Abstract
This overview of the 4th edition of the WHO Classification of thymic tumors has two aims. First, to comprehensively list the established and new tumour entities and variants that are described in the new WHO Classification of thymic epithelial tumors, germ cell tumors, lymphomas, dendritic cell and myeloid neoplasms, and soft tissue tumors of the thymus and mediastinum; second, to highlight major differences in the new WHO Classification that result from the progress that has been made since the 3rd edition in 2004 at immunohistochemical, genetic and conceptual levels. Refined diagnostic criteria for type A, AB, B1–B3 thymomas and thymic squamous cell carcinoma are given and will hopefully improve the reproducibility of the classification and its clinical relevance. The clinical perspective of the classification has been strengthened by involving experts from radiology, thoracic surgery and oncology; by incorporating state-of-the-art PET/CT images; and by depicting prototypic cytological specimens. This makes the thymus section of the new WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart a valuable tool for pathologists, cytologists and clinicians alike. The impact of the new WHO Classification on therapeutic decisions is exemplified in this overview for thymic epithelial tumors and mediastinal lymphomas, and future perspectives and challenges are discussed.
INRODUCTION
The 4th edition of the WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart is largely a revision of the 3rd edition that was published in 2004 under the editorship of William D. Travis, Elizabeth Brambilla, Hans Konrad Müller-Hermelink and Curtis C. Harris.1 It was for the first time that the WHO Classification gathered all thoracic tumors in one book. In contrast, the previous 2nd edition of the WHO Classification of Tumours of the Thymus, edited by Dr. Juan Rosai was published in 1999 and addressed only thymic tumors. In this edition the concept of type A, AB, B1–B3 nomenclature was introduced for thymomas.2 The 3rd and the new 4th editions3 perpetuate this unique nomenclature for thymomas since it has achieved world-wide acceptance and has not been challenged seriously by new data.4 In the 3rd edition the histopathology of thymic tumors was complemented with descriptions of their clinical symptoms, macroscopic, immunohistological and genetic features, and prognostic data.
In the 4th edition of the WHO Classification of thoracic tumors edited by William D. Travis, Elizabeth Brambilla, Allen Burke, Alexander Marx and Andrew G. Nicholson, this interdisciplinary perspective on thymic tumors is further strengthened by involving clinical experts from radiology, thoracic surgery and oncology as co-authors, and by the incorporation of state-of-the-art CT and PET/CT images (Fig. 1) and cytology (Fig. 2). The foundations for this interdisciplinary approach and the broad consensus on conceptual changes and histological criteria for improved thymoma subtyping were greatly aided by two international interdisciplinary conferences organized by the International Thymic Malignancy Interest Group (ITMIG) in New York City, U.S.A., and Mannheim, Germany and convened experts from North America, Asia and Europe. Another focus of the 4th edition was the revision and refinement of histological and immunohistochemical diagnostic criteria for a more robust and reproducible subtyping of thymomas and for the distinction between thymomas and thymic carcinomas. The necessary preparatory work for this refinement of histological criteria was achieved at the ITMIG consensus meetings in New York and Mannheim5 and is reflected in the 4th edition not only in the ‘histopathology” but also ‘differential diagnosis’ paragraphs. Furthermore, the epidemiological and prognostic data in the chapters on thymic epithelial tumors were for the first time not only based on single-center experience or small meta-analysis, but on recent data derived from the world-wide, retrospective database of the ITMIG that has compiled over 6000 cases of thymomas, thymic carcinomas and thymic neuroendocrine neoplasms.6–9
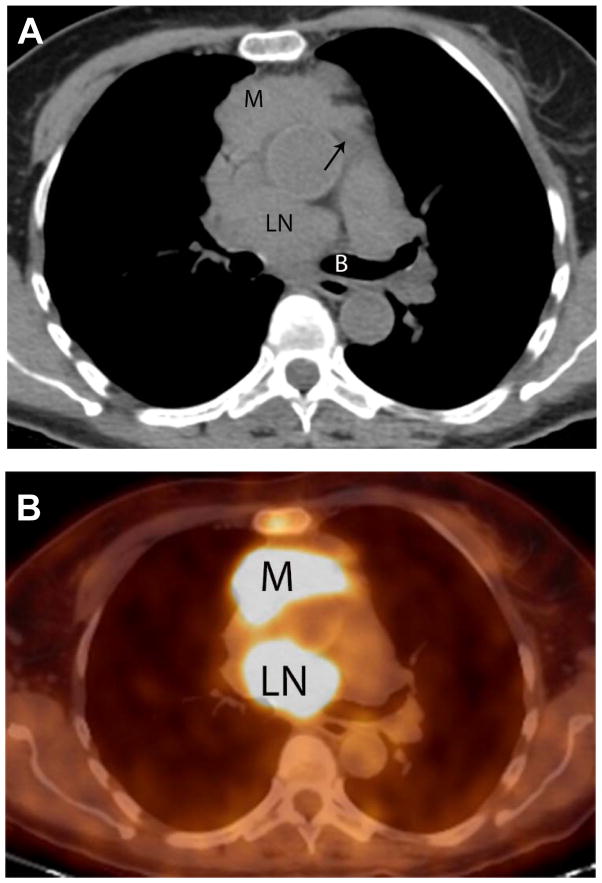
Fig. 1.
Images of a fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) of a 71-year-old woman with type B3 thymoma. A. Axial CT image at the level of the left main bronchus (B) demonstrates an anterior mediastinal mass (M) and low right paratracheal lympadenopathy. Infiltration of superior recess of the pericardium (arrow). B. Axial fused image at the same level demonstrates that both the primary tumor and the metastasis are FDG avid.
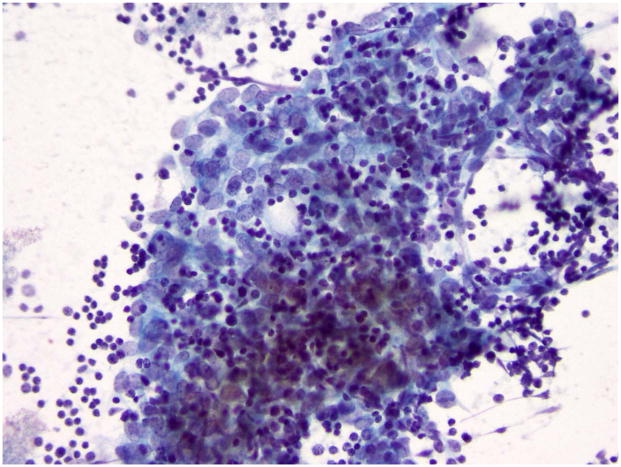
Fig. 2.
Cytology of WHO type B2 thymoma (fine needle aspirate). Large tumor cells with elongated or round nuclei and nucleoli intermingled with small lymphocytes.
In the chapters on germ cell tumors, lymphoid, hematopoietic and soft tissue neoplasms, there are no changes of concept or diagnostic criteria in the 4th compared to the 3rd edition. Minor revisions concern new immunohistochemical and genetic data and the adaptation of nomenclature and definitions to the WHO Classifications of Tumours of the Haematopoietic and Lymphoid Tissue,10 Tumours of Soft Tissues and Bone11 and Tumours of the Urinary System and Male Genital Organs [Moch H. et al., forthcoming].
The following ‘overview’ focuses on differences between the 3rd and 4th edition of the WHO Classification of tumors of the thymus rather than providing comprehensive description of the tumors.
NOVELTIES IN THE NEW WHO CLASSIFICATION OF MEDIASTINAL TUMORS
THYMOMAS
Conceptual continuity
The nomenclature of the major thymoma subtypes based on letters and numbers (type A, AB, B1–B3)2 is maintained in the 4th edition (Table 1), as is the recommendation to use the modified Masaoka-Koga system for the staging of thymomas.12 A new TNM staging system is currently being jointly developed by the International Association for the Study of Lung Cancer (IASLC) and ITMIG, but preliminary staging proposals13, 14 should not be used in clinical settings prior to the approval by the International Union against Cancer (UICC) and the American Joint Committee on Cancer (AJCC).15
Table 1.
