Total ω-6c | 7.51 | 5.26 | 3.83 | 6.43 | 9.73 | Biscuits/muffins, French fries/fried potatoes, butter, mayonnaise/salad dressings |
| LA | 7.44 | 5.24 | 3.80 | 6.35 | 9.66 | Biscuits/muffins, French fries/fried potatoes, butter, mayonnaise/salad dressings, safflower/corn oil |
| AA | 0.07 | 0.05 | 0.04 | 0.06 | 0.09 | Eggs, fish, chicken, ham/lunch meats, shellfish |
| ω-3/ω-6 | 0.15 | 0.11 | 0.11 | 0.13 | 0.17 | N/A |
| Fish (g/day)d | ||||||
| Tuna | 8.13 | 10.61 | 1.40 | 4.77 | 12.40 | N/A |
| Shell fish | 3.30 | 6.01 | 0.00 | 0.00 | 4.62 | N/A |
| Other (broiled/baked) | 7.88 | 14.70 | 0.00 | 3.85 | 10.77 | N/A |
Note:
Total PUFA = ALA + DPA + DHA + EPA + LA + AA
Total ω-3 = ALA + DPA + DHA + EPA
Total ω-6 = LA + AA
Cases with null values for tuna (N=343), shell fish (N=750), and other (N=505) are included in calculations.
