Abstract
Purpose
To characterize the risk and risk factors for intraocular pressure (IOP) elevation in pediatric non-infectious uveitis.
Design
Multi-center retrospective cohort study.
Participants
Nine hundred sixteen children (1593 eyes) <18 years old at presentation with non-infectious uveitis followed between January 1978 through December 2007 at five academic uveitis centers in United States.
Methods
Medical records review by trained, certified experts.
Main outcome measures
Prevalence and incidence of IOP≥21 and ≥30mmHg and incidence of a rise in IOP by ≥10mmHg. To avoid under ascertainment, outcomes were counted as present when IOP-lowering therapies were in use.
Results
Initially 251 (15.8%) and 46 eyes (2.9%) had IOP≥21 and ≥30mmHg, respectively. Factors associated with presenting IOP elevation included age 6–12 years (versus other pediatric ages), prior cataract surgery (adjusted odds ratio≥21mmHg [aOR21]=2.42, P=0.01), pars plana vitrectomy (adjusted odds ratio≥30mmHg[aOR30]=11.1, P=0.03), duration of uveitis ≥6 months (aORs30 up to 11.8, P<0.001), contralateral IOP elevation (aOR21=16.9, aOR30=8.29; each P<0.001), visual acuity worse than 20/40 (aORs21 up to 1.73, P=0.02; aORs30 up to 2.81 P=0.03), and topical corticosteroid use (aORs up to 8.92, P<0.001 in a dose-response relationship).
The median follow-up was 1.25 years (interquartile range 0.4–3.66). The estimated risk of any observed IOP elevation to ≥21 mmHg, ≥30 mmHg and of a rise in IOP by ≥10mmHg was 33.4%, 14.8% and 24.4% respectively within 2 years. Factors associated with IOP elevation included pars plana vitrectomy (adjusted hazard ratio≥21mmHg[aHR21]=3.36, P<0.001), contralateral IOP elevation (aHRs up to 9.54, P<0.001), the use of topical (aHRs up to 8.77 that followed a dose-response relationship, P<0.001), periocular (aHRs up to 7.96, P<0.001) and intraocular (aHRs up to 19.7, P<0.001) corticosteroids.
Conclusions
IOP elevation affects a large minority of children with non-infectious uveitis. Statistically significant risk factors include IOP elevation or use of IOP-lowering treatment in the contralateral eye and local corticosteroid use – that demonstrated a dose-and route of administration-dependent relationship. In contrast, use of immunosuppressive drug therapy did not increase such risk. Pediatric eyes with non-infectious uveitis should be followed closely for IOP elevation when strong risk factors such as the use of local corticosteroids and contralateral IOP elevation are present.
Keywords: pediatric, uveitis, intraocular pressure, risk, risk factor, glaucoma, ocular hypertension, corticosteroid, incidence, prevalence
Introduction
Since Joseph Beers first reported an association between uveitis and glaucoma in 1813, 1 the irreversible blindness caused by uveitic glaucoma has remained an important complication of pediatric uveitis impacting the length of morbidity-indicative disability adjusted life years. 2 Although several potential factors contributing to optic nerve damage are under investigation, 3–6 intraocular pressure (IOP) elevation is a predominant modifiable risk factor. The risk of glaucomatous optic neuropathy in open angle glaucoma has been reported to increase in a non-linear fashion at higher pressures, approaching 100% when IOP was ≥35mmHg. 7,8 The Multicenter Uveitis Steroid Treatment (MUST) Trial has demonstrated that 24% of uveitic eyes that manifested IOP rise by ≥10mmHg, developed glaucomatous optic neuropathy within 2 years. 9
Available information regarding the prevalence and incidence of IOP elevation in pediatric uveitis cases varies widely; estimates among various pediatric uveitic subpopulations were found to range between 3–51%. 10–12 Synthesis of these reports has limitations given that the existing data involved smaller subpopulations with a limited study power, variable lengths of follow-up, disparate criteria for age limits and discordant definitions for IOP elevation. None of the reports exclusively describes the risk of IOP elevation in pediatric non-infectious uveitic populations.
To better characterize the potential risk of IOP elevation in pediatric non-infectious uveitis and to describe potentially predictive risk factors thereof, we conducted a study in children<18 years of age at presentation belonging to the large Systemic Immunosuppressive Therapy for Eye Diseases (SITE) non-infectious uveitis study cohort that received subspecialty care over a 30-year period in the United States.
Methods
The study adhered to tenets of the Declaration of Helsinki. Governing Institutional Review Boards of participating institutions approved inclusion of patients both living and deceased with waiver of informed consent for this retrospective review.
Study Population
Data for this report were abstracted from the parent SITE Cohort Study database–the study design of which has been described elsewhere. 13 Patients with infectious uveitis & AIDS had been excluded from the parent cohort. For this analysis, all patients <18 years of age at presentation with non-infectious uveitis followed at five academic ocular-inflammation centers in the United States between January 1978 through December 2007 were included.
Data collection
Comprehensive patient records were reviewed by trained certified experts to obtain pertinent data that related to each eye at every visit recorded based on external, slit-lamp and dilated fundus examinations, tonometric measurements and methods, angiography and optical coherence tomography (when indicated), each medication in use and operative records (each used when indicated per clinical judgment) that were entered into a customized, real-time, error-correcting Microsoft Access database (Microsoft Corporation, Redmond, WA).
Main outcome measures
The primary outcomes assessed were prevalence of IOP≥21 and ≥30mmHg. In outcome-naïve eyes at presentation, the incidence of IOP≥21 and ≥30, and a rise by ≥10mmHg were evaluated over time. To avoid under-ascertainment, all of these outcomes were counted as present when IOP-lowering therapy was found to be in active use at any subsequent follow-up visit, on the presumption that these eyes had exhibited IOP elevation during the interim that required treatment with IOP-lowering medications or surgery by collaborating ophthalmologists. 14
Potential associations were evaluated between outcomes and age, gender, race, smoking status, relevant systemic inflammatory and non-inflammatory diseases, intraocular surgery, anatomical location of inflammation according to the International Uveitis Study Group criteria, 15,16 duration and laterality of uveitis, contralateral IOP elevation, hypotony (ipsilateral and contralateral), visual acuity, markers of ocular inflammatory activity graded in a manner similar to Standardization of Uveitis Nomenclature working group and the National Eye Institute grading criteria for vitreous haze, 17–19 uveitic structural complications and use of immunosuppressive drug therapy and corticosteroids. Prednisone- and topical prednisolone acetate 1%-equivalent dosages of all alternative systemic and topical corticosteroids used during the course of management were calculated to facilitate comparisons. Periocular and intraocular corticosteroids typically included dosages of 40 mg and 4 mg of triamcinolone acetonide, respectively.
Covariates missing at any visit were imputed by carrying the last recorded value forward. Visits lacking IOP records were censored, because the outcome could not be studied. Because cataract is objectively difficult to define in a retrospective chart review due to incomplete and non-standardized documentation, we limited our attention to cataract surgery, which can be well ascertained by chart review and is a reasonable surrogate for cataract. Due to concerns that the pathophysiology of IOP elevation may differ during early postoperative periods, data obtained from eyes within one month of the post cataract, pars plana vitrectomy and retinal detachment surgical period were excluded; however subsequent data were retained.
Based on pathogenesis considerations; IOP elevation in the contralateral eye and the presence of uveitis in the contralateral eye, each might be predictive of IOP elevation in the ipsilateral eye independent of each other. Hence these variables were analyzed separately.
When appropriate, time-updated covariates were used: for the level of current inflammatory activity, use of treatments, and the presence of complications of inflammation. For instance, the time-updated level of activity would reflect something like the instantaneous effect of activity on IOP, rather than the cumulative effects of inflammation over the years (the latter perhaps better captured by the presence of complications of inflammation). Regarding such complications, once peripheral anterior synechia, posterior synechia and band keratopathy were noted, we considered them to be present throughout the course of follow up (time-updated covariates which could only transition from absent to present). The rationale for this approach was that even if some of these were clinically noted to be absent at subsequent visits (such as after cataract surgery or EDTA chelation), the damage to the eye already had occurred. The short-run effects of local corticosteroid injections on IOP elevation are observed infrequently after a period of three months subsequent to the procedure. Hence outcomes that occurred only within three months of application of periocular and intravitreous corticosteroids were attributed to the procedure.
Statistical method and analyses
The prevalence of IOP elevation was evaluated based on the proportion with each event of interest. The relationships between prevalent IOP elevation and potentially associated factors were evaluated using crude and adjusted odds ratios (ORs) calculated using logistic regression that incorporated generalizations of generalized estimating equations to account for correlation between the eyes of individual patients. 20 The relationships between incident IOP elevation and potentially associated factors were evaluated using crude and adjusted hazard ratios (HRs) calculated using Cox proportional hazards models with a robust sandwich estimate to account for correlation between the eyes of individual patients. 21 Final multiple logistic regression and Cox regression models adjusted for covariates that were associated with the outcome in two or more of the five crude analyses (two for prevalence, three for incidence). The same covariates were used in multiple regression for prevalence and incidence models, except that time-updated variables requiring knowledge of the past (e.g., prior corticosteroid injections) were available only for the incidence models. The values of time-updated variables other than periocular and intravitreous corticosteroids were updated at every observation and their value at the time of the outcome observation was used. Since intravitreous corticosteroids were rarely applied, the fraction of eye-years associated with outcomes was of insufficient magnitude to be used as an adjustment factor in the statistical model.
Two-year incidence estimates were evaluated by calculating the cumulative incidence estimated from the crude Cox regression hazard function that allowed nonlinear IOP elevation rates and confidence intervals consistent with the hazard ratios and accounting for correlation between eyes of the same patient. Each outcome (≥21, ≥30, rise by ≥10mmHg) was modeled independently for both prevalence and incidence analyses; 95% confidence intervals were presented for all point estimates following the convention where the lower and upper bounds of the confidence interval are presented as subscripts before and after the estimate respectively.22 All statistical analyses were performed with SAS software version 9.4 (SAS Inc., Cary, NC).
Results
IOP elevation at presentation and potential risk factors
Among 1593 eyes (916 children) at cohort entry, 251 (15.8%) and 46 (2.9%) eyes presented with IOP≥21 and ≥30mmHg, respectively. IOP measurements were recorded using Goldmann applanation tonometer, pneumotonometer, Tonopen, Schiotz and other methods in 56%, 30%, 13.7%, 0.04% and 0.29% of eyes, respectively.
