Abstract
Epi-illuminescence intravital fluorescence microscopy has been employed to study leukocyte-endothelial interactions in a number of brain pathologies. Historically, dyes such as Rhodamine 6G have been injected intravenously. However, intravenous injections can predispose experimental animals to a multitude of complications and requires a high degree of technical skill. Here we study the efficacy of injecting Rhodamine 6G into the peritoneum (IP) for the purpose of analyzing leukocyte-endothelial interactions through a cranial window during real time intravital microscopy. After examining the number of rolling and adherent leukocytes through a cranial window we found no advantage to the intravenous injection (IV). Additionally, we tested blood from both routes of injection by flow cytometry to gain a very precise picture of the two methods. The two routes of administration failed to show any difference in the ability to detect cells. The study supports the notion that IP Rhodamine 6G works as efficaciously as IV and should be considered a viable alternative in experimental design for investigations employing intravital microscopy. Facilitated intravital studies will allow for more exploration into cerebral pathologies and allow for more rapid translation from the laboratory to the patient with less chance of experimental error from failed IV access.
Keywords: Rhodamine 6G, Epi-illuminescence intravital fluorescence microscopy, Intraperitoneal injection, Cranial window, Leukocyte Rolling, Leukocyte Adhesion
Introduction
A number of pathologies including cerebral ischemia, multiple sclerosis, infection, and traumatic brain injury all cause peripheral leukocytes to enter the brain. Rolling and adhesion of peripheral leukocytes remains a necessary process in this entry (Bauer and others, 2009; Goldsby and others, 2000; Jin and others, 2010; Kawakami and others, 2012; Kenne and others, 2012; Lakhan and others, 2009; Lustig and others, 1992; Zhang and others, 2009a; Zhang and others, 2009b). Epi-illuminescence intravital fluorescence microscopy allows for visualization and quantification of this process in pial blood vessels and has been used to study the process in EAE (Experimental Autoimmune Encephalomyelitis), stroke, and other neuroinflammatory conditions (Ramirez and others, 2012; Zhang and others, 2009a; Zhang and others, 2009b).
Clear visualization of leukocytes and platelets, but not erythrocytes, can be easily achieved with administration of fluorescent dyes such as Rhodamine 6G (Gear, 1974; Johnson and others, 1980; Ross and Pawlina, 2006). Rhodamine has traditionally been administered through IV access (Levene and others, 2007; Zhang and others, 2009b). However, IV access in mice poses multiple difficulties and drawbacks. Regardless of the vein accessed, the technician must be able to properly cannulate the vessel and no universal method has been developed to evaluate this procedure (Groman and Reinhardt, 2004). In mice, the tail vein is small and consistent and reliable injections remain difficult to achieve with a failure rate of as much as 40% (Groman and Reinhardt, 2004; Levene and others, 2007). Injections to vessels in the inner thigh (femoral, saphenous) or neck (jugular) could affect traditional motor analysis and gait scoring schemes essential to neuromuscular analysis (Levene and others, 2007). Injection to the facial vein avoids many of these risks and if performed correctly should prohibit any gait abnormalities (Levene and others, 2007).
However, facial vein injection, as with many other injections sites, requires the use of anesthesia to gain surgical access. Anesthetics can induce significant bradycardia as well as fluctuations in blood pressure, body temperature, and respiratory rate leading to breathing problems (Cox and others, 1994; Olson and others, 1994; Stoenica and others, 2006). Age, sex, temperature, nutritional status, and overall health of the animal can also vary the effect of anesthesia and therefore experimental findings (Barnes and Eltherington, 1973). While anesthesia must be administered for the purpose of intravital microscopy, failed IV cannulations lead to longer time under anesthetics as well as bleeding and ultimately increases the risk of adverse outcomes and the chance of impacting results. Thus, a method to administer rhodamine that minimized these risks but also provided reliable and effective delivery to allow for epi-illuminescence intravital fluorescence microscopy would be highly advantageous.
IP injection satisfied the objectives of relieving the adverse side effects associated with surgical IV access. To our knowledge, no one has examined the efficacy of IP rhodamine and in particular, for use in intravital microscopy. The IP route had been utilized for administration of both fluorescein and Evans Blue dye (Manaenko and others, 2011; Morrey and others, 2008). A study comparing intravenous and intraperitoneal Evans Blue dye found the results were independent of the administration route (Manaenko and others, 2011). In the present study, Rhodamine 6G was administered by both routes and leukocyte-endothelial interactions examined through implanted cranial windows. Rolling and adhesion following both methods of injection was quantified and showed no difference. Flow cytometry was also used to compare staining efficacy following both methods of administration. The overall percentage of stained cells did not vary among the samples but IV administration had a significantly higher fluorescent mean. However, this difference was eradicated by increasing the dose of Rhodamine 6G used for IP injection.