SD = standard deviation
N/A = not applicable
LIBCSP = Long Island Breast Cancer Study Project
PUFAs and Mortality
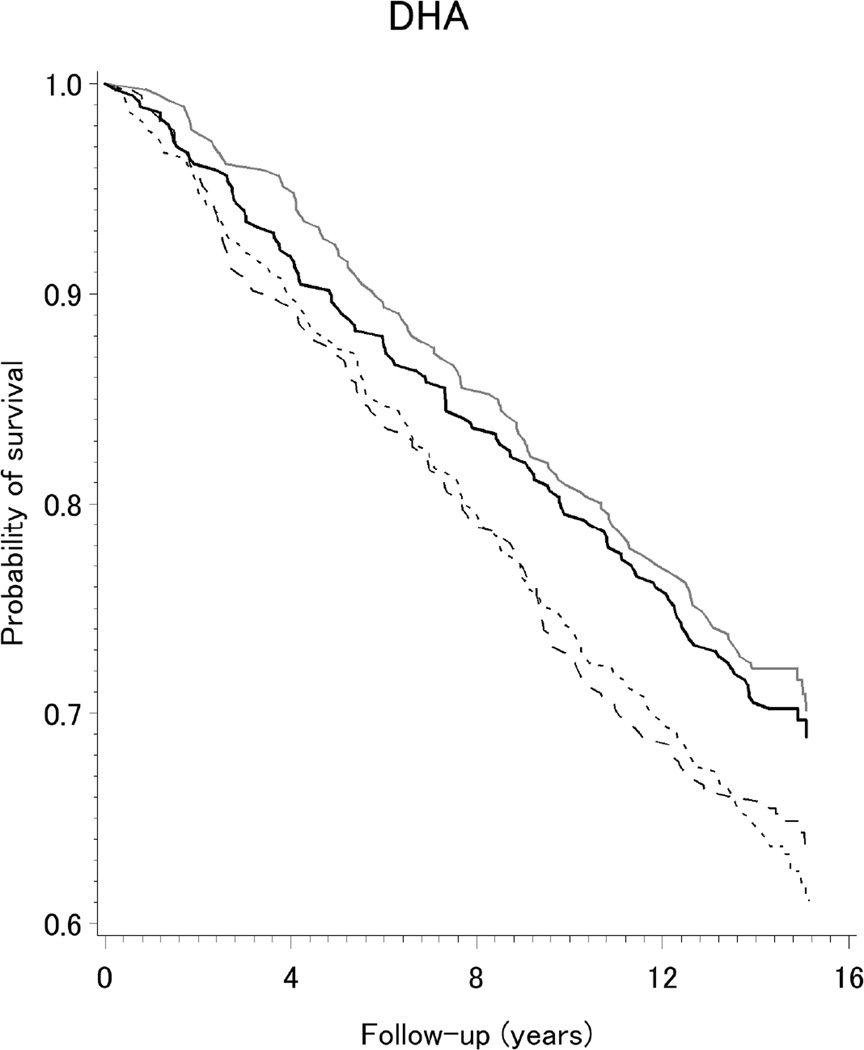
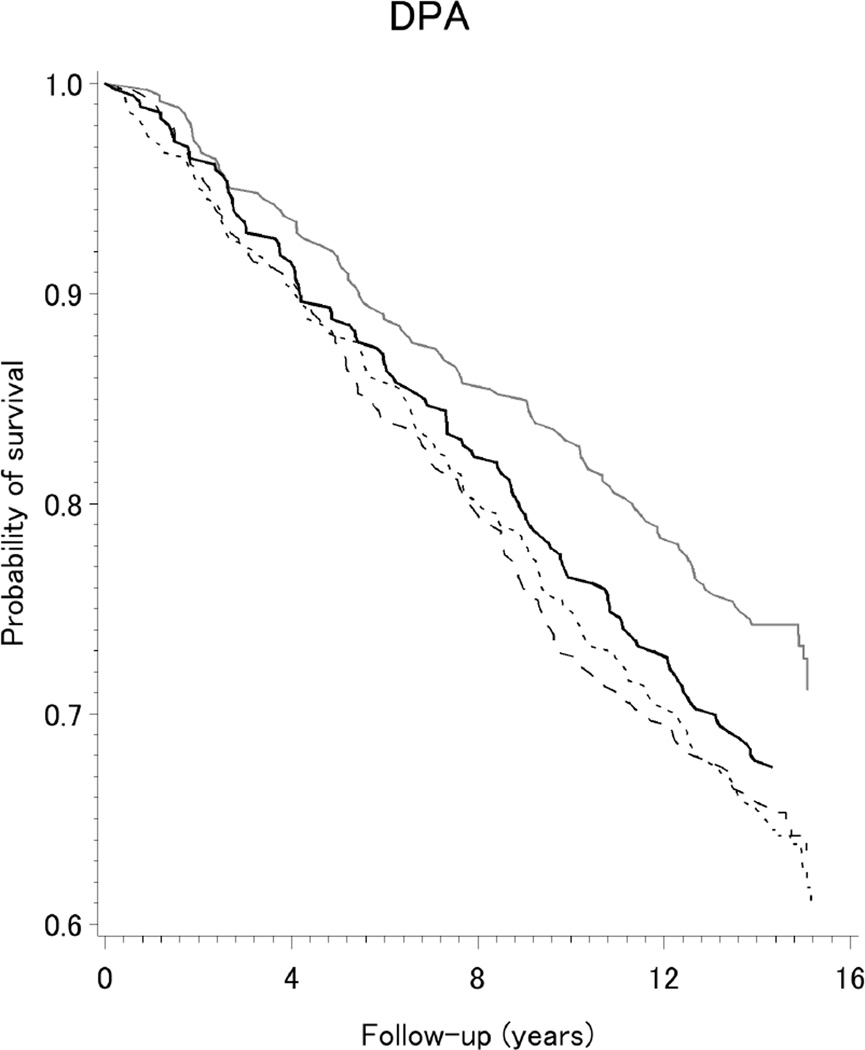
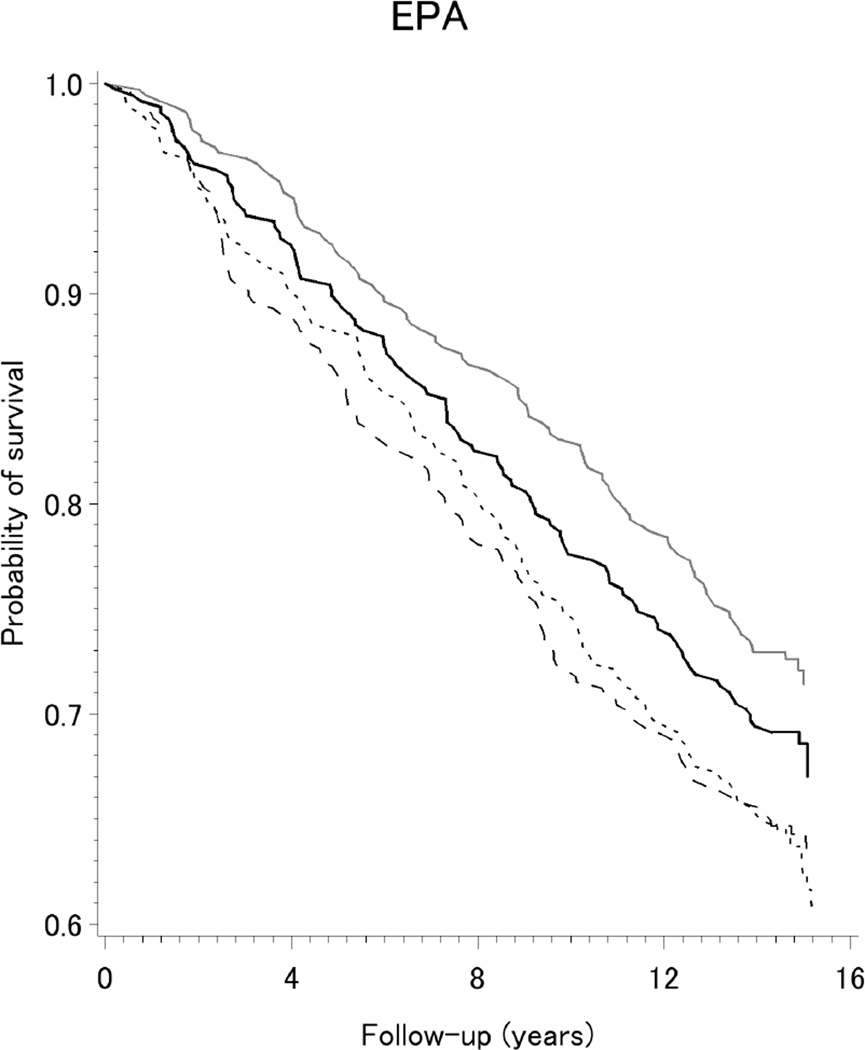
The Kaplan-Meier curves shown in Figure 1 indicate survival was improved among our population-based sample of women with breast cancer reporting higher intake (Q3 and Q4) of the long-chain ω-3 fatty acids DPA, DHA, and EPA. As presented in Table 2, reductions of 16–34% in all-cause mortality were observed for higher intake of long-chain ω-3 fatty acids. Specifically, lower death rates were observed for the highest two quartiles of intake (Q3 or Q4), compared to the lowest quartile (Q1), of DHA (HRQ3vs.Q1=0.73, 95%CI=0.56,0.94; and HRQ4vs.Q1=0.71, 95% CI=0.55,0.92), EPA (HRQ3vs.Q1=0.70, 95% CI=0.54,0.91; and HRQ4vs.Q1=0.75, 95% CI=0.58,0.97); and DPA (HRQ3vs.Q1=0.66, 95%CI=0.51,0.86; and HRQ4vs.Q1=0.84, 95%CI=0.64,1.10). The corresponding hazard for ω-3/ω-6 ratio was modestly decreased by 15%, but the confidence interval included the null value. As also shown in Table 2, adjusted hazards for all-cause mortality were increased by 14–30% for higher intakes of total ω-6, LA, AA, and ALA but confidence intervals were wide. Patterns were similar, but less precise, for PUFA intake from all dietary sources in relation to breast cancer-specific mortality after 15 years of follow-up (Table 4), and when we considered all-cause mortality after 5-years of follow-up (Supplement Table S1).