The distribution of particular covariates as noted at presentation, that we judged were of most clinical interest or were adjusted for in the statistical model have been summarized in Table 1. Eye-specific data are used, given that most characteristics are eye-specific. The distributions from a patient level perspective were similar. A comprehensive summary of all covariates studied is given as Table 2, available at http://aaojournal.org, for those interested in association results for other variables.
Table 1.
Prevalence of IOP* elevation (IOP* ≥ 21, 30mmHg) in pediatric non-infectious uveitis cases at the time of presentation to subspecialty care: Absolute risk, relative odds and risk factors
| IOP*≥21mmHg | IOP*≥30mmHg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | IOP* mmHg: n eyes (%) | Univariate | Multivariate* | Univariate | Multivariate* | |||||||
| <21 n=1342 |
21 – <30 n=205 |
≥30 n=46 |
Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI) |
P* | ||
| Age at presentation | <6 years | 115 (84.6%) | 19 (14.0%) | 2 (1.5%) | 0.69 (0.39, 1.22) | 0.01 | 0.77 (0.40, 1.47) | 0.07 | 0.51 (0.22, 1.18) | 0.06 | 0.30 (0.11, 0.88) | 0.007 |
| 6–12 years | 657 (81.8%) | 119 (14.8%) | 27 (3.4%) | 1 | 1 | 1 | 1 | |||||
| 13–17 years | 570 (87.2%) | 67 (10.2%) | 17 (2.6%) | 0.63 (0.46, 0.87) | 0.67 (0.47, 0.95) | 0.64 (0.42, 0.98) | 0.45 (0.25, 0.82) | |||||
| Juvenile idiopathic arthritis | No | 1038 (85.1%) | 151 (12.4%) | 31 (2.5%) | 1 | 0.01 | 1 | 0.68 | 1 | 0.003 | 1 | 0.20 |
| Yes | 304 (81.5%) | 54 (14.5%) | 15 (4.0%) | 1.54 (1.11, 2.13) | 0.91 (0.59, 1.41) | 1.88 (1.24, 2.84) | 0.58 (0.26, 1.32) | |||||
| Cataract surgery prior to presentation | Never | 1276 (85.2%) | 187 (12.5%) | 35 (2.3%) | 1 | <0.001 | 1 | 0.01 | 1 | <0.001 | 1 | 0.14 |
| Prior to SITE | 66 (69.5%) | 18 (18.9%) | 11 (11.6%) | 5.44 (3.33, 8.88) | 2.42 (1.19, 4.92) | 9.16 (5.46, 15.4) | 2.99 (0.69, 13.0) | |||||
| Pars plana vitrectomy (not retinal detachment) | No | 1317 (84.5%) | 200 (12.8%) | 41 (2.6%) | 1 | 0.02 | 1 | 0.53 | 1 | <0.001 | 1 | 0.03 |
| Yes | 25 (71.4%) | 5 (14.3%) | 5 (14.3%) | 2.57 (1.17, 5.64) | 0.71 (0.25, 2.04) | 4.35 (1.91, 9.87) | 11.1 (1.30, 94.6) | |||||
| Uveitis category | Anterior | 621 (83.0%) | 99 (13.2%) | 28 (3.7%) | 1 | 0.02 | 1 | 0.62 | 1 | 0.001 | 1 | 0.87 |
| Intermediate | 491 (86.9%) | 62 (11.0%) | 12 (2.1%) | 0.59 (0.41, 0.83) | 0.89 (0.57, 1.39) | 0.36 (0.22, 0.61) | 0.84 (0.38, 1.85) | |||||
| Posterior | 90 (79.6%) | 21 (18.6%) | 2 (1.8%) | 1.05 (0.61, 1.81) | 1.30 (0.71, 2.37) | 0.70 (0.32, 1.50) | 0.75 (0.28, 1.97) | |||||
| Panuveitis | 140 (83.8%) | 23 (13.8%) | 4 (2.4%) | 1.001 (0.61, 1.64) | 0.86 (0.51, 1.48) | 1.03 (0.57, 1.87) | 1.23 (0.42, 3.55) | |||||
| Bilateral uveitis | No | 129 (79.6%) | 24 (14.8%) | 9 (5.6%) | 1 | 0.08 | 1 | 0.003 | 1 | 0.09 | 1 | 0.004 |
| Yes | 1213 (84.8%) | 181 (12.6%) | 37 (2.6%) | 0.71 (0.48, 1.04) | 0.51 (0.33, 0.80) | 0.66 (0.41, 1.07) | 0.32 (0.15, 0.69) | |||||
| Duration of uveitis prior to presentation | <6 Months | 571 (88.0%) | 70 (10.8%) | 8 (1.2%) | 1 | <0.001 | 1 | 0.33 | 1 | <0.001 | 1 | <0.001 |
| 6 Months to <2 Years | 393 (83.6%) | 64 (13.6%) | 13 (2.8%) | 1.52 (1.04, 2.22) | 1.16 (0.78, 1.73) | 2.35 (1.32, 4.17) | 4.05 (1.97, 8.33) | |||||
| 2 to <5 Years | 215 (76.8%) | 47 (16.8%) | 18 (6.4%) | 2.27 (1.51, 3.41) | 1.43 (0.89, 2.28) | 4.22 (2.38, 7.48) | 6.18 (2.62, 14.6) | |||||
| 5+ Years | 163 (84.0%) | 24 (12.4%) | 7 (3.6%) | 1.96 (1.23, 3.11) | 0.88 (0.48, 1.63) | 4.41 (2.37, 8.18) | 11.8 (3.32, 41.9) | |||||
| Other eye history of IOP* elevation | No Drops/Surgery | 1253 (86.5%) | 168 (11.6%) | 28 (1.9%) | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 |
| Drops/Surgery | 89 (61.8%) | 37 (25.7%) | 18 (12.5%) | 21.1 (12.9, 34.7) | 16.9 (9.46, 30.1) | 64.7 (36.4, 115) | 8.29 (3.16, 21.7) | |||||
| Visual acuity | 20/40 or better | 906 (85.8%) | 128 (12.1%) | 22 (2.1%) | 1 | 0.002 | 1 | 0.02 | 1 | 0.006 | 1 | 0.03 |
| >20/40 – <20/200 | 306 (82.5%) | 51 (13.7%) | 14 (3.8%) | 1.48 (1.09, 2.01) | 1.48 (1.03, 2.11) | 1.58 (1.07, 2.31) | 1.99 (1.07, 3.71) | |||||
| 20/200 or worse | 123 (77.4%) | 26 (16.4%) | 10 (6.3%) | 1.85 (1.26, 2.72) | 1.73 (1.08, 2.77) | 2.07 (1.28, 3.33) | 2.81 (1.04, 7.57) | |||||
| Anterior chamber cells | Quiet | 511 (81.9%) | 88 (14.1%) | 25 (4.0%) | 1 | <0.001 | 1 | <0.001 | 1 | 0.22 | 1 | 0.02 |
| 0.5+ | 255 (77.7%) | 62 (18.9%) | 11 (3.4%) | 1.15 (0.81, 1.63) | 1.06 (0.70, 1.59) | 0.90 (0.56, 1.45) | 1.03 (0.49, 2.14) | |||||
| 1+ | 259 (87.8%) | 30 (10.2%) | 6 (2.0%) | 0.71 (0.48, 1.06) | 0.56 (0.35, 0.87) | 0.82 (0.50, 1.34) | 2.09 (0.95, 4.59) | |||||
| 2+ or worse | 314 (91.5%) | 25 (7.3%) | 4 (1.2%) | 0.48 (0.32, 0.73) | 0.37 (0.23, 0.59) | 0.57 (0.34, 0.97) | 0.44 (0.20, 0.97) | |||||
| Snowballs | No | 1154 (83.5%) | 184 (13.3%) | 44 (3.2%) | 1 | 0.007 | 1 | 0.87 | 1 | <0.001 | 1 | 0.30 |
| Yes | 188 (89.1%) | 21 (10.0%) | 2 (0.9%) | 0.51 (0.32, 0.83) | 1.05 (0.59, 1.85) | 0.20 (0.09, 0.47) | 0.56 (0.19, 1.65) | |||||
| Peripheral anterior synechia | No | 1326 (84.5%) | 202 (12.9%) | 41 (2.6%) | 1 | 0.02 | 1 | 0.20 | 1 | <0.001 | 1 | 0.13 |
| Yes | 15 (65.2%) | 3 (13.0%) | 5 (21.7%) | 2.94 (1.20, 7.22) | 1.62 (0.77, 3.37) | 5.29 (2.12, 13.2) | 4.65 (0.62, 34.7) | |||||
| Band keratopathy | No | 1044 (84.7%) | 159 (12.9%) | 29 (2.4%) | 1 | <0.001 | 1 | 0.52 | 1 | <0.001 | 1 | 0.55 |
| Yes | 149 (76.4%) | 36 (18.5%) | 10 (5.1%) | 2.32 (1.60, 3.37) | 1.11 (0.68, 1.81) | 2.92 (1.85, 4.60) | 0.89 (0.38, 2.11) | |||||
| Missing | 149 (89.8%) | 10 (6.0%) | 7 (4.2%) | 0.55 (0.31, 0.99) | 0.73 (0.40, 1.34) | 0.65 (0.31, 1.39) | 1.68 (0.64, 4.43) | |||||
| Immunosuppressive therapy/Biologics | No | 1095 (85.7%) | 147 (11.5%) | 35 (2.7%) | 1 | <0.001 | 1 | 0.18 | 1 | <0.001 | 1 | 0.31 |
| Yes | 247 (78.2%) | 58 (18.4%) | 11 (3.5%) | 2.18 (1.55, 3.07) | 1.32 (0.88, 1.98) | 2.80 (1.84, 4.26) | 1.46 (0.70, 3.01) | |||||
| Systemic corticosteroids | No | 1137 (85.2%) | 160 (12.0%) | 37 (2.8%) | 1 | 0.03 | 1 | 0.24 | 1 | 0.41 | 1 | 0.89 |
| Yes | 205 (79.2%) | 45 (17.4%) | 9 (3.5%) | 1.53 (1.05, 2.24) | 1.31 (0.84, 2.05) | 1.24 (0.75, 2.06) | 0.94 (0.41, 2.18) | |||||
| Topical corticosteroids | None/day | 563 (90.2%) | 52 (8.3%) | 9 (1.4%) | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 |
| 1 drop/day | 114 (85.7%) | 15 (11.3%) | 4 (3.0%) | 1.65 (0.89, 3.04) | 1.47 (0.79, 2.73) | 3.38 (1.46, 7.80) | 3.47 (1.04, 11.6) | |||||
| 2 drops/day | 109 (79.0%) | 23 (16.7%) | 6 (4.3%) | 4.02 (2.40, 6.74) | 2.78 (1.44, 5.35) | 7.64 (3.72, 15.7) | 4.29 (1.11, 16.6) | |||||
| 3 drops/day | 118 (83.1%) | 21 (14.8%) | 3 (2.1%) | 2.93 (1.74, 4.92) | 2.89 (1.59, 5.23) | 4.71 (2.35, 9.42) | 5.09 (1.72, 15.1) | |||||
| 4 drops/day | 145 (75.1%) | 42 (21.8%) | 6 (3.1%) | 4.32 (2.68, 6.97) | 3.81 (2.18, 6.67) | 6.36 (3.17, 12.7) | 8.64 (3.15, 23.6) | |||||
| 5–8 drops/day | 161 (78.2%) | 34 (16.5%) | 11 (5.3%) | 3.34 (2.08, 5.35) | 3.07 (1.78, 5.29) | 5.88 (2.97, 11.6) | 6.36 (2.55, 15.9) | |||||
| >8 drops/day | 132 (84.1%) | 18 (11.5%) | 7 (4.5%) | 2.75 (1.61, 4.68) | 2.94 (1.58, 5.47) | 5.44 (2.64, 11.2) | 8.92 (3.12, 25.5) | |||||
IOP-Intraocular Pressure, n eyes-number of eyes, CI-Confidence Interval, P-Probability, SITE-Systemic Immunosuppressive Therapy in Eye Diseases Study, n/a-Not Applicable
Multivariate: The variables by which risk was adjusted for were: Juvenile idiopathic arthritis, Cataract surgery performed prior to presentation, Pars plana vitrectomy (not retinal detachment), Uveitis category, Bilateral uveitis, Duration of uveitis prior to presentation, Other eye history of IOP elevation, Visual acuity, Anterior chamber cells, Snowballs, Peripheral anterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids and Topical corticosteroids.
A more comprehensive version of this table including other variables such as: Sex, Race category, Sarcoidosis, HLA-B27, Adamantiades-Behçet’s disease, Hypertension, Retinal detachment Surgery, Inflammatory activity, Vitreous cells, Vitreous haze, Keratic precipitates, Posterior synechia can be found as Table 2-available online at http://aaojournal.org
Table 2.
Prevalence of IOP* elevation (IOP* ≥21, 30mmHg) in pediatric non-infectious uveitis cases at the time of presentation to subspecialty care: Absolute risk, relative odds and risk factors
| IOP*≥21mmHg | IOP*≥30mmHg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | IOP* mmHg: n eyes (%) | Univariate | Multivariate* | Univariate | Multivariate* | |||||||
| <21 n=1342 |
21 – <30 n=205 |
≥30 n=46 |
Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI*) |
P* | Odds Ratio (95% CI) |
P* | ||
| Age at presentation | <6 years | 115 (84.6%) | 19 (14.0%) | 2 (1.5%) | 0.69 (0.39, 1.22) | 0.01 | 0.77 (0.40, 1.47) | 0.07 | 0.51 (0.22, 1.18) | 0.06 | 0.30 (0.11, 0.88) | 0.007 |
| 6–12 years | 657 (81.8%) | 119 (14.8%) | 27 (3.4%) | 1 | 1 | 1 | 1 | |||||
| 13–17 years | 570 (87.2%) | 67 (10.2%) | 17 (2.6%) | 0.63 (0.46, 0.87) | 0.67 (0.47, 0.95) | 0.64 (0.42, 0.98) | 0.45 (0.25, 0.82) | |||||
| Sex | Male | 613 (84.3%) | 97 (13.3%) | 17 (2.3%) | 1 | 0.62 | 1 | 0.62 | 1 | 0.27 | 1 | 0.58 |
| Female | 729 (84.2%) | 108 (12.5%) | 29 (3.3%) | 1.08 (0.80, 1.45) | 0.92 (0.67, 1.27) | 1.25 (0.84, 1.86) | 1.16 (0.68, 1.98) | |||||
| Race category | White | 1047 (85.2%) | 149 (12.1%) | 33 (2.7%) | 1 | 0.48 | 1 | 0.47 | 1 | 0.20 | 1 | 0.33 |
| Black | 84 (84.0%) | 10 (10.0%) | 6 (6.0%) | 1.33 (0.74, 2.41) | 0.89 (0.50, 1.58) | 1.94 (0.97, 3.87) | 1.75 (0.61, 4.98) | |||||
| Hispanic | 97 (83.6%) | 17 (14.7%) | 2 (1.7%) | 1.15 (0.65, 2.06) | 0.99 (0.55, 1.78) | 1.52 (0.75, 3.07) | 1.90 (0.66, 5.47) | |||||
| Other | 114 (77.0%) | 29 (19.6%) | 5 (3.4%) | 1.38 (0.85, 2.24) | 1.47 (0.88, 2.46) | 1.30 (0.71, 2.40) | 0.72 (0.31, 1.64) | |||||
| Juvenile idiopathic arthritis | No | 1038 (85.1%) | 151 (12.4%) | 31 (2.5%) | 1 | 0.01 | 1 | 0.68 | 1 | 0.003 | 1 | 0.20 |
| Yes | 304 (81.5%) | 54 (14.5%) | 15 (4.0%) | 1.54 (1.11, 2.13) | 0.91 (0.59, 1.41) | 1.88 (1.24, 2.84) | 0.58 (0.26, 1.32) | |||||
| Sarcoidosis | No | 1323 (84.1%) | 205 (13.0%) | 45 (2.9%) | 1 | 0.60 | 1 | 0.002 | 1 | 0.67 | 1 | 0.41 |
| Yes | 19 (95.0%) | 0 (0.0%) | 1 (5.0%) | 0.66 (0.14, 3.15) | 0.20 (0.07, 0.55) | 1.40 (0.29, 6.72) | 0.57 (0.15, 2.17) | |||||
| HLA-B27 | No | 1278 (84.0%) | 197 (13.0%) | 46 (3.0%) | 1 | 0.78 | 1 | 0.06 | 1 | 0.56 | 1 | 0.06 |
| Yes | 64 (88.9%) | 8 (11.1%) | 0 (0.0%) | 0.90 (0.44, 1.84) | 0.49 (0.23, 1.03) | 1.29 (0.55, 3.01) | 0.35 (0.12, 1.04) | |||||
| Adamantiades-Behçet’s disease | No | 1333 (84.4%) | 203 (12.8%) | 44 (2.8%) | 1 | 0.47 | 1 | 0.30 | 1 | 0.15 | 1 | 0.57 |
| Yes | 9 (69.2%) | 2 (15.4%) | 2 (15.4%) | 1.68 (0.41, 6.80) | 1.95 (0.56, 6.84) | 2.39 (0.73, 7.89) | 0.62 (0.12, 3.18) | |||||
| Hypertension | No | 1329 (84.4%) | 203 (12.9%) | 43 (2.7%) | 1 | 0.59 | 1 | 0.47 | 1 | 0.56 | 1 | 0.64 |
| Yes | 13 (72.2%) | 2 (11.1%) | 3 (16.7%) | 1.45 (0.38, 5.57) | 1.82 (0.36, 9.16) | 1.59 (0.33, 7.64) | 0.78 (0.27, 2.22) | |||||
| Cataract surgery prior to presentation | Never | 1276 (85.2%) | 187 (12.5%) | 35 (2.3%) | 1 | <0.001 | 1 | 0.01 | 1 | <0.001 | 1 | 0.14 |
| Prior to SITE | 66 (69.5%) | 18 (18.9%) | 11 (11.6%) | 5.44 (3.33, 8.88) | 2.42 (1.19, 4.92) | 9.16 (5.46, 15.4) | 2.99 (0.69, 13.0) | |||||
| Pars plana vitrectomy (not retinal detachment) | No | 1317 (84.5%) | 200 (12.8%) | 41 (2.6%) | 1 | 0.02 | 1 | 0.53 | 1 | <0.001 | 1 | 0.03 |
| Yes | 25 (71.4%) | 5 (14.3%) | 5 (14.3%) | 2.57 (1.17, 5.64) | 0.71 (0.25, 2.04) | 4.35 (1.91, 9.87) | 11.1 (1.30, 94.6) | |||||
| Retinal detachment surgery | No | 1335 (84.4%) | 202 (12.8%) | 45 (2.8%) | 1 | 0.06 | 1 | 0.68 | 1 | 0.47 | 1 | 0.49 |
| Yes | 7 (63.6%) | 3 (27.3%) | 1 (9.1%) | 3.16 (0.96, 10.4) | 1.38 (0.30, 6.37) | 1.76 (0.38, 8.20) | 0.56 (0.11, 2.93) | |||||
| Uveitis category | Anterior | 621 (83.0%) | 99 (13.2%) | 28 (3.7%) | 1 | 0.02 | 1 | 0.62 | 1 | 0.001 | 1 | 0.87 |
| Intermediate | 491 (86.9%) | 62 (11.0%) | 12 (2.1%) | 0.59 (0.41, 0.83) | 0.89 (0.57, 1.39) | 0.36 (0.22, 0.61) | 0.84 (0.38, 1.85) | |||||
| Posterior | 90 (79.6%) | 21 (18.6%) | 2 (1.8%) | 1.05 (0.61, 1.81) | 1.30 (0.71, 2.37) | 0.70 (0.32, 1.50) | 0.75 (0.28, 1.97) | |||||
| Panuveitis | 140 (83.8%) | 23 (13.8%) | 4 (2.4%) | 1.001 (0.61, 1.64) | 0.86 (0.51, 1.48) | 1.03 (0.57, 1.87) | 1.23 (0.42, 3.55) | |||||
| Bilateral uveitis | No | 129 (79.6%) | 24 (14.8%) | 9 (5.6%) | 1 | 0.08 | 1 | 0.003 | 1 | 0.09 | 1 | 0.004 |
| Yes | 1213 (84.8%) | 181 (12.6%) | 37 (2.6%) | 0.71 (0.48, 1.04) | 0.51 (0.33, 0.80) | 0.66 (0.41, 1.07) | 0.32 (0.15, 0.69) | |||||