Epithelial tumours
| Thymoma | |
| Type A thymoma, including atypical variant | 8581/3* |
| Type AB thymoma | 8582/3* |
| Type B1 thymoma | 8583/3* |
| Type B2 thymoma | 8584/3* |
| Type B3 thymoma | 8585/3* |
| Micronodular thymoma with lymphoid stroma | 8580/1* |
| Metaplastic thymoma | 8580/3 |
| Other rare thymomas | |
| Microscopic thymoma | 8580/0 |
| Sclerosing thymoma | 8580/3 |
| Lipofibroadenoma | 9010/0* |
| Thymic carcinoma | |
| Squamous cell carcinoma | 8070/3 |
| Basaloid carcinoma | 8123/3 |
| Mucoepidermoid carcinoma | 8430/3 |
| Lymphoepithelioma-like carcinoma | 8082/3 |
| Clear cell carcinoma | 8310/3 |
| Sarcomatoid carcinoma | 8033/3 |
| Adenocarcinomas | |
| Papillary adenocarcinoma | 8260/3 |
| Thymic carcinoma with adenoid cystic carcinoma-like features | 8200/3* |
| Mucinous adenocarcinoma | 8480/3 |
| Adenocarcinoma, NOS | 8140/3 |
| NUT carcinoma | 8023/3* |
| Undifferentiated carcinoma | 8020/3 |
| Other rare thymic carcinomas | |
| Adenosquamous carcinoma | 8560/3 |
| Hepatoid carcinoma | 8576/3 |
| Thymic carcinoma, NOS | 8586/3 |
| Thymic neuroendocrine tumours | |
| Carcinoid tumors | |
| Typical carcinoid | 8240/3 |
| Atypical carcinoid | 8249/3 |
| Large cell neuroendocrine carcinoma | 8013/3 |
| Combined large cell neuroendocrine carcinoma | 8013/3 |
| Small cell carcinoma | 8041/3 |
| Combined small cell carcinoma | 8045/3 |
| Combined thymic carcinomas | |
| Germ cell tumours of the mediastinum | |
| Seminoma | 9061/3 |
| Embryonal carcinoma | 9070/3 |
| Yolk sac tumour | 9071/3 |
| Choriocarcinoma | 9100/3 |
| Teratoma | |
| Teratoma, mature | 9080/0 |
| Teratoma, immature | 9080/1 |
| Mixed germ cell tumours | 9085/3 |
| Germ cell tumours with somatic-type solid malignancy | 9084/3 |
| Germ cell tumours with associated haematological malignancy | 9086/3* |
| Lymphomas of the mediastinum | |
| Primary mediastinal large B-cell lymphoma | 9679/3 |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) | 9699/3 |
| Other mature B-cell lymphomas** | |
| T lymphoblastic leukaemia / lymphoma | 9837/3 |
| Anaplastic large cell lymphoma (ALCL) and other rare mature T- and NK-cell lyphomas | |
| ALCL, ALK-positive (ALK+) | 9714/3 |
| ALCL, ALK-negative (ALK−) | 9702/3 |
| Hodgkin lymphoma | 9650/3 |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell and classical Hodgkin lymphoma | 9596/3 |
| Histiocytic and dendritic cell neoplasms of the mediastinum | |
| Langerhans cell lesions | |
| Thymic Langerhans cell histocytosis | 9751/1 |
| Langerhans cell sarcoma | 9756/3 |
| Histiocytic sarcoma | 9755/3 |
| Follicular dendritic cell sarcoma | 9758/3 |
| Interdigitating dendritic cell sarcoma | 9757/3 |
| Fibroblastic reticular cell tumour | 9759/3 |
| Indeterminate dendritic cell tumour | 9757/3 |
| Myeloid sarcoma and extramedullary acute myeloid leukaemia | 9930/3 |
| Soft tissue tumours of the mediastinum | |
| Thymolipoma | 8850/0 |
| Lipoma | 8850/0 |
| Liposarcoma | |
| Well-differentiated | 8850/3 |
| Dedifferentiated | 8858/3 |
| Myxoid | 8852/3 |
| Pleomorphic | 8854/3 |
| Solitary fibrous tumour | 8815/1 |
| Malignant | 8815/3 |
| Synovial sarcoma | |
| Synovial sarcoma, NOS | 9040/3 |
| Synovial sarcoma, spindle cell | 9041/3 |
| Synovial sarcoma, epithelioid cell | 9042/3 |
| Synovial sarcoma, biphasic | 9043/3 |
| Vascular neoplasms | |
| Lymphangioma | 9170/0 |
| Haemangioma | 9120/0 |
| Epitheloid hemangioendothelioma | 9133/3 |
| Angiosarcoma | 9120/3 |
| Neurogenic tumours | |
| Tumours of peripheral nerves | |
| Ganglioneuroma | 9490/0 |
| Ganglioneuroblastoma | 9490/3 |
| Neuroblastoma | 9500/3 |
| Ectopic tumours of the thymus | |
| Ectopic thyroid tumours | |
| Ectopic parathyroid tumours | |
| Other rare ectopic tumours | |
The morphology codes are from the International Classification of Diseases for Oncology (ICD-O). Behaviour is coded / 0 for benign tumours; / 1 for unspecified, borderline, or uncertain behaviour; / 2 for carcinoma in situ and grade III intraepithelial neoplasia; and / 3 for malignant tumors.
The classification ist modified from the previous WHO classification,1 taking into account changes in our understanding of these lesion.
These new codes were approved by the IARC/WHO Committee for ICD-0.
This can be any mature B cell lymphoma and the respective ICD-O code from the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.10
Conceptual changes
One new feature of the 4th edition is that some histological criteria for the characterization of thymomas using H&E stained specimens are now considered “obligatory/indispensible”, and others as “optional” according to their importance for diagnosis (Table 2).5 It is hoped that these clarifications will reduce previous ambiguity and may help to overcome the unsatisfactory reproducibility of the WHO classification reported by some studies.16–18
Table 2.
Refinement of diagnostic criteria of thymomas in the 2015 WHO classification of thymic tumors3 by stressing obligatory and optional features. The atypical type A thymoma variant (in bold) is the only new addition to the ‘historic’ list of thymoma subtypes.1
| Thymoma subtype | Obligatory criteria | Optional criteria |
|---|---|---|
| Type A | Occurrence of bland, spindle shaped epithelial cells (at least focally); paucitya or absence of immature (TdT+) T cells throughout the tumor | Polygonal epithelial cells CD20+ epithelial cells |
| Atypical type A variant | Criteria of type A thymoma; in addition: comedo-type tumor necrosis; increased mitotic count (>4/2mm2); nuclear crowding | Polygonal epithelial cells CD20+ epithelial cells |
| Type AB | Occurrence of bland, spindle shaped epithelial cells (at least focally); abundancea of immature (TdT+) T cells focally or throughout tumor | Polygonal epithelial cells CD20+epithelial cells |
| Type B1 | Thymus-like architecture and cytology: abundance of immature T cells, areas of medullary differentiation (medullary islands); paucity of polygonal or dendritic epithelia cells without clustering (i.e.<3 contiguous epithelial cells) | Hassall’s corpuscles; perivascular spaces |
| Type B2 | Increased numbers of single or clustered polygonal or dendritic epithelial cells intermingled with abundant immature T cells | Medullary islands; Hassall’s corpuscles; perivascular spaces |
| Type B3 | Sheets of polygonal slightly to moderately atypical epithelial cells; absent or rare intercellular bridges; paucity or absence of intermingled TdT+ T cells | Hassall’s corpuscles; perivascular spaces |
| MNTb | Nodules of bland spindle or oval epithelial cells surrounded by an epithelial cell-free lymphoid stroma | Lymphoid follicles; monoclonal B cells and/or plasma cells (rare) |
| Metaplastic thymoma | Biphasic tumor composed of solid areas of epithelial cells in a background of bland-looking spindle cells; absence of immature T cells | Pleomorphism of epithelial cells; actin, keratin, or EMA-positive spindle cells |
| Rare othersc |
Paucity versus abundance: any area of crowded immature T cells or moderate numbers of immature T cells in >10% of the investigated tumor are indicative of “abundance”;
MNT, micronodular thymoma with lymphoid stroma;
Microscopic thymoma; sclerosing thymoma, lipofibroadenoma
Another conceptual change concerns the reporting of the >30% of thymomas that show more than one histological pattern (some authors19 find >50% on extensive sampling).19, 20 Instead of using the term ‘combined thymic epithelial tumors’,1 the diagnosis should list all the histological thymoma types that are encountered, starting with the most prominent component; minor components should be reported subsequently and quantified in 10% increments.5 This rule is not applicable to type AB thymoma (a distinct thymoma entity) and tumors with a carcinoma component of whatever size (see ‘combined carcinomas’ below).