Figure 1.
Kaplan-Meier survival curves for dietary intake (quartiles) of long-chain ω-3 fatty acids DPA, DHA, and EPA, among a population-based sample of women with breast cancer, LIBCSP, 1996/1997 through 2011(an average of 14.7 years of follow-up)
Table 2.
Multivariablea-adjusted HRs and 95% CI for the association between dietary PUFA intake and all-cause mortality among a population-based sample of women with breast cancer (N=1,463), LIBCSP, 1996/1997 through 2011(an average of 14.7 years of follow-up)
| PUFA | Q1 |
Q2 |
Q3 |
Q4 |
p for linear trend |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | HR | D | HR | 95% CI | D | HR | 95% CI | D | HR | 95% CI | ||
| Total PUFA | 124 | 1.00 | 128 | 1.12 | 0.87, 1.44 | 110 | 0.97 | 0.73, 1.27 | 123 | 1.16 | 0.84, 1.61 | 0.37 |
| Total ω-3 | 124 | 1.00 | 116 | 0.93 | 0.71, 1.21 | 124 | 1.07 | 0.82, 1.41 | 121 | 1.00 | 0.72, 1.38 | 0.45 |
| ALA | 114 | 1.00 | 129 | 1.18 | 0.91, 1.52 | 121 | 1.18 | 0.90, 1.55 | 121 | 1.14 | 0.83, 1.57 | 0.16 |
| DPA | 136 | 1.00 | 131 | 0.93 | 0.73, 1.19 | 99 | 0.66 | 0.51, 0.86 | 119 | 0.84 | 0.64, 1.10 | 0.09 |
| DHA | 139 | 1.00 | 130 | 0.93 | 0.73, 1.18 | 105 | 0.73 | 0.56, 0.94 | 111 | 0.71 | 0.55, 0.92 | 0.04 |
| EPA | 136 | 1.00 | 131 | 0.93 | 0.73, 1.18 | 102 | 0.70 | 0.54, 0.91 | 116 | 0.75 | 0.58, 0.97 | 0.08 |
| Total ω-6 | 122 | 1.00 | 124 | 1.10 | 0.85, 1.42 | 112 | 1.04 | 0.79, 1.38 | 127 | 1.27 | 0.92, 1.76 | 0.38 |
| LA | 121 | 1.00 | 126 | 1.14 | 0.88, 1.47 | 111 | 1.05 | 0.79, 1.39 | 127 | 1.30 | 0.94, 1.79 | 0.38 |
| AA | 122 | 1.00 | 120 | 1.00 | 0.78, 1.30 | 113 | 0.89 | 0.68, 1.16 | 130 | 1.15 | 0.87, 1.52 | 0.54 |
| ω-3/ω-6 | 124 | 1.00 | 113 | 0.89 | 0.69, 1.14 | 128 | 0.93 | 0.73, 1.19 | 120 | 0.85 | 0.66, 1.09 | 0.27 |
Note:
D=deaths, LIBCSP=Long Island Breast Cancer Study Project
Multivariable-adjusted HRs and 95% CI adjusted age (5-year age group) and total energy intake (kcal/day)
Table 4.