| Duration of uveitis prior to presentation | <6 Months | 571 (88.0%) | 70 (10.8%) | 8 (1.2%) | 1 | <0.001 | 1 | 0.33 | 1 | <0.001 | 1 | <0.001 |
| 6 Months to <2 Years | 393 (83.6%) | 64 (13.6%) | 13 (2.8%) | 1.52 (1.04, 2.22) | 1.16 (0.78, 1.73) | 2.35 (1.32, 4.17) | 4.05 (1.97, 8.33) | |||||
| 2 to <5 Years | 215 (76.8%) | 47 (16.8%) | 18 (6.4%) | 2.27 (1.51, 3.41) | 1.43 (0.89, 2.28) | 4.22 (2.38, 7.48) | 6.18 (2.62, 14.6) | |||||
| 5+ Years | 163 (84.0%) | 24 (12.4%) | 7 (3.6%) | 1.96 (1.23, 3.11) | 0.88 (0.48, 1.63) | 4.41 (2.37, 8.18) | 11.8 (3.32, 41.9) | |||||
| Other eye history of IOP* elevation | No Drops/Surgery | 1253 (86.5%) | 168 (11.6%) | 28 (1.9%) | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 |
| Drops/Surgery | 89 (61.8%) | 37 (25.7%) | 18 (12.5%) | 21.1 (12.9, 34.7) | 16.9 (9.46, 30.1) | 64.7 (36.4, 115) | 8.29 (3.16, 21.7) | |||||
| Visual acuity | 20/40 or better | 906 (85.8%) | 128 (12.1%) | 22 (2.1%) | 1 | 0.002 | 1 | 0.02 | 1 | 0.006 | 1 | 0.03 |
| >20/40 – <20/200 | 306 (82.5%) | 51 (13.7%) | 14 (3.8%) | 1.48 (1.09, 2.01) | 1.48 (1.03, 2.11) | 1.58 (1.07, 2.31) | 1.99 (1.07, 3.71) | |||||
| 20/200 or worse | 123 (77.4%) | 26 (16.4%) | 10 (6.3%) | 1.85 (1.26, 2.72) | 1.73 (1.08, 2.77) | 2.07 (1.28, 3.33) | 2.81 (1.04, 7.57) | |||||
| Inflammatory activity | Inactive | 335 (78.8%) | 72 (16.9%) | 18 (4.2%) | 1 | <0.001 | 1 | 0.66 | 1 | <0.001 | 1 | 0.009 |
| Slightly active | 165 (82.1%) | 29 (14.4%) | 7 (3.5%) | 0.77 (0.49, 1.21) | 0.88 (0.52, 1.48) | 0.86 (0.50, 1.49) | 2.47 (0.86, 7.11) | |||||
| Active | 840 (87.0%) | 104 (10.8%) | 21 (2.2%) | 0.48 (0.35, 0.66) | 0.80 (0.49, 1.30) | 0.39 (0.26, 0.59) | 0.42 (0.17, 1.08) | |||||
| Anterior chamber cells | Quiet | 511 (81.9%) | 88 (14.1%) | 25 (4.0%) | 1 | <0.001 | 1 | <0.001 | 1 | 0.22 | 1 | 0.02 |
| 0.5+ | 255 (77.7%) | 62 (18.9%) | 11 (3.4%) | 1.15 (0.81, 1.63) | 1.06 (0.70, 1.59) | 0.90 (0.56, 1.45) | 1.03 (0.49, 2.14) | |||||
| 1+ | 259 (87.8%) | 30 (10.2%) | 6 (2.0%) | 0.71 (0.48, 1.06) | 0.56 (0.35, 0.87) | 0.82 (0.50, 1.34) | 2.09 (0.95, 4.59) | |||||
| 2+ or worse | 314 (91.5%) | 25 (7.3%) | 4 (1.2%) | 0.48 (0.32, 0.73) | 0.37 (0.23, 0.59) | 0.57 (0.34, 0.97) | 0.44 (0.20, 0.97) | |||||
| Vitreous cells | Quiet | 536 (81.8%) | 89 (13.6%) | 30 (4.6%) | 1 | 0.001 | 1 | <0.001 | 1 | 0.009 | 1 | 0.36 |
| 0.5+ | 183 (78.2%) | 48 (20.5%) | 3 (1.3%) | 1.12 (0.75, 1.66) | 1.43 (0.91, 2.24) | 0.71 (0.40, 1.23) | 1.68 (0.65, 4.31) | |||||
| 1+ | 295 (92.2%) | 21 (6.6%) | 4 (1.3%) | 0.52 (0.34, 0.79) | 0.49 (0.33, 0.74) | 0.63 (0.37, 1.07) | 0.80 (0.34, 1.87) | |||||
| 2+ or worse | 285 (86.9%) | 38 (11.6%) | 5 (1.5%) | 0.58 (0.38, 0.89) | 0.66 (0.41, 1.09) | 0.36 (0.19, 0.68) | 0.58 (0.22, 1.49) | |||||
| Vitreous haze | Quiet | 883 (83.5%) | 141 (13.3%) | 34 (3.2%) | 1 | 0.08 | 1 | 0.96 | 1 | 0.001 | 1 | 0.73 |
| 1+ | 116 (86.6%) | 14 (10.4%) | 4 (3.0%) | 0.72 (0.43, 1.22) | 1.01 (0.54, 1.89) | 0.50 (0.23, 1.11) | 0.82 (0.26, 2.57) | |||||
| 2+ or worse | 96 (84.2%) | 11 (9.6%) | 7 (6.1%) | 0.79 (0.45, 1.40) | 0.83 (0.42, 1.67) | 0.67 (0.34, 1.29) | 0.50 (0.15, 1.67) | |||||
| Missing | 247 (86.1%) | 39 (13.6%) | 1 (0.3%) | 0.60 (0.39, 0.91) | 1.01 (0.62, 1.63) | 0.27 (0.13, 0.55) | 0.84 (0.31, 2.25) | |||||
| Snowballs | No | 1154 (83.5%) | 184 (13.3%) | 44 (3.2%) | 1 | 0.007 | 1 | 0.87 | 1 | <0.001 | 1 | 0.30 |
| Yes | 188 (89.1%) | 21 (10.0%) | 2 (0.9%) | 0.51 (0.32, 0.83) | 1.05 (0.59, 1.85) | 0.20 (0.09, 0.47) | 0.56 (0.19, 1.65) | |||||
| Keratic precipitates | No | 962 (81.6%) | 180 (15.3%) | 37 (3.1%) | 1 | <0.001 | 1 | <0.001 | 1 | 0.009 | 1 | 0.055 |
| Yes | 231 (93.1%) | 15 (6.0%) | 2 (0.8%) | 0.38 (0.24, 0.60) | 0.31 (0.19, 0.50) | 0.43 (0.23, 0.82) | 0.39 (0.15, 1.02) | |||||
| Missing | 149 (89.8%) | 10 (6.0%) | 7 (4.2%) | 0.42 (0.24, 0.75) | 0.61 (0.33, 1.13) | 0.48 (0.23, 1.005) | 1.27 (0.44, 3.64) | |||||
| Peripheral anterior synechia | No | 1326 (84.5%) | 202 (12.9%) | 41 (2.6%) | 1 | 0.02 | 1 | 0.20 | 1 | <0.001 | 1 | 0.13 |
| Yes | 15 (65.2%) | 3 (13.0%) | 5 (21.7%) | 2.94 (1.20, 7.22) | 1.62 (0.77, 3.37) | 5.29 (2.12, 13.2) | 4.65 (0.62, 34.7) | |||||
| Posterior synechia | No | 1104 (85.1%) | 156 (12.0%) | 37 (2.9%) | 1 | 0.01 | 1 | 0.42 | 1 | 0.06 | 1 | 0.80 |
| Yes | 238 (80.4%) | 49 (16.6%) | 9 (3.0%) | 1.55 (1.11, 2.15) | 1.18 (0.79, 1.76) | 1.50 (0.99, 2.28) | 1.09 (0.55, 2.18) | |||||
| Band keratopathy | No | 1044 (84.7%) | 159 (12.9%) | 29 (2.4%) | 1 | <0.001 | 1 | 0.52 | 1 | <0.001 | 1 | 0.55 |
| Yes | 149 (76.4%) | 36 (18.5%) | 10 (5.1%) | 2.32 (1.60, 3.37) | 1.11 (0.68, 1.81) | 2.92 (1.85, 4.60) | 0.89 (0.38, 2.11) | |||||
| Missing | 149 (89.8%) | 10 (6.0%) | 7 (4.2%) | 0.55 (0.31, 0.99) | 0.73 (0.40, 1.34) | 0.65 (0.31, 1.39) | 1.68 (0.64, 4.43) | |||||
| Immunosuppressive therapy/Biologics | No | 1095 (85.7%) | 147 (11.5%) | 35 (2.7%) | 1 | <0.001 | 1 | 0.18 | 1 | <0.001 | 1 | 0.31 |
| Yes | 247 (78.2%) | 58 (18.4%) | 11 (3.5%) | 2.18 (1.55, 3.07) | 1.32 (0.88, 1.98) | 2.80 (1.84, 4.26) | 1.46 (0.70, 3.01) | |||||
| Systemic corticosteroids | No | 1137 (85.2%) | 160 (12.0%) | 37 (2.8%) | 1 | 0.03 | 1 | 0.24 | 1 | 0.41 | 1 | 0.89 |
| Yes | 205 (79.2%) | 45 (17.4%) | 9 (3.5%) | 1.53 (1.05, 2.24) | 1.31 (0.84, 2.05) | 1.24 (0.75, 2.06) | 0.94 (0.41, 2.18) | |||||
| Topical corticosteroids | None/day | 563 (90.2%) | 52 (8.3%) | 9 (1.4%) | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 | 1 | <0.001 |
| 1 drop/day | 114 (85.7%) | 15 (11.3%) | 4 (3.0%) | 1.65 (0.89, 3.04) | 1.47 (0.79, 2.73) | 3.38 (1.46, 7.80) | 3.47 (1.04, 11.6) | |||||
| 2 drops/day | 109 (79.0%) | 23 (16.7%) | 6 (4.3%) | 4.02 (2.40, 6.74) | 2.78 (1.44, 5.35) | 7.64 (3.72, 15.7) | 4.29 (1.11, 16.6) | |||||
| 3 drops/day | 118 (83.1%) | 21 (14.8%) | 3 (2.1%) | 2.93 (1.74, 4.92) | 2.89 (1.59, 5.23) | 4.71 (2.35, 9.42) | 5.09 (1.72, 15.1) | |||||
| 4 drops/day | 145 (75.1%) | 42 (21.8%) | 6 (3.1%) | 4.32 (2.68, 6.97) | 3.81 (2.18, 6.67) | 6.36 (3.17, 12.7) | 8.64 (3.15, 23.6) | |||||
| 5–8 drops/day | 161 (78.2%) | 34 (16.5%) | 11 (5.3%) | 3.34 (2.08, 5.35) | 3.07 (1.78, 5.29) | 5.88 (2.97, 11.6) | 6.36 (2.55, 15.9) | |||||
| >8 drops/day | 132 (84.1%) | 18 (11.5%) | 7 (4.5%) | 2.75 (1.61, 4.68) | 2.94 (1.58, 5.47) | 5.44 (2.64, 11.2) | 8.92 (3.12, 25.5) | |||||
IOP-Intraocular Pressure, n eyes-number of eyes, CI-Confidence Interval, P-Probability, SITE-Systemic Immunosuppressive Therapy in Eye Diseases Study, n/a-Not Applicable
Multivariate: The variables by which risk was adjusted for were: Juvenile idiopathic arthritis, Cataract surgery performed prior to presentation, Pars plana vitrectomy (not retinal detachment), Uveitis category, Bilateral uveitis, Duration of uveitis prior to presentation, Other eye history of IOP elevation, Visual acuity, Anterior chamber cells, Snowballs, Peripheral anterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids and Topical corticosteroids.
Demographic characteristics demonstrated little association with IOP outcomes except that eyes of children 6–12 years of age were more likely to present with IOP≥21mmHg (adjusted overall P=0.07) and IOP≥30mmHg (adjusted overall P=0.007) when compared with eyes in other age groups. Regarding systemic disease associations, eyes of uveitic children did not demonstrate any risk of presenting IOP elevation or else the risk was not consistently present in both the crude and adjusted analysis (see Table 2, available at http://aaojournal.org for more details).
Regarding clinical characteristics, 30.5% of pediatric eyes that previously had undergone cataract surgery were noted to have a 2.5 fold risk of presenting with IOP≥21mmHg (adjusted odds ratio≥21mmHg [aOR21]=1.192.424.92, P=0.01) when compared those eyes that did not have cataract surgery performed prior to referral. The higher odds associated with presenting IOP≥30mmHg in eyes that had prior cataract surgery was not found to be significant on adjusting for other factors, although the pattern of association was similar to that for the IOP≥21 mmHg outcome. In eyes that had undergone pars plana vitrectomy (other than for retinal detachment), the statistical significance of the crude association with presenting IOP≥21mmHg did not persist after accounting for other factors; but eyes of uveitic children were 11-fold more likely to present with IOP≥30mmHg (adjusted odds ratio≥30mmHg [aOR30]=1.3011.194.6, P=0.03). Pediatric eyes that had undergone retinal detachment surgery prior to presentation did not demonstrate such risk (all adjusted P values>0.1).
IOP≥21mmHg was noted in 16.9%, 13.1%, 20.4% and 16.2% of eyes, and IOP≥30mmHg in 3.7%, 2.1% 1.8% and 2.4% of eyes with anterior, intermediate, posterior and panuveitis, respectively. Although these differences were overall statistically significant in the crude analysis, the association was abrogated on adjusting for other factors. Adjusted for other factors, pediatric eyes with bilateral uveitis had one-half and one-third less odds of presenting with IOP≥21 and ≥30mmHg, respectively (aOR21=0.330.510.80, P=0.003; aOR30=0.150.320.69, P=0.004), when compared with eyes with unilateral involvement. When compared with those having a uveitis duration <6 months, children with a longer duration (≥6 months) of uveitis had higher risk of presenting IOP≥21mmHg that was not found to be statistically significant on accounting for other factors. However, the risk associated with presenting IOP≥30mmHg was more pronounced, progressively increasing with longer duration of uveitis; reaching up to 11.8-fold in those eyes with uveitis of ≥5year duration (aORs30 up to3.3211.841.9, P<0.001).
Uveitic eyes of children that were on IOP-lowering treatment in the contralateral eye had a strong risk of presenting with IOP elevation: 38.2% and 12.5% of such uveitic eyes had IOP≥21 (aOR21=9.4616.930.1, P<0.001) and ≥30 mmHg (aOR30=3.168.2921.7, P<0.001), respectively.
Pediatric uveitic eyes with worse visual acuity were more likely to present with IOP elevation; each category of progressively worse visual acuity was associated with progressively higher IOP (see Tables 1).
Current activity of inflammation as manifested by: anterior chamber cells was associated with lower odds of IOP≥21 and ≥30mmHg and vitreous cells associated with lower odds of IOP≥21mmHg; in a dose-response relationship. Eyes with vitreous snowballs had lower crude odds of presenting IOP elevation, but this association did not persist on adjusting for other covariates. Other inflammatory markers followed a similar pattern, but most specific associations were not significant after adjusting for other factors. Presence of keratic precipitates (aOR21=0.190.310.5, P<0.001, aOR30=0.150.391.02, P=0.055) was associated with one-third lower odds of presenting IOP elevation. Regarding structural complications of uveitis, the higher crude odds of presenting IOP elevation associated with eyes having peripheral anterior synechiae, posterior synechiae and band keratopathy were no longer found to be significant after adjusting for other factors.
Use of systemic immunosuppressive therapy and systemic corticosteroids were not significantly associated with a higher risk of presenting with IOP elevation after adjusting for other factors including use of topical corticosteroids. However, use of topical corticosteroids was a strong risk factor for presenting IOP elevation–even doses of 1–2 drops/day were associated with substantially higher risk, and higher doses tended to have progressively higher risk that reached up to ~9-fold (see Table 1).
A sensitivity analysis regarding presenting IOP elevation limited to eyes where IOP had been measured using applanation tonometry yielded similar results.
Incidence of IOP elevation over 2 years and potential risk factors
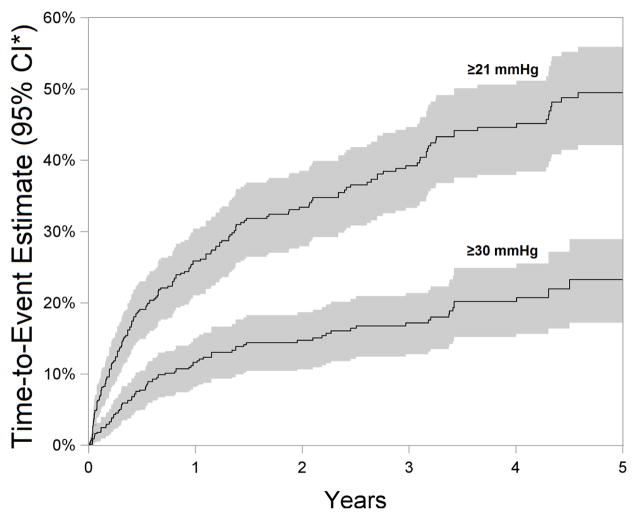
Eyes of children that were outcome-naïve at presentation were followed for a median of 1.25 years (interquartile range 0.4–3.66). During follow-up, a high frequency of IOP elevation events were observed; 27.933.438.5% eyes developed IOP≥21mmHg, 10.714.818.7% developed IOP≥30mmHg and 19.724.428.8% of eyes developed a rise by ≥10mmHg within 2 years of presentation to uveitis sub-specialty care. The proportion of eyes with IOP elevation (cumulative incidence) continued to rise throughout follow-up (see Figure 1), but the rate of rise (reflecting the incidence rate) tended to decline over follow-up time.
