Abstract
Introduction:
Due to the complications associated with the use of non-native biomaterials and the lack of local tissues, bioengineered tissues are required for surgical reconstruction of complex urinary tract diseases, including those of the urinary bladder. The self-assembly method of matrix formation using autologous stromal cells obviates the need for exogenous biomaterials. We aimed at creating novel ex-vivo multilayer urinary tissue from a single bladder biopsy.
Methods:
After isolating urothelial, bladder stromal and smooth muscle cells from bladder biopsies, we produced 2 models of urinary equivalents: (1) the original one with dermal fibroblasts and (2) the new one with bladder stromal cells. Dermal fibroblasts and bladder stromal cells were stimulated to form an extracellular matrix, followed by sequential seeding of smooth muscle cells and urothelial cells. Stratification and cellular differentiation were assessed by histology, immunostaining and electron microscopy. Barrier function was checked with the permeability test. Biomechanical properties were assessed with uniaxinal tensile strength, elastic modulus, and failure strain.
Results:
Both urinary equivalents could be handled easily and did not contract. Stratified epithelium, intact basement membrane, fused matrix, and prominent muscle layer were detected in both urinary equivalents. Bladder stromal cell-based constructs had terminally differentiated urothelium and more elasticity than dermal fibroblasts-based equivalents. Permeation studies showed that both equivalents were comparable to native tissues.
Conclusions:
Organ-specific stromal cells produced urinary tissues with more terminally differentiated urothelium and better biomechanical characteristics than non-specific stromal cells. Smooth muscle cells could be incorporated into the self-assembled tissues effectively. This multilayer tissue can be used as a urethral graft or as a bladder model for disease modelling and pharmacotherapeutic testing.
Introduction
Studying the development and differentiation of the urothelium is important to understand many bladder pathologies, urothelium healing, and regenerative processes. Although there are many animal and ex vivo models to study the urinary bladder, none of them allow proper investigation of the normal development and differentiation of the human urothelium. Due to slow turnover of the umbrella cells, post-injury inflammation and hyperplasia, ethical drawbacks and animal cost, the in vivo study of urothelial differentiation and development is difficult.1–4 The investigation of preor postnatal differentiation in rodents does not follow the same pathways in human.5 In vitro studies can avoid these obstacles; however, these studies have limitations. The monolayer models lack the complex three-dimensional (3D) structure, the variety of cell types, and differentiation state present in 3D environment. Although tissue explants models have been created,6,7 they have certain deficiencies, including limited availability, insufficient viability of thawn tissues and potential transfer of infectious agents. The current 3D cell culture models8,9 lack the structural architecture of functional tissue and use only one cell type. Also, the use of exogenous matrices would alter the signaling pathways and the process of differentiation and leads to inflammatory response and fibrosis if grafted.
To avoid these problems, we have previously created an in vitro model of urinary bladder with dermal fibroblasts (DF) and urothelial cells (UCs).10–12 As smooth muscle cells (SMCs) are responsible for urine transport and evacuation, their inclusion in any tissue-engineered graft for urinary tract is required.13 Therefore, we aimed in this study to produce a more biomimetic urinary tissue that closely simulated urinary tissue by using organ-specific stromal cells to produce urinary ECM and incorporating SMCs.
Methods
The Ethical Research Committee of our research centre approved this study. Two different types of urinary equivalents were constructed and evaluated: one using dermal fibroblasts (originally published) and the other new model using bladder stromal cells (BSCs). In both models, UCs and SMCs were seeded.