A third conceptual change is the introduction of immunohistochemical features as criteria for diagnosis of thymomas with ambiguous histology. Examples are the distinction of type A and AB thymomas by the paucity versus abundance of immature TdT+ T cells; or the different patterns of coarse versus delicate cytokeratin networks in type AB and B1 thymomas, respectively (see below). Routine immunohistochemical markers that are recommended in otherwise difficult-to-classify thymomas and thymic carcinomas are given in Table 3.3
Table 3.
Markers considered to be useful for the routine immunohistochemical characterization of otherwise difficult to classify thymomas and thymic carcinomas.3
| Marker | Cellular and subcellular targets of mediastinal tumors |
|---|---|
| Cytokeratins | Epithelial cells of normal thymus, thymomasa, thymic carcinomas, neuroendocrine tumors, many germ cell tumors, rare sarcomas and dendritic cell tumors; metatases to the mediastinum |
| Cytokeratin 19 | Epithelial cells of normal thymus, thymomasa and thymic carcinomas |
| Cytokeratin 20 | Negative in normal thymus and thymomas. May be positive in rare thymic adenocarcinomas, teratomas or metastases |
| P63 | Nuclei of normal and neoplastic thymic epithelial cells, squamous epithelial cells (e.g. in teratoma, metastasis), primary mediastinal large B-cell lymphoma |
| P40 | Nuclei of normal and neoplastic thymic epithelial cells, squamous epithelial cells (e.g. in teratoma; metastasis) |
| TdT | Immature T cells of normal thymus, >90% of thymomas and neoplastic T cells of T lymphoblastic lymphoma |
| CD5 | Immature and mature T cells of thymus and >90% of thymomas Neoplastic T cells of many T lymphoblastic lymphomas Epithelial cells in70% of thymic carcinomasb |
| CD20 | Normal and neoplastic B cells Epithelial cells in 50% of cases of type A and AB thymoma |
| CD117 | Epithelial cells in 80% of thymic carcinomas Neoplastic cells in most seminomas |
Beware of rare cytokeratin-negative thymomas (that typically maintain expression of p63/p40)21;
beware of adenocarcinoma metastases to the mediastinum that can be CD5+ as well
A final conceptual change concerns the appreciation that all major thymoma subtypes can behave in a clinically aggressive fashion19 and, therefore, should no longer be called benign tumors, irrespective of tumor stage. Accordingly, their ICD-O codes now have a /3 suffix, labelling them as malignant (Table 1). Exceptions are micronodular and microscopic thymomas, for which fatal outcomes are not on record.
Key new discoveries
Improved genetic22–24, epigenetic25, 26 and transcriptomic24, 27 analyses have augmented our knowledge about the distinct molecular basis of thymomas and thymic carcinomas. The latter show mutations of epigenetic regulatory genes,22 methylation patterns26 and expression profiles of anti-apoptotic genes27 that clearly distinguish them from thymomas. On the other hand, the identification of a highly recurrent point mutation in the GTF2I oncogene in all major thymoma subtypes and thymic carcinomas23 is a seminal finding that underlines the unique tumor biology of thymic epithelial tumors and lends strong support to the WHO-based subtyping of thymic tumors.28 Nevertheless, new predictive markers with therapeutic perspective are still awaited.29, 30
Individual changes concerning thymoma subtypes
Atypical type A thymoma
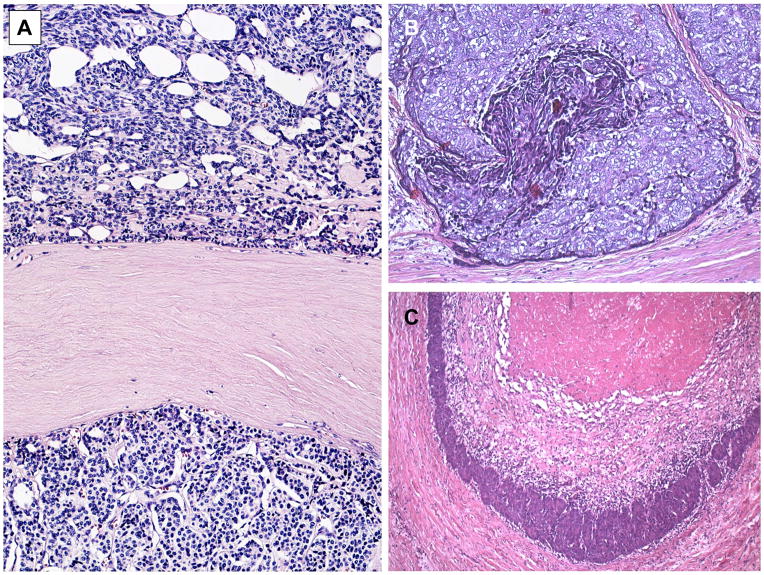
A new addition in the 4th edition is the delineation of an “atypical type A thymoma variant” from conventional type A thymomas (Tables 1). The new term reflects the experience that rare type A thymomas can show hypercellularity, increased mitotic activity and necrosis (Fig. 3). Necrosis in particular appears to correlate with advanced stage, including metastasis.5, 31–33 Rare type AB thymomas can show similar features.31 The clinical significance of this variant needs further studies.
Fig. 3.
Conventional type A thymoma versus atypical type A thymoma variant. A. Conventional type A thymoma exhibiting areas composed of spindle cells (upper half) and polygonal cells (lower half) separated by a broad fibrouis septum. B. and C. Atypical type A thymoma variant showing various degrees of spontaneous necrosis in polygonal cell areas. This tumor invaded the lung and showed a missense mutation (chromosome 7 c.74146970T>A) in the GTF2I gene that is common in type A thymomas but rare in type B thymomas and thymic carcinomas.23
Type A versus AB thymoma
The distinction between type A and AB thymoma has been notoriously difficult.16–18 It is now stressed that both thymoma types share the occurrence of bland-looking spindle epithelial cells (that may optionally be accompanied by oval or polygonal tumor cells), and are distinguished from each other by a low and high content of immature T cells, respectively (Fig. 4a–c). Any lymphocyte-dense areas (with crowded TdT+ cells that are ‘impossible to count’ in ‘type B-like’ areas) or >10% tumor areas with a moderate infiltrate of immature T cells should prompt classification as type AB thymoma.5 The close relationship between type A and AB thymomas is strongly supported by largely overlapping genetic alterations.23 However, it will be interesting to study whether this genetic overlap applies to lymphocyte-poor and lymphocyte-rich areas in type AB thymoma alike.
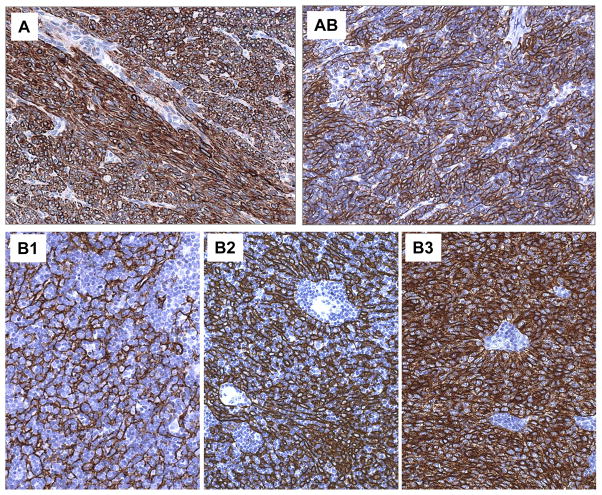
Fig. 4.
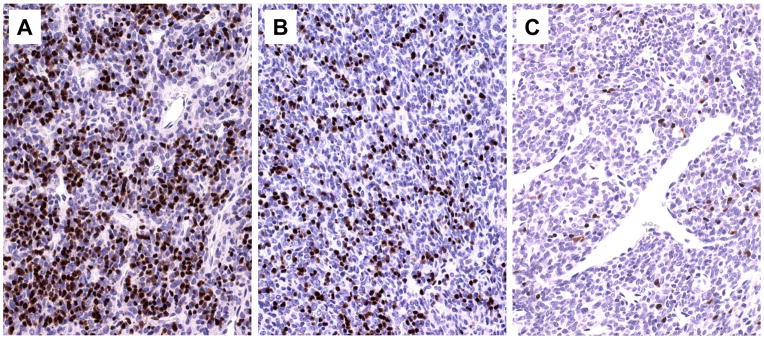
Immunohistochemistry: an important tool for the diagnosis of difficult-to-classify type A versus AB thymomas. A. Abundance of TdT+ immature T cells in type AB thymoma; any such crowding of TdT+ T cells excludes the diagnosis of type A thymoma; B. Moderate numbers of TdT+ immature T cells in a spindle cell thymoma imply a diagnosis of type AB thymoma when occurring in >10% of the tumor: if occurring in ≤10% of the tumor, a diagnosis of type A thymoma should be rendered. C. Near absence of TdT+ immature T cells in type A thymoma.