Figure 1.
Time-to-event estimate demonstrating the cumulative incidence (95% CI*) of IOP elevation in pediatric non-infectious uveitis over a 5-year follow-up period. >20% of eyes were estimated to have a visit with IOP≥30 mmHg within five years. Although the rate of incidence of IOP elevation in pediatric non-infectious uveitic eyes declines with time under subspecialty care, the cumulative incidence increases over time. The shaded areas around the time-to-event curves represent 95% confidence intervals.
CI*-Confidence interval
The relationship between potential risk factors (that were of clinical interest or adjusted for in the statistical model) and IOP elevation events is given as Table 3, and a complete summary of all the variables studied is given as Table 4 (available at http://aaojournal.org). There were several differences in the risk factor association pattern between the prevalence and incidence analyses regarding demographic and diagnostic features. During follow-up, none of the demographic characteristics or systemic disease associations were associated with an alteration in risk of developing IOP elevation or the statistical significance of the association was found to be inconsistent across the crude and adjusted analyses.
Table 3.
Incidence of IOP* elevation (IOP* ≥ 21, 30mmHg) in pediatric eyes with non-infectious uveitis over 2 years: Absolute risk, relative hazard and risk factors
| IOP*≥21mmHg | IOP* ≥30mmHg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate* | Univariate | Multivariate* | |||||||
| 2-year Incidence Estimate (95% CI*) |
Hazard Ratio (95% CI*) |
P* | Hazard Ratio (95% CI) |
P* | 2-year Incidence Estimate (95% CI*) |
Hazard Ratio (95% CI*) |
P* | Hazard Ratio (95% CI) |
P* | ||
| Age at presentation | <6 years | 0.39 (0.21, 0.54) | 1.19 (0.67, 2.12) | 0.57 | 1.23 (0.69, 2.18) | 0.74 | 0.16 (0.02, 0.29) | 1.11 (0.45, 2.77) | 0.93 | 1.20 (0.48, 3.01) | 0.92 |
| 6–12 years | 0.34 (0.27, 0.41) | 1 | 1 | 0.15 (0.10, 0.20) | 1 | 1 | |||||
| 13–17 years | 0.31 (0.23, 0.38) | 0.88 (0.61, 1.27) | 0.95 (0.64, 1.42) | 0.14 (0.08, 0.19) | 0.94 (0.56, 1.58) | 1.0003 (0.57, 1.77) | |||||
| Juvenile idiopathic arthritis | No | 0.33 (0.27, 0.39) | 1 | 0.80 | 1 | 0.59 | 0.14 (0.10, 0.19) | 1 | 0.62 | 1 | 0.11 |
| Yes | 0.34 (0.25, 0.43) | 1.05 (0.73, 1.50) | 0.87 (0.53, 1.44) | 0.16 (0.09, 0.23) | 1.14 (0.68, 1.91) | 0.57 (0.29, 1.14) | |||||
| Cataract surgery | Never | 0.32 (0.27, 0.37) | 1 | 0.03 | 1 | 0.54 | 0.14 (0.10, 0.18) | 1 | 0.051 | 1 | 0.87 |
| Prior to SITE | 0.35 (0.07, 0.54) | 1.09 (0.47, 2.51) | 0.59 (0.18, 1.90) | 0.32 (0.05, 0.52) | 2.63 (1.07, 6.48) | 1.19 (0.36, 3.95) | |||||
| During SITE | 0.59 (0.31, 0.75) | 2.27 (1.23, 4.19) | 0.67 (0.28, 1.59) | 0.26 (0.06, 0.42) | 2.06 (0.91, 4.65) | 0.85 (0.30, 2.42) | |||||
| Pars plana vitrectomy (not retinal detachment) | No | 0.32 (0.26, 0.37) | 1 | <0.001 | 1 | <0.001 | 0.14 (0.10, 0.18) | 1 | 0.15 | 1 | 0.52 |
| Yes | 0.68 (0.42, 0.82) | 2.98 (1.74, 5.11) | 3.36 (1.75, 6.45) | 0.24 (0.07, 0.37) | 1.74 (0.81, 3.72) | 1.40 (0.50, 3.93) | |||||
| Uveitis category | Anterior | 0.36 (0.28, 0.43) | 1 | 0.06 | 1 | 0.92 | 0.16 (0.10, 0.22) | 1 | 0.84 | 1 | 0.33 |
| Intermediate | 0.28 (0.20, 0.35) | 0.75 (0.51, 1.10) | 0.98 (0.60, 1.62) | 0.14 (0.08, 0.19) | 0.86 (0.50, 1.46) | 1.09 (0.58, 2.06) | |||||
| Posterior | 0.25 (0.06, 0.40) | 0.64 (0.28, 1.47) | 0.84 (0.36, 1.96) | 0.10 (0, 0.20) | 0.62 (0.20, 1.91) | 1.15 (0.35, 3.81) | |||||
| Panuveitis | 0.48 (0.30, 0.61) | 1.48 (0.90, 2.43) | 1.15 (0.63, 2.09) | 0.14 (0.03, 0.24) | 0.88 (0.37, 2.08) | 0.35 (0.11, 1.12) | |||||
| Bilateral uveitis | No | 0.48 (0.33, 0.60) | 1 | 0.01 | 1 | 0.29 | 0.16 (0.06, 0.25) | 1 | 0.73 | 1 | 0.59 |
| Yes | 0.32 (0.26, 0.37) | 0.59 (0.39, 0.90) | 0.76 (0.45, 1.27) | 0.15 (0.10, 0.19) | 0.89 (0.45, 1.75) | 1.26 (0.54, 2.94) | |||||
| Duration of uveitis prior to presentation | <6 Months | 0.39 (0.31, 0.47) | 1 | 0.14 | 1 | 0.01 | 0.15 (0.09, 0.20) | 1 | 0.83 | 1 | 0.81 |
| 6 Months to <2 Years | 0.30 (0.22, 0.38) | 0.72 (0.49, 1.07) | 0.63 (0.43, 0.94) | 0.16 (0.09, 0.22) | 1.08 (0.62, 1.89) | 1.11 (0.64, 1.92) | |||||
| 2 to <5 Years | 0.25 (0.12, 0.36) | 0.57 (0.31, 1.04) | 0.48 (0.24, 0.97) | 0.12 (0.03, 0.20) | 0.80 (0.35, 1.81) | 0.65 (0.23, 1.80) | |||||
| 5+ Years | 0.28 (0.14, 0.40) | 0.65 (0.36, 1.17) | 0.52 (0.26, 1.01) | 0.18 (0.05, 0.28) | 1.25 (0.57, 2.70) | 0.85 (0.31, 2.35) | |||||
| Other eye history of IOP* elevation | Normal | 0.30 (0.24, 0.35) | 1 | <0.001 | 1 | <0.001 | 0.10 (0.07, 0.14) | 1 | <0.001 | 1 | <0.001 |
| 21 – <30 | 0.60 (0.45, 0.72) | 2.63 (1.76, 3.92) | 2.85 (1.88, 4.31) | 0.27 (0.16, 0.36) | 2.92 (1.72, 4.96) | 3.36 (2.00, 5.63) | |||||
| ≥30 | 0.64 (0, 0.91) | 2.92 (0.78, 11.0) | 2.46 (0.57, 10.6) | 0.58 (0, 0.88) | 8.04 (1.91, 33.8) | 9.54 (1.95, 46.7) | |||||
| Drops/Surgery | 0.43 (0.20, 0.59) | 1.59 (0.86, 2.92) | 1.83 (0.88, 3.81) | 0.39 (0.18, 0.54) | 4.55 (2.41, 8.58) | 6.22 (2.69, 14.4) | |||||
| Visual acuity | 20/40 or better | 0.30 (0.23, 0.36) | 1 | 0.02 | 1 | 0.13 | 0.11 (0.07, 0.15) | 1 | 0.004 | 1 | 0.12 |
| >20/40 – <20/200 | 0.44 (0.33, 0.52) | 1.62 (1.13, 2.32) | 1.35 (0.90, 2.03) | 0.23 (0.14, 0.31) | 2.34 (1.40, 3.91) | 1.89 (1.03, 3.47) | |||||
| 20/200 or worse | 0.31 (0.16, 0.42) | 1.03 (0.59, 1.78) | 0.81 (0.42, 1.55) | 0.20 (0.08, 0.30) | 1.94 (0.98, 3.82) | 1.52 (0.60, 3.84) | |||||
| Anterior chamber cells | Quiet | 0.35 (0.27, 0.41) | 1 | 0.01 | 1 | <0.001 | 0.15 (0.10, 0.20) | 1 | 0.01 | 1 | 0.005 |
| 0.5+ | 0.43 (0.32, 0.52) | 1.32 (0.90, 1.93) | 0.88 (0.61, 1.27) | 0.23 (0.14, 0.31) | 1.59 (0.95, 2.66) | 1.23 (0.73, 2.09) | |||||
| 1+ | 0.28 (0.16, 0.39) | 0.78 (0.47, 1.31) | 0.46 (0.26, 0.81) | 0.11 (0.04, 0.18) | 0.73 (0.36, 1.48) | 0.40 (0.18, 0.87) | |||||
| 2+ or worse | 0.20 (0.10, 0.29) | 0.53 (0.31, 0.93) | 0.30 (0.17, 0.52) | 0.06 (0.004, 0.10) | 0.35 (0.14, 0.92) | 0.19 (0.06, 0.61) | |||||
| Snowballs | No | 0.35 (0.29, 0.40) | 1 | 0.04 | 1 | 0.25 | 0.16 (0.11, 0.20) | 1 | 0.10 | 1 | 0.18 |
| Yes | 0.19 (0.06, 0.29) | 0.48 (0.24, 0.96) | 0.66 (0.32, 1.35) | 0.06 (0, 0.13) | 0.37 (0.11, 1.22) | 0.39 (0.10, 1.56) | |||||
| Peripheral anterior synechia | No | 0.33 (0.28, 0.38) | 1 | 0.44 | 1 | 0.42 | 0.15 (0.10, 0.18) | 1 | 0.26 | 1 | 0.47 |
| Yes | 0.45 (0.01, 0.69) | 1.48 (0.54, 4.03) | 1.64 (0.49, 5.49) | 0.26 (0, 0.47) | 1.91 (0.62, 5.86) | 1.50 (0.50, 4.50) | |||||
| Band keratopathy | No | 0.32 (0.26, 0.38) | 1 | 0.03 | 1 | 0.71 | 0.12 (0.08, 0.16) | 1 | 0.003 | 1 | 0.02 |
| Yes | 0.44 (0.33, 0.53) | 1.51 (1.06, 2.14) | 1.20 (0.77, 1.87) | 0.26 (0.16, 0.34) | 2.28 (1.41, 3.68) | 2.06 (1.08, 3.90) | |||||
| Missing | 0.22 (0.02, 0.38) | 0.64 (0.25, 1.64) | 0.98 (0.36, 2.66) | 0.17 (0, 0.31) | 1.44 (0.50, 4.16) | 2.87 (1.01, 8.21) | |||||
| Immunosuppressive therapy/Biologics | No | 0.30 (0.23, 0.36) | 1 | 0.06 | 1 | 0.31 | 0.14 (0.09, 0.18) | 1 | 0.47 | 1 | 0.58 |
| Yes | 0.39 (0.30, 0.46) | 1.39 (0.99, 1.95) | 1.21 (0.84, 1.76) | 0.16 (0.10, 0.22) | 1.19 (0.74, 1.93) | 0.86 (0.49, 1.49) | |||||
| Systemic corticosteroids | None | 0.31 (0.25, 0.36) | 1 | 0.01 | 1 | 0.38 | 0.15 (0.10, 0.19) | 1 | 0.02 | 1 | 0.08 |
| ≤7.5mg/day | 0.33 (0.15, 0.47) | 1.07 (0.58, 1.99) | 1.05 (0.53, 2.07) | 0.02 (0, 0.06) | 0.12 (0.02, 0.88) | 0.11 (0.01, 0.94) | |||||
| >7.5mg/day | 0.50 (0.35, 0.62) | 1.90 (1.23, 2.95) | 1.39 (0.87, 2.22) | 0.25 (0.11, 0.38) | 1.88 (0.97, 3.63) | 1.43 (0.68, 3.03) | |||||
| Topical corticosteroids | None/day | 0.18 (0.13, 0.24) | 1 | <0.001 | 1 | <0.001 | 0.07 (0.04, 0.11) | 1 | <0.001 | 1 | <0.001 |
| 1 drop/day | 0.13 (0.01, 0.24) | 0.70 (0.27, 1.80) | 0.75 (0.28, 2.03) | 0.08 (0, 0.15) | 1.07 (0.36, 3.17) | 0.97 (0.29, 3.23) | |||||
| 2 drops/day | 0.36 (0.19, 0.49) | 2.15 (1.16, 4.01) | 3.06 (1.67, 5.60) | 0.11 (0.01, 0.20) | 1.56 (0.59, 4.11) | 1.88 (0.66, 5.38) | |||||
| 3 drops/day | 0.46 (0.25, 0.62) | 3.03 (1.62, 5.68) | 3.57 (1.82, 7.02) | 0.15 (0.02, 0.27) | 2.15 (0.79, 5.85) | 2.75 (1.03, 7.32) | |||||
| 4 drops/day | 0.57 (0.39, 0.69) | 4.09 (2.50, 6.69) | 4.61 (2.81, 7.57) | 0.31 (0.17, 0.43) | 4.76 (2.45, 9.24) | 6.07 (2.79, 13.2) | |||||
| 5–8 drops/day | 0.60 (0.40, 0.73) | 4.51 (2.68, 7.60) | 5.55 (3.22, 9.59) | 0.32 (0.12, 0.48) | 5.00 (2.34, 10.7) | 7.76 (3.03, 19.9) | |||||
| >8 drops/day | 0.54 (0.31, 0.69) | 3.77 (2.05, 6.91) | 5.15 (2.52, 10.5) | 0.36 (0.16, 0.52) | 5.85 (2.77, 12.3) | 8.77 (3.45, 22.3) | |||||
| Periocular corticosteroids | No | 0.32 (0.26, 0.37) | 1 | <0.001 | 1 | <0.001 | 0.14 (0.10, 0.18) | 1 | <0.001 | 1 | <0.001 |
| Yes | 0.99 (0.90, 0.9997) | 13.5 (7.37, 24.8) | 7.96 (4.29, 14.7) | 0.81 (0.22, 0.95) | 10.9 (4.48, 26.4) | 7.54 (2.71, 20.9) | |||||
| Intraocular corticosteroids | No | 0.33 (0.28, 0.38) | 1 | <0.001 | 1 | 0.02 | 0.15 (0.11, 0.19) | 1 | <0.001 | 1 | 0.004 |
| Yes | >0.99 (0, 1) | 69.6 (20.4, 238) | 6.96 (1.41, 34.2) | >0.99 (0, 1) | 113 (31.0, 412) | 18.1 (2.55, 129) | |||||
IOP-Intraocular Pressure, CI-Confidence Interval, P-Probability, SITE-Systemic Immunosuppressive Therapy in Eye Diseases Study, n/a-Not Applicable, Hypotony was noted if any eye exhibited an episode of IOP ≤5mmHg.
Time-updated variables include: Cataract surgery, Pars plana vitrectomy (not retinal detachment), Retinal detachment surgery, Other eye history of IOP elevation, History of hypotony, Other eye history of hypotony, Visual acuity, Inflammatory activity, Anterior chamber cells, Vitreous cells, Vitreous haze, Snowballs, Keratic precipitates, Peripheral anterior synechia, Posterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids, Topical corticosteroids, Periocular corticosteroids and Intraocular corticosteroids. For time-varying characteristics, eyes potentially contribute partial time to more than one level of the covariate, so the numbers in each category cannot be calculated.
