Abstract
Prior research has shown an increase in GLP-1 concentrations during exercise but this exercise bout was conducted postprandially. The purpose of this study was to examine the incretin response to a meal following an exercise bout of different intensities in obese subjects. Eleven women (BMI>37.3±7.0 kg/m2; Age 24.3±4.6 y) participated in 3 counter-balanced study days where a standardized meal was preceded by: 1) No exercise (NoEx), 2) ModEx (55% VO2max), and 3) IntEx(4 min (80% VO2max)/3 min (50% VO2max). Frequent blood samples were analyzed for glucose, lactate, insulin, glucagon, glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and C-peptide concentrations throughout 280 min of testing. Glucose concentrations were not different between conditions during exercise or meals. There were no differences between conditions in insulin levels during exercise and recovery, but postprandial insulin incremental area under the curve was lower in ModEx vs. NoEx (p<0.01). GIP and GLP-1 levels were not different between conditions during exercise, but during exercise recovery, GLP-1 concentrations were higher in ModEx vs. NoEx (p=0.03). The meal increased the incretin responses (P<0.01) but this response was not affected by prior exercise. Glucagon concentrations increased with exercise (P<0.05) and continued to be elevated during recovery, with the greatest increase with IntEx compared with NoEx (P<0.05). No differences between conditions were detected for hepatic insulin extraction, insulin secretion, or insulin sensitivity. Exercise prior to an evening meal has no impact on the incretin response to the subsequent meal, yet insulin concentrations were lower during the meals that followed exercise. Exercise intensity had no impact on this response.
Keywords: Glucose metabolism, high intensity exercise, insulin sensitivity
1.0 Introduction
Postprandial hyperglycemia and hyperinsulinemia have been proposed to be a greater predictor of cardiovascular- and diabetes-related complications than fasting glucose and hemoglobin Alc (4). Lifestyle interventions, such as moderate intensity exercise are recommended to improve postprandial glucose metabolism, as exercise improves insulin action in muscle and liver (25). Although insulin responses to exercise have been well studied, the effect of prior exercise on the gut peptides that indirectly contribute to whole-body glycemic control has not been studied extensively. In response to nutrient ingestion, the glucoregulatory effects of glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are to stimulate insulin secretion (2, 6). However, exercise, a strong physiologically relevant stimuli, may alter the incretin response to nutrient ingestion if exercise precedes the meal. Most studies to date have shown that circulating plasma GIP levels do not change during exercise of varying intensity and duration (3, 5, 15) while plasma GLP-1 levels showed slight increases in the latter stages of running in athletes (15). Two studies in healthy, normal weight individual (13, 19) reported increases in plasma GLP-1 concentrations during both high (70% VO2max) and moderate (50% VO2max) intensity cycling exercise, but this exercise was conducted one hour postprandially which may have altered the exercise response (13, 19). No studies to date, however, have studied the effect of a prior exercise bout on the incretin response at a subsequent meal.
Therefore, this study examined the incretin response (GLP-1 and GIP) to a meal following an exercise bout in young, healthy obese women. Most of the previous research has examined the exercise/meal response following 12 h of fasting, however, many individuals exercise late in the day when they have not eaten in the previous 3-4 hours. Thus we have examined this response to a dinner meal when it is known that due to diurnal variation the body is not as glucose tolerant (21). Additionally the recent work by Little et al (11) reported that a single session of high intensity interval training resulted in greater postprandial improvement in glucose in the 24 h period following the exercise bout compared to moderate exercise. Thus we compared study days that had a preceding single bout of moderate-intensity aerobic continuous exercise vs. high-intensity aerobic interval exercise on the incretin (GLP-1 and GIP) responses during the subsequent meal. We hypothesized that GLP-1 and GIP responses to the meal would be greater when exercise preceded the meal and these responses would augment the insulin response, lowering the glucose levels. Secondly we speculated that this response would be greater following a bout of high-intensity aerobic interval exercise compared to moderate-intensity continuous aerobic exercise.
2.0 Materials and Methods
2.1 Subjects
Eleven young, obese, sedentary women signed an informed consent form that was approved by the University of Missouri Institutional Review Board prior to participation in this study. Preliminary screening excluded individuals on the basis of pre-existing conditions of cardiovascular disease, diabetes, and/or musculoskeletal injuries hindering participation in exercise. Inclusion criteria included individuals between 18-35 y of age, a body mass index (BMI) > 30 kg/m2, (obese), weight stable over prior 3 months, not regular exercisers, no prior history of lung, kidney, endocrine, or gastrointestinal disease, and not taking any medications known to alter glucose metabolism.