Type B thymomas and distinction from thymic carcinoma
Type B1 and B2 thymomas are, by definition, both lymphocyte-rich tumors. To improve their distinction from one another, which can be difficult,17, 18 the thymus-like architecture and cytology of B1 thymomas are highlighted as obligatory criteria, including presence of medullary islands as a ‘must’, and absence of epithelial cell clusters. By contrast, type B2 thymomas must show a higher than normal number of polygonal (non-spindle) neoplastic epithelial cells that commonly occur in clusters (defined as 3 or more contiguous epithelial cells). Medullary islands optionally occur in type B2 thymomas, while Hassall’s corpuscles occur as optional feature commonly in type B1 and rarely in B2 thymomas (Table 2). Cytokeratin (and p40/p63) expression patterns may be helpful to distinguish type B1 from type B2 (and AB) thymoma (Fig. 5).
Fig. 5.
Immunohistochemical staining of keratin across the spectrum of type A to B3 thymomas. Of note, among the lymphocyte-rich thymomas a dense epithelial cell networks is typical of type AB and type B2 thymoma, while a delicate network is an obligatory feature of type B1 thymoma.
Type B3 thymoma is a lymphocyte-poor, epithelial-rich tumor but distinction from type B2 thymomas may be difficult.5 Because of the lack of distinguishing markers, the differential diagnosis rests on the visual impression that type B2 thymomas look blue on H&E staining (due to the presence of many admixed lymphocytes), while type B3 thymomas look pink (due to sheets of confluent tumor cells).
The distinction of type B3 thymoma from thymic squamous cell carcinoma (TSQCC) can be a challenge when rare tumors with a type B3 thymoma morphology show focal expression of “TSQCC-markers” (such as CD5 and CD117) and/or lack of “type B3 thymoma markers” (such as TdT+ T cells); or when tumors with a TSQCC morphology harbour (usually rare) TdT+ immature T cells.5 In the 4th edition it is now stated that tumors that look like type B3 thymoma on H&E staining should be diagnosed as type B3 thymoma, and tumors with TSQCC morphology should be labelled as TSQCC, irrespective of immunohistochemistry.
Other thymomas
No major changes have been introduced. In metaplastic thymomas, exclusive staining of the polygonal but not the spindle cell component for p63 or p40 may be diagnostically helpful.
THYMIC CARCINOMAS, INCLUDING COMBINED THYMIC CARCINOMAS
Conceptual continuity
With few exceptions (e.g. NUT carcinomas; adenocarcinomas), the nomenclature and diagnostic criteria of almost all thymic carcinoma subtypes remain unchanged. The Masaoka-Koga staging system may still be used for thymic carcinomas till publication of the new UICC/ AJCC approved TNM system (supposedly in 2016).
Conceptual changes
The thymic adenocarcinomas are gathered in one chapter and labelled according to their H&E histology, abandoning the distinction between papillary and non-papillary adenocarcinomas.
While the term “combined thymic epithelial tumors” was abandoned in the 4th edition (see above), the term Combined thymic carcinomas was introduced for tumors that are either composed of different types of thymic carcinomas (extremely rare), or of a thymic carcinoma and any type of thymoma or carcinoid (rare). However, in analogy to the lung, heterogeneous thymic epithelial tumors with a small cell carcinoma component or a large cell neuroendocrine carcinoma component are not counted among the combined thymic carcinomas but among the neuroendocrine carcinomas (see below). The reporting of a combined thymic carcinoma must start with the carcinoma component irrespective of its proportion, followed by the thymoma component.5 In case of two or more carcinoma components, the dominant component is reported first.
Changes concerning individual thymic carcinoma subtypes
Thymic squamous cell carcinomas (TSQCC)
FoxN1 and CD205 are novel markers32 that complement CD5 and CD117 as markers that are expressed in most TSQCC but not pulmonary squamous cell carcinomas. In 10–20% of mediastinal squamous cell carcinomas without expression of these markers, staging remains paramount to distinguish thymic from pulmonary primaries. Among the many new molecular findings in thymic carcinoma (reviewed in 28) significant predictive markers remain to be identified.
Basaloid carcinoma of the thymus
In the lung, carcinomas with basaloid features, including prominent palisading as hallmark, are termed “basaloid squamous cell carcinoma” and considered a high grade variant of squamous cell carcinoma with distinct genetic features.34 In the thymus, cancers with basaloid features show a more varied histology (including a wide range of proliferative activities, common macropapillary growth pattern and association with cysts) and a broader spectrum of clinical aggressiveness.35 Genetic data are sparse and not overlapping with those of pulmonary basaloid squamous cell carcinoma.36 Therefore, the term ‘basaloid carcinoma of the thymus’ is maintained in the 4th edition (Table 1).
Mucoepidermoid carcinoma (MEC)
Translocation of the MAML2 gene is characteristic of almost all low-grade MECs and many high-grade MECs of salivary glands,37 bronchi and lung.38 This translocation has recently been observed in thymic MECs as well,39 allowing their distinction from adenosquamous carcinomas and adenocarcinomas.
Sarcomatoid carcinoma
The microscopic description of sarcomatoid carcinoma has been refined and now distinguishes 1) spindle cell carcinoma (malignant transformation of type A thymoma); 2) sarcomatoid transformation of preexisting thymic carcinoma; and 3) true carcinosarcoma with heterologous component(s).
Adenocarcinomas
Papillary adenocarcinomas of the thymus commonly co-occur with type A and type AB thymomas and are now defined as low-grade carcinomas, while high-grade adenocarcinomas with (usually focal) papillary growth are counted among the “adenocarcinomas, NOS”
Thymic carcinomas that were called “adenoid-cystic carcinomas (ACC)” in the 3rd edition are now labelled as ‘thymic carcinomas with adenoid cystic carcinoma-like features’ in the 4th edition since they lack the immunohistochemical features of true ACC in other organs.40 Enteric differentiation (as shown by immunohistochemistry) occurs in a subset of mucinous carcinomas and adenocarcinomas, NOS, and is currently of unknown clinical significance. Such cases require clinical staging to exclude metastasis from a colorectal primary.41
NUT carcinoma
These highly aggressive cancers that were first observed as midline thoracic tumors in children and young adults and harboured a unique t(15;19)-translocation with generation of a BRD4-NUT fusion oncogene were called ‘carcinoma with t(15;19) translocation’ in the 3rd edition. These tumors are now known to occur at all ages, outside the thorax (and rarely outside the midline) in ~40%, and to show variant NUT (nuclear protein in testis) translocations in ~30% of cases.42, 43 Therefore, these tumors are now labelled NUT carcinomas irrespective of anatomic location. Analysis for NUT overexpression by immunohistochemistry is now possible and highly sensitive, and should be considered in any undifferentiated cancer, particularly if there is focal squamous differentiation.
Undifferentiated carcinoma
This tumor shows epithelial differentiation but is otherwise a diagnosis of exclusion. Poorly differentiated squamous cell carcinoma, lymphoepithelioma-like carcinoma, sarcomatoid carcinoma, NUT carcinoma, small and large cell neuroendocrine carcinoma, and adenocarcinoma of the thymus, germ cell tumor, extension from the lung and metastasis must be excluded. By definition, immunohistochemical markers (e.g. CK5/6; p63, CD5) of any of the aforementioned tumors are absent, but CD117 and PAX8 may be expressed.