Multivariate: The variables by which risk was adjusted for were: Juvenile idiopathic arthritis, Cataract surgery, Pars plana vitrectomy (not retinal detachment), Uveitis category, Bilateral uveitis, Duration of uveitis prior to presentation, Other eye History of IOP elevation, Visual Acuity, Anterior chamber cells, Snowballs, Peripheral anterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids, Topical corticosteroids and Periocular corticosteroids.
A more comprehensive version of this table including other outcomes such as ≥ IOP 10mmHg rise and variables such as: Sex, Race category, Sarcoidosis, HLA-B27, Adamantiades-Behçet’s disease, Hypertension, Retinal detachment surgery, History of hypotony*, Other eye history of hypotony*, Inflammatory Activity, Vitreous cells, Vitreous haze, Keratic precipitates, Posterior synechia can be found as Table 4-available online at http://aaojournal.org
Table 4.
Incidence of IOP* elevation (IOP* ≥ 21, 30, 10mmHg rise) in pediatric eyes with non-infectious uveitis over 2 years: Absolute risk, relative hazard and risk factors
| IOP*≥21mmHg | IOP*≥ 30mmHg | IOP*≥10mmHg rise | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariate | Multivariate* | Univariate | Multivariate* | Univariate | Multivariate* | ||||||||||
| 2-year Incidence Estimate (95% CI*) |
Hazard Ratio (95% CI*) |
P* | Hazard Ratio (95% CI) |
P* | 2-year Incidence Estimate (95% CI*) |
Hazard Ratio (95% CI*) |
P* | Hazard Ratio (95% CI) |
P* | 2-year Incidence Estimate (95% CI*) |
Hazard Ratio (95% CI*) |
P* | Hazard Ratio (95% CI) |
P* | ||
| Age at presentation | <6 years | 0.39 (0.21, 0.54) | 1.19 (0.67, 2.12) | 0.57 | 1.23 (0.69, 2.18) | 0.74 | 0.16 (0.02, 0.29) | 1.11 (0.45, 2.77) | 0.93 | 1.20 (0.48, 3.01) | 0.92 | 0.29 (0.10, 0.43) | 1.22 (0.60, 2.49) | 0.82 | 1.32 (0.60, 2.90) | 0.78 |
| 6–12 years | 0.34 (0.27, 0.41) | 1 | 1 | 0.15 (0.10, 0.20) | 1 | 1 | 0.24 (0.18, 0.30) | 1 | 1 | |||||||
| 13–17 years | 0.31 (0.23, 0.38) | 0.88 (0.61, 1.27) | 0.95 (0.64, 1.42) | 0.14 (0.08, 0.19) | 0.94 (0.56, 1.58) | 1.0003 (0.57, 1.77) | 0.24 (0.17, 0.30) | 0.96 (0.65, 1.43) | 1.01 (0.65, 1.56) | |||||||
| Sex | Male | 0.35 (0.27, 0.42) | 1 | 0.62 | 1 | 0.54 | 0.13 (0.08, 0.18) | 1 | 0.33 | 1 | 0.40 | 0.25 (0.19, 0.31) | 1 | 0.70 | 1 | 0.58 |
| Female | 0.32 (0.26, 0.39) | 0.92 (0.65, 1.30) | 0.89 (0.62, 1.28) | 0.16 (0.11, 0.21) | 1.28 (0.78, 2.11) | 1.26 (0.74, 2.14) | 0.24 (0.18, 0.29) | 0.93 (0.64, 1.35) | 0.89 (0.58, 1.35) | |||||||
| Race category | White | 0.31 (0.25, 0.37) | 1 | 0.39 | 1 | 0.98 | 0.13 (0.09, 0.17) | 1 | 0.27 | 1 | 0.78 | 0.23 (0.18, 0.27) | 1 | 0.06 | 1 | 0.69 |
| Black | 0.41 (0.21, 0.56) | 1.39 (0.78, 2.49) | 0.97 (0.55, 1.71) | 0.26 (0.07, 0.42) | 2.13 (0.96, 4.71) | 1.59 (0.59, 4.28) | 0.40 (0.22, 0.53) | 1.97 (1.16, 3.35) | 1.12 (0.53, 2.36) | |||||||
| Hispanic | 0.45 (0.20, 0.62) | 1.58 (0.83, 3.01) | 1.14 (0.60, 2.18) | 0.19 (0.04, 0.32) | 1.47 (0.61, 3.51) | 1.21 (0.49, 3.01) | 0.33 (0.13, 0.48) | 1.54 (0.78, 3.01) | 1.52 (0.74, 3.11) | |||||||
| Other | 0.36 (0.16, 0.52) | 1.20 (0.63, 2.28) | 1.01 (0.53, 1.90) | 0.14 (0, 0.27) | 1.07 (0.35, 3.25) | 1.27 (0.42, 3.81) | 0.20 (0.03, 0.34) | 0.86 (0.35, 2.11) | 0.93 (0.38, 2.31) | |||||||
| Juvenile idiopathic arthritis | No | 0.33 (0.27, 0.39) | 1 | 0.80 | 1 | 0.59 | 0.14 (0.10, 0.19) | 1 | 0.62 | 1 | 0.11 | 0.24 (0.19, 0.29) | 1 | 0.76 | 1 | 0.13 |
| Yes | 0.34 (0.25, 0.43) | 1.05 (0.73, 1.50) | 0.87 (0.53, 1.44) | 0.16 (0.09, 0.23) | 1.14 (0.68, 1.91) | 0.57 (0.29, 1.14) | 0.25 (0.17, 0.33) | 1.07 (0.71, 1.59) | 0.67 (0.39, 1.13) | |||||||
| Sarcoidosis | No | 0.34 (0.28, 0.39) | 1 | 0.66 | 1 | 0.13 | 0.15 (0.11, 0.19) | 1 | n/a | 1 | n/a | 0.25 (0.20, 0.29) | 1 | 0.29 | 1 | 0.09 |
| Yes | 0.27 (0, 0.49) | 0.78 (0.26, 2.36) | 0.54 (0.25, 1.19) | 0 (n/a) | 0 (n/a) | 0 (n/a) | 0.15 (0, 0.28) | 0.57 (0.20, 1.62) | 0.40 (0.13, 1.17) | |||||||
| HLA-B27 | No | 0.34 (0.28, 0.39) | 1 | 0.13 | 1 | 0.03 | 0.15 (0.11, 0.19) | 1 | 0.42 | 1 | 0.07 | 0.25 (0.20, 0.29) | 1 | 0.21 | 1 | 0.02 |
| Yes | 0.21 (0.06, 0.34) | 0.57 (0.27, 1.18) | 0.35 (0.13, 0.91) | 0.10 (0.002, 0.19) | 0.66 (0.24, 1.82) | 0.38 (0.13, 1.09) | 0.16 (0.04, 0.26) | 0.61 (0.28, 1.32) | 0.35 (0.15, 0.85) | |||||||
| Adamantiades-Behçet’s disease | No | 0.34 (0.28, 0.39) | 1 | 0.47 | 1 | 0.24 | 0.15 (0.11, 0.19) | 1 | 0.92 | 1 | 0.03 | 0.24 (0.20, 0.29) | 1 | 0.72 | 1 | 0.83 |
| Yes | 0.17 (0, 0.44) | 0.46 (0.06, 3.75) | 0.45 (0.12, 1.71) | 0.16 (0, 0.42) | 1.11 (0.13, 9.30) | 4.75 (1.15, 19.6) | 0.17 (0, 0.44) | 0.67 (0.08, 5.61) | 1.15 (0.32, 4.16) | |||||||
| Hypertension | No | 0.34 (0.28, 0.39) | 1 | 0.17 | 1 | 0.12 | 0.15 (0.11, 0.19) | 1 | 0.46 | 1 | 0.10 | 0.25 (0.20, 0.29) | 1 | 0.53 | 1 | 0.11 |
| Yes | 0.10 (0, 0.26) | 0.25 (0.03, 1.78) | 0.21 (0.03, 1.49) | 0.07 (0, 0.20) | 0.48 (0.07, 3.41) | 0.19 (0.02, 1.41) | 0.17 (0, 0.35) | 0.64 (0.16, 2.53) | 0.41 (0.14, 1.23) | |||||||
| Cataract surgery | Never | 0.32 (0.27, 0.37) | 1 | 0.03 | 1 | 0.54 | 0.14 (0.10, 0.18) | 1 | 0.051 | 1 | 0.87 | 0.23 (0.18, 0.27) | 1 | 0.005 | 1 | 0.85 |
| Prior to SITE | 0.35 (0.07, 0.54) | 1.09 (0.47, 2.51) | 0.59 (0.18, 1.90) | 0.32 (0.05, 0.52) | 2.63 (1.07, 6.48) | 1.19 (0.36, 3.95) | 0.42 (0.22, 0.57) | 2.14 (1.20, 3.82) | 0.79 (0.28, 2.17) | |||||||
| During SITE | 0.59 (0.31, 0.75) | 2.27 (1.23, 4.19) | 0.67 (0.28, 1.59) | 0.26 (0.06, 0.42) | 2.06 (0.91, 4.65) | 0.85 (0.30, 2.42) | 0.46 (0.19, 0.64) | 2.37 (1.21, 4.66) | 1.05 (0.39, 2.81) | |||||||
| Pars plana vitrectomy (not retinal detachment) | No | 0.32 (0.26, 0.37) | 1 | <0.001 | 1 | <0.001 | 0.14 (0.10, 0.18) | 1 | 0.15 | 1 | 0.52 | 0.23 (0.19, 0.28) | 1 | 0.01 | 1 | 0.20 |
| Yes | 0.68 (0.42, 0.82) | 2.98 (1.74, 5.11) | 3.36 (1.75, 6.45) | 0.24 (0.07, 0.37) | 1.74 (0.81, 3.72) | 1.40 (0.50, 3.93) | 0.45 (0.21, 0.62) | 2.24 (1.20, 4.19) | 1.72 (0.75, 3.93) | |||||||
| Retinal detachment surgery | No | 0.34 (0.28, 0.39) | 1 | n/a | 1 | n/a | 0.15 (0.11, 0.19) | 1 | 0.93 | 1 | 0.42 | 0.24 (0.20, 0.29) | 1 | 0.98 | 1 | 0.60 |
| Yes | 0 (n/a) | 0 (n/a) | 0 (n/a) | 0.14 (0, 0.37) | 0.91 (0.10, 8.10) | 2.92 (0.22, 39.2) | 0.25 (0, 0.55) | 1.03 (0.18, 5.87) | 1.71 (0.23, 13.0) | |||||||
| Uveitis category | Anterior | 0.36 (0.28, 0.43) | 1 | 0.06 | 1 | 0.92 | 0.16 (0.10, 0.22) | 1 | 0.84 | 1 | 0.33 | 0.27 (0.20, 0.34) | 1 | 0.30 | 1 | 0.41 |
| Intermediate | 0.28 (0.20, 0.35) | 0.75 (0.51, 1.10) | 0.98 (0.60, 1.62) | 0.14 (0.08, 0.19) | 0.86 (0.50, 1.46) | 1.09 (0.58, 2.06) | 0.22 (0.15, 0.28) | 0.76 (0.50, 1.16) | 0.93 (0.55, 1.57) | |||||||
| Posterior | 0.25 (0.06, 0.40) | 0.64 (0.28, 1.47) | 0.84 (0.36, 1.96) | 0.10 (0, 0.20) | 0.62 (0.20, 1.91) | 1.15 (0.35, 3.81) | 0.15 (0.03, 0.26) | 0.51 (0.21, 1.24) | 0.60 (0.23, 1.53) | |||||||
| Panuveitis | 0.48 (0.30, 0.61) | 1.48 (0.90, 2.43) | 1.15 (0.63, 2.09) | 0.14 (0.03, 0.24) | 0.88 (0.37, 2.08) | 0.35 (0.11, 1.12) | 0.29 (0.15, 0.40) | 1.05 (0.59, 1.90) | 0.58 (0.27, 1.25) | |||||||
| Bilateral uveitis | No | 0.48 (0.33, 0.60) | 1 | 0.01 | 1 | 0.29 | 0.16 (0.06, 0.25) | 1 | 0.73 | 1 | 0.59 | 0.35 (0.21, 0.47) | 1 | 0.049 | 1 | 0.84 |
| Yes | 0.32 (0.26, 0.37) | 0.59 (0.39, 0.90) | 0.76 (0.45, 1.27) | 0.15 (0.10, 0.19) | 0.89 (0.45, 1.75) | 1.26 (0.54, 2.94) | 0.23 (0.18, 0.28) | 0.61 (0.38, 0.998) | 0.94 (0.51, 1.73) | |||||||
| Duration of uveitis prior to presentation | <6 Months | 0.39 (0.31, 0.47) | 1 | 0.14 | 1 | 0.01 | 0.15 (0.09, 0.20) | 1 | 0.83 | 1 | 0.81 | 0.27 (0.20, 0.33) | 1 | 0.48 | 1 | 0.65 |
| 6 Months to <2 Years | 0.30 (0.22, 0.38) | 0.72 (0.49, 1.07) | 0.63 (0.43, 0.94) | 0.16 (0.09, 0.22) | 1.08 (0.62, 1.89) | 1.11 (0.64, 1.92) | 0.22 (0.15, 0.29) | 0.80 (0.52, 1.25) | 0.82 (0.51, 1.32) | |||||||
| 2 to <5 Years | 0.25 (0.12, 0.36) | 0.57 (0.31, 1.04) | 0.48 (0.24, 0.97) | 0.12 (0.03, 0.20) | 0.80 (0.35, 1.81) | 0.65 (0.23, 1.80) | 0.18 (0.08, 0.28) | 0.65 (0.33, 1.25) | 0.66 (0.30, 1.46) | |||||||
| 5+ Years | 0.28 (0.14, 0.40) | 0.65 (0.36, 1.17) | 0.52 (0.26, 1.01) | 0.18 (0.05, 0.28) | 1.25 (0.57, 2.70) | 0.85 (0.31, 2.35) | 0.28 (0.14, 0.39) | 1.04 (0.59, 1.86) | 0.79 (0.39, 1.59) | |||||||
| Other eye history of IOP* elevation | Normal | 0.30 (0.24, 0.35) | 1 | <0.001 | 1 | <0.001 | 0.10 (0.07, 0.14) | 1 | <0.001 | 1 | <0.001 | 0.22 (0.17, 0.27) | 1 | 0.009 | 1 | 0.03 |
| 21 – <30 | 0.60 (0.45, 0.72) | 2.63 (1.76, 3.92) | 2.85 (1.88, 4.31) | 0.27 (0.16, 0.36) | 2.92 (1.72, 4.96) | 3.36 (2.00, 5.63) | 0.31 (0.19, 0.40) | 1.46 (0.93, 2.30) | 1.74 (1.11, 2.75) | |||||||
| ≥30 | 0.64 (0, 0.91) | 2.92 (0.78, 11.0) | 2.46 (0.57, 10.6) | 0.58 (0, 0.88) | 8.04 (1.91, 33.8) | 9.54 (1.95, 46.7) | 0.57 (0.06, 0.81) | 3.42 (1.35, 8.65) | 2.34 (0.60, 9.07) | |||||||
| Drops/Surgery | 0.43 (0.20, 0.59) | 1.59 (0.86, 2.92) | 1.83 (0.88, 3.81) | 0.39 (0.18, 0.54) | 4.55 (2.41, 8.58) | 6.22 (2.69, 14.4) | 0.37 (0.19, 0.52) | 1.88 (1.04, 3.43) | 2.22 (1.09, 4.55) | |||||||
| History of hypotony* | No | 0.33 (0.28, 0.39) | 1 | 0.75 | 1 | 0.07 | 0.15 (0.11, 0.19) | 1 | 0.40 | 1 | 0.09 | 0.24 (0.20, 0.29) | 1 | 0.52 | 1 | 0.35 |
| Yes | 0.29 (0, 0.50) | 0.85 (0.30, 2.41) | 0.40 (0.15, 1.07) | 0.06 (0, 0.18) | 0.41 (0.05, 3.21) | 0.13 (0.01, 1.38) | 0.31 (0.05, 0.50) | 1.34 (0.55, 3.22) | 0.66 (0.28, 1.57) | |||||||