2.2 Experimental design
Following screening, all subjects completed an exercise familiarization session, and three study days, which included an exercise bout followed by a standardized meal test given at ~1800 h. In a counter-balanced order, subjects completed: 1) control day (NoEx), 2) moderate-intensity continuous aerobic exercise (ModEx), and 3) high-intensity aerobic interval exercise (IntEx). All subjects completed all study days but the the days were done in varying order to minimize the confounding effect of treatment order. Both ModEx and IntEx exercise sessions were matched for caloric-expenditure (400 kcal). Blood was sampled throughout the 280 min testing day. Study days were performed at least seven days apart. All subjects were studied between days 1-10 of their menstrual cycle.
2.3 Screening day
Height, weight and resting blood pressure were measured. Fasting blood glucose was measured, and subjects with elevated fasting blood glucose measurements (>110 mg/dL) were excluded from the study. Medical history and physical activity questionnaires were completed on the screening day to determine habitual activity levels.
2.4 Body composition and VO2 max testing
Fat mass, fat-free mass and percent body fat was assessed using a BOD POD® (Cosmed Inc, Rome, Italy). Subjects completed a maximal aerobic capacity test on a Quinton Q-Stress TM55 (Cardiac Science, Bothell, WA) treadmill using a continuous exercise test as previous published (8). The speed began at 2.5 mph and grade at 0% for two minutes and then increased to 3.0 mph for 2 min. Speed then remained constant at 3.0 mph while the grade was incrementally increased 2.5% every two min until the subject reached volitional fatigue. Indirect calorimetry was used to measure oxygen consumption (Parvomedics TrueOne 2400 metabolic cart, Salt Lake City, UT). The results of this test were used to determine relative intensities for the two exercise study days.
2.5 Familiarization day
Subjects were familiarized with the high-intensity aerobic interval protocol based on the findings of the VO2max test; the specific speed and grade needed to elicit 50% and 80% VO2max workloads were determined. The familiarization day was used to establish the exercise duration necessary to expend 400 kcal. Oxygen consumption was measured throughout the entire exercise bout to ensure the appropriate workload. Adjustments to speed and grade of the treadmill were made if necessary.
2.6 Day prior to study day or morning of study day
A three-day, 24 h dietary recall was collected and analyzed using Nutritionist Pro Diet Analysis (Axxya Systems, LLC, 2009) to determine average caloric intake throughout a typical day. The average caloric intake of the three days was used to place the subject in either a 2000, 2300, or 2600 kcal/day eating plan for each of the three study days. The evening prior to the study day, subjects picked up a pack-out breakfast (~0700 h) and lunch (~1200 h) (65% carbohydrate, 20% fat, 15% protein). Subjects were allowed ad libitum water each study day. Subjects were asked to abstain from exercise or alcohol consumption for the 24 h prior to the study day.
2.7 Study day
Subjects reported to the laboratory at 1600 h and a venous catheter was inserted into the antecubital vein of the forearm. After ~20 min of rest, two venous blood baseline samples (t = −10, 0 min) were drawn. Subjects then underwent one of the 3 experimental conditions: 1) no exercise (NoEx) - the subject remained seated quietly in the laboratory for a time equivalent to exercise, 2) ModEx – the subject walked on the treadmill at 55% VO2max, and 3) IntEx - the subject walked on the treadmill at 80% VO2max for 4 min interspersed with 3 min recovery at 50% VO2max. This high-intensity interval protocol has been used previously in untrained or overweight individuals (12, 26). Exercise duration was determined by the time to expend 400 kcal on both exercise days, which was measured continuously by indirect calorimetry. Following a 45-minute recovery, a standardized meal (800 kcal; 65% carbohydrate, 20% fat, 15% protein) was provided and consumed within 20 min. Blood samples were taken every 10 min during exercise and recovery, as well as every ten minutes following the meal for the first hour, and then samples were drawn every 30 min for the final two hours.