Cell isolation
Bladder biopsies were cut into small pieces and incubated overnight at 4 ºC in solution containing 500 mg/mL thermolysin (Sigma). The urothelial layer was then gently scraped off the rest of the biopsy to isolate UCs with trypsinization. The remaining part of the biopsy included the bladder submucosa (white colour) and SMCs (pink colour). The submucosa was dissected away from the SMC layer with sharp scissors; they were separately processed to isolate BSCs and SMCs with collagenase H solution (0.125 U/mL; Boehringer Mannheim, Laval, QC) as previously described.10,14 The medium used to grow and expand UCs was DHc (composed of DMEM-Ham [Dulbecco modified Eagle medium-Ham 12] containing 10% fetal bovine serum [FBS, Invitrogen], 5 mg/mL insulin [Sigma], 0.4 mg/mL hydrocortisone [Calbiochem, San Diego, CA], 10-10 M cholera toxin [ICN, St-Laurent, QC], 10 mg/mL epidermal growth factor [Austral Biologicals, San Ramon, CA]), and antibiotics (100 U/mL penicillin and 25 mg/mL gentamicin [Sigma]). The culture medium used for culture of BSCs and SMCs was DMEM, containing 10% FBS and antibiotics. The phenotype of the isolated cells was checked with phase contract microscopy and immunofluorescence (pancytokeratins [AE] 1/AE3 for UCs and smooth muscle actin [SMA] and calponin for BSCs and SMCs).
DF were isolated from human skin biopsies as previously described using 0.2 U/mL collagenase H and cultured in DMEM medium supplemented with 10% FBS and antibiotics.15
Construction of urinary equivalents
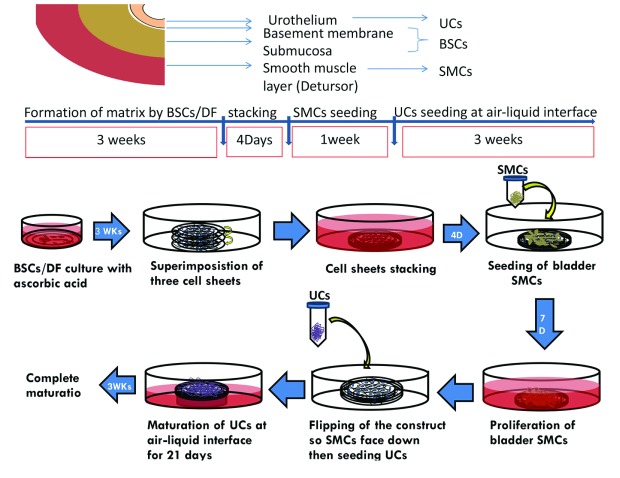
DF and BSCs were seeded separately in 6 well-cultured plates using DH medium with 10% FBS, antibiotics, and 50 μg/mL ascorbate for 21 days to form a collagen sheet. Three stromal sheets were superimposed to form one sheet. SMCs were then seeded on top of stromal sheets for 7 days. The construct was flipped and UCs were seeded on the other stromal side using differentiating medium (DHc, and keratinocyte serum free medium with bovine pituitary extract and epidermal growth factor [Gibco/Life Technology, Ontario] using a ratio of 60%:40%) plus 50 μg/mL ascorbate for 21 days at the air-liquid interface (Fig. 1).
Fig. 1.
The study plan. In the upper panel, each cell type is extracted from a different portion within the same single bladder biopsy where urothelial cells (UCs) were extracted from mucosa, bladder stroma cells (BSCs) mainly from submucosa and smooth muscles (SMCs) from detrusor muscle. The main steps of urinary equivalent formation with time scale for each step is shown in middle panel. Illustrations for the steps of equivalent production with self-assembly technique are seen in the lower panel.
Histological evaluation
Samples were fixed in 3.7% neutral buffered formaldehyde solution and embedded in paraffin. Histological sections (5 μm) were cut and stained using Masson’s trichrome (MT) and Hematoxylin and eosin (H&E).
Samples were embedded in frozen tissue medium (OCT compound). The primary antibodies used are described in Table 1. Secondary antibodies were coupled with Alexa 594 or Alexa 488 fluorochrome. For controls, the primary antibody was omitted. The specimens were then examined with a Nikon eclipse E600 epifluorescence microscope (Nikon, Tokyo, Japan) and images were processed with AxioVs 40 V4.8.2.0 (Carl Zeiss MicroImaging GmbH).
Table 1.