| Other eye history of hypotony* | No | 0.34 (0.28, 0.39) | 1 | 0.70 | 1 | 0.06 | 0.15 (0.11, 0.19) | 1 | 0.90 | 1 | 0.15 | 0.24 (0.20, 0.29) | 1 | 0.17 | 1 | 0.30 |
| Yes | 0.29 (0.05, 0.48) | 0.85 (0.36, 2.00) | 0.39 (0.15, 1.04) | 0.14 (0, 0.30) | 0.92 (0.23, 3.66) | 0.28 (0.05, 1.60) | 0.37 (0.12, 0.55) | 1.65 (0.81, 3.37) | 0.66 (0.30, 1.44) | |||||||
| Visual acuity | 20/40 or better | 0.30 (0.23, 0.36) | 1 | 0.02 | 1 | 0.13 | 0.11 (0.07, 0.15) | 1 | 0.004 | 1 | 0.12 | 0.19 (0.14, 0.24) | 1 | 0.003 | 1 | 0.27 |
| >20/40 – <20/200 | 0.44 (0.33, 0.52) | 1.62 (1.13, 2.32) | 1.35 (0.90, 2.03) | 0.23 (0.14, 0.31) | 2.34 (1.40, 3.91) | 1.89 (1.03, 3.47) | 0.32 (0.23, 0.40) | 1.81 (1.21, 2.71) | 1.45 (0.89, 2.34) | |||||||
| 20/200 or worse | 0.31 (0.16, 0.42) | 1.03 (0.59, 1.78) | 0.81 (0.42, 1.55) | 0.20 (0.08, 0.30) | 1.94 (0.98, 3.82) | 1.52 (0.60, 3.84) | 0.35 (0.21, 0.47) | 2.02 (1.22, 3.34) | 1.54 (0.80, 2.94) | |||||||
| Inflammatory activity | Inactive | 0.36 (0.28, 0.43) | 1 | 0.59 | 1 | 0.34 | 0.16 (0.10, 0.22) | 1 | 0.22 | 1 | 0.83 | 0.26 (0.20, 0.32) | 1 | 0.15 | 1 | 0.66 |
| Slightly active | 0.33 (0.20, 0.44) | 0.90 (0.56, 1.46) | 1.13 (0.66, 1.92) | 0.20 (0.09, 0.30) | 1.26 (0.66, 2.40) | 1.03 (0.48, 2.23) | 0.31 (0.19, 0.42) | 1.22 (0.75, 1.97) | 1.26 (0.70, 2.26) | |||||||
| Active | 0.31 (0.23, 0.38) | 0.83 (0.58, 1.18) | 1.42 (0.89, 2.27) | 0.11 (0.06, 0.16) | 0.69 (0.39, 1.21) | 0.80 (0.35, 1.84) | 0.20 (0.14, 0.26) | 0.73 (0.47, 1.13) | 0.97 (0.51, 1.86) | |||||||
| Anterior chamber cells | Quiet | 0.35 (0.27, 0.41) | 1 | 0.01 | 1 | <0.001 | 0.15 (0.10, 0.20) | 1 | 0.01 | 1 | 0.005 | 0.24 (0.18, 0.30) | 1 | 0.01 | 1 | 0.004 |
| 0.5+ | 0.43 (0.32, 0.52) | 1.32 (0.90, 1.93) | 0.88 (0.61, 1.27) | 0.23 (0.14, 0.31) | 1.59 (0.95, 2.66) | 1.23 (0.73, 2.09) | 0.34 (0.24, 0.43) | 1.51 (1.005, 2.28) | 1.08 (0.71, 1.64) | |||||||
| 1+ | 0.28 (0.16, 0.39) | 0.78 (0.47, 1.31) | 0.46 (0.26, 0.81) | 0.11 (0.04, 0.18) | 0.73 (0.36, 1.48) | 0.40 (0.18, 0.87) | 0.20 (0.11, 0.29) | 0.83 (0.47, 1.48) | 0.50 (0.27, 0.94) | |||||||
| 2+ or worse | 0.20 (0.10, 0.29) | 0.53 (0.31, 0.93) | 0.30 (0.17, 0.52) | 0.06 (0.004, 0.10) | 0.35 (0.14, 0.92) | 0.19 (0.06, 0.61) | 0.15 (0.06, 0.22) | 0.58 (0.31, 1.08) | 0.36 (0.19, 0.68) | |||||||
| Vitreous cells | Quiet | 0.36 (0.29, 0.42) | 1 | 0.24 | 1 | 0.43 | 0.16 (0.11, 0.22) | 1 | 0.11 | 1 | 0.07 | 0.26 (0.20, 0.32) | 1 | 0.29 | 1 | 0.25 |
| 0.5+ | 0.26 (0.14, 0.36) | 0.68 (0.41, 1.13) | 0.70 (0.40, 1.25) | 0.06 (0.009, 0.11) | 0.34 (0.14, 0.80) | 0.26 (0.10, 0.73) | 0.16 (0.07, 0.24) | 0.55 (0.30, 1.01) | 0.49 (0.23, 1.03) | |||||||
| 1+ | 0.37 (0.23, 0.48) | 1.03 (0.64, 1.65) | 1.16 (0.70, 1.91) | 0.15 (0.06, 0.23) | 0.88 (0.43, 1.78) | 0.82 (0.38, 1.79) | 0.24 (0.14, 0.33) | 0.88 (0.52, 1.48) | 0.80 (0.43, 1.48) | |||||||
| 2+ or worse | 0.25 (0.12, 0.36) | 0.65 (0.37, 1.14) | 0.94 (0.51, 1.73) | 0.15 (0.05, 0.23) | 0.89 (0.44, 1.83) | 0.93 (0.40, 2.16) | 0.23 (0.13, 0.32) | 0.86 (0.50, 1.48) | 0.96 (0.50, 1.86) | |||||||
| Vitreous haze | Quiet | 0.35 (0.29, 0.40) | 1 | 0.73 | 1 | 0.52 | 0.15 (0.10, 0.19) | 1 | 0.98 | 1 | 0.93 | 0.24 (0.19, 0.29) | 1 | 0.91 | 1 | 0.80 |
| 1+ | 0.29 (0.12, 0.43) | 0.81 (0.42, 1.56) | 0.60 (0.28, 1.26) | 0.14 (0.01, 0.25) | 0.90 (0.35, 2.35) | 0.75 (0.27, 2.03) | 0.28 (0.12, 0.41) | 1.16 (0.60, 2.23) | 0.68 (0.31, 1.48) | |||||||
| 2+ or worse | 0.34 (0.08, 0.53) | 0.98 (0.43, 2.23) | 1.24 (0.53, 2.91) | 0.17 (0.01, 0.30) | 1.12 (0.42, 3.01) | 0.90 (0.33, 2.40) | 0.26 (0.09, 0.40) | 1.08 (0.52, 2.24) | 0.82 (0.32, 2.13) | |||||||
| Missing | 0.27 (0.12, 0.39) | 0.74 (0.41, 1.34) | 0.91 (0.49, 1.69) | 0.13 (0.02, 0.23) | 0.87 (0.36, 2.08) | 0.82 (0.30, 2.26) | 0.21 (0.07, 0.33) | 0.83 (0.41, 1.68) | 0.93 (0.41, 2.11) | |||||||
| Snowballs | No | 0.35 (0.29, 0.40) | 1 | 0.04 | 1 | 0.25 | 0.16 (0.11, 0.20) | 1 | 0.10 | 1 | 0.18 | 0.26 (0.21, 0.31) | 1 | 0.01 | 1 | 0.07 |
| Yes | 0.19 (0.06, 0.29) | 0.48 (0.24, 0.96) | 0.66 (0.32, 1.35) | 0.06 (0, 0.13) | 0.37 (0.11, 1.22) | 0.39 (0.10, 1.56) | 0.08 (0, 0.15) | 0.27 (0.10, 0.77) | 0.35 (0.11, 1.08) | |||||||
| Keratic precipitates | No | 0.35 (0.29, 0.40) | 1 | 0.40 | 1 | 0.43 | 0.15 (0.11, 0.19) | 1 | 0.92 | 1 | 0.30 | 0.25 (0.20, 0.30) | 1 | 0.68 | 1 | 0.42 |
| Yes | 0.29 (0.14, 0.42) | 0.81 (0.46, 1.44) | 0.69 (0.37, 1.27) | 0.13 (0.02, 0.23) | 0.86 (0.35, 2.10) | 0.70 (0.23, 2.12) | 0.19 (0.06, 0.30) | 0.72 (0.35, 1.49) | 0.64 (0.29, 1.40) | |||||||
| Missing | 0.22 (0.02, 0.37) | 0.57 (0.22, 1.45) | 0.74 (0.26, 2.10) | 0.16 (0, 0.31) | 1.12 (0.39, 3.21) | 2.04 (0.72, 5.81) | 0.23 (0.04, 0.39) | 0.93 (0.39, 2.19) | 1.38 (0.53, 3.58) | |||||||
| Peripheral anterior synechia | No | 0.33 (0.28, 0.38) | 1 | 0.44 | 1 | 0.42 | 0.15 (0.10, 0.18) | 1 | 0.26 | 1 | 0.47 | 0.24 (0.19, 0.28) | 1 | 0.07 | 1 | 0.13 |
| Yes | 0.45 (0.01, 0.69) | 1.48 (0.54, 4.03) | 1.64 (0.49, 5.49) | 0.26 (0, 0.47) | 1.91 (0.62, 5.86) | 1.50 (0.50, 4.50) | 0.43 (0.13, 0.62) | 2.04 (0.95, 4.36) | 2.00 (0.81, 4.95) | |||||||
| Posterior synechia | No | 0.33 (0.27, 0.38) | 1 | 0.53 | 1 | 0.005 | 0.14 (0.09, 0.18) | 1 | 0.23 | 1 | 0.0501 | 0.23 (0.18, 0.28) | 1 | 0.18 | 1 | 0.04 |
| Yes | 0.36 (0.27, 0.43) | 1.11 (0.80, 1.53) | 0.56 (0.38, 0.84) | 0.18 (0.11, 0.24) | 1.31 (0.84, 2.04) | 0.61 (0.37, 1.0003) | 0.28 (0.21, 0.35) | 1.27 (0.89, 1.80) | 0.64 (0.42, 0.99) | |||||||
| Band keratopathy | No | 0.32 (0.26, 0.38) | 1 | 0.03 | 1 | 0.71 | 0.12 (0.08, 0.16) | 1 | 0.003 | 1 | 0.02 | 0.21 (0.16, 0.26) | 1 | 0.004 | 1 | 0.21 |
| Yes | 0.44 (0.33, 0.53) | 1.51 (1.06, 2.14) | 1.20 (0.77, 1.87) | 0.26 (0.16, 0.34) | 2.28 (1.41, 3.68) | 2.06 (1.08, 3.90) | 0.37 (0.27, 0.45) | 1.90 (1.30, 2.77) | 1.35 (0.85, 2.15) | |||||||
| Missing | 0.22 (0.02, 0.38) | 0.64 (0.25, 1.64) | 0.98 (0.36, 2.66) | 0.17 (0, 0.31) | 1.44 (0.50, 4.16) | 2.87 (1.01, 8.21) | 0.24 (0.04, 0.39) | 1.13 (0.48, 2.68) | 1.92 (0.77, 4.76) | |||||||
| Immunosuppressive therapy/Biologics | No | 0.30 (0.23, 0.36) | 1 | 0.06 | 1 | 0.31 | 0.14 (0.09, 0.18) | 1 | 0.47 | 1 | 0.58 | 0.23 (0.17, 0.28) | 1 | 0.41 | 1 | 1.00 |
| Yes | 0.39 (0.30, 0.46) | 1.39 (0.99, 1.95) | 1.21 (0.84, 1.76) | 0.16 (0.10, 0.22) | 1.19 (0.74, 1.93) | 0.86 (0.49, 1.49) | 0.26 (0.19, 0.33) | 1.17 (0.80, 1.72) | 0.999 (0.64, 1.56) | |||||||
| Systemic corticosteroids | None | 0.31 (0.25, 0.36) | 1 | 0.01 | 1 | 0.38 | 0.15 (0.10, 0.19) | 1 | 0.02 | 1 | 0.08 | 0.23 (0.17, 0.27) | 1 | 0.003 | 1 | 0.14 |
| ≤7.5mg/day | 0.33 (0.15, 0.47) | 1.07 (0.58, 1.99) | 1.05 (0.53, 2.07) | 0.02 (0, 0.06) | 0.12 (0.02, 0.88) | 0.11 (0.01, 0.94) | 0.19 (0.06, 0.30) | 0.83 (0.40, 1.71) | 0.85 (0.37, 1.91) | |||||||
| >7.5mg/day | 0.50 (0.35, 0.62) | 1.90 (1.23, 2.95) | 1.39 (0.87, 2.22) | 0.25 (0.11, 0.38) | 1.88 (0.97, 3.63) | 1.43 (0.68, 3.03) | 0.41 (0.27, 0.53) | 2.08 (1.31, 3.31) | 1.69 (0.98, 2.92) | |||||||
| Topical corticosteroids | None/day | 0.18 (0.13, 0.24) | 1 | <0.001 | 1 | <0.001 | 0.07 (0.04, 0.11) | 1 | <0.001 | 1 | <0.001 | 0.12 (0.08, 0.16) | 1 | <0.001 | 1 | <0.001 |
| 1 drop/day | 0.13 (0.01, 0.24) | 0.70 (0.27, 1.80) | 0.75 (0.28, 2.03) | 0.08 (0, 0.15) | 1.07 (0.36, 3.17) | 0.97 (0.29, 3.23) | 0.07 (0, 0.15) | 0.59 (0.18, 1.88) | 0.58 (0.17, 2.01) | |||||||
| 2 drops/day | 0.36 (0.19, 0.49) | 2.15 (1.16, 4.01) | 3.06 (1.67, 5.60) | 0.11 (0.01, 0.20) | 1.56 (0.59, 4.11) | 1.88 (0.66, 5.38) | 0.29 (0.14, 0.41) | 2.70 (1.45, 5.05) | 3.10 (1.60, 6.01) | |||||||
| 3 drops/day | 0.46 (0.25, 0.62) | 3.03 (1.62, 5.68) | 3.57 (1.82, 7.02) | 0.15 (0.02, 0.27) | 2.15 (0.79, 5.85) | 2.75 (1.03, 7.32) | 0.20 (0.04, 0.34) | 1.81 (0.75, 4.35) | 2.35 (0.91, 6.07) | |||||||
| 4 drops/day | 0.57 (0.39, 0.69) | 4.09 (2.50, 6.69) | 4.61 (2.81, 7.57) | 0.31 (0.17, 0.43) | 4.76 (2.45, 9.24) | 6.07 (2.79, 13.2) | 0.53 (0.37, 0.66) | 6.10 (3.61, 10.3) | 6.67 (3.72, 12.0) | |||||||
| 5–8 drops/day | 0.60 (0.40, 0.73) | 4.51 (2.68, 7.60) | 5.55 (3.22, 9.59) | 0.32 (0.12, 0.48) | 5.00 (2.34, 10.7) | 7.76 (3.03, 19.9) | 0.44 (0.25, 0.58) | 4.62 (2.54, 8.38) | 5.21 (2.49, 10.9) | |||||||
| >8 drops/day | 0.54 (0.31, 0.69) | 3.77 (2.05, 6.91) | 5.15 (2.52, 10.5) | 0.36 (0.16, 0.52) | 5.85 (2.77, 12.3) | 8.77 (3.45, 22.3) | 0.50 (0.29, 0.65) | 5.53 (2.99, 10.2) | 6.26 (3.08, 12.7) | |||||||
| Periocular corticosteroids | No | 0.32 (0.26, 0.37) | 1 | <0.001 | 1 | <0.001 | 0.14 (0.10, 0.18) | 1 | <0.001 | 1 | <0.001 | 0.23 (0.19, 0.28) | 1 | <0.001 | 1 | <0.001 |
| Yes | 0.99 (0.90, 0.9997) | 13.5 (7.37, 24.8) | 7.96 (4.29, 14.7) | 0.81 (0.22, 0.95) | 10.9 (4.48, 26.4) | 7.54 (2.71, 20.9) | 0.97 (0.68, 0.996) | 12.9 (6.40, 26.0) | 7.31 (3.28, 16.3) | |||||||
| Intraocular corticosteroids | No | 0.33 (0.28, 0.38) | 1 | <0.001 | 1 | 0.02 | 0.15 (0.11, 0.19) | 1 | <0.001 | 1 | 0.004 | 0.24 (0.19, 0.28) | 1 | <0.001 | 1 | <0.001 |
| Yes | >0.99 (0, 1) | 69.6 (20.4, 238) | 6.96 (1.41, 34.2) | >0.99 (0, 1) | 113 (31.0, 412) | 18.1 (2.55, 129) | >0.99 (0.89, 1) | 92.0 (34.5, 245) | 19.7 (4.45, 86.8) | |||||||
IOP-Intraocular Pressure, CI-Confidence Interval, P-Probability, SITE-Systemic Immunosuppressive Therapy in Eye Diseases Study, n/a-Not Applicable, Hypotony was noted if any eye exhibited an episode of IOP ≤5mmHg.