2.8 Plasma Analysis
Blood was collected in chilled EDTA tubes (3 mL) pretreated with dipeptidyl peptidase-4 (DPP-IV) inhibitor (10 μL/mL blood; Millipore, Billerica, MA), and immediately analyzed for glucose and lactate using a YSI2300 STAT Plus (YSI, Yellow Springs, OH). Samples were centrifuged and plasma aliquots were stored at −80°C until analysis. Plasma insulin, C-peptide, glucagon, GLP-1, GIP were analyzed using a MILLIPLEX magnetic bead-based quantitative multiplex immunoassay with the MAGPIX instrumentation (Millipore, Billerica, MA). The intra-assay coefficients of variability (CV) were 5.6%, 5.1%, 5.8%, 7.2% and 4.7% for c-peptide, GIP, GLP-1, glucagon and insulin, respectively. The inter-assay CV was 14.7%, 8.7%, 12.1%, 9.8%and 12.8% for c-peptide, GIP, GLP-1, glucagon and insulin, respectively.
2.9 Statistics
All data was tested for normal distribution and are presented as means ± SE. An analysis of variance (ANOVA) with repeated measures was used to test for differences between mean baseline concentrations of glucose, lactate, C-peptide, GIP, GLP-1, glucagon, and insulin. To examine the effects of exercise on the metabolic and hormonal response, two-way ANOVAs with repeated measures (condition, time, and condition × time interactions) were performed for time intervals corresponding to exercise, or equivalent exercise (t=10-60), recovery (t=60-100) and meal response (t=100-280). If statistical significance was detected, post-hoc multiple pairwise comparisons (Tukey-Kramer) was performed. Incremental area under the curve (iAUC) was calculated using the trapezoidal rule to assess total changes in each hormone for exercise, recovery, and meal response in each condition and analyzed using repeated measures ANOVA. Insulin sensitivity was calculated using the Matsuda index (14). The insulin secretion rates (ISR) used in the model were calculated by deconvoluting c–peptide concentrations (22). Hepatic insulin extraction was calculated as (1-(Insulin (pmol/L) /C-Peptide (pmol/L)) ×100 at individual time points (t=0, 40, 70, 100, 130, 160, 220). β-cell function following the meal was calculated as (ΔInsulin 130-100 min)/(ΔGlucose 130-100 min).
3.0 Results
Eleven young (age 24.3±1.4 y), obese (BMI 37.3±2.1 kg/m2; body fat 42.1±2.5%), sedentary women were included in this study. All subjects had a normal fasting glucose level of 95.5±2.3 mg/dL and a mean VO2peak of 25.2±1.4 ml/kg/min. While equal in caloric expenditure (400 kcal), the ModEx sessions were significantly longer than the IntEx sessions (55.4±1.7 vs 50.5±1.6 min; p<0.001).
Baseline glucose, GIP, GLP-1 and glucagon levels were not significantly different between study days (p>0.05). Baseline lactate levels were significantly higher on the ModEx day vs. NoEx day (p=0.01), but there were no differences in baseline lactate levels between the ModEx and IntEx days. Baseline insulin and C-peptide levels were significantly lower on the NoEx vs. ModEx study day (p<0.05) (Table 1).
Table 1.
Fasting Glucose & Hormone Levels
| NoEx | ModEx | IntEx | |
|---|---|---|---|
| Glucose, mg/dL | 72.0 ±3.4 | 75.6±1.7 | 78.0±3.4 |
| Lactate, mg/dL | 5.3 ±0.55 | 6.8±0.63* | 6.9±0.86 |
| Insulin, pg/mL | 974±154 | 1213±179* | 1085±158 |
| C-Peptide, pg/mL | 1986±111 | 2353±192* | 2328±242 |
| GIP, pg/mL | 78.1±14.3 | 70.5±8.9 | 83.1±14.9 |
| GLP-1, pg/mL | 25.7±2.3 | 33.4±6.5 | 31.2±4.4 |
| Glucagon, pg/mL | 26.9±5.5 | 19.2±3.3 | 23.2±4.2 |
p<0.05 NoEx vs. ModEx, n=11 subjects
3.1 Exercise Response
In response to exercise (t=10-60) and recovery (t=60-100), lactate concentrations were significantly increased during exercise on both ModEx and IntEx days (p=0.001) compared to NoEx condition and lactate levels on the IntEx day were greater than the ModEx condition (p<0.01, data not shown).