Primary antibodies used in the study
| Component | Antibody | Dilution | Source |
|---|---|---|---|
| Epithelium | AE 1,3 | 1:400 | Chemicon, Cambridge, MA, USA |
| CK20 | 1:100 | Abcam Inc., Cambridge, MA, USA | |
| CK14 | 1:100 | Sigma, St Louis, MO, USA | |
| K5 | 1:200 | Abcam Inc., Cambridge, MA, USA | |
| Ki-67 | 1/500 | Abcam Inc., Cambridge, MA, USA | |
| UPIII | 1:50 | Santa Cruz, Santa Cruz, CA, USA | |
| E-cadherin | 1:100 | Abcam Inc., Cambridge, MA, USA | |
| Occludin | 1:500 | Mybiosource, San Diego, CA, USA | |
| Basement membrane | Laminin V | 1:500 | Chemicon, Cambridge, MA, USA |
| Collagen IV | 1:800 | Abcam Inc., Cambridge, MA, USA | |
| Matrix | Collagen III | 1:200 | Cederlane, Burlington, ON, Canada |
| Fibronectin | 1:100 | ATCC, Manassas, VA, USA | |
| Laminin | 1:200 | Abcam Inc., Cambridge, MA, USA | |
| Elastin | 1:400 | Santa Cruz, Santa Cruz, CA, USA | |
| Smooth muscle cells | SMA | 1:1000 | Chemicon, Cambridge, MA, USA |
| Calponin | 1:500 | Santa Cruz, Santa Cruz, CA, USA | |
| Smoothelin | 1:500 | Affinity BioReagents, 3-Flow, CO, USA | |
| Desmin | 1:500 | Abcam Inc., Cambridge, MA, USA |
Functional studies
Tissue permeability was evaluated using Franz cells and radioactively labelled urea14 C at multiple time points for a maximum of 8 hours, as previously described.10,16 Both urinary equivalents were compared to native bladders.
Mechanical testing
Using a mechanical tester tester (Tytron 250, MTS Systems Corporation, MN), we measured the mechanical properties of both urinary equivalents by uniaxial tensile testing (UTS), elastic modulus, and failure strain. Sample thickness was measured on histological cuts using the Image J software (NIH, Bethesda, MD). Data were analyzed using Minitab (Minitab, State College, PA). Statistical comparison of the mechanical properties of both urinary equivalents was done using Student’s t-test. Results are presented as mean ± standard error (SE), with p < 0.05 considered statistically significant.
Electron microscopy
Samples of BSCs-SMCs equivalents were fixed with 2.5% glutaraldehyde in cacodylate buffer at 4 °C, rinsed with cacodylate buffer and post-fixed in 1% osmium tetroxide. Biopsies were dehydrated, dried, and sprayed with gold to be viewed with a Jeol JSM-63060LV (Tokyo, Japan).
Results
Cell isolation
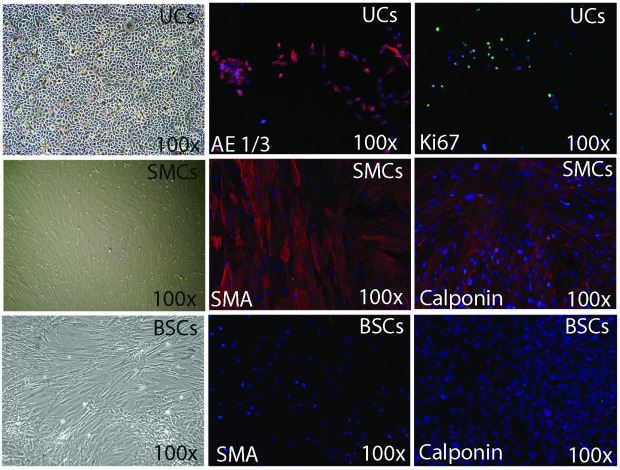
The 3 vesical cell types were successfully isolated and exhibited their characteristic phenotypes. Polygonal UCs expressed AE1/3 and KI-67 indicating high proliferative capacity. SMCs showed the typical spindle shape appearance with positive staining for SMCs markers. BSCs and DF showed different morphology with phase contrast microscopy, weak expression of SMA, and lack of expression for calponin (Fig. 2).
Fig. 2.
Phenotype characterization of 3 cell types extracted from bladder biopsy with immunofluorescence. Urothelial cells (UCs) had the characteristic appearance under phase contrast microscopy and their phenotype with phase contrast microscopy, expressed epithelial markers (AE1/AE3) with immunofluorescence. Smooth muscle cells (SMCs) exhibited SMCs markers; smooth muscle actin and calponin. Bladder stromal cells (BSCs) manifested weak expression for SMA and no expression for calponin.