Multivariablea-adjusted HRs and 95% CI for the association between dietary PUFA intake and breast cancer-specific mortality among a population-based sample of women with breast cancer (N=1,463), LIBCSP, 1996/1997 through 2011(an average of 14.7 years of follow-up)
| PUFA | Q1 |
Q2 |
Q3 |
Q4 |
p for linear trend |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | HR | D | HR | 95% CI | D | HR | 95% CI | D | HR | 95% CI | ||
| Total PUFA | 38 | 1.00 | 62 | 1.56 | 1.03, 2.36 | 53 | 1.25 | 0.80, 1.95 | 57 | 1.25 | 0.74, 2.11 | 0.63 |
| Total ω-3 | 43 | 1.00 | 51 | 1.08 | 0.71, 1.64 | 63 | 1.32 | 0.87, 2.01 | 53 | 0.96 | 0.57, 1.61 | 0.80 |
| ALA | 46 | 1.00 | 50 | 1.06 | 0.71, 1.60 | 57 | 1.17 | 0.77, 1.77 | 57 | 1.03 | 0.64, 1.68 | 0.64 |
| DPA | 55 | 1.00 | 50 | 0.85 | 0.58, 1.25 | 44 | 0.69 | 0.46, 1.03 | 61 | 0.93 | 0.63, 1.38 | 0.52 |
| DHA | 55 | 1.00 | 50 | 0.88 | 0.58, 1.25 | 50 | 0.81 | 0.55, 1.19 | 55 | 0.86 | 0.59, 1.27 | 0.53 |
| EPA | 53 | 1.00 | 50 | 0.92 | 0.62, 1.35 | 51 | 0.84 | 0.57, 1.25 | 56 | 0.92 | 0.63, 1.36 | 0.57 |
| Total ω-6 | 37 | 1.00 | 61 | 1.59 | 1.05, 2.42 | 54 | 1.34 | 0.86, 2.11 | 58 | 1.36 | 0.81, 2.30 | 0.62 |
| LA | 37 | 1.00 | 61 | 1.60 | 1.05, 2.43 | 54 | 1.35 | 0.86, 2.11 | 58 | 1.36 | 0.81, 2.29 | 0.63 |
| AA | 47 | 1.00 | 48 | 0.98 | 0.65, 1.47 | 52 | 1.01 | 0.67, 1.52 | 63 | 1.22 | 0.80, 1.86 | 0.40 |
| ω-3/ω-6 | 63 | 1.00 | 44 | 0.65 | 0.44, 0.95 | 53 | 0.79 | 0.55, 1.15 | 50 | 0.79 | 0.54, 1.14 | 0.13 |
Note:
D=deaths, LIBCSP=Long Island Breast Cancer Study Project
Multivariable-adjusted HRs and 95% CI adjusted age (5-year age group) and total energy intake (kcal/day)
We also considered an interaction between ω-3 and ω-6, and all-cause mortality; however, as shown in Supplement Table S2, no interaction on an additive scale was observed. Although there was statistical evidence for interaction on a multiplicative scale when considering the association between PUFA and all-cause mortality stratified by BMI (Supplement Tables S3 and S4), there was no consistent pattern. Additionally, the inverse probability estimates for the direct association of PUFA on survival accounting for tumor size (Supplement Table S5) were similar to the estimates that did not account for tumor size.
Despite our novel biologic hypothesis, we observed little or no association between select putatively functional genetic polymorphisms involved in inflammation, oxidative stress, and estrogen metabolism and all-cause mortality (Supplement Table S6); nor did we observe notable interactions between ω-3/ω-6 ratio and polymorphisms in multiple biologic pathways on overall mortality (Supplement Table S7).
Fish and Mortality
As shown in Table 3, fish intake was associated with a 25–34% reduction in all-cause mortality. Specifically, lower rates of death were observed for: the highest quartile of intake for those in the highest quartile of tuna intake, compared to no intake (HRQ4=0.71, 95% CI=0.55,0.92); and the highest two quartiles for other fish (broiled/baked) (HRQ3=0.66, 95% CI=0.51,0.85; and HRQ4=0.75, 95% CI=0.58,0.97). There was little or no evidence of an association between all-cause mortality and shell fish intake. Adjusted estimates for breast cancer-specific mortality showed pronounced but imprecise reductions when we considered tuna and other fish in relation to 5 years of follow-up (Supplement Table S1). Estimates, however, were closer to the null when we considered 15 years of follow up [tuna (HRQ4=0.81, 95% CI=0.54,1.21) and other baked/broiled fish (HRQ4=1.04, 95% CI=0.71,1.52)].
Table 3.