Clinical relevance of refined thymoma and thymic carcinoma diagnosis
The first step when assessing a mediastinal mass is to establish a differential diagnosis between thymic epithelial tumors, lymphomas, and other neoplasms, including metastasis. This relies on clinical presentation, including neurological examination, tumor marker profiles and imaging features.44 In thymic epithelial lesions next steps are the differentiation of thymic malignancy from hyperplasia or non-involuted thymus and the assessment of upfront resectability of the tumor, as complete resection is the most significant prognostic variable. In a postoperative setting, precise histopathological subtyping is of major importance for treatment decisions. In this setting, postoperative radiotherapy may be considered after complete resection of stage II thymomas with more aggressive histology, including B2 and B3 subtypes. Chemoradiotherapy is a major component of the therapeutic strategy of thymic carcinomas, as perioperative or exclusive treatment modality.45 In advanced stage refractory disease, KIT mutations have exclusively been reported to occur in thymic carcinomas, but their potential role as biomarkers needs further studies; meanwhile, targeting angiogenesis using multikinase inhibitors is of higher relevance in carcinomas irrespective of their KIT mutational status.46 By contrast, the histone deacetylase inhibitor, belinostat,47 the insulin-like growth factor 1 receptor-targeting antibody, cixutumumab,48 and somatostatin analogues49 are more effective in thymomas. The importance of diagnosing NUT carcinoma results from the ineffectiveness of conventional chemotherapeutic regimens and availability of promising new targeted agents.50
THYMIC NEUROENDOCRINE TUMORS
Conceptual continuity
These chapters cover neuroendocrine epithelial tumors (NETs), but not paraganglioma and neurogenic tumors. In the 4th edition, the nomenclature and criteria (see Table 4) of thymic typical and atypical carcinoids, large cell neuroendocrine carcinoma (LCNEC) and small cell carcinoma remain the same as in the 3rd edition.1 The definition of ‘neuroendocrine differentiation’ in the carcinoids and large cell neuroendocrine carcinomas, i.e. strong and diffuse expression of usually more than one of four neuroendocrine marker (chromogranin A, synaptophysin, CD56 and NSE) in >50% of tumor cells has been maintained. As before, small cell carcinoma remains a histological diagnosis; expression of neuroendocrine markers is often present but not required.
Table 4.
Thymic neuroendocrine tumors covered by the 4th edition of the WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart.3 Conceptual changes are in bold (these tumors were counted among the combined thymic epithelial tumors in the 3rd edition).
| Distinguishing histological criteria | |
|---|---|
| Carcinoid tumors | |
| Typical carcinoid | <2 mitoses/2mm2; no necrosis |
| Atypical carcinoid | <2 mitoses/2mm2; with necrosis; or 2–10 mitoses/2mm2; + or − necrosis |
| Large cell neuroendocrine carcinoma (LCNEC) | >10 mitoses/2mm2; no small cell features |
| Combined LCNEC | LCNEC combined with any thymoma(s) or thymic carcinoma(s) |
| Small cell carcinoma (SCC) | Typical SCC histology |
| Combined small cell carcinoma | SCC combined with any thymoma(s) or thymic carcinoma(s) |
Conceptual changes
The descriptive terms “well differentiated neuroendocrine carcinoma” (referring to carcinoids) and “poorly differentiated neuroendocrine carcinoma” (referrring to LCNEC and small cell carcinoma) of the 3rd edition were abandoned, since LCNECs and even SCC may be highly differentiated in terms of neuroendocrine features. Following the strategy in the lung, the 4th edition instead separates typical and atypical carcinoids as low and intermediate grade neuroendocrine tumors, respectively, from high grade neuroendocrine carcinomas that comprise LCNEC and small cell carcinoma.
Key new findings
A major advance has been the elucidation of genetic alterations at the CGH level by an international consortium.51 The progressive increase and overlap of genetic gains and losses from typical carcinoids through atypical carcinoids to LCNECs and small cell carcinomas lends support to the WHO classification. Genetic alterations in thymic and pulmonary carcinoids are significantly different from each other, while the genetics of high grade neuroendocrine tumors from thymus and lung are indistinguishable.51 It is the currently favored concept that different molecular pathways lead to thymic carcinoids and thymic high grade neuroendocrine carcinomas.51 The recent description of shared genetic alterations in thymic carcinoids and associated synchronous or metachronous high grade neuroendocrine carcinomas challenge this view52 and suggest that transformation of carcinoids might be an alternative pathway leading to high grade neuroendocrine carcinomas.52,53 There are currently no immunohistological markers that can unequivocally distinguish between thymic and pulmonary primaries, although a TTF1-/PAX8 (polyclonal)+ profile appears to be more common in thymic carcinoids.54 Clinical and radiological correlation remains the mainstay for the distinction between thymic and pulmonary neuroendocrine tumors.
Individual changes in subtypes of thymic NETs
Except for new genetic data, there are no further changes in individual thymic NETs.
GERM CELL TUMORS (GCTs) OF THE MEDIASTINUM, INCLUDING GCTs WITH SOMATIC TYPE MALIGNANCY
Conceptual continuity
The nomenclature and histological criteria defining the main mediastinal GCT categories are maintained in the 4th edition of the WHO Classification with one minor exception: GCT with somatic-type malignancy that is either a clonally related sarcoma, carcinoma or both is now called ‘GCT with somatic-type solid malignancy’ to stress the distinction from GCT with associated clonally related hematological malignancies. GCTs with the latter variant of somatic type malignancy are unique to the mediastinum and called ‘GCTs with associated hematological malignancy’ (Table 5)
Table 5.
Germ cell tumors covered by the 4th edition of the WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart,3 and useful immunohistological markers that were not mentioned in the 3rd edition. In bold is the new addendum ‘solid’ to the name of GCTs that are composed of a GCT component and associated sarcoma and/or carcinoma.
| OCT4 | SALL4 | Glypican 3 | SOX2 | SOX17 | |
|---|---|---|---|---|---|
| Seminoma | + | + | --- | --- | + |
| Embryonal carcinoma | + | + | --- | + | --- |
| Yolk sac tumor | --- | + | + | --- | --- |
| Choriocarcinoma | + | +a | +b | --- | --- |
| Mature teratoma | --- | −/+ | +/− | --- | --- |
| Immature teratoma | --- | −/+ | +/− | --- | --- |
| Mixed GCTs | |||||
| GCTs with associated somatic-type solid malignancy | |||||
| GCTs with associated hematological malignancy | |||||
mononuclear trophoblast;
syncytiotrophoblast
Conceptual changes
There have been no conceptual changes.
Individual changes of GCT subtypes
The classical immunohistological markers (keratins, PLAP, CD117; CD30; AFP; beta-subunit of HCG) are usually sufficient to recognize even complex GCTs. Several new markers, such as OCT4, glypican 3 and SOX2 (Table 5) may help to more precisely delineate the individual components.55–57
LYMPHOMAS OF THE MEDIASTINUM
Conceptual continuity, no conceptual changes
The ‘new’ classification of mediastinal lymphomas was streamlined according to the 4th edition of the WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues.10 There have been no conceptual changes (Table 1).
Individual changes of lymphoma subtypes
Primary mediastinal large B-cell lymphomas (PMBL)
The typical immunophenotype of PMBL as reported in the 3rd edition1(CD20+ CD79a+ PAX5+ CD30+/− CD15− CD10− IRF4/MUM1+/− HLADR−) has been complemented, among others, by CD23 (positive in 70%) and p63 (positive in >90%), while p40 is absent.58 As a caveat, p63 is also expressed in normal and neoplastic thymic epithelial cells,59 although it is typically accompanied by p40 expression.60 In spite of a characteristic immunohistochemical profile, and a gene expression profile that extensively overlaps with that of Hodgkin lymphoma but not other B cell lymphomas,61 the diagnosis of PMBL in daily practice still requires clinical data (particularly absence of extensive extrathoracic lymph node infiltration) to exclude systemic diffuse large B-cell lymphoma with mediastinal involvement.
Among the novel genetic alterations reported since 2004, the following are of special interest from a biological and potentially immunotherapeutic perspective: translocations of the HLA class II transactivator CIITA at 16p13.13 associated with down-regulation of HLA class II molecules; and the almost specific amplification and/or translocation of the 9p24.1 region including the loci coding for the immune checkpoint molecules PD-L1 and PD-L2,62, 63 which are overexpressed and helpful immunohistochemical markers in PMBL.64
MALT lymphoma
New genetic findings include a high frequency of trisomy 3, low incidence of trisomy 18, and no translocations involving MALT1 and IGH.65, 66
T lymphoblastic leukemia/lymphoma
The new edition highlights a subset of TdT-negative cases.67 The latter might be related to early thymic progenitor T-ALLs that co-express early myeloid markers, and stem cell markers (CD34 and CD117). These are considered high risk subgroups.68, 69
Among many novel genetic alterations detected, mutations of NOTCH1/FXBW are associated with a favourable outcome,70 while loss of heterozygocity (LOH) at 6q has a negative impact on prognosis.71
Anaplastic large cell lymphoma (ALCL)
In contrast to the 3rd edition, the 4th edition (like the WHO book on hematological malignancies10) emphasizes the difference between ALK(+) and ALK(−) ALCL. Both arise only exceptionally in the mediastinum. By immunohistochemistry, ALCL must be distinguished from other CD30-positive lymphomas and EMA-positive solid tumors, including carcinomas, and from melanomas. However, the diagnosis of ALK(−) ALCL versus CD30-positive peripheral T cell lymphoma, NOS, still rests on the identification of ‘hallmark cells’ in a background of large cell lymphoma, but the distinction may sometimes be arbitrary.