Structure and architecture of urinary equivalent formation
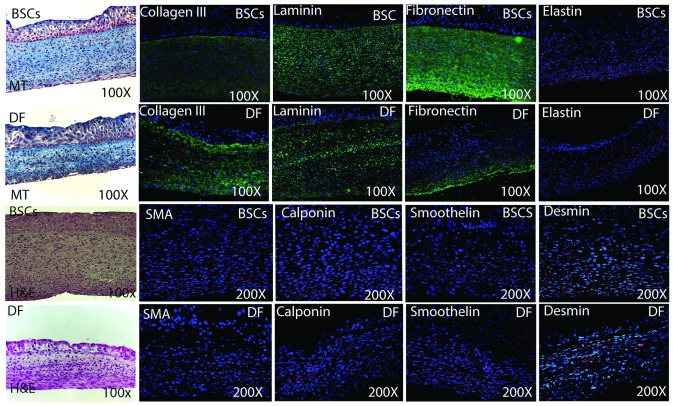
Both equivalents did not shrink and could be handled easily. On MT and H&E, the sections demonstrated architecture similar to the urinary tract tissue composed of multilayer, well-stratified epithelium overlying a well-defined basement membrane, thick stroma, and a muscle layer (Fig. 3). The epithelial layer had normal cell polarization with the columnar cells forming the basal layer, the flat cells forming the superficial layer and rounded or polyhedral cells in-between. SMCs were aligned on the outer surface of the equivalent.
Fig. 3.
Showing the tissue architecture with histochemical staining, different extracellular matrix (ECM) proteins (3A) and smooth muscle cells (SMCs) content and phenotype (3B) for both urinary constructs; bladder stroma cells (BSCs)-based and DF-based equivalents with immunofluorescence. Masson Trichrome (MT) and hematoxylin and eosin (H&E) revealed that both equivalents are tri-layered with multiple layers of urothelial cells, well fused stroma and smooth muscle layer. Although the different ECM proteins were detected in both equivalents but they were more expressed in BSCs=based equivalents. SMCs cells displayed different markers of SMCs maturation including those of terminal differentiation (SMA, calponin, smoothelin and desmin).
Characterization of equivalents by immunoflourescence staining
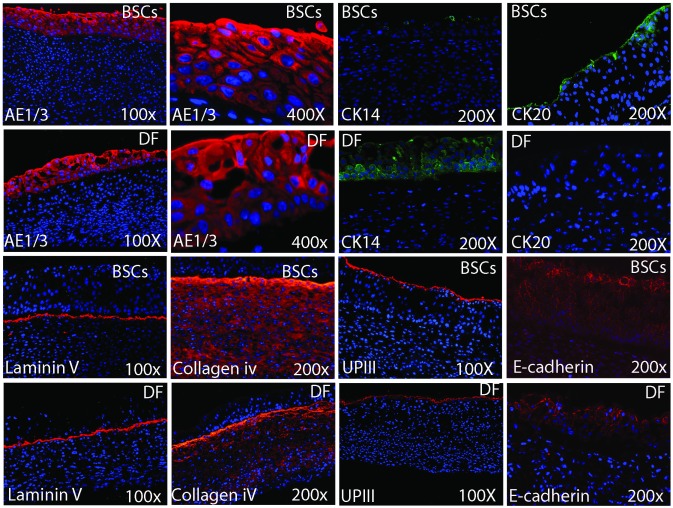
Both equivalents showed positive staining for the main ECM proteins: collagen I &III, laminin, fibronectin and elastin, with greater expression of the last 3 for the BSCs equivalents (Fig. 3, part A). The expression of SMCs markers was evident with different distribution in both equivalents (Fig. 3, part B). Both equivalents presented UCs with positive staining to AE1/AE3. However, with regards to terminal differentiation, there was a remarkable difference in favour of the organ-specific BSCs equivalents, which showed minimal expression of CK14 and obvious expression of CK20, while DF-SMCs equivalents revealed remarkable expression of CK14, mainly in the basal cells with no expression of CK20. Both equivalents demonstrated positive staining for laminin V and collagen IV, matrix proteins of the basement membrane. Uroplakin III and E-cadherin were also detected (Fig. 4).