Multivariablea-adjusted HRs and 95% CI for the association between fish intake and all-cause mortality among a population-based sample of women with breast cancer (N=1,463), LIBCSP, 1996/1997 through 2011(an average of 14.7 years of follow-up)
| Fish | Never |
Q1 |
Q2 |
Q3 |
Q4 |
p for linear trend |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | HR | D | HR | 95% CI | D | HR | 95% CI | D | HR | 95% CI | D | HR | 95% CI | ||
| Tuna | 125 | 1.00 | 86 | 0.98 | 0.74, 1.29 | 84 | 0.93 | 0.70, 1.22 | 78 | 1.06 | 0.80, 1.41 | 112 | 0.71 | 0.55, 0.92 | 0.31 |
| Shell fish | 268 | 1.00 | 23 | 0.71 | 0.46, 1.09 | 84 | 0.98 | 0.76, 1.25 | 46 | 0.79 | 0.57, 1.08 | 64 | 1.05 | 0.79, 1.39 | 0.42 |
| Other fish (broiled/baked) | 319 | 1.00 | 145 | 0.95 | 0.72, 1.24 | 87 | 0.97 | 0.71, 1.33 | 91 | 0.66 | 0.51, 0.85 | 83 | 0.75 | 0.58, 0.97 | 0.03 |
Note:
D=deaths, LIBCSP=Long Island Breast Cancer Study Project
Multivariable-adjusted HRs and 95% CI adjusted age (5-year age group) and total energy intake (kcal/day)
Discussion
In this population-based follow-up study of women with breast cancer, we observed reduced hazards of 16–34% for all-cause mortality after 15 years of follow-up with consumption of higher levels, compared to the lowest level, of long-chain ω-3 fatty acids (DPA, DHA, and EPA). Similarly, higher intake of tuna and other baked/broiled fish was associated with 25–34% decreased all-cause mortality (and an imprecise 19% reduction in breast cancer-specific mortality in association with tuna intake only). As shown in the Supplement, we found limited evidence for interaction between ω-3 and ω-6, or between PUFAs and most genotypes considered in three related pathways.
Our population-based results are consistent with one previous study on PUFA and/or fish intake in relation to survival among women with breast cancer that is relatively comparable to our own 11. Patterson and colleagues 11 examined marine food sources of EPA and DHA in the WHEL study, and reported reductions of 28% for recurrence and 41% for all-cause mortality. The study methods for the WHEL study differ from ours in that only marine ω-3 sources were assessed using repeated 24-hour recalls after varying lengths of time since diagnosis among women recruited for a dietary intervention aimed to reduce breast cancer recurrence; whereas, we considered marine and other dietary PUFA sources that were assessed using a 101-item FFQ administered within three months of diagnosis to a population-based sample of women newly diagnosed with breast cancer. The robustness of the results across studies, despite the methodological differences in our LIBCSP study and the WHEL study, support the possibility of long-chain ω-3 as a potential risk reduction strategy for breast cancer survivors, even in the presence of potential measurement error. Further research is needed to confirm these findings.
Our reported results for long-chain ω-3 fatty acids (DPA, DHA, EPA), total ω-6, LA, AA, and the ratio of ω-3/ω-6 are consistent with the biologic mechanism of a PUFA-induced cytotoxic environment via lipid peroxidation, and the potential benefit of this environment on reducing apoptosis and cell growth in cancer cells 5, 6. Long-chain ω-3 fatty acids contain more double bonds within the fatty acid chain compared to ALA and ω-6. These double bonds provide additional opportunities for lipid peroxidation and thus could help promote a cytotoxic environment within the cell. Consequently, this cytotoxic environment could provide a beneficial environment for women with breast cancer.