Materials and Methods
Animal treatment methods
This study was carried out in accordance with the National Institutes of Health guidelines for the treatment of animals and was approved by the Animal Care and Use Committee at Temple University. Male C57Bl/6 (Jackson Laboratories) weighing 20–25g were housed with a 12-h light/dark cycle with access to water and food ad libitum. The animals were anesthetized with mixture of ketamine (100 mg/mL) and xylazine (20mg/mL) mixed (1:1 by volume) at a dose of 1 mL/kg. Body temperature during all surgical procedures was maintained with a heating pad and lamp.
Administration of Rhodamine 6G
Freshly prepared 0.05% Rhodamine 6G was administered in an amount of 100 uL except for the last set of flow cytometry studies in which 200 uL was administered IP. After the depth of anesthesia was confirmed by a lack of toe pinch response, the skin over the inner thigh was shaved and prepped for IV injection by swabbing with isopropyl alcohol. A 1 cm incision was made to visualize the femoral and saphenous veins. Under a dissecting scope, a 30 gauge needle was advanced into the lumen of the vein at a distal location. Upon injection of rhodamine, displacement of blood in the vessel and visualization of dye in the vessel confirmed entry and placement of the needle. Pressure was applied until bleeding stopped and the skin was closed with staples.
For IP injection, the needle was inserted at a 30° angle on an imaginary line drawn across the abdomen just above the knees of the mice. The needle was inserted along this line on the right side of the midline to a depth of 5mm (2005). Aspiration was performed prior to injection of Rhodamine 6G to ensure proper placement.
Implant of cranial windows
The head was shaved and positioned in a stereotactic holder as described in Zhang (Zhang and others, 2009a; Zhang and others, 2012). The skin over the right cortical hemisphere was swabbed with isopropyl alcohol twice and then removed as well as the periosteum. A 4 mm diameter circular craniotomy was performed using an electric high speed drill (Fine Science Tools) over the right parietal cortex. It was bounded anteriorly by the coronal suture, medially by the sagittal suture, laterally by the attachment of the temporal muscle, and posteriorly by the lambdoid suture. The window was positioned in the middle of the sagittal suture to include some terminal branches of the middle cerebral artery. Sterile isotonic saline was dripped over the cranium to avoid thermal injury of the cortex. The dura was also removed and exposed brain kept moist with saline as well. A 5 mm diameter coverglass was then implanted over the exposed brain and an airtight seal formed with Nexaband Quick Seal. The coverglass provided adequate mechanical protection from infection or contamination. A recovery period of 4 days was provided between the implant of the windows and the examination of rhodamine application (Zhang and others, 2009a; Zhang and others, 2012).
Stimulation of Inflammation and Intravital Microscopy
Since animals in the study lacked pathology, neuroinflammation needed to be initiated to quantify rolling and adhesion. Lipopolysaccharide (LPS) from E. coli O111:B4 (Calbiochem) was dissolved in 1XPBS to a concentration of 1 μg/mL and injected intraperitoneally at 5 mg/kg in each animal at the time of Rhodamine 6G administrations for studies involving visualization of cerebral vasculature.
Intravital microscopy was carried out as previously described by our laboratory (Zhang and others, 2009a; Zhang and others, 2012). Anesthesia was provided and after a lack of toe pinch response, the animal was fixed in a stereotactic head holder. An epi-illuminiscence microscope with a dichroic mirror (BX10, Olympus, Japan) in combination with a black and white digital camera, (Cooke, 1600, Cooke Corporation, Romulus, MI) and a TRITC filter (excitation 540 nm, emission 605 nm) was employed to gain a final magnification of 200x through the windows. One image was captured every hour for 8 hours to prevent photo-bleaching and phototoxicity by the mercury lamp. When possible the same vessel was examined each time. The collection time was limited to 30 seconds. The images recorded by Camware software at a video frame rate of 25 frames/sec and saved as mpeg files for offline analysis (Zhang and others, 2009a; Zhang and others, 2012).