Fig. 4.
Showing the expression of different epithelial and basement membrane markers among the 2 equivalents (bladder stroma cells [BSCs]-based and DF-based equivalents). Although both equivalents revealed expression of general epithelial markers (AE 1,3), uroplakin III (UPIII) and tight junctions E-cadherin, however they had different expression of cytokeratin 20 and 14. BSCs-based equivalents had terminally differentiated urothelium with evident expression of CK 20 and minimal expression of CK14, while DF-based equivalents showed the opposite. There was well-defined intact basement membrane as shown by positive staining for Laminin V and Collagen IV.
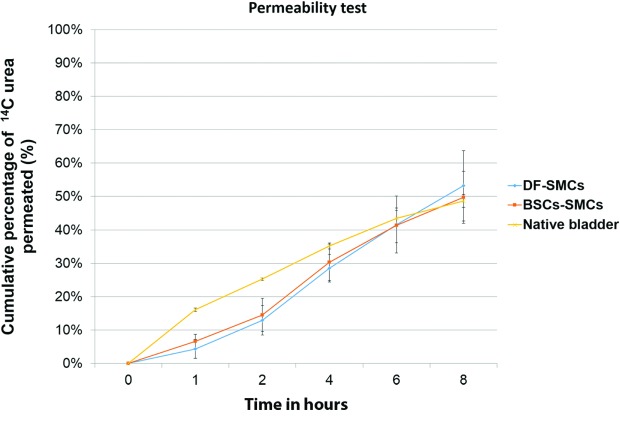
Permeability function of the urinary equivalents
Both the BSCs-based and DF-based equivalents revealed permeability comparable to native bladders, with no major difference after 8 hours (Fig. 5).
Fig. 5.
Permeability test using 14C-urea. Both equivalents were comparable to native tissues after 8 hours with no significant difference among them.
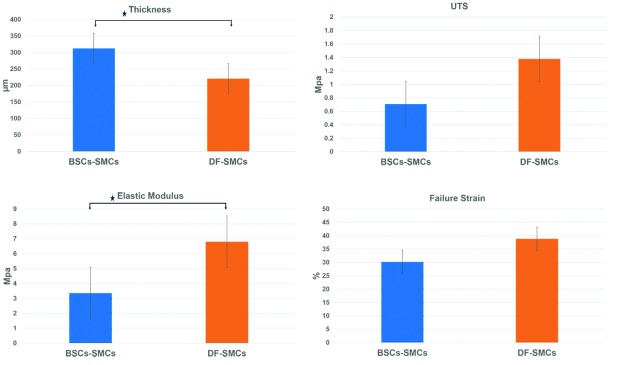
Mechanical properties of urinary equivalents
Delamination was observed in all DF-SMCs equivalents. DF-SMCs had significantly higher elastic modulus, while BCSs-SMCs had significantly higher stromal thickness. There was no significant difference between both urinary equivalents regarding failure strain and UTS (Fig. 6).
Fig. 6.
Biomechanical characteristics of the urinary equivalents in regard to uniaxial tensile strength (UTS), elastic modulus and failure strain. Bladder stroma cells-based equivalents had thicker tissue and less elastic modulus No significant difference between UTS and failure strain was detected between the 2 equivalents. Each column represents mean ± standard error, with p < 0.05 indicating significance: *p < 0.05.
Electron microscopy
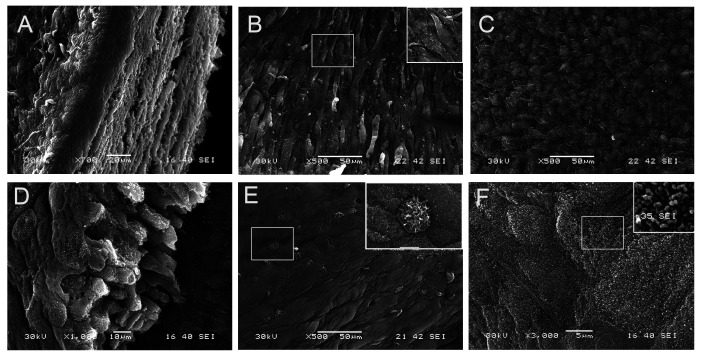
In 3D, electron microscopy defined the multilayered structure of BSCs-based equivalents. SMCs were clearly aligned. The cut section through the epithelium was well-stratified, with the most upper layer being flat, covered with urothelial plaques formed of uroplakins. Multiple microvillus cells were scattered among the UCs. Cell boundaries and tight junctions were identified (Fig. 7).