Hodgkin lymphoma (HL)
The diagnostic criteria of HL (virtually all mediastinal cases are nodular sclerosis classical HL) have remained unchanged. A number of inactivating mutations, amplifications, and translocations have been described that affect NF-kB, JAK-STAT, DNA damage and apoptosis pathways as well as epigenetic homeostasis [reviewed in72]. Recognition that genetic and transcriptomic variation of the tumor cells, and tumor-associated T cells and macrophages have prognostic relevance is also new.73–75 Nonetheless, stage remains the single most important prognostic factor in mediastinal HL.
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma
In the 3rd edition, this aggressive lymphoma was included in the “Grey zone between HL and NHL”. A current synonym is “mediastinal grey zone lymphoma, MGZL”. Refined diagnostic criteria include a sheet-like growth pattern of large, pleomorphic tumor cells including Hodgkin cells; a less prominent inflammatory background than cHL; usually preserved B-cell program (expression of CD20, PAX5, OCT2, BOB.1; clonal immunoglobulin gene rearrangement); expression of CD30 (consistently) and CD15 (variably); and lack of CD10 and ALK1 expression. There are distinctive genetic and epigenetic features of MGZL in addition to features that overlap with cHL and PMBL (Fig. 6).76–78
Fig. 6.
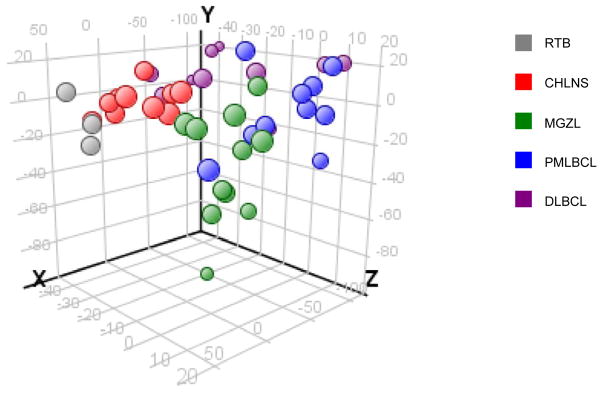
Distinct epigenetic profile of mediastinal grey zone lymphoma (MGZL) as assessed by principal component analysis. The methylation data for 1421 CpG targets from various lymphomas and reactive tonsillar B cells (RTB) were subjected to principal component analysis and projected onto the first three principal components. MGZL has a distinct epigenetic profile intermediate between classical Hodgkin lymphoma, nodular sclerosis subtype (CHLNS) and primary mediastinal large B-cell lymphoma (PMLBCL), but different from that of diffuse large B-cell lymphoma (DLBCL) and RTB. Adapted from Haematologica,74 with permission.
Clinical relevance of refined mediastinal lymphoma diagnosis
Accurate diagnosis is essential for the differential treatment of mediastinal classical Hodgkin lymphoma (cHL), primary mediastinal large B-cell lymphoma (PMBL) and mediastinal grey zone lymphoma (MGZL). Patients with cHL currently receive polychemotherapy that seems to require inclusion of bleomycin and dacarbazin (e.g. ABVD) followed by involved field irradiation for optimal outcome.79 PMBL, inspite of its close molecular relationship to cHL, has been treated very differently, e.g. with CHOP and rituximab (R-CHOP) followed by radiotherapy. However, a recent study shows that radiation may be dispensible in PMBL when R-CHOP is replaced by the DA-EPOCH-R regimen.80 This might be advantageous in the commonly young female patients with PMBL since mediastinal radiotherapy has potentially serious late effects, such as cardiovascular disease, lung cancer, and breast cancer.81 For patients with MGZL, DA-EPOCH-R treatment alone may not be sufficient since they more often required additional radiation to achieve long term complete remission than patients with PMBL.80
HISTIOCYTIC AND DENDRITIC CELL NEOPLASMS OF THE MEDISATINUM
Conceptual continuity
The classification of mediastinal histiocytic and dendritic cell tumors was streamlined according to the 4th edition of the WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues.10
Conceptual changes
The only changes are i) The deletion of the terms “follicular dendritic cell (FDC) tumour” and “interdigitating dendritic cell (IDC) tumour” (i.e. tumors of unknown malignant potential: ICD-O codes: 9758/1 and 9757/1, respectively), since all FDC and IDC neoplasias are now considered malignant; ii) the addition of the previously neglected ‘fibroblastic reticular cell tumors’, ‘indeterminate dendritic cell tumors’ and rare dendritic cell tumors with mixed phenotypes (‘hybrid dendritic cell tumors’).
Individual changes of histiocytic and dendritic cell tumors
A new finding is the detection of BRAF mutations in about 50% of all cases of Langerhans cell histiocytosis, including mediastinal cases.82
In terms of differential diagnosis, the new WHO classification of tumors of the thymus clarifies some important diagnostic challenges that need careful immunohistochemical analysis:
S100-positive Langerhans cell lesions, indeterminate dendritic cell tumors and interdigitating dendritic cell sarcomas must be distinguished from the much more common metastatic melanoma, nerve sheath tumors and myoepithelial lesions. CD1a+ Langerin+ Langerhans cell neoplasms need to be distinguished from CD1a+ Langerin- indeterminate dendritic cell tumors.
The EMA-positive and/or D2-40-positive subset of follicular dendritic cell sarcomas must not be confused with cytokeratin-deficient thymomas,21 meningioma and mesothelioma.
The cytokeratin-positive, actin-positive or desmin-positive subsets of fibroblastic reticular cell tumors need to be distinguished from spindle cell epithelial tumors (e.g. atypical type A thymomas; sarcomatoid carcinomas), smooth muscle and myofibroblastic tumors, as well as angiomatoid fibrous histiocytoma.
MYELOID SARCOMA AND EXTRAMEDULLARY LEUKEMIA
There have been no conceptual or individual changes.
SOFT TISUE TUMORS OF THE MEDIASTINUM
Conceptual continuity, no conceptual changes
The classification of mediastinal soft tissue tumors was streamlined according to the 4th edition of the WHO Classification of Tumours of Soft Tissue and Bone.11 There have been no conceptual changes since the 3rd edition of the WHO book (Table 1).
Individual changes in subtypes of soft tissue tumors
Diagnostic criteria of all entities are maintained. New, diagnostically useful genetic alterations as compared to the 3rd edition are STAT6 translocation in solitary fibrous tumors83 and MYC gene amplification in radiation-induced (angio-)sarcomas.84, 85
In contrast to the WHO Classification of Tumours of Soft Tissue and Bone,11 the 3rd and 4th editions of the WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart cover neurogenic tumors that are among the most common tumors in the posterior mediastinum. The exceedingly rare neuroblastomas and ganglioneuroblastomas of the thymic region are unusual, since they occur in elderly patients.86, 87 It is unknown, whether they are genetically distinct from their childhood counterparts.
ECTOPIC TUMORS OF THE THYMUS AND METASTASIS TO THE THYMUS
There have been no conceptual or individual changes
PERSPECTIVES AND CHALLENGES
The molecular revolution has just begun to approach thymic epithelial tumors (TETs),23, 25 but the understanding of their biology is far away from the level reached in many hematological and solid tumors. However, The Cancer Genome Atlas (TCGA) has recently included TETs for analysis of genetic, transcriptomic, and epigenetic changes in relation to clinicopathologic and follow-up data. Exploiting the databases compiled by the International Thymic Malignancy Interest Group (ITMIG)6–9 might further promote our understanding of TETs and open perspectives for targeted therapies. In addition to this bench-to-bedside perspective, new clues to the molecular and cellular pathogenesis of thymic tumors can also be expected through clinical trials23 that segregate patients into responders and non-responders and, thus open new avenues for bedside-to-bench studies of unknown underlying molecular mechanisms.30
An intriguing new molecular finding that may give a first hint to the etiology of TETs is the recent description of human polyomavirus 7 in the epithelial cells of the majority of TETs, of some hyperplastic thymuses of adults but not of fetal thymic tissue.88 If confirmed, this might have an impact on the classification of TETs and their clinical management.