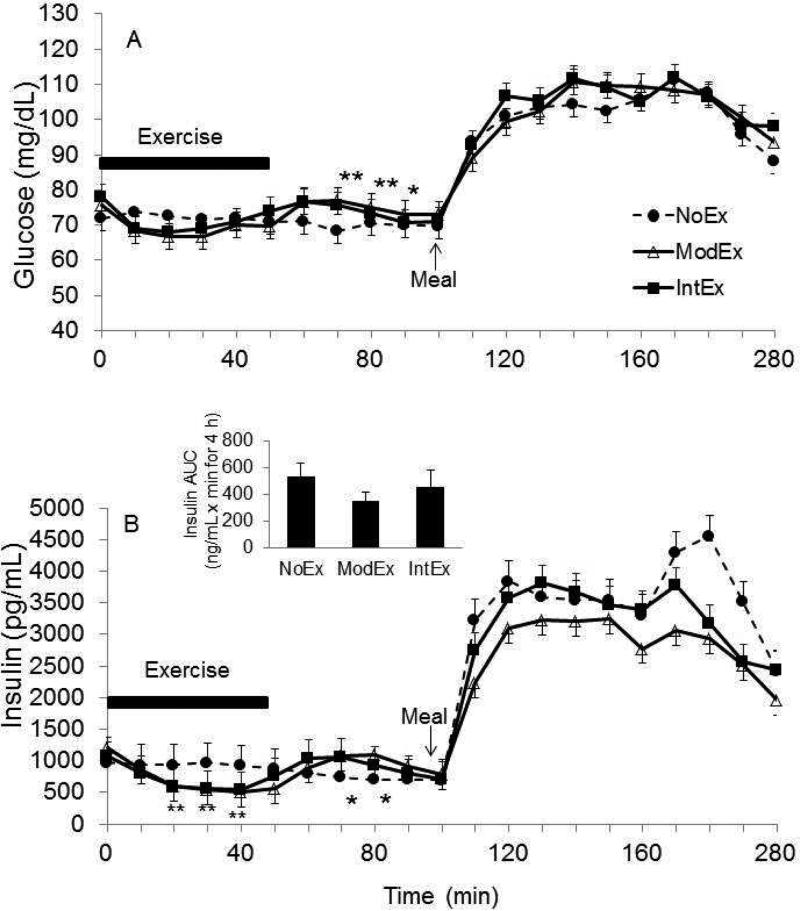
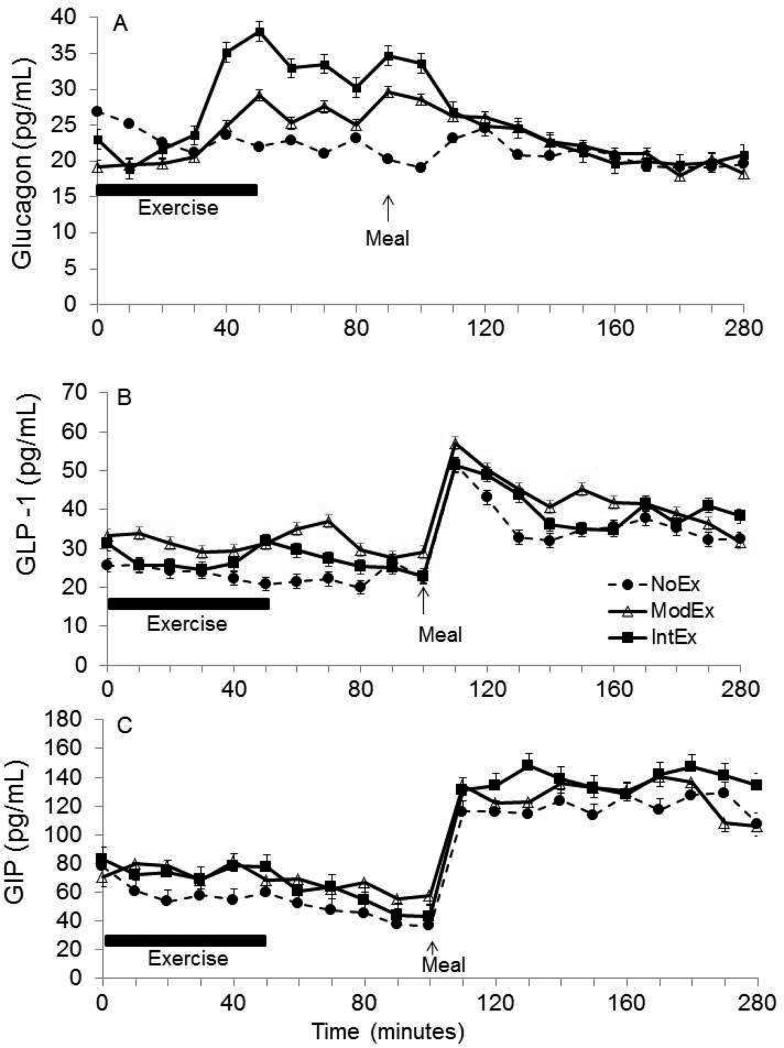
A time × condition interaction (p=0.005) was detected for glucose concentrations during the exercise time period, with slightly lower glucose concentrations during exercise conditions (Figure 1a). During recovery, glucose concentrations rebounded and were higher with the IntEx and ModEx conditions compared to NoEx condition (p<0.01), but they were not different from each other. In the NoEx condition, there was no change in glucose levels during exercise and recovery periods. Insulin concentrations decreased with both the ModEx and IntEx condition compared to the NoEx condition (time × condition interaction, p<0.001) (Figure 1b), and then increased during recovery (p<0.001). On the NoEx day, there was no change in insulin levels over this time period. In response to exercise, glucagon concentration increased during both exercise conditions, and peak glucagon concentrations occurred near the end of exercise (ModEx 29.4±2.5, IntEx 47.1±2.1 pg/ml, p<0.05) (Figure 2a). During recovery, glucagon levels remained elevated, and glucagon iAUC was greater during the IntEx condition than the NoEx condition (p<0.05).