Fig. 7.
Scanning electron microscopy for bladder stroma cells- smooth muscle cells (SMCs) reconstructs. A: Cut section through the whole equivalent showing the different layers and thee thick stroma. B: SMC surface with aligned smooth muscle cells. The box in the upper right corner emphasizing the spindle shaped appearance of SMCs. C: The upper surface of the stroma with tightly packed collagen. D: Cut section though the urotheium with multiple layers resting on basement membrane (BM). E: the apical surface of urothelium formed of umbrella cells with well-defined borders and scattered microvillous cells (magnified picture in the upper right corner). F: the apical membrane of umbrella cells with uroplakins particles (magnified picture in the upper right corner).
Discussion
Tissue engineering (TE) approaches include either the use of acellular matrices or the use of cell-seeded matrices.17 In the genitourinary field, most of the strategies for TE require cell-seeded matrices.18 Clinical trials have been initiated for a number of diseases with varying degrees of success.19–23 To avoid the use of non-autologous tissues incorporated in these studies, a tissue-engineered construct was developed using UCs and DF, applying the principle of self-assembly.24,25 Consequently, as the lamina propria (LP) of the bladder is composed of ECM containing several types of cells, mainly fibroblasts,26 stimulating the stromal cells from LP would give rise to ECM with many characteristics similar to native urinary tissues. In one experiment, when stromal cells from LP were cocultured with UCs or SMCs, BSCs sped up the growth of both cells.27 In our results, sufficient BSCs could be easily isolated and they could maintain high proliferation capacity over passages. This makes them available for reconstructing urethral long segments and large vesical patches and makes them suitable for large-scale diagnostic experiments. ECMs were similar in both equivalents; they were thicker, better and more comparable to those of the bladder with BSCs equivalents.28
Our results showed that both equivalents had a well-stratified urothelium. However, terminal differentiation of urothelium was obtained only with BSCs-based equivalents, as CK20 is specific for umbrella cells in normal bladder and the marker of final differentiation for superficial cells. CK14 does not stain on normal urothelium and signifies vulnerability of the bladder to future disease.29–31 It is detected in pathologic bladders, denoting squamoid phenotype change of urothelium. It is observed before morphologic squamoid differentiation and is considered a reactive response to urothelial stress or injury. This pattern of CK expression for DF-based equivalents simulates reactive urothelium with tendency towards squamous metaplasia.
As the culture conditions were the same for both types of equivalents, the difference in urothelial differentiation can be attributed to the effects of organ-specific stromal cells on urothelium development and terminal differentiation. The fibroblasts are diverse in their phenotype due to the multiple embryonic origins and the epigenetic program that reflects their location.32,33 In skin equivalents, DF of human palmoplantar skin induced the formation of an epidermis with a palmoplantar phenotype even when nonpalmoplantar epidermal cells were used.34 This indicates that site-specific differentiation of fibroblasts empowers the differentiation of overlying epithelial cells. Consequently, our BSCs-equivalent can act as the correct or suitable microenvironment for urothelial growth and differentiation for progenitor or stem cells.
BSCs-SMCs equivalents were more elastic than DF-SMCs with no significant difference in strength. The elasticity of BSCs-SMCs tissues is a favourable feature as these tissues should be able to distend during urine filling or expulsion, without a major increase in their intraluminal pressure. BSCs-based constructs showed mechanical characteristics similar to one-layer small intestinal submucosa, bladder acellular matrix graft (BAMG), and normal urethra.35 In engineered tissues, there is a tradeoff between improved mechanical strength and decreased extensibility, which can affect their effectiveness and how well they match the mechanical properties of native tissue.