Finally, a new staging system for thymomas and thymic carcinomas is on the horizon15 but awaits approval by UICC and AJCC.
Taken together, the 4th edition of the WHO Classification of thymic tumors has added important new data to the previous edition, but can be expected to require amendments or additions due to significant new discoveries before long.
What are challenges that need to be tackled in the years ahead to translate a foreseeable better understanding of thymoma and thymic carcinoma biology into improved management of patients? A so far unresolved key problem that has strongly inhibited functional in vitro and preclinical in vivo studies is the paucity of permanent bona fide thymoma and thymic carcinoma cell lines27, 89, 90 and the virtual absence of relevant spontaneous and xenograft-based animal models.91 Except for the rarity of thymomas and thymic carcinomas, the lack of cell lines and functional xenografts is likely due to the essential need of neoplastic thymic epithelial cells for crosstalk with stromal cells and particularly developing T cells in the case of thymomas to prevent senescence in vitro.27 Therefore, systems biology approaches will likely be necessary to decipher the complex ligand-based and paracrine networks that help to sustain the survival of neoplastic epithelial cells in vivo and might be essential in vitro as well. This knowledge might be a prerequisite to establish relevant models for preclinical functional studies and, ultimately, high-throughput state-of-the-art drug testing.
References
- 1.Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press; 2004. [Google Scholar]
- 2.Rosai J, Sobin L. Histological Typing of Tumours of the Thymus. 1999 [Google Scholar]
- 3.Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumours of the lung, pleura, thymus and heart. IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 4.Strobel P, Hartmann E, Rosenwald A, et al. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology. 2014;64:557–566. doi: 10.1111/his.12279. [DOI] [PubMed] [Google Scholar]
- 5.Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 6.Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:367–372. doi: 10.1097/JTO.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. The Journal of thoracic and cardiovascular surgery. 2015;149:95–100. 101.e101–102. doi: 10.1016/j.jtcvs.2014.09.124. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:1573–1578. doi: 10.1097/JTO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 9.Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. The Journal of thoracic and cardiovascular surgery. 2015;149:103–109. e102. doi: 10.1016/j.jtcvs.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; 2008. [Google Scholar]
- 11.Fletcher CDM, Bridge JA, Hogendorn PCW, et al. WHO Classification of tumours of soft tissue and bone. IARC Press; 2014. [Google Scholar]
- 12.Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:S1710–1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 13.Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:S81–87. doi: 10.1097/JTO.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:S73–80. doi: 10.1097/JTO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 15.Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:S65–72. doi: 10.1097/JTO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 16.Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka Stage and Size Are Independent Prognostic Predictors in Thymoma and Modified Masaoka Stage is Superior to Histopathologic Classifications. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015 doi: 10.1097/JTO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 17.Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematology/oncology clinics of North America. 2008;22:381–392. doi: 10.1016/j.hoc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Detterbeck FC. Clinical value of the WHO classification system of thymoma. The Annals of thoracic surgery. 2006;81:2328–2334. doi: 10.1016/j.athoracsur.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 19.Moran CA, Weissferdt A, Kalhor N, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. American journal of clinical pathology. 2012;137:444–450. doi: 10.1309/AJCP76KEGWQKWOKA. [DOI] [PubMed] [Google Scholar]
- 20.Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:1501–1509. doi: 10.1200/JCO.2004.10.113. [DOI] [PubMed] [Google Scholar]
- 21.Adam P, Hakroush S, Hofmann I, et al. Thymoma with loss of keratin expression (and giant cells): a potential diagnostic pitfall. Virchows Archiv: an international journal of pathology. 2014 doi: 10.1007/s00428-014-1606-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Scientific reports. 2014;4:7336. doi: 10.1038/srep07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nature genetics. 2014;46:844–849. doi: 10.1038/ng.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:6790–6799. doi: 10.1158/1078-0432.CCR-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrini I, Wang Y, Zucali PA, et al. Copy number aberrations of genes regulating normal thymus development in thymic epithelial tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:1960–1971. doi: 10.1158/1078-0432.CCR-12-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Yin B, Wei Q, et al. Aberrant DNA methylation in thymic epithelial tumors. Cancer investigation. 2009;27:582–591. doi: 10.1080/07357900802620869. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Belharazem D, Li L, et al. Anti-Apoptotic Signature in Thymic Squamous Cell Carcinomas - Functional Relevance of Anti-Apoptotic BIRC3 Expression in the Thymic Carcinoma Cell Line 1889c. Frontiers in oncology. 2013;3:316. doi: 10.3389/fonc.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan A, Girard N, Marx A. State of the art of genetic alterations in thymic epithelial tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014;9:S131–136. doi: 10.1097/JTO.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Chavez A, Thomas A, Rajan A, et al. Molecular Profiling and Targeted Therapy for Advanced Thoracic Malignancies: A Biomarker-Derived, Multiarm, Multihistology Phase II Basket Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.58.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marx A, Weis CA. Sunitinib in thymic carcinoma: enigmas still unresolved. The Lancet Oncology. 2015;16:124–125. doi: 10.1016/S1470-2045(15)70010-0. [DOI] [PubMed] [Google Scholar]
- 31.Green AC, Marx A, Strobel P, et al. Type A and AB thymomas: histological features associated with increased stage. Histopathology. 2014 doi: 10.1111/his.12512. [DOI] [PubMed] [Google Scholar]
- 32.Nonaka D, Henley JD, Chiriboga L, et al. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. The American journal of surgical pathology. 2007;31:1038–1044. doi: 10.1097/PAS.0b013e31802b4917. [DOI] [PubMed] [Google Scholar]
- 33.Vladislav IT, Gokmen-Polar Y, Kesler KA, et al. The role of histology in predicting recurrence of type A thymomas: a clinicopathologic correlation of 23 cases. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1059–1064. doi: 10.1038/modpathol.2013.49. [DOI] [PubMed] [Google Scholar]
- 34.Brambilla C, Laffaire J, Lantuejoul S, et al. Lung squamous cell carcinomas with basaloid histology represent a specific molecular entity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:5777–5786. doi: 10.1158/1078-0432.CCR-14-0459. [DOI] [PubMed] [Google Scholar]
- 35.Brown JG, Familiari U, Papotti M, et al. Thymic basaloid carcinoma: a clinicopathologic study of 12 cases, with a general discussion of basaloid carcinoma and its relationship with adenoid cystic carcinoma. The American journal of surgical pathology. 2009;33:1113–1124. doi: 10.1097/PAS.0b013e3181a2443b. [DOI] [PubMed] [Google Scholar]
- 36.Zhou R, Zettl A, Strobel P, et al. Thymic epithelial tumors can develop along two different pathogenetic pathways. The American journal of pathology. 2001;159:1853–1860. doi: 10.1016/s0002-9440(10)63031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nature genetics. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 38.de Achcar RO, Nikiforova MN, Dacic S, et al. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Human pathology. 2009;40:854–860. doi: 10.1016/j.humpath.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Roden AC, Erickson-Johnson MR, Yi ES, et al. Analysis of MAML2 rearrangement in mucoepidermoid carcinoma of the thymus. Human pathology. 2013;44:2799–2805. doi: 10.1016/j.humpath.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Di Tommaso L, Kuhn E, Kurrer M, et al. Thymic tumor with adenoid cystic carcinomalike features: a clinicopathologic study of 4 cases. The American journal of surgical pathology. 2007;31:1161–1167. doi: 10.1097/PAS.0b013e3180555ba8. [DOI] [PubMed] [Google Scholar]