Measurement of Leukocyte/Endothelial Interactions
The number of rolling leukocytes was considered to be the total number of leukocytes moving at a significantly slower rate than the midline velocity of blood cells as described in Zhang (Zhang and others, 2009a; Zhang and others, 2012). They were counted as they passed an arbitrary line drawn perpendicular to the long axis of the vessel for 30 seconds. This count was repeated 3 times for each vessel with the arbitrary line repositioned each time. The average of the 3 counts was reported and normalized to 1 minute with results reported as the number of rolling leukocytes/minute.
Adherent leukocytes were defined as the total number of leukocytes that were firmly attached to the endothelium and did not change position during the course of 30 seconds of observation (Zhang and others, 2009a; Zhang and others, 2012). Adherent leukocytes were reported as the number of cells/mm2. The vascular surface area for each vessel was calculated to accomplish this by utilizing ImageJ software (NIH).
Flow Cytometry
To gain a very precise picture of fluorescent activity in individual cells, we utilized flow cytometry. Four hours after the administration of Rhodamine 6G, a lancet was utilized to perforate the facial vein of the mice and a few drops of blood were collected in 1 mL of PBS containing Heparin (750 ng/mL). The samples were centrifuged and resuspended in 2 mL RBC Lysis Buffer (eBioSciences). Cells were washed and finally resuspended in 200 μL 1X PBS. The samples were analyzed with a BD FACSCalibur flow cytometer and analysis performed with Cell Quest Pro software.
Statistical Analysis
Rolling and Adhesions studies, were analyzed with a 2 way analysis of variance for repeated measures with Bonferroni correction. Flow cytometry studies were subjected to a Student’s t-Test. Data is reported as the Mean ± SEM. A p-value < 0.05 was used for statistical significance.
Results
Effect of Injection Method on Number of Observed Rolling and Adherent Leukocytes, Figures 1–3
Figure 1.
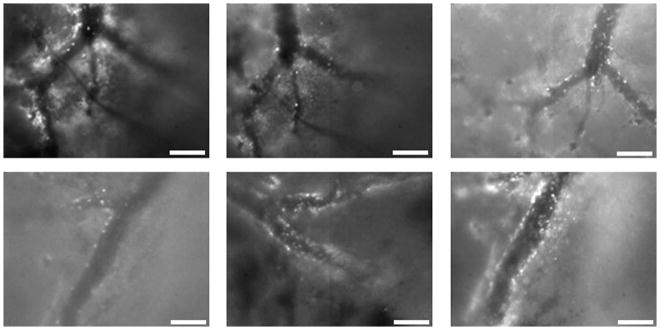
Still images obtained from movies of cerebral blood flow captured by intravital microscopy. Top row: Intravenous administration at 1, 4, and 8 hours respectively. Bottom row: Intraperitoneal administration of Rhodamine 6G at the same time points. Scale bar is 100 μM.
Figure 3.
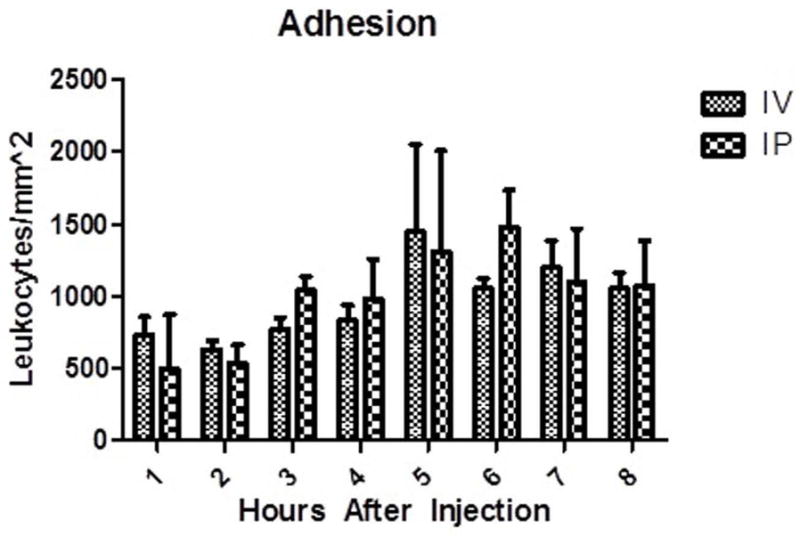
Adherent leukocytes on pial endothelial cells. There was no difference between either group at matched time points. Data displayed as Mean ± SEM. p < 0.05. n =3 mice in each group.