In both types of equivalent, SMCs attached, proliferated and kept their phenotype after seeding them on collagen sheets. This denotes the applicability of adding SMCs to our self-assembled constructs. SMCs reduced inflammatory response and enhanced smooth muscle regeneration and neovascularization.13 The electron microscopy observations are consistent with healthy adult urothelium.36 The presence of microvilous cells enlarges the surface area and affects the bacterial attachment and fluid movement across the cell membrane.37
Although both self-assembled urinary tissues were similar in many aspects, including permeability function, BSCs-SMCs had more ideal biomimetic urinary tissue because they were fully organ-specific, including stromal cells and matrix, and had thicker and elastic tissue with terminally differentiated epithelium. The limitations to our study include the long duration of equivalent formation and the lack of in vivo implantation. The replacement of urinary tract tissues is an elective procedure that does not require emergency reconstructive surgery. In vivo implantation is our next step.
Conclusions
Urinary tract specific stromal cells can give rise to ECMs using the self-assembly technique, with better physiological and mechanical characteristics than non-specific stromal cells. Our urinary equivalent is suitable as urethral graft or as bladder model for disease modelling and drug testing.
Footnotes
Competing interests: The authors all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Hatina J, Schulz WA. Stem cells in the biology of normal urothelium and urothelial carcinoma. Neoplasma. 2012;59:728–36. doi: 10.4149/neo_2012_089. [DOI] [PubMed] [Google Scholar]
- 2.Veranic P, Romih R, Jezernik K. What determines differentiation of urothelial umbrella cells? Eur J Cell Biol. 2004;83:27–34. doi: 10.1078/0171-9335-00351. [DOI] [PubMed] [Google Scholar]
- 3.Veranic P, Erman A, Kerec-Kos M, et al. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem Cell Biol. 2009;131:129–39. doi: 10.1007/s00418-008-0492-x. [DOI] [PubMed] [Google Scholar]
- 4.Romih R, Koprivec D, Martincic DS, et al. Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int. 2001;25:531–7. doi: 10.1006/cbir.2000.0658. [DOI] [PubMed] [Google Scholar]
- 5.Erman A, Jezernik K, Stiblar-Martincic D, et al. Postnatal restoration of the mouse urinary bladder urothelium. Histochem Cell Biol. 2001;115:309–16. doi: 10.1007/s004180000240. [DOI] [PubMed] [Google Scholar]
- 6.Kreft ME, Sterle M, Veranic P, et al. Urothelial injuries and the early wound healing response: Tight junctions and urothelial cytodifferentiation. Histochem Cell Biol. 2005;123:529–39. doi: 10.1007/s00418-005-0770-9. [DOI] [PubMed] [Google Scholar]
- 7.Janssen DA, Geutjes PJ, Odenthal J, et al. A new, straightforward ex vivo organoid bladder mucosal model for preclinical research. J Urol. 2013;190:341–9. doi: 10.1016/j.juro.2012.12.103. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Kropp BP, Moore P, et al. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J Urol. 2000;164:928–34. doi: 10.1097/00005392-200009020-00004. discussion 934–5. [DOI] [PubMed] [Google Scholar]
- 9.Varley CL, Southgate J. Organotypic and 3D reconstructed cultures of the human bladder and urinary tract. Methods Mol Biol. 2011;695:197. doi: 10.1007/978-1-60761-984-0_13. [DOI] [PubMed] [Google Scholar]
- 10.Bouhout S, Perron E, Gauvin R, et al. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Engineering. 2010;16:1539–48. doi: 10.1089/ten.tea.2009.0473. [DOI] [PubMed] [Google Scholar]
- 11.Bouhout S, Gauvin R, Gibot L, et al. Bladder substitute reconstructed in a physiological pressure environment. Journal of Pediatric Urology. 2011;7:276–82. doi: 10.1016/j.jpurol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Cattan V, Bernard G, Rousseau A, et al. Mechanical stimuli induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol. 2011;60:1291–8. doi: 10.1016/j.eururo.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 13.Arenas da Silva LF, Micol L, Tiemessen D, et al. Is there a need for smooth muscle cell transplantation in urethral reconstruction? Tissue Eng Part A. 2014;20:1542–9. doi: 10.1089/ten.tea.2013.0185. [DOI] [PubMed] [Google Scholar]