- 41.Moser B, Schiefer AI, Janik S, et al. Adenocarcinoma of the Thymus, Enteric Type: Report of 2 Cases, and Proposal for a Novel Subtype of Thymic Carcinoma. The American journal of surgical pathology. 2014 doi: 10.1097/PAS.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 42.French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer discovery. 2014;4:928–941. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.den Bakker MA, Roden AC, Marx A, et al. Histologic Classification of Thymoma: A Practical Guide for Routine Cases. Journal of thorax oncology. 2014;9:S125–S130. doi: 10.1097/JTO.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 45.Girard N. Thymic epithelial tumours: from basic principles to individualised treatment strategies. European respiratory review: an official journal of the European Respiratory Society. 2013;22:75–87. doi: 10.1183/09059180.00007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. The Lancet Oncology. 2015;16:177–186. doi: 10.1016/S1470-2045(14)71181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas A, Rajan A, Szabo E, et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: a clinical and translational study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20:5392–5402. doi: 10.1158/1078-0432.CCR-14-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajan A, Carter CA, Berman A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. The Lancet Oncology. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loehrer PJ, Sr, Wang W, Johnson DH, et al. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:293–299. doi: 10.1200/JCO.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 50.French C. NUT midline carcinoma. Nature reviews Cancer. 2014;14:149–150. doi: 10.1038/nrc3659. [DOI] [PubMed] [Google Scholar]
- 51.Strobel P, Zettl A, Shilo K, et al. Tumor genetics and survival of thymic neuroendocrine neoplasms: A multi-institutional clinicopathologic study. Genes, chromosomes & cancer. 2014;53:738–749. doi: 10.1002/gcc.22183. [DOI] [PubMed] [Google Scholar]
- 52.Pelosi G, Bimbatti M, Fabbri A, et al. Thymic neuroendocrine neoplasms (T-NENs) with CTNNB1 (beta-catenin) gene mutation show components of well-differentiated tumor and poorly-differentiated carcinoma, challenging the concept of secondary high-grade neuroendocrine carcinoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(Suppl 2):487A. (#1956) [Google Scholar]
- 53.Moran S, Suster S. Thymic neuroendocrine carcinomas with combined features ranging from well-differentiated (carcinoid) to small cell carcinoma. A clinicopathologic and immunohistochemical study of 11 cases. American journal of clinical pathology. 2000;113:345–350. doi: 10.1309/Q01U-60BL-VEV4-TWR1. [DOI] [PubMed] [Google Scholar]
- 54.Weissferdt A, Tang X, Wistuba II, et al. Comparative immunohistochemical analysis of pulmonary and thymic neuroendocrine carcinomas using PAX8 and TTF-1. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1554–1560. doi: 10.1038/modpathol.2013.111. [DOI] [PubMed] [Google Scholar]
- 55.de Jong J, Stoop H, Gillis AJ, et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. The Journal of pathology. 2008;215:21–30. doi: 10.1002/path.2332. [DOI] [PubMed] [Google Scholar]
- 56.Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer research. 2003;63:2244–2250. [PubMed] [Google Scholar]
- 57.Zynger DL, Everton MJ, Dimov ND, et al. Expression of glypican 3 in ovarian and extragonadal germ cell tumors. American journal of clinical pathology. 2008;130:224–230. doi: 10.1309/8DN7DQRDFB4QNH3N. [DOI] [PubMed] [Google Scholar]
- 58.Zamo A, Malpeli G, Scarpa A, et al. Expression of TP73L is a helpful diagnostic marker of primary mediastinal large B-cell lymphomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18:1448–1453. doi: 10.1038/modpathol.3800440. [DOI] [PubMed] [Google Scholar]
- 59.Dotto J, Pelosi G, Rosai J. Expression of p63 in thymomas and normal thymus. American journal of clinical pathology. 2007;127:415–420. doi: 10.1309/2GAYKPDDM85P2VEW. [DOI] [PubMed] [Google Scholar]
- 60.Chilosi M, Zamo A, Brighenti A, et al. Constitutive expression of DeltaN-p63alpha isoform in human thymus and thymic epithelial tumours. Virchows Archiv: an international journal of pathology. 2003;443:175–183. doi: 10.1007/s00428-003-0857-4. [DOI] [PubMed] [Google Scholar]
- 61.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. The Journal of experimental medicine. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 63.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi M, Roemer MG, Chapuy B, et al. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. The American journal of surgical pathology. 2014;38:1715–1723. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kominato S, Nakayama T, Sato F, et al. Characterization of chromosomal aberrations in thymic MALT lymphoma. Pathology international. 2012;62:93–98. doi: 10.1111/j.1440-1827.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 66.Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leukemia & lymphoma. 2011;52:2276–2283. doi: 10.3109/10428194.2011.596968. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Fan X, Routbort M, et al. Absence of terminal deoxynucleotidyl transferase expression identifies a subset of high-risk adult T-lymphoblastic leukemia/lymphoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1338–1345. doi: 10.1038/modpathol.2013.78. [DOI] [PubMed] [Google Scholar]
- 68.Bonn BR, Rohde M, Zimmermann M, et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121:3153–3160. doi: 10.1182/blood-2012-12-474148. [DOI] [PubMed] [Google Scholar]
- 69.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. The Lancet Oncology. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:1966–1973. doi: 10.1200/JCO.2011.39.7661. [DOI] [PubMed] [Google Scholar]
- 71.Burkhardt B, Bruch J, Zimmermann M, et al. Loss of heterozygosity on chromosome 6q14–q24 is associated with poor outcome in children and adolescents with T-cell lymphoblastic lymphoma. Leukemia. 2006;20:1422–1429. doi: 10.1038/sj.leu.2404275. [DOI] [PubMed] [Google Scholar]
- 72.Kuppers R. New insights in the biology of Hodgkin lymphoma. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2012;2012:328–334. doi: 10.1182/asheducation-2012.1.328. [DOI] [PubMed] [Google Scholar]
- 73.Greaves P, Clear A, Owen A, et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122:2856–2863. doi: 10.1182/blood-2013-06-508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steidl C, Diepstra A, Lee T, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120:3530–3540. doi: 10.1182/blood-2012-06-439570. [DOI] [PubMed] [Google Scholar]
- 75.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. The New England journal of medicine. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eberle FC, Rodriguez-Canales J, Wei L, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011;96:558–566. doi: 10.3324/haematol.2010.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1586–1597. doi: 10.1038/modpathol.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanhentenrijk V, Vanden Bempt I, Dierickx D, et al. Relationship between classic Hodgkin lymphoma and overlapping large cell lymphoma investigated by comparative expressed sequence hybridization expression profiling. The Journal of pathology. 2006;210:155–162. doi: 10.1002/path.2043. [DOI] [PubMed] [Google Scholar]
- 79.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 80.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. The New England journal of medicine. 2013;368:1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeMieux MH, Solanki AA, Mahmood U, et al. Risk of second malignancies in patients with early-stage classical Hodgkin’s lymphoma treated in a modern era. Cancer medicine. 2015 doi: 10.1002/cam4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
- 83.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nature genetics. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Käcker C, Marx A, Mössinger K, et al. High frequency of MYC gene amplification is a common feature of radiation-induced sarcomas. Further results from EORTC STBSG TL 01/01 Genes, chromosomes & cancer. 2013;52:93–98. doi: 10.1002/gcc.22009. [DOI] [PubMed] [Google Scholar]
- 85.Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. The American journal of pathology. 2010;176:34–39. doi: 10.2353/ajpath.2010.090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ueda Y, Omasa M, Taki T, et al. Thymic Neuroblastoma within a Thymic Cyst in an Adult. Case reports in oncology. 2012;5:459–463. doi: 10.1159/000342357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellegrino M, Gianotti L, Cassibba S, et al. Neuroblastoma in the Elderly and SIADH: Case Report and Review of the Literature. Case reports in medicine. 2012;2012:952645. doi: 10.1155/2012/952645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rennspiess D, Pujari S, Keijzers M, et al. Detection of human polyomavirus 7 in human thymic epithelial tumors. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:360–366. doi: 10.1097/JTO.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ehemann V, Kern MA, Breinig M, et al. Establishment, characterization and drug sensitivity testing in primary cultures of human thymoma and thymic carcinoma. International journal of cancer Journal international du cancer. 2008;122:2719–2725. doi: 10.1002/ijc.23335. [DOI] [PubMed] [Google Scholar]
- 90.Gokmen-Polar Y, Sanders KL, Goswami CP, et al. Establishment and characterization of a novel cell line derived from human thymoma AB tumor. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1564–1573. doi: 10.1038/labinvest.2012.115. [DOI] [PubMed] [Google Scholar]
- 91.Marx A, Porubsky S, Belharazem D, et al. Thymoma related myasthenia gravis in humans and potential animal models. Experimental neurology. 2015 doi: 10.1016/j.expneurol.2015.02.010. [DOI] [PubMed] [Google Scholar]