Rolling leukocytes were assessed through the cranial windows every hour for 8 hours, Figures 1 and 2. In the IV group, a mean of 43 ± 11 cells/min were observed rolling at 1 hour and declined to 38 ± 13 cells/min at 8 hours. The IP group exhibited a slightly higher mean number of observable cells with 80 ± 33 cells/min at 1 hour and finally 70 ± 25 cells/min at 8 hours, Figure 2. Two way ANOVA revealed no main effect of route [F(1,66) = 1.77, ns], no main effect of time [F(7,66) = 0.41, ns], and no significant interaction [F(7,66) = 0.51, ns]. There was no statistically significant difference between any matched time points. Adherent leukocytes were also evaluated between the two injection methods, Figure 3. In the IV group, at 1 hour a mean of 732 ± 124 cells/mm2 were found to be adherent compared to a mean of 1051 ± 113 cells/mm2 at 8 hours. In the IP group, adherent cells had a mean number of 497 ± 153 cells/mm2 at 1 hour and rose to a mean of 1075 ± 138 cells/mm2 at 8 hours. Two way ANOVA revealed no main effect of route [F(1,66) = 0.14, ns], a main effect of time [F(7,66) = 4.01, p < 0.05], and no significant interaction [F(7,66) = 0.62, ns]. In comparing the groups, no statistically significant difference was found among any of the corresponding time points.
Figure 2.
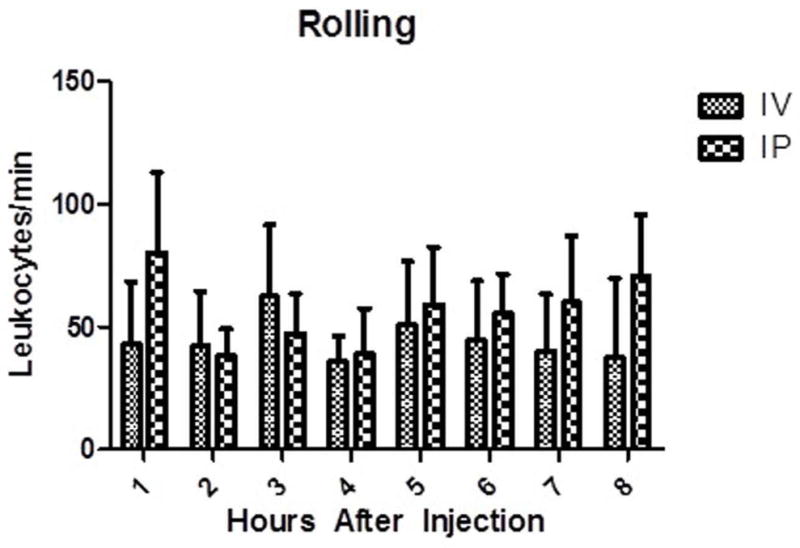
Rolling leukocytes on pial endothelial cells. There was no difference between either group at matched time points. Data displayed as Mean ± SEM. p < 0.05. n =3 mice in each group.
Effect of Injection Method on Flow Cytometry, Figure 4
Figure 4.
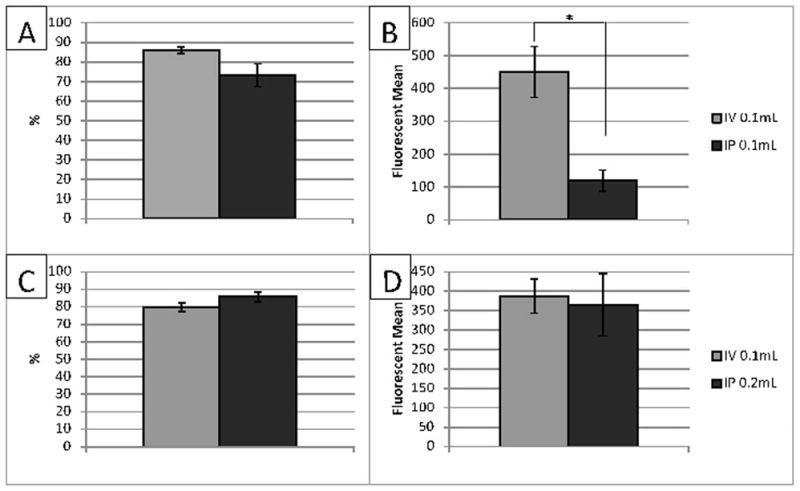
Flow cytometry at 4 hours. Panel A and B, overall percentage of stained cells (A) and fluorescent mean (B) with 100 μL of Rhodamine 6G given. There was no difference in the overall percent of stained cells, however, there was a statistically significant difference among the fluorescent mean. Panel C and D, overall percentage of stained cells (C) and fluorescent mean (D) when 200 μL of Rhodamine 6G given. Data expressed as Mean ± SEM. p < 0.05. n =5 mice in each group.