- 14.Magnan M, Berthod F, Champigny MF, et al. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J Pediatr Urol. 2006;2:261–70. doi: 10.1016/j.jpurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Auger FA, Lopez Valle CA, Guignard R, et al. Skin equivalent produced with human collagen. In Vitro Cell Dev Biol Anim. 1995;31:432–9. doi: 10.1007/BF02634255. [DOI] [PubMed] [Google Scholar]
- 16.Franz TJ. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol. 1975;64:190–5. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- 17.Atala A. Recent developments in tissue engineering and regenerative medicine. Curr Opin Pediatr. 2006;18:167–71. doi: 10.1097/01.mop.0000193294.94646.be. [DOI] [PubMed] [Google Scholar]
- 18.Orabi H, Bouhout S, Morissette A, et al. Tissue engineering of urinary bladder and urethra: Advances from bench to patients. Scientific World Journal. 2013;2013:154564. doi: 10.1155/2013/154564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Kassaby A1, AbouShwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol. 2008;179:1432–6. doi: 10.1016/j.juro.2007.11.101. [DOI] [PubMed] [Google Scholar]
- 20.Orabi H, Safwat AS, Shahat A, et al. The use of small intestinal submucosa graft for hypospadias repair: Pilot study. Arab J Urol. 2013;11:415–20. doi: 10.1016/j.aju.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 22.Raya-Rivera A, Esquiliano DR, Yoo JJ, et al. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet. 2011;377:1175–82. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, et al. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet. 2014;384:329–36. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- 24.Bouhout S, Perron E, Gauvin R, et al. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng Part A. 2010;16:1539–48. doi: 10.1089/ten.tea.2009.0473. [DOI] [PubMed] [Google Scholar]
- 25.Magnan M, Lévesque P, Gauvin R, et al. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng Part A. 2009;15:197–202. doi: 10.1089/ten.tea.2007.0303. [DOI] [PubMed] [Google Scholar]
- 26.Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol. 2009;6:596–611. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 27.Soler R, Fullhase C, Guimaraes-Souza N, et al. The effect of lamina propria cells on the growth of urothelial and smooth muscle cells. J Urol. 2009;181:78. doi: 10.1016/S0022-5347(09)60225-1. [DOI] [Google Scholar]
- 28.Macarak EJ, Howard PS. The collagens and their urologic significance. Scand J Urol Nephrol Suppl. 1997;184:25–33. [PubMed] [Google Scholar]
- 29.Vaidyanathan S, McDicken IW, Ikin AJ, et al. A study of cytokeratin 20 immunostaining in urothelium of neuropathic bladder of patients with spinal cord injury. BMC Urol. 2002;2:7. doi: 10.1186/1471-2490-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romih P, Vennic P, Jezernik K. Appraisal of differentiation markers in urothelial cells. Appl Immunohistochem Mol Morphol. 2002;10:339–43. doi: 10.1097/00129039-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Vaidyanathan S, McDicken IW, Son BM, et al. Detection of early squamous metaplasia in bladder biopsies of spinal cord injury patients by immunostaining for cytokeratin 14. Spinal Cord. 2003;41:432–4. doi: 10.1038/sj.sc.3101464. [DOI] [PubMed] [Google Scholar]
- 32.Sorrell JM, Caplan Al. Fibroblasts A diverse population at the center of it all. In: Jeon Kwang W., editor. International Review of Cell and Molecular Biology. Burlington, MA: Academic Press; 2009. pp. 161–214. [DOI] [PubMed] [Google Scholar]
- 33.Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol. 2004;200:134–45. doi: 10.1002/jcp.10474. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Itami S, Tarutani M, et al. Regulation of keratin 9 in nonpalmoplantar keratinocytes by palmoplantar fibroblasts through epithelial–mesenchymal interactions. J Invest Dermatol. 1999;112:483–8. doi: 10.1046/j.1523-1747.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 35.Feng C, Xu YM, Fu Q, et al. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J Biomed Mater Res A. 2010;94:317–25. doi: 10.1002/jbm.a.32729. [DOI] [PubMed] [Google Scholar]
- 36.Al-Motabagani Mohamed Akram. Age-related changes in the urinary bladder of the female albino rats. Int J Morphol. 2005;23:309–16. [Google Scholar]
- 37.Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–80. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]