Time-updated variables include: Cataract surgery, Pars plana vitrectomy (not retinal detachment), Retinal detachment surgery, Other eye history of IOP elevation, History of hypotony, Other eye history of hypotony, Visual acuity, Inflammatory activity, Anterior chamber cells, Vitreous cells, Vitreous haze, Snowballs, Keratic precipitates, Peripheral anterior synechia, Posterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids, Topical corticosteroids, Periocular corticosteroids and Intraocular corticosteroids. For time-varying characteristics, eyes potentially contribute partial time to more than one level of the covariate, so the numbers in each category cannot be calculated.
Multivariate: The variables by which risk was adjusted for were: Juvenile idiopathic arthritis, Cataract surgery, Pars plana vitrectomy (not retinal detachment), Uveitis category, Bilateral uveitis, Duration of uveitis prior to presentation, Other eye history of IOP elevation, Visual acuity, Anterior chamber cells, Snowballs, Peripheral anterior synechia, Band keratopathy, Immunosuppressive therapy/Biologics, Systemic corticosteroids, Topical corticosteroids and Periocular corticosteroids.
In contrast to the results of the prevalence analysis, the higher crude hazard noted in eyes that had undergone cataract surgery prior to or during the course of the study was no longer significant after accounting for other variables. Eyes that had pars plana vitrectomy performed either prior to or during the course of management at a participating center, had ~3-fold higher adjusted incidence (adjusted hazard ratio≥21mmHg[aHR21]=1.753.366.45, P<0.001) of IOP≥21mmHg; did not demonstrate a higher risk for developing IOP elevation of ≥30mmHg and the higher crude hazard noted with ≥10mmHg rise was not found to be significant after adjusting for other variables (adjusted P values>0.1). On average, eyes that had undergone retinal detachment surgery did not have increased incidence of IOP elevation.
The risk of developing IOP elevation by anatomical location, laterality and duration of uveitis either did not vary or else did not show consistent association across crude and adjusted analyses.
Eyes of patients in whom the fellow eye was found to have IOP elevation or needed IOP-lowering medications or surgery were associated with ~9.5-fold higher adjusted incidence (aHRs up to 1.959.5446.7, P<0.001) of IOP elevation. Similar to the pattern observed at presentation, progressively higher levels of contralateral IOP elevation were associated with increasing propensity for developing progressively worse ipsilateral IOP elevation in a step-wise manner (see Figure 2). The absolute risks of developing IOP elevations of ≥21, ≥30 and rise by ≥10mmHg in the ipsilateral eye when the contralateral eye had IOP≥30mmHg were 64%, 58%, and 57% respectively within 2 years. In eyes with a history of hypotony (≤5mmHg) in the ipsilateral or contralateral eye the lowered trend to develop IOP elevation was either not found or was inconsistent across both crude and adjusted analyses.
Figure 2.
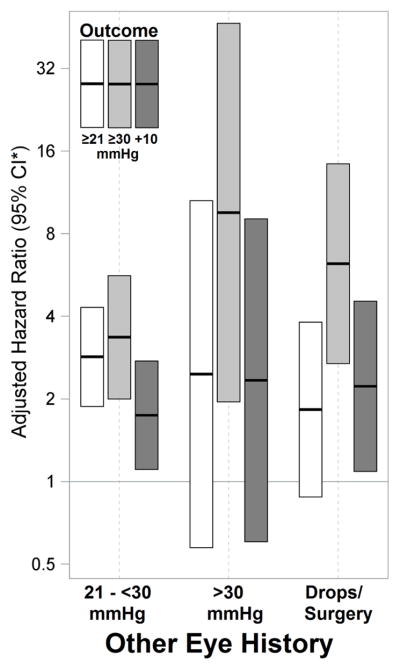
Risk of incident IOP elevation in pediatric non-infectious uveitis when the contralateral eye experienced IOP elevation or was on active IOP-lowering drops or surgery. A dose-dependent pattern was noted.
CI*-Confidence interval
In contrast to the results of the prevalence analysis, eyes that developed diminished visual acuity during the course of follow-up were observed to have a minimally higher crude hazard of developing IOP elevation but the significance of the association was not sustained when other factors were accounted for (all adjusted P values>0.1).
As in the prevalence analysis, higher grade current activity of inflammation was inversely associated with IOP elevation, such that pediatric eyes with no or low grade (+0.5) anterior chamber cells demonstrated a similar hazard of incident IOP elevation whereas eyes with increasing grades of anterior chamber cells (≥1+) were progressively less likely to demonstrate such risk (all P values≤ 0.005). The association of IOP elevation with vitreous cells and haze and keratic precipitates was not found to be significant in children. Although eyes of children having snowballs were noted to be less likely to develop IOP≥21and 10mmHg rise in the crude analyses, this association did not persist after accounting for other factors. Regarding structural complications of uveitis, eyes with peripheral anterior and posterior synechia were not consistently associated with incident IOP elevation. Eyes with band keratopathy were ~2-fold more likely to develop IOP≥30mmHg (adjusted hazard ratio≥30mmHg[aHR30]=1.082.063.90, P=0.02) and were found to have a higher crude hazard to develop IOP elevations of ≥21 and 10mmHg rise (the latter two associations became non-significant when other variables were accounted for).
Eyes of children on immunosuppressive therapy did not demonstrate increased hazard of IOP elevation (all adjusted P values>0.1). Regarding systemic corticosteroids, eyes of children receiving >7.5mg/day of prednisone (or equivalent) had a higher crude risk of developing IOP elevation when compared with eyes of children receiving ≤7.5mg/day or not on systemic corticosteroids, but the difference was no longer significant on accounting for other covariates.
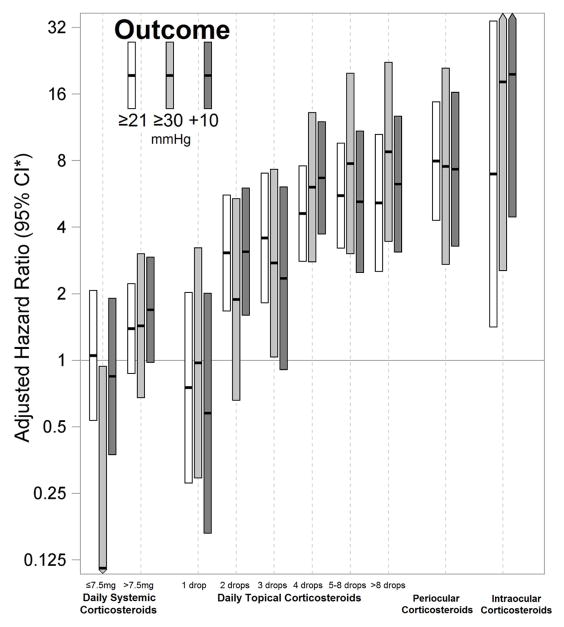
Eyes treated with local corticosteroids were noted to have a strong risk for developing IOP elevation in a dose- and route of administration-dependent manner. Even eyes on 2–3 drops/day of prednisolone acetate 1% (or equivalent alternative topical corticosteroids) demonstrated a 2- to 3-fold increased incidence of IOP elevation, the relative hazard reaching up to 4- to 9-fold when drops were being instilled ≥4 times/day (P<0.001). The risk of IOP elevation was progressively higher with increased frequency of drop instillation similar to that noted during prevalence analysis. Eyes that had received periocular corticosteroid injections had a 7-to 8-fold hazard of IOP elevation (aHR21=4.297.9614.7, aHR30=2.717.5420.9, aHR10=3.287.3116.3; each P<0.001); the risk being similar to that observed with the application of higher frequency of topical prednisolone acetate 1% (or equivalent alternative topical corticosteroid) drops. Use of intraocular corticosteroids was rare in this cohort, but when observed was associated with a very high risk of IOP elevation (aHR21=1.416.9634.2, P=0.02; aHR30=2.5518.1129, P=0.004; aHR10=4.4519.786.8, P<0.001) (See figure 3).
Figure 3.
Risk of incident IOP elevation associated with corticosteroid use in pediatric non-infectious uveitis demonstrating the dose-and route of administration-dependent response. Systemic corticosteroids refer to doses for prednisone or an equipotent dose of an alternative corticosteroid. Topical corticosteroids refer to the number of drops per day of prednisolone acetate 1% or an equipotent dose of an alternative topical corticosteroid. Periocular and intraocular corticosteroids typically included dosages of 40 mg and 4 mg of triamcinolone acetonide, respectively.
CI*-Confidence interval
A sensitivity analysis regarding incident IOP elevation limited to eyes where IOP had been measured using applanation tonometry yielded similar results.
Discussion
Our results demonstrate that IOP elevation affects a large minority of children with non-infectious uveitis, and identifies several risk factors. These observations potentially allow clinicians to minimize exposure to avoidable risk factors and to closely monitor high-risk eyes with risk factors that cannot be modified. The strongest risk factors identified for IOP elevation by this analysis, included eyes of children with contralateral IOP elevation and the use of local corticosteroids.
The observation that uveitic eyes of children whose contralateral eyes exhibited IOP elevation and/or required IOP-lowering therapy had a several-fold risk of IOP elevation may be the result of shared genetic and environmental exposures between the two eyes.
The high risk of IOP elevation in uveitic eyes of children receiving local corticosteroids noted in the study, is consistent with reports from other studies. 9,23,24 Our study further characterized the degree of risk across a range of topical corticosteroid dosages as well across alternative methods of local corticosteroid administration, adjusting for other factors including the current grade of anterior chamber inflammatory cells (which might be affected by use of corticosteroids). Our results suggest that the risk of IOP elevation on the equivalent of one drop of prednisolone acetate 1% daily is not especially high in children, as it was elevated only in the prevalence analysis for IOP≥30 mmHg, and not in the incidence analyses. However, even dosages equivalent to 2 drops daily of prednisolone acetate 1% consistently were associated with 2- to 4-fold increased risk, and risk progressively increased with increasing dose, with ~9-fold increase noted with frequency of instillation greater than 8 drops per day. The risk associated with periocular corticosteroid use was similar to or marginally more than that associated with high dose topical prednisolone acetate 1% (or equivalent alternative topical corticosteroid) therapy. This might reflect a similar or slightly higher degree of drug delivery to the region of the trabecular meshwork when a depot is placed deep in the orbit, even though a large amount of corticosteroids are administered with longer bioavailability. Intraocular corticosteroid therapy was associated with a very high level of risk of IOP elevation (~100% by two years), probably because of a high level of access of the drug to the trabecular meshwork over a longer period of time. In contrast, use of systemic corticosteroids was not strongly associated with elevated IOP, particularly if the daily dose was 7.5 mg/day (prednisone or prednisone equivalent) or less, in which case the risk was similar to or less than that of patients not taking oral corticosteroids, probably because of low levels of drug delivery to the trabecular meshwork.
IOP elevation with corticosteroid use has been reported to occur within hours, but typically takes weeks to develop. The cumulative effect of local and systemic corticosteroids that might cause an IOP elevation in the long-run (possibly take years to develop) could not studied by us. 24–27 Corticosteroids have been known to induce changes in trabeculocytes and functional and structural (F-actin CLAN cytoskeletal structure, extracellular matrix myocilin deposition trabecular meshwork-induced glucocorticoid response protein-1 causing cell death) changes in the trabecular meshwork that increase outflow resistance. 15,24,25 Also glucocorticoid responsiveness in corticosteroid responders has been attributed to overexpression of glucocorticoid receptor GFβ isoform when compared to GFα. 28 Corticosteroids have been reported to cause accelerated ocular hypertension with a rapidly pronounced, severe and more frequent IOP elevation in children, the analysis of which was outside the scope of this study. 29,30 Varying the placement of corticosteroids in and around the eye and the route of administration might determine their differential bioavailability in the aqueous and vitreous and might affect IOP elevation differently. 31 Perhaps the proximity of corticosteroids to the trabecular meshwork (depending on whether the periocular corticosteroids were injected by the subconjunctival or peribulbar route), 32 duration of contact time with the trabecular meshwork and repository nature of the formulation itself may determine the extent and duration of IOP elevation. Our retrospective study did not allow direct assessment of these hypotheses.
In contrast to corticosteroids, immunosuppressive drug therapy was not associated with IOP elevation, even though one might have expected a selection bias in that more severely affected eyes would be more likely to receive such therapy. This observation suggests that use of corticosteroid-sparing therapy could be a useful strategy to help to avoid IOP elevation in eyes that require long-term therapy for intraocular inflammation. However, because of the slower onset of action of immunosuppressive agents and the morbidity associated with ocular inflammation; corticosteroids likely will continue to play an important therapeutic role. Our results suggest that corticosteroid use requires careful monitoring at appropriate intervals to detect IOP elevation at a sufficiently early stage to allow timely intervention, and that long-term corticosteroid use should be minimized.
Regarding other potential risk factors, the absolute risk of IOP elevation in JIA-associated uveitic eyes was 34% by two years, a level near the high end of the distribution in our study’s overall pediatric uveitis population. Previous reports from subsets of patients at one of our participating centers have had similar results (most patients from which and several others were included in the current analysis). 33–35 However, JIA itself did not seem to have large direct effects on the risk of IOP elevation, suggesting that the presence of the other factors identified would be more predictive of risk in this population than the presence of JIA itself. Some previous reports that included adults, had observed eyes with Adamantiades-Behcet’s Disease-associated uveitis 36 to have a higher and HLA-B27 positivity related uveitis 37,38 to have a lower incidence of IOP elevation. We did not observe any consistent associations of IOP elevation with HLA-B27, Adamantiades-Behcet’s Disease, hypertension and sarcoidosis. All of these are diseases typically of adult onset, 36–38 and were observed to occur infrequently in the current study, limiting the power to detect moderate associations.
Eyes that had undergone cataract surgery prior to presentation for subspecialty care were more likely to present with IOP elevation, but eyes that had cataract surgery performed either previous to or during follow-up were not found to be associated with increased risk; a pattern for which several potential explanations are possible. Perhaps good perioperative inflammatory control and the availability of resources to manage intra- and post-operative complications under subspecialty care might explain this difference. Alternatively, cataract (leading to cataract surgery) might have served as an indicator of a greater extent of inflammatory damage at presentation, 39 which may not have been detectable after those eyes presenting with IOP elevation were removed from the at risk pool of the incidence analyses. It also could reflect regression to the mean if IOP elevation was a factor provoking referral. Eyes that had undergone pars plana vitrectomy tended to have both a higher prevalence and incidence of IOP elevation, which might be explained on the basis of increased disease severity among eyes requiring vitrectomy. The same relationship might underlie the association between band keratopathy and increased risk of IOP elevation.
Several eye-specific factors such as worse visual acuity, unilateral uveitis and uveitis of duration ≥6 months were associated with presenting IOP elevation but were not associated with increased incidence of IOP elevation during follow-up. These differences might reflect regression to mean if IOP was the reason for referral in these subsets. Also, all eyes that had an outcome at baseline (potentially eyes with worse disease on average) were not included in the incidence analysis, which may explain this difference between associations with prevalent and incident IOP elevation. Alternatively, ongoing inflammatory damage might have been stabilized by tertiary care, which could explain why we did not observe ongoing increased risk during follow-up.