Our results for ALA intake, which suggest a modest increase in the rate of overall death, are not consistent with a biologic hypothesis via inflammatory pathways. This discrepancy may reflect the foods that are contributing to high ALA intake in our Long Island population; namely, we observed that the foods containing butter and fried foods are contributing to high ALA intake. The similarity in foods contributing to high intake for ALA and ω-6 intake, may explain the potentially spurious increased rate of overall death observed for greater intake of ALA. Also, in vivo conversion of ALA into long-chain ω-3 PUFAs is inefficient in populations with high ω-6 23, which may possibly explain the modest increase in the overall rate of death observed here.
This prospective, population-based study has multiple strengths. We are the first to examine the potential relation between PUFA intake and breast cancer survival, while simultaneously considering both ω-3 and ω-6 PUFAs. Additionally, we examined multiple ω-3 (e.g., ALA, DPA, DHA, and EPA) and ω-6 (e.g., LA, AA) subtypes in relation to all-cause mortality, thus our additional assessments complement and move beyond what was examined in WHEL, a low-fat dietary intervention for which examination of ω-6 PUFAs was not reported. Consideration of non-marine sources of PUFAs may be critical for some U.S. populations that consume low amounts of fish 24. Further, we are the first to explore PUFA interactions with genes in multiple genetic pathways using a population-based design (discussed in more detail in the Supplement).
However, our follow-up study also has limitations. Despite our relatively large sample size, estimates for the associations with breast cancer-specific mortality were imprecise for both the 5-year and 15-year follow-up periods, and less pronounced compared to all-cause mortality for the entire 15-year follow-up period only. It is possible that long-chain ω-3 intake has the potential to reduce 15-year mortality from other outcomes, such as cardiovascular disease mortality, which could potentially lead to more pronounced estimates for all-cause compared to breast-cancer specific mortality after 15 years of follow-up. Nonetheless, during the first five years following a breast cancer diagnosis, most deaths are due to breast cancer. Thus, our results suggest that ω-3 intake is associated with both all-cause and breast cancer-specific mortality within the first five years after diagnosis. Additionally, we were able to capture dietary intake close to the time of diagnosis, and thus this exposure window may be more relevant for the 5-year follow period, given the more pronounced estimates observed for this time period. However, the imprecision for all breast cancer-specific estimates, regardless of the time period, is likely due to PUFA measurement error. For example, it is possible that a one-time baseline FFQ measurement of diet may not accurately reflect dietary intake throughout the 15-year period following diagnosis. One recent study compared pre-diagnosis versus post-diagnosis dietary intake, and reported dietary increases in oily fish and fish oil consumption post breast cancer diagnosis 25. However, this repeated measure study enrolled women 9–15 months after diagnosis, and asked them to concurrently recall dietary intake one year prior to diagnosis as well as changes in diet following diagnosis; thus, the reported changes in dietary intake are subject to errors in recall. Nonetheless, it is possible that the estimates of long-chain ω-3 PUFA intake in our study population are conservative. Further, long-chain ω-3 PUFA levels differ by fish species, with tuna being among the highest 26. Thus, future, larger studies should consider repeated PUFA measurements (self-reported intake of specific fish species, fish oil, and/or biomarkers) throughout follow-up to enhance our examination of the potential association between long-chain ω-3 PUFAs and breast cancer survival.
In addition, although our study is population-based and reflects the racial distribution of the target population on Long Island, which improves study validity, the LIBCSP population includes predominantly Caucasian women; therefore, examination of racial differences was not possible. However, our results are generalizable to Caucasian-American women for whom the rates of breast cancer remain high 1. Future studies may consider exploring possible heterogeneity between PUFA and survival by race, or by breast cancer tumor subtypes.
In conclusion, in our population-based follow-up study of women with breast cancer on Long Island, NY, we observed 16–34% reductions in all-cause mortality after 15 years of follow-up for high intake of fish, and long-chain ω-3 (DPA, DHA, and EPA), which is consistent with laboratory evidence and the one other U.S.-based epidemiologic study considering this issue 11. Thus, pending additional replication, dietary intake of fish and other sources of long-chain ω-3 fatty acids may provide an additional strategy to improve survival following breast cancer.