Blood was extracted from both groups 4 hours after injection and analyzed utilizing flow cytometry. When 100 μL was administered, overall staining of leukocytes in the IV group had a mean of 85.88% ± 1.70% while in the IP group it was 73.28% ± 5.84%, Figure 4. This difference was not statistically significant. The fluorescent mean of the IV group was found to be 450.29 ± 173.46 compared to 119.182 ± 72.76 in the IP group, Figure 4. This difference was statistically significant, p = 0.0123.
Since the fluorescent mean was lower with the IP method, the amount of rhodamine injected IP was doubled (200 μL) and compared to the original dose of intravenous rhodamine (100 μL). In this study, intravenous rhodamine stained a mean of 79.89% ± 5.79% of all leukocytes compared to 85.62% ± 6.40% of leukocytes from mice given a double dose intraperitoneally, Figure 4. The fluorescent mean was 387.35 ± 44.15 in the IV group and 365.58 ± 80.49 in the IP group, Figure 4. No statistically significant difference was found.
Discussion
The purpose of the study was to determine if administration of Rhodamine 6G through an IP injection would be as efficacious as an IV injection for intravital microscopic observations of leukocyte rolling and adhesion. In brief, IP injection proved to be as effective as IV injection. Figure 1 clearly demonstrates the ease of identification of leukocytes in each method. The importance of this study stems from the high failure rates of IV injection (Groman and Reinhardt, 2004). This technique simplifies experimental design and minimizes complications.
The number of cells detected was quantified to determine the number of rolling and adherent leukocytes. LPS had been injected to create inflammation above baseline and provide a large enough sampling of leukocytes. IV administration of rhodamine has been the standard used in other publications and it was possible that IP injection would not provide adequate staining and underscore the number of rolling cells (Zhang and others, 2009a; Zhang and others, 2009b). This was not the case as the IP injection never differed significantly from the IV injection method in the number of rolling leukocytes detected. The results were the same for measurements of adherent leukocytes. The IP method, again, did not differ in its ability to stain and identify cells compared to IV injection. Thus, both methods of injection yield no difference in the quantification of rolling and adherent leukocytes and the simplified IP method may be beneficial in some experimental designs.
We next performed flow cytometry on blood samples at 4 hours to evaluate the effect of the two injection methods on the staining of leukocytes. Our selection of 4 hours was based on the fact that it represented the halfway point of our study. Administration of equal amounts of rhodamine produced no statistically significant difference in overall stained leukocytes but it did produce differences in the fluorescent means with the IV method yielding higher values. Despite the difference in fluorescent means, the flow cytometry was still able to detect an equivalent number of cells regardless of the injection method. When we doubled the amount of rhodamine injected into the peritoneum, 200 μL, no differences were found in the overall number of cells stained or the fluorescent mean. Higher amounts of rhodamine in IP injections could perhaps increase the concentration gradient and amount of diffusion leading to more dye in circulation and ultimately in each leukocyte.
In summary, it has been shown that regardless of the injection method used, the number of rolling and adherent leukocytes in cranial windows remain unchanged based on the injection route. Neither method creates a statistically significant different percentage of overall stained leukocytes. However, at equal injection volumes, the IV method generates greater fluorescent means. This difference is easily averted when the dose administered in the peritoneum is doubled. With no difference by either method, the much simpler IP method can be considered a viable substitute in some protocols and experimental designs.
Conclusion
Considering the complications associated with IV injection from both the administration of anesthesia and the high rate of failure in cannulating the vessels, IP injection should be considered as an alternative and equally efficacious method to administer Rhodamine 6G for analysis of leukocyte-endothelial interactions in cranial windows during intravital microscopy.
Acknowledgments
In particular we would like to thank Dr. Doina Ganea, Dr. Jimmy Yen, Dr. Wenmen Wu, Dr. Weimin Kong, and Dr. Sara Ward for their assistance and technical advice. This work was supported in part by Grant from the National Institute of Drugs of Abuse, T32 DA007237.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Zachary Wilmer Reichenbach, Email: zachr@temple.edu, Temple University School of Medicine, Center for Substance Abuse Research, 3500 North Broad Street, 8th Floor, Philadelphia, PA 19140, +1-610-751-6820.