We did not note a consistent predilection to IOP elevation based on the anatomical location of uveitis, other than a weak indication suggesting lower risk with intermediate uveitis, which may reflect similar risk over the long-run across the various sites of inflammation. However, the inverse relationship between current anterior segment inflammatory findings and risk of IOP elevation may reflect a short-run anatomical effect in that lower IOP may be caused by current ciliary body inflammation. Our results suggest that in children current vitreous inflammation activity is not strongly associated with an alteration in the risk of IOP elevation. IOP elevation or hypotony might occur in relationship to the chronicity and severity of uveitic inflammatory processes that affect aqueous humor dynamics, 40 as indicated by associations noted with structural markers of inflammation. Also, eyes that had experienced hypotony either in the same or the contralateral eye inconsistently demonstrated a lower risk of IOP elevation. Perhaps hypotony, which might reflect damage to the ciliary body in excess of damage to the outflow pathways, is a long-run effect hence observed infrequently in our study.
All limitations of a retrospective design are applicable to our study, including potential selection bias and cohort effects. Our results are likely representative of IOP elevation patterns at tertiary centers (referral centers for complicated pediatric uveitis patients) but might overestimate the risk in a ‘general’ population. Data to study the risk of IOP elevation associated with presence of aqueous flare, which has been reported elsewhere as a risk factor, 41 were not available. The effect of measurement error related to inter-tonometric method variability and IOP correction factors based on age, 42 ocular characteristics in children and anesthesia among others also should be considered. However, these limitations seem unlikely to bias results sufficiently to result in false associations of the large magnitude observed. If the high dose corticosteroid effect on IOP persists for a few weeks, the risk associated with lower doses of corticosteroids might have been overestimated somewhat: eyes receiving higher doses shortly before the point of observation might have had higher risk than eyes consistently receiving the same dose, but both would be counted as on the same dose at the time of observation using the method that was possible with our retrospective data. If so, the consistent dose-response relationship observed might have been even more pronounced. The study did not evaluate the effect of difluprednate 0.005%, which was not available at the time of the study, but may be associated with higher risk of IOP elevation than alternative corticosteroid drops. 43 Not all eyes that required IOP-lowering therapy might have experienced IOP elevations ≥30 and ≥10mmHg rise; thus the risk for these IOP outcomes may have been IOP over-estimated, although the approach would provide a reasonable estimate of the number of cases requiring treatment for IOP elevation. The rationale for our approach is that many outcomes likely would have occurred without treatment, and ignoring this possibility would have resulted in highly informative censoring, an important violation of the assumptions of the survival analysis method. Although with multiple comparisons and a Type 1 error threshold of 5%, some associations could have been due to chance, in an exploratory observational study, penalization of the Type 1 error probability for multiple comparisons is not recommended, 44 and in any case the strength of the relationship (P<0.001) noted with most positive associations would be robust to multiplicity concerns. Strengths of the study include the statistical advantages of its large sample size and data collection under a common study protocol across multiple centers.
In summary, IOP elevation affects a large minority of pediatric uveitis cases, suggesting that such eyes should be monitored for IOP elevation. Patients who experience such elevation in one uveitic eye are highly likely to experience it in the other eye, and should be monitored especially closely. The use of topical corticosteroids at a dose of more than 1 drop of prednisolone acetate 1% per day, as well as periocular and particularly intraocular corticosteroids, are associated with large increases in the risk of IOP elevation, with very high risks associated with high dosages of topical corticosteroids, periocular and intravitreous corticosteroids. Particularly close follow-up when treating active inflammation is warranted, as our results suggest that the use of large amounts of corticosteroids and the resolution of anterior chamber inflammation both are associated with an increased risk of IOP elevation. Since topical corticosteroids are associated with increased risk in a dose-response relationship, it is important to balance use of sufficient doses to quickly quell inflammation with use of a low enough dose (or sufficiently short duration of high dose therapy) to minimize the risk of IOP elevation. Prompt inflammatory control, corticosteroid-sparing strategies when chronic corticosteroid therapy is required, and early subspecialty involvement for complex cases might help improve outcomes. Further research regarding the functional outcomes of IOP elevation in this context would help to elucidate these results and determine whether certain interventions are more effective than others.
Supplementary Material
Acknowledgments
Financial Support:
This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness, the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C previously was supported by and RBN and HNS continue to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Research Group
University of Pennsylvania/Scheie Eye Institute
John H. Kempen, MD, PhD (Principal Investigator, Penn Center Director)
Marshall M. Joffe, MD, PhD (Statistical Methodologist)
Kurt A. Dreger, BS (Database Construction and Management)
Tonetta D. Fitzgerald, BA, COA, CCRC (Project Coordinator)
Craig W. Newcomb, MS (Biostatistician)
Maxwell Pistilli, MS (Biostatistician)
Srishti Kothari, MBBS, DOMS, DNB (Post-doctoral Fellow)
Naira Khacharyan, MD, MPH, DrPH (Post-doctoral Fellow)
Former Members:
Pichaporn Artornsombudh, MD (Post-doctoral Fellow)
Asaf Hanish, MPH (Biostatistical Programmer)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Johns Hopkins University/Wilmer Eye Institute
Jennifer E. Thorne, MD, PhD (Center Director, 2007-present)
Hosne Begum, MBBS, MPH (Study Coordinator)
Kurt A. Dreger, BS (Database Construction and Management)
Former Members:
Ebenezer Daniel, MBBS, MPH, PhD (Post-doctoral Fellow)
James P. Dunn, MD (Major Clinician)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Douglas A. Jabs, MD, MBA (Clinic Founder, Former Clinic Director)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Mercy Medical Center, Baltimore
Kathy J. Helzlsouer, MD, MHS (Cancer Epidemiologist)
Icahn School of Medicine at Mount Sinai
Douglas A. Jabs, MD, MBA (Founder of JHU Clinic, JHU Clinic Director 2005–2007)
Massachusetts Eye Research & Surgery Institution/Ocular Immunology &Uveitis Foundation
C. Stephen Foster, MD, FACS, FACR (Center Director and Founder, and Director and Founder of previous Massachusetts Eye & Ear Infirmary Center, 2004–2005)
Srishti Kothari, MBBS, DOMS, DNB (Post-doctoral Fellow)
Naira Khacharyan, MD, MPH, DrPH (Post-doctoral Fellow)
Former Members:
Pichaporn Artornsombudh, MD (Post-doctoral Fellow)
R. Oktay Kaçmaz, MD, MPH (Post-doctoral Fellow)
Abhishek Payal, MBBS, MPH (Post-doctoral Fellow)
Siddharth S. Pujari, MBBS, MPH (Post-doctoral Fellow)
National Eye Institute, Laboratory of Immunology
H. Nida Sen, MD, MS (NEI Center Director)
Robert B. Nussenblatt, MD, MPH (Center Founder and Co-Director)
Former Members:
Grace A. Levy-Clarke, MD (Former Center Director)
Sapna S. Gangaputra, MBBS, MPH (Post-doctoral Fellow)
Oregon Health & Sciences University/Casey Eye Institute
Eric B. Suhler, MD, MPH (Center Director)
James T. Rosenbaum, MD, FACR (Center Founder and Co-Director)
Teresa Liesegang, COT, CRC (Project Coordinator)
University of Pittsburgh
Jeanine Buchanich, PhD (Epidemiologist)
Terri L. Washington (Project Coordinator)
Footnotes
Presented at:
ARVO, May 4–8, 2014, Orlando, Florida
Conflict of interest:
The following potential conflicts of interest were reported: C. Stephen Foster serves as a consultant for AbbVie, Alcon Laboratories, Allergan, Inc., Ista Pharmaceuticals, Lux Biosciences, Novartis Pharmaceutical, Bausch & Lomb Surgical and as an equity owner for EyeGate. Jennifer E. Thorne serves a consultant for AbbVie, Gilead and Xoma. Douglas A. Jabs serves on Data and Safety Monitoring committees for Applied Genetic Technologies Corporation and Novartis Pharmaceutical Corporation and as a consultant to Santen, Inc. Grace A. Levy-Clarke was employed by Johnson & Johnson Vision Care. James T. Rosenbaum serves or has served as a consultant for AbbVie, Amgen, Allergan, EMD Serono, Genentech, Lux, Novartis, Regeneron, Sanofi, Santen, UCB, and Xoma and has been involved in clinical trials for Abbott Laboratories, Genentech, Lux Biosciences, and Eyegate Pharma. Scott D. Lawrence has received payment for lectures, including service on speakers’ bureaus–Alcon. John H. Kempen serves or has served as a consultant for AbbVie, Alcon, Allergan, Can-Fite, Clearside, Lux Biosciences, Roche, Sanofi-Pasteur, and Xoma.
Contributions:
Design and conduct of the study (SK, MP, CSF, JHK); collection, management, analysis and interpretation of the data (all authors); preparation, review, or approval of the manuscript (all authors)
Institutional Review Board Approval:
The project was conducted in accordance with the principles of the Declaration of Helsinki, with the approval of the governing Institutional Review Boards of each institution, each of which has granted waiver of consent, allowing all living and deceased patients to be included.
This article contains additional online-only material. The following should appear online-only: Tables 2, 4, and Appendix.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beer GJ. Die Lehre v. d. Augenkrankheiten. Vienna. 1813;1:633. [Google Scholar]
- 2.Arnesen T, Nord E. The value of DALY life: Problems with ethics and validity of disability adjusted life years. BMJ. 1999;319(7222):1423–5. doi: 10.1136/bmj.319.7222.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013;13(1):43–9. doi: 10.1016/j.coph.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Rieck J. The pathogenesis of glaucoma in the interplay with the immune system. Invest Ophthalmol Vis Sci. 2013;54(3):2393–409. doi: 10.1167/iovs.12-9781. [DOI] [PubMed] [Google Scholar]
- 5.Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007;52 (Suppl 2):S162–73. doi: 10.1016/j.survophthal.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013;36:199–216. doi: 10.1016/j.preteyeres.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Spaeth GL. Valid relevance in medical practice: The inadequacy of the linear model of health and disease: The Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2011;52(9):6250–6. doi: 10.1167/iovs.10-7134. [DOI] [PubMed] [Google Scholar]
- 8.Davanger M, Ringvold A, Blika S. The probability of having glaucoma at different IOP levels. Acta Ophthalmol (Copenh) 1991;69(5):565–8. doi: 10.1111/j.1755-3768.1991.tb04840.x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DS, Holbrook JT, Ansari H, et al. Risk of elevated intraocular pressure and glaucoma in patients with uveitis: Results of the multicenter uveitis steroid treatment trial. Ophthalmology. 2013;120(8):1571–9. doi: 10.1016/j.ophtha.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116(8):1544–51. 1551.e1. doi: 10.1016/j.ophtha.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panek WC, Holland GN, Lee DA, Christensen RE. Glaucoma in patients with uveitis. Br J Ophthalmol. 1990;74(4):223–7. doi: 10.1136/bjo.74.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi T, Ohtani S, Miyata K, et al. A clinical evaluation of uveitis-associated secondary glaucoma. Jpn J Ophthalmol. 2002;46(5):556–62. doi: 10.1016/s0021-5155(02)00549-x. [DOI] [PubMed] [Google Scholar]
- 13.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: The systemic immunosuppressive therapy for eye diseases (SITE) cohort study. Ophthalmic Epidemiol. 2008;15(1):47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA. Improving the reporting of clinical case series. Am J Ophthalmol. 2005;139(5):900–5. doi: 10.1016/j.ajo.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster CS, Vitale AT. Diagnosis and treatment of uveitis. 2. India: JP Medical Ltd; 2013. [Google Scholar]
- 16.Deschenes J, Murray PI, Rao NA, Nussenblatt RB International Uveitis Study Group. International uveitis study group (IUSG): Clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi: 10.1080/09273940801899822. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trusko B, Thorne J, Jabs D, et al. The standardization of uveitis nomenclature (SUN) project. development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med. 2013;52(3):259–65. S1–6. doi: 10.3414/ME12-01-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Lin D, Weissfeld L. Regression analysis of multivariate incomplete failure time data by using the marginal distribution. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 22.Joffe MM, Pistilli M, Kempen JH. Marginal structural models for comparing alternative treatment strategies in ophthalmology using observational data. Ophthalmic Epidemiol. 2013;20(4):197–200. doi: 10.3109/09286586.2013.792939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiddee W, Trope GE, Sheng L, et al. Intraocular pressure monitoring post intravitreal steroids: A systematic review. Surv Ophthalmol. 2013;58(4):291–310. doi: 10.1016/j.survophthal.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Albert D, Miller J, Azar D, Blodi B. Section11: Glaucoma, Albert & Jakobiec’s Principles & practice of ophthalmology. 3. Saunders: Elsevier; 2008. [Google Scholar]
- 25.Allingham R, Damji K, Freedman S, et al. Shields’ textbook of glaucoma. 6. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 26.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: A review of the literature. Eye (Lond) 2006;20(4):407–16. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 27.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr Opin Ophthalmol. 2006;17(2):163–7. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46(12):4607–16. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- 29.Ohji M, Kinoshita S, Ohmi E, Kuwayama Y. Marked intraocular pressure response to instillation of corticosteroids in children. Am J Ophthalmol. 1991;112(4):450–4. doi: 10.1016/s0002-9394(14)76256-7. [DOI] [PubMed] [Google Scholar]
- 30.Lam DS, Kwok AK, Chew S. Accelerated ocular hypertensive response to topical steroids in children. Br J Ophthalmol. 1997;81(5):422–3. doi: 10.1136/bjo.81.5.421d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weijtens O, Schoemaker RC, Romijn FP, et al. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109(10):1887–91. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- 32.Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999;128(2):192–7. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 33.Tugal-Tutkun I, Havrlikova K, Power WJ, Foster CS. Changing patterns in uveitis of childhood. Ophthalmology. 1996;103(3):375–83. doi: 10.1016/s0161-6420(96)30682-9. [DOI] [PubMed] [Google Scholar]
- 34.Foster CS, Havrlikova K, Baltatzis S, et al. Secondary glaucoma in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand. 2000;78(5):576–9. doi: 10.1034/j.1600-0420.2000.078005576.x. [DOI] [PubMed] [Google Scholar]
- 35.Merayo-Lloves J, Power WJ, Rodriguez A, et al. Secondary glaucoma in patients with uveitis. Ophthalmologica. 1999;213(5):300–4. doi: 10.1159/000027443. [DOI] [PubMed] [Google Scholar]
- 36.Elgin U, Berker N, Batman A. Incidence of secondary glaucoma in Behcet disease. J Glaucoma. 2004;13(6):441–4. doi: 10.1097/00061198-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Tay-Kearney ML, Schwam BL, Lowder C, et al. Clinical features and associated systemic diseases of HLA-B27 uveitis. Am J Ophthalmol. 1996;121(1):47–56. doi: 10.1016/s0002-9394(14)70533-1. [DOI] [PubMed] [Google Scholar]
- 38.Braakenburg AM, de Valk HW, de Boer J, Rothova A. Human leukocyte antigen-B27-associated uveitis: Long-term follow-up and gender differences. Am J Ophthalmol. 2008;145(3):472–9. doi: 10.1016/j.ajo.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: Clinical characteristics and complications. Am J Ophthalmol. 2009;147(4):667–78. e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Daniel E, Pistilli M, Pujari SS, et al. Risk of hypotony in noninfectious uveitis. Ophthalmology. 2012;119(11):2377–85. doi: 10.1016/j.ophtha.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland GN. A reconsideration of anterior chamber flare and its clinical relevance for children with chronic anterior uveitis (An American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:344–64. [PMC free article] [PubMed] [Google Scholar]
- 42.Sampaolesi R, Zarate J, Sampaolesi J. The glaucomas. Berlin: Springer-Verlag; 2009. Volume I-Pediatric glaucomas. [Google Scholar]
- 43.Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012;153(5):932–8. doi: 10.1016/j.ajo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine-Reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.