Figure 1.
The pattern of glucose (a) and insulin (b) responses over time (t0-280 min). *P<0.05 different from the no exercise day (NoEx); **P<0.05 different from the (NoEx).
Figure 2.
The pattern of glucagon (a), GLP-1 (b) and GIP (c) responses over time (t0-280 min).
GLP-1 concentrations decreased slightly during the initial stages of exercise and reached a nadir at 30 min into exercise for both the ModEx and IntEx study days (Figure 2b). GLP-1 concentrations showed no response to exercise except a slight non-significant increase as exercise was terminated in both the ModEx and IntEx conditions before decreasing gradually during recovery. Mean GLP-1 concentrations tended to be higher on both ModEx (31.7±5.9 pg/mL) and IntEx (27.2±4.2 pg/mL) days compared to NoEx (23.1±2.6 pg/mL), and a condition effect approached significance (p=0.06). During recovery, mean GLP-1 concentrations during ModEx condition (31.7±5.3 pg/mL) was greater than the NoEx condition (22.6±3.3 pg/mL) (p=0.03), but IntEx condition (26.1±3.4 pg/mL) was not different from NoEx condition (p=0.16). We found no difference in GIP levels between trials (Figure 2c). Although there was no statistically significant difference in GIP responses to exercise, the GIP concentrations remained stable before slightly decreasing during recovery, while during the NoEx condition, GIP levels tended to decrease during both exercise and recovery time periods.
3.2 Meal Response
Glucose concentrations increased in response to the meal in all conditions (p<0.05), reaching peak values at ~110 mg/dL. Interestingly, the postprandial glucose response remained elevated above preprandial levels for the entire 3 h sampling period for all conditions (Figure 1a). In response to changes in glucose, insulin concentrations also increased following the meal in all conditions (p<0.05). There was an effect of exercise intensity on postprandial insulin iAUC (p<0.03) but only ModEx was significantly attenuated compared to the NoEx condition (p=0.005) (Figure 1b inset). There was no difference between the IntEx condition and ModEx or NoEx condition. Glucagon concentrations were suppressed postprandially in all conditions (p<0.05; Figure 2a). Glucagon iAUCMeal was significantly lower in the ModEx condition (−1,305±558 pg/ml × min, p=0.01) compared to NoEx condition (215±254 pg/ml × min), whereas no differences were found between the IntEx (−2,200±1,300 pg/ml × min) and NoEx condition. Both GIP and GLP-1 concentrations were increased in response to the meal in all conditions with a 2.5-3 fold increase within 20 min of meal consumption. GLP-1 levels peaked at t=10-20 min postprandially and then declined slightly. There was no effect of the prior exercise on either GLP-1 or GIP concentrations; both GIP and GLP-1 remained elevated above preprandial levels for entire 3 h post-meal sampling period.
We calculated hepatic insulin extraction (%) at baseline (t=0), middle of exercise (t=40), late recovery (t=70), pre-meal (t=100), as well as early (t=130) and late (t=160, 220) postprandial response. In the middle of exercise (t=40), hepatic insulin extraction was significantly greater during both the ModEx and IntEx conditions compared to NoEx (p<0.001), as well as during recovery (t=70) (p<0.05). On the ModEx day, insulin extraction was significantly greater at 2 h postprandial compared to NoEx (p<0.05; Table 2). Insulin secretion following the meal (t=100-280) was not different between study days. Insulin sensitivity was not significant either 2 h postprandial (t=100-220) (p=0.70) or 3 h postprandial (t=100-280) between study conditions (p=0.74).