Hongbo Li, Email: hongboli@temple.edu, Temple University School of Medicine, Center for Substance Abuse Research, 3500 North Broad Street, 8th Floor, Philadelphia, PA 19140.
John P. Gaughan, Email: jgaughan@temple.edu, Temple University School of Medicine, Department of Cellular and Molecular Physiology, 3500 North Broad Street, 10th Floor, Philadelphia, PA 19140.
Melanie Elliott, Email: Melanie.elliott@jefferson.edu, Department of Neurosurgery, Thomas Jefferson University Hospital, 1025 Walnut Street, Philadelphia, PA 19107.
Ronald Tuma, Email: Ronald.tuma@temple.edu, Temple University School of Medicine, Center for Substance Abuse Research, 3500 North Broad Street, 8th Floor, Philadelphia, PA 19140.
References
- 1.AALAS Learning Library Animal Care and Use in Research and Education.
- 2.Barnes CD, Eltherington LG. Drug dosage in laboratory animals, a handbook. Berkeley: University of California Press; 1973. p. 341. [Google Scholar]
- 3.Bauer M, Brakebusch C, Coisne C, Sixt M, Wekerle H, Engelhardt B, Fässler R. Beta1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proc Natl Acad Sci U S A. 2009;106(6):1920–5. doi: 10.1073/pnas.0808909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox AK, Morck DW, Olson ME. Evaluation of detomidine and ketamine-detomidine for anesthesia in laboratory rats. Contemp Top Lab Anim Sci. 1994;33(2):52–5. [PubMed] [Google Scholar]
- 5.Gear AR. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974;249(11):3628–37. [PubMed] [Google Scholar]
- 6.Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Kuby immunology. xxv. New York: W.H. Freeman; 2000. p. 670. [Google Scholar]
- 7.Groman EV, Reinhardt CP. Method to quantify tail vein injection technique in small animals. Contemp Top Lab Anim Sci. 2004;43(1):35–8. [PubMed] [Google Scholar]
- 8.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980;77(2):990–4. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami N, Bartholomäus I, Pesic M, Mues M. An autoimmunity odyssey: how autoreactive T cells infiltrate into the CNS. Immunol Rev. 2012;248(1):140–55. doi: 10.1111/j.1600-065X.2012.01133.x. [DOI] [PubMed] [Google Scholar]
- 11.Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J Neuroinflammation. 2012;9:17. doi: 10.1186/1742-2094-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levene HB, Zhang M, Erb CJ, Jallo JI, Loftus CM, Tuma RF. Method to perform IV injections on mice using the facial vein. J Neurosci Methods. 2007;164(2):304–7. doi: 10.1016/j.jneumeth.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Lustig S, Danenberg HD, Kafri Y, Kobiler D, Ben-Nathan D. Viral neuroinvasion and encephalitis induced by lipopolysaccharide and its mediators. J Exp Med. 1992;176(3):707–12. doi: 10.1084/jem.176.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195(2):206–10. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrey JD, Olsen AL, Siddharthan V, Motter NE, Wang H, Taro BS, Chen D, Ruffner D, Hall JO. Increased blood-brain barrier permeability is not a primary determinant for lethality of West Nile virus infection in rodents. J Gen Virol. 2008;89(Pt 2):467–73. doi: 10.1099/vir.0.83345-0. [DOI] [PubMed] [Google Scholar]
- 17.Olson ME, Vizzutti D, Morck DW, Cox AK. The parasympatholytic effects of atropine sulfate and glycopyrrolate in rats and rabbits. Can J Vet Res. 1994;58(4):254–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32(12):4004–16. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross MH, Pawlina W. Histology: a text and atlas: with correlated cell and molecular biology. Baltimore, MD: Lippincott Wiliams & Wilkins; 2006. p. xvii.p. 906. [Google Scholar]
- 20.Stoenica L, Senkov O, Gerardy-Schahn R, Weinhold B, Schachner M, Dityatev A. In vivo synaptic plasticity in the dentate gyrus of mice deficient in the neural cell adhesion molecule NCAM or its polysialic acid. Eur J Neurosci. 2006;23(9):2255–64. doi: 10.1111/j.1460-9568.2006.04771.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009a;78(1):86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Mahadevan A, Amere M, Li H, Ganea D, Tuma RF. Unique Effects of Compounds Active at Both Cannabinoid and Serotonin Receptors During Stroke. Translational Stroke Research. 2012;3(3):348–356. doi: 10.1007/s12975-012-0197-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, Tuma RF. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009b;4(2):249–59. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