Supplementary Material
Acknowledgments
Financial support: This study was supported in part with grants from the National Institutes of Health (CA/ES 66572, CA58233, ES10126, ES009089); the Department of Defense (U.S. Army BC972005); and the Breast Cancer Research Foundation.
Footnotes
Conflict Disclosure: The authors declare no financial or non-financial conflicts.
References
- 1.Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin [serial online] 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat [serial online] 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw PT, Ibrahim JG, Stevens J, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology [serial online] 2012;23:320–327. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland RJ, Eng SM, Stevens J, et al. Influence of prediagnostic recreational physical activity on survival from breast cancer. Eur J Cancer Prev [serial online] 2012;21:46–54. doi: 10.1097/CEJ.0b013e3283498dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenais B, Blanckaert V. The janus face of lipids in human breast cancer: How polyunsaturated fatty acids affect tumor cell hallmarks. Int J Breast Cancer [serial online] 2012;2012:712536. doi: 10.1155/2012/712536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res [serial online] 2010;49:76–86. doi: 10.1016/j.plipres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br J Cancer [serial online] 2009;101:1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki S, Horacsek M, Kesteloot H. An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev Med [serial online] 1993;22:187–202. doi: 10.1006/pmed.1993.1016. [DOI] [PubMed] [Google Scholar]
- 9.Ishimoto H, Nakamura H, Miyoshi T. Epidemiological study on relationship between breast cancer mortality and dietary factors. Tokushima J Exp Med [serial online] 1994;41:103–114. [PubMed] [Google Scholar]
- 10.Petrek JA, Hudgins LC, Ho M, Bajorunas DR, Hirsch J. Fatty acid composition of adipose tissue, an indication of dietary fatty acids, and breast cancer prognosis. J Clin Oncol [serial online] 1997;15:1377–1384. doi: 10.1200/JCO.1997.15.4.1377. [DOI] [PubMed] [Google Scholar]
- 11.Patterson RE, Flatt SW, Newman VA, et al. Marine fatty acid intake is associated with breast cancer prognosis. J Nutr [serial online] 2011;141:201–206. doi: 10.3945/jn.110.128777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyogoku S, Hirohata T, Nomura Y, Shigematsu T, Takeshita S, Hirohata I. Diet and prognosis of breast cancer. Nutr Cancer [serial online] 1992;17:271–277. doi: 10.1080/01635589209514196. [DOI] [PubMed] [Google Scholar]
- 13.Gammon MD, Neugut AI, Santella RM, et al. The long island breast cancer study project: Description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat [serial online] 2002;74:235–254. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol [serial online] 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol [serial online] 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 16.Potischman N, Swanson CA, Coates RJ, et al. Dietary relationships with early onset (under age 45) breast cancer in a case-control study in the united states: Influence of chemotherapy treatment. Cancer Causes Control [serial online] 1997;8:713–721. doi: 10.1023/a:1018475203820. [DOI] [PubMed] [Google Scholar]
- 17.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol [serial online] 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Agriculture, Agricultural Research Service, USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Home Page. Available from URL: http://www.ars.usda.gov.libproxy.lib.unc.edu/nutrientdata.
- 19.Allison PD SAS Institute. Survival Analysis using SAS: A Practical Guide. 2nd ed. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 20.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. 3, [thoroughly rev a updat ed. Available from URL: http://www.loc.gov/catdir/enhancements/fy0828/2007036316-t.html. [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology [serial online] 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 23.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res [serial online] 1998;68:159–173. [PubMed] [Google Scholar]
- 24.Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the united states. Am J Clin Nutr [serial online] 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 25.Velentzis LS, Keshtgar MR, Woodside JV, et al. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat [serial online] 2011;128:473–482. doi: 10.1007/s10549-010-1238-8. [DOI] [PubMed] [Google Scholar]
- 26.Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the omega-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: Risks and benefits. Environ Res [serial online] 2004;95:414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.