Table 2.
Hepatic Insulin Extraction (%) Calculations
| NoEx | ModEx | IntEx | |
|---|---|---|---|
| Baseline (t=0) | 71.4±4.0 | 68.7±4.0 | 71.2±4.2 |
| Exercise (t=40) | 71.8±3.7 | 78.0±3.3* | 79.4±3.1* |
| Recovery (t=70) | 74.6±3.4 | 68.0±3.7‡ | 67.7±4.2‡ |
| Pre-Meal (t=100) | 72.4±4.1 | 74.2±4.5 | 74.2±4.1 |
| Early Postprandial (t=130) | 44.8±5.0 | 52.1±4.6 | 48.7±6.6 |
| Late Postprandial (t=160) | 51.3±4.8 | 57.2±4.0 | 52.5±7.2 |
| 2 h Postprandial (t=220) | 47.4±6.5 | 54.9±5.8‡ | 50.9±9.4 |
p<0.001 vs NoEx
p<0.05 vs NoEx
4.0 Discussion
The novel findings of this study were that: 1) a prior exercise bout does not alter the GLP-1 and GIP concentrations to a subsequent meal, 2) in contrast with previous reports (13, 20) regardless of exercise intensity GLP-1 and GIP concentrations did not increase when subjects had been previously fasted, and 3) moderate-intensity aerobic exercise lowered insulin levels to a subsequent meal more so than shorter, higher-intensity interval exercise despite no differences in the incretin response.
4.1 Exercise Responses
During exercise in healthy subjects, increased glucose uptake by muscle is balanced by an equal rise in hepatic glucose production, resulting in relatively unchanged blood glucose levels (1). In the present study, ModEx and IntEx caused a 45% and 33% reduction in plasma insulin concentrations, respectively, with only slight changes in plasma glucose levels during exercise. In the present study the augmented glucagon response during exercise is to support peripheral glucose demands, and this response was enhanced more so in IntEx condition. Likewise as expected insulin concentrations decreased to prevent a dramatic drop in blood glucose levels
Few studies (13, 15, 19) have examined the incretin response to exercise. We found no differences in GIP and GLP-1 between trials. Our findings are similar to earlier reports which have reported that circulating plasma GIP levels do not change during 3 h of cycling exercise at 40% VO2 max (5), or during cycling at 75% VO2 max until exhaustion (5). Likewise, our GLP-1 findings were similar to an earlier study (15) which showed a slight increase in GLP-1 levels in the latter stages of the exercise, but contrast the findings of Ueda et. al (19) and Martins et. al (13). The findings of these authors are frequently cited and they report an increase in GLP-1 concentrations during exercise that persisted into recovery; however, in these studies they measured the hormonal response to moderate-intensity cycling exercise in a postprandial state (a meal was consumed ~60 min prior to exercise). Thus the apparent exercise response they noted may be partly due to the prior meal stimulus. Further close examination of the exercise responses in the Martin study reveal that the increases in these concentrations are very slight, and could be due to hemoconcentration as a result of the exercise. Our result of a slight decrease in GLP-1 concentrations during exercise is most likely due to the glucose-dependent nature of incretin release and the increasing length of time from the prior meal, or that incretins respond in a similar manner as insulin during exercise (2). When plasma glucose is readily used for energy during exercise, this may suppress the incretin secretion resulting in lower insulin levels.
4.2 Meal Responses
This is one of the few studies examining the meal response later in the day and examining the effect of prior exercise. This is the first study to show that prior exercise, regardless of intensity, had no impact on the incretin response to a subsequent meal, supporting the fact that the incretins need the nutrient stimulus to be released. Our data show a 2.5-3 fold increase in GIP and GLP-1 concentrations, respectively, in response to a 800 kcal mixed meal (with no prior exercise) which is similar to the findings of others that showed the incretins are elevated for 3-4 hours after intake of a mixed meal in healthy volunteers (6), and is directly proportional to meal size (24). We showed that the meal stimulus, which was twice the caloric intake than caloric expenditure of exercise, was the most potent stimulator of incretin hormones. Prior exercise of either continuous or interval nature did not impact the post-meal incretin response.
Despite no differences in the GIP and GLP-1 responses to the meal, a lower peak insulin response to the meal ingestion occurred only after the ModEx condition, which was sustained throughout the postprandial period. These findings demonstrate that the intensity of prior exercise differentially impacts postprandial insulin concentrations in obese females, most likely due to different capacities to impact peripheral insulin sensitivity, and not due to differences in the incretin response. The mechanisms responsible for a lower insulin response to a post-exercise meal may include: 1) diminished insulin secretion due to high α-adrenergic tone, 2) improved insulin receptor activation by insulin in muscle and fat tissue following interval training (16), or 3) increased clearance of insulin from circulation by the liver (18), however, we found no difference in insulin sensitivity or β-cell function in response to the mixed meal following either IntEx or ModEx. We did note that hepatic insulin extraction was greater in ModEx than in NoEx. In the present study, the failure of a high-intensity exercise bout to reduce postprandial insulin suggests that acute, moderate intensity exercise, but not interval exercise, through repetitive and consistent prior muscle contraction, may be more effective in increasing peripheral insulin action. That being said the meal stimulated a similar glucose response on all study days.
In these healthy obese women we noted one surprising observation in the glucose levels. Although none of the postprandial glucose levels increased to dramatically high levels, in all conditions the postprandial glucose levels were elevated above preprandial glucose levels for the entire 3 h postprandial meal sampling period, and did not return to the premeal concentrations. It should be noted these glucose levels were well within a normal range but seem to reflect in a different post-meal pattern of response than is seen following the breakfast meal which is usually studied. This finding is also supported by unpublished data that we have where we see a similar trend in the glucose response to the dinner meal. In a study in endurance trained men studied in the mid-afternoon/early evening hours ~2 h following a small meal, O'Conner et al (15) observed a more sustained elevation of plasma glucose following ingestion of a 75 g glucose drink post-exercise that was accompanied by a decrease and delayed insulin response. This is similar to the response seen in our ModEx condition. Although postprandial glucose levels were well within the normal range, it is interesting that by 3 h, glucose levels were not back to pre-exercise or pre-meal levels. It was expected that exercise would result in a lower postprandial glucose excursion due to increases in blood flow, glucose transport and oxidation in the working muscles (18), but prior research demonstrating this phenomenon has used fasted, morning exercise. Potentially diurnal differences in the postprandial pattern of meal glucose appearance and insulin secretion may account for the differences noted (17). Glucose tolerance is lower in the evening than in the morning due to a slower rate of glucose disappearance following an evening meal compared to a morning meal (7, 9). Some investigators have proposed a reduction in insulin secretion in the evening (7), and others have suggested diurnal differences in insulin sensitivity (9, 23). One study (17) recently reported a lower postprandial glucose excursion at breakfast than at lunch and dinner; suggesting the presence of a diurnal pattern to glucose tolerance in healthy, non-diabetic subjects. This was characterized by a trend of reduced β-cell responsiveness and insulin action with increasing hepatic insulin extraction after lunch and dinner than at breakfast. Lower glucose tolerance and insulin action in response to an evening meal may partly explain why in our study glucose concentrations remained elevated over preprandial glucose concentrations throughout the 3 h post-meal sampling period in all conditions; regardless of prior exercise.
A further complexity is a potential diurnal variation in incretin response to a meal. Since diurnal variation exists for the insulin response to oral glucose or meal ingestion (17), a corresponding diurnal variation in the release and/or action of GIP and GLP-1 may potentially exist. Recent evidence in rodents has demonstrated a circadian pattern in GLP-1 secretion which was synchronized with insulin secretion. In addition, Lindgren et al (10) recently reported that the peak in GIP and GLP-1 secretion over the first 30 min after a mixed meal (524 kcal) was ~80% higher in the morning than in the afternoon, but diurnal differences in incretin secretion are restricted to the early phase. The mechanism behind this is not known.
In conclusion, exercise prior to an evening meal has no impact on the incretin response to the subsequent meal, and exercise intensity had no impact on this response. While neither acute exercise condition differentially affects the postprandial incretin response, ModEX reduced postprandial insulin more so than IntEx (and NoEX) which may be attributed to enhanced peripheral insulin action and increased hepatic extraction of insulin. Lastly, unlike previous reports conducted in the postprandial state, exercise in a fasted state does not induce an incretin response.
Highlights.
Moderate nor vigorous interval exercise bout does not alter the GLP-1 and GIP concentrations to a subsequent meal
The exercises did not increase GLP-1 or GIP responses
Moderate exercise lowered insulin levels to a subsequent meal more so than vigorous interval exercise
Acknowledgement
This project was partially supported by NIH R21DK084467, and the J.R. Albert foundation grant. NIH had no role in the design, analysis or writing of this article
Abbreviations
- ModEx
moderate-intensity aerobic continuous exercise
- IntEx
high-intensity aerobic interval exercise
- BMI
body mass index
- NoEX
no exercise
- VO2max
maximal oxygen uptake
- GLP
1 - glucagon-like peptide-1
- GIP
glucose-dependent insulinotropic polypeptide
- OGTT
oral glucose tolerance test
- DPP
IV - dipeptidyl peptidase-4
- CV
coefficients of variability
- ANOVA
analysis of variance
- iAUC
Incremental area under the curve
- ISR
insulin secretion rates
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
LMN: Conception of the study, designed research, conducted research, analyzed data, edited final version of paper, had responsibility for final content of paper
TDH: Conducted research and analyzed data, edited final version of paper.
HJL: Designed research, data interpretation, edited final version of paper
Y-MP: Conducted research and analyzed data, edited final version of paper
NCW: Conducted research and analyzed data, edited final version of paper
JPT: Designed research, data interpretation, edited final version of paper
JAK: Conception of the study, designed research, conducted research, analyzed data, edited final version of paper, had primary responsibility for final content of paper
Declaration of interest: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
References
- 1.Adams OP. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes. 2013;6:113–122. doi: 10.2147/DMSO.S29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggio L, Drucker D. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Blom PC, Hostmark AT, Flaten O, Hermansen L. Modification by exercise of the plasma gastric inhibitory polypeptide response to glucose ingestion in young men. Acta Physiol Scand. 1985;123:367–368. doi: 10.1111/j.1748-1716.1985.tb07602.x. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Hilsted J, Galbo H, Sonne B, Schwartz T, Fahrenkrug J, de Muckadell OB, Lauritsen KB, Tronier B. Gastroenteropancreatic hormonal changes during exercise. Am J Physiol. 1980;239:G136–140. doi: 10.1152/ajpgi.1980.239.3.G136. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ, Deacon CF, Vilsboll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–168. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Jarret tR, Baker I, Keen H, Oakley N. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1:199–201. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaley JA, Goulopoulou S, Franklin RM, Baynard T, Holmstrup ME, Carhart R, Jr., Weinstock RS, Fernhall B. Plasticity of heart rate signalling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obes (Lond) 2009;33:1198–1206. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee A, Ader M, Bray G, Bergman R. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41:750–759. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren O, Mari A, Deacon CF, Carr RD, Winzell MS, Vikman J, Ahren B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab. 2009;94:2887–2892. doi: 10.1210/jc.2009-0366. [DOI] [PubMed] [Google Scholar]
- 11.Little JP, Jung ME, Wright AE, Wright W, Manders RJ. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Physiol Nutr Metab. 2014;39:835–841. doi: 10.1139/apnm-2013-0512. [DOI] [PubMed] [Google Scholar]
- 12.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007;193:251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor AM, Pola S, Ward BM, Fillmore D, Buchanan KD, Kirwan JP. The gastroenteroinsular response to glucose ingestion during postexercise recovery. Am J Physiol Endocrinol Metab. 2006;290:E1155–1161. doi: 10.1152/ajpendo.00500.2005. [DOI] [PubMed] [Google Scholar]
- 16.Pestell RG, Ward GM, Galvin P, Best JD, Alford FP. Impaired glucose tolerance after endurance exercise is associated with reduced insulin secretion rather than altered insulin sensitivity. Metabolism. 1993;42:277–282. doi: 10.1016/0026-0495(93)90074-x. [DOI] [PubMed] [Google Scholar]
- 17.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuominen J, Ebeling P, Koivisto V. Exercise increases insulin clearance in healthy man and insulin-dependent diabetes mellitus patients. Clin Physiol. 1997;17:19–30. doi: 10.1046/j.1365-2281.1997.01717.x. [DOI] [PubMed] [Google Scholar]
- 19.Ueda S-Y, Yoshikawa T, Katsura Y, Usui T, Fujimoto S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. Journal of Endocrinology J Endocrinology. 2009;203:357–364. doi: 10.1677/JOE-09-0190. [DOI] [PubMed] [Google Scholar]
- 20.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol. 2009;201:151–159. doi: 10.1677/JOE-08-0500. [DOI] [PubMed] [Google Scholar]
- 21.Van Cauter E, Desir D, Decoster C, Fery F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69:604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 22.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 23.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 24.Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman DH. Regulation of glucose fluxes during exercise in the postabsorptive state. Annual review of physiology. 1995;57:191–218. doi: 10.1146/annurev.ph.57.030195.001203. [DOI] [PubMed] [Google Scholar]
- 